Abstract
Surgical resection is the only potentially curative treatment for patients with cholangiocarcinoma. For both perihilar cholangiocarcinoma (pCCA) and intrahepatic cholangiocarcinoma (iCCA), 5‐year overall survival of about 30% has been reported in large series. This review addresses several challenges in surgical management of cholangiocarcinoma. The first challenge is diagnosis: a biopsy is typically avoided because of the risk of seeding metastases and the low yield of a brush of the bile duct. However, about 15% of patients with suspected pCCA are found to have a benign diagnosis after resection. The second challenge is staging; even with the best preoperative imaging, a substantial percentage of patients has occult metastatic disease detected at staging laparoscopy or early recurrence after resection. The third challenge is an adequate volume and function of the future liver remnant, which may require preoperative biliary drainage and portal vein embolization. The fourth challenge is a complete resection: a positive bile duct margin is not uncommon because the microscopic biliary extent of disease may be more extensive than perceived on imaging. The fifth challenge is the high post‐operative mortality that has decreased in very high volume Asian centres, but remains about 10% in many Western referral centres. The sixth challenge is that even after a complete resection most patients develop recurrent disease. Recent randomized controlled trials found conflicting results regarding the benefit of adjuvant chemotherapy. The final challenge is to determine which patients with cholangiocarcinoma should undergo liver transplantation rather than resection.
Keywords: cholangiocarcinoma, hepatectomy, klatskin tumor, liver transplantation, surgery
Abbreviations
- AJCC
American Joint Committee on Cancer
- ALPPS
associating liver partition and portal vein ligation for staged hepatectomy
- CRT
chemoradiotherapy
- dCCA
distal cholangiocarcinoma
- DFS
disease‐free survival
- EBD
endoscopic biliary drainage
- ENBD
endoscopic nasobiliary drainage
- ENSCCA
European Network for the Study of Cholangiocarcinoma
- ERCP
endoscopic retrograde cholangio‐pancreatography
- FISH
fluorescent in situ hybridization
- FLR
future liver remnant
- HAIP
hepatic arterial infusion pump
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- iCCA
intrahepatic cholangiocarcinoma
- IRE
irreversible electroporation
- LT
liver transplantation
- OS
overall survival
- PBD
preoperative biliary drainage
- pCCA
perihilar cholangiocarcinoma
- PDT
photodynamic therapy
- PET
positron emission tomography
- PHLF
post‐hepatectomy liver failure
- PSC
primary sclerosing cholangitis
- PTBD
percutaneous transhepatic biliary drainage
- PVE
portal vein embolization
- RCT
randomized controlled trial
- TACE
transarterial chemo‐embolization
Key points.
Pathological confirmation before surgery is not mandatory in patients with cholangiocarcinoma.
All imaging for cholangiocarcinoma must be performed before biliary drainage. After drainage it is harder to determine resectability.
Preoperative biliary drainage may do more harm than good in patients with an FLR above 50%. A hepatobiliary surgeon experienced with cholangiocarcinoma should be consulted prior to biliary drainage.
Portal vein embolization (PVE) is recommended in patients with an FLR of less than 40%.
Multiple tumors and positive lymph nodes are poor prognostic factors for iCCA, but do not preclude 5‐year survival after resection.
1. INTRODUCTION
Surgical resection is the only potentially curative treatment for patients with cholangiocarcinoma. Cholangiocarcinoma can arise anywhere along the biliary tree. Intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA) cholangiocarcinoma differ in tumour characteristics and each has a separate American Joint Committee on Cancer (AJCC) staging system.1, 2 Consequently, they require a different treatment approach. dCCA is beyond the scope of this review because diagnostic work‐up and treatment are more similar to cancer of the head of the pancreas.
Most patients with cholangiocarcinoma are ineligible for surgical resection because of metastatic or locally advanced disease at the time of presentation. Only about one in five patients with pCCA is eligible for surgery at the time of presentation.3 Therefore, even high‐volume Western centres rarely perform more than two resection per month for pCCA.4, 5 The main goal of surgery for cholangiocarcinoma is a complete (R0) resection with low post‐operative mortality. This goal requires a multidisciplinary team with dedicated radiologists, interventional radiologists, endoscopists, anaesthesiologists, intensivists, hepatologists, pathologists and hepatobiliary surgeons with both surgical oncology and vascular or transplant skills. Outcomes are best in high‐volume centres.6
The aetiology, pathogenesis, risk factors and epidemiology of cholangiocarcinoma are reviewed in separate contributions in this issue. The aim of this review is to summarize the diagnostic work‐up and treatment of patients with resectable pCCA or iCCA.
2. PERIHILAR CHOLANGIOCARCINOMA
2.1. Diagnostic work‐up
In this review, we focus on the diagnostic work‐up pertaining to surgical treatment options (ie, resection and liver transplantation). The diagnosis pCCA continues to pose a challenge. The early symptoms are non‐specific; abdominal discomfort or pain, anorexia and weight loss. Most patients present with a high serum bilirubin. Sometimes patients complain of pruritus, which may precede jaundice by a few weeks. In some patients, pCCA is incidentally discovered because of abnormal liver function tests.7
The main goal of the diagnostic work‐up is not only to confirm the presence of cancer, but also to assess the extent of bile duct involvement, vascular involvement (portal vein and the branches, hepatic proper artery and the branches) and distant metastases.8 It is crucial to perform all imaging before biliary drainage, because ascertainment of the biliary and vascular extent of pCCA is more challenging after endoscopic stent placement. Moreover, the extent of the tumour determines the future liver remnant (FLR), which determines the segments to be drained (see also section on biliary drainage).
Initial US examination shows intrahepatic biliary tree dilatation, typically without dilatation of the gallbladder and the common bile duct. CT of the chest and abdomen is the standard modality for staging and assessment of vascular involvement. MRI with MRCP and diffusion‐weighted imaging can be of additional value to assess the biliary extent of the tumour. Positron emission tomography (PET) CT should not be routinely used in the diagnostic work‐up because of low sensitivity and specificity. However, in selected patients (eg, with increased surgical risk or more advanced disease), PET CT may be justified with a 10% yield of occult metastatic disease.9, 10 Studies comparing imaging modalities are sparse.11
The diagnosis of pCCA requires the presence of a malignant appearing stricture in the liver hilum and at least one of the following:
Biopsy or cytology positive for cancer cells
Polysomy by fluorescent in situ hybridization (FISH)
Mass forming lession on CT or MRI at the stricture
CA 19.9 elevated above 100 U/ml
Preoperative biopsies and intraluminal brushings have a high false‐negative rate. A negative brush or biopsy should not exclude the diagnosis of pCCA or delay proper treatment.12 Endoscopic ultrasound‐guided fine needle aspiration or biopsy may be also useful for obtaining a diagnosis. For patients eligible for surgery, a biopsy is not mandatory. A biopsy should be restricted to selected cases, because of the risk of tumour seeding and tract recurrences. The indication for biopsy should always be discussed at a hepatobiliary multidisciplinary team meeting that should include a transplant surgeon. A preoperative biopsy is contraindicated if a patient is eligible for liver transplantation.
MRCP has replaced endoscopic retrograde cholangio‐pancreatography (ERCP) to determine the biliary extent of the tumour. ERCP with endoluminal biopsy may play a role in selected patients when pathological confirmation is justifed. The differential diagnosis of pCCA includes: choledocholithiasis, benign focal stenosis of the hepatic ducts, Mirizzi syndrome, gallbladder cancer, primary sclerosing cholangitis (PSC), autoimmune cholangitis and metastatic disease to the bile duct or hepatoduodenal lymph nodes (eg, colorectal cancer). In about 15% of patients who underwent a resection for apparent pCCA, final pathological examination found benign disease.13
2.2. Indication to resection
Given that surgery provides the only chance for cure of pCCA, hepatobiliary resection is indicated when it is both anatomically and physiologically feasible. The goal for surgery is to achieve an R0 resection while preserving adequate vascular inflow to and function of the FLR. This can be very difficult, given the localization of these tumours in the hepatic hilum in intimate relation with the portal vein and hepatic artery.
pCCA is typically stratified according to the Bismuth‐Corlette based on the biliary extent of the tumour14 and the AJCC classification systems1 that considers local (biliary and vascular) and distant tumour extent. The Blumgart system also considers hepatic lobar atrophy.15 Regardless of the staging system, it is difficult to accurately evaluate resectability preoperatively, and the definitive decision to resect or not is made at surgical exploration. For example, the biliary extent of the tumour towards the segmental bile ducts is often more extensive at final pathology, than judged on preoperative imaging.
In the majority of patients, radical surgical resection of pCCA requires an (extended) hemi‐hepatectomy, and not only the patient's functional status (ie, co‐morbid conditions, nutrition, performance status) but also the volume and function FLR needs to be considered. Traditionally, a “safe” liver resection has been considered one leaving an FLR of at least 25% of the preoperative liver volume in patients with normal liver parenchyma or at least 30% to 40% in livers that are compromised by steatosis, chronic cholestasis, cirrhosis or chemotherapy.16, 17 FLR function can be assessed more formally by examining hepatocellular uptake and excretion (indocyanine green clearance), uptake and biotransformation (13C‐methacetin breath test, LiMAx), and uptake (hepatobiliary scintigraphy). These function tests can be combined with SPECT‐CT to differentiate functional from non‐functional liver tissue.18 When the FLR is insufficient, strategies to increase the FLR should be considered (see sections below on portal vein embolization).
2.3. Preoperative biliary drainage
Obstructive cholangitis is an absolute indication for preoperative biliary drainage (PBD). In the absence of cholangitis, PBD of the FLR is debated, because it may cause cholangitis that is associated with post‐operative liver failure and mortality. The indication for PBD must therefore be cautiously evaluated by a hepatobiliary multidisciplinary team.
Obstructive jaundice is associated with a pro‐inflammatory state.19 Some series reported an association between serum bilirubin and post‐operative complications,20, 21 and the authors therefore advocated routine PBD. However, a randomized controlled trial (RCT) for patients with periampullary tumours found an increase in perioperative complications after PBD.22 Consequently, patients with resectable dCCA and a bilirubin level below 15 mL/dL should undergo a pancreatoduodenectomy without preoperative biliary drainage. Because liver resection in jaundiced patients was judged at higher surgical risk, most surgeons continued PBD in jaundiced patients with pCCA.6 Several retrospective studies reported that PBD is associated with infectious complications, and cholangitis is an independent prognostic factor for post‐operative mortality.23, 24 Another drawback of PBD is the risk of clinical deterioration or progressive disease because of complications secondary to PBD in about 15% of pCCA patients.25
In pCCA patients with an insufficient FLR (ie, below 40%), PBD of the FLR is required prior to portal vein embolization (PVE), because biliary obstruction impairs liver regeneration. For patients with a FLR volume above 50% (eg, requiring a left hemihepatectomy), the risk of cholangitis and related mortality after drainage does not justify the potential benefit of biliary decompression.4, 24
Two procedures to drain the bile ducts are available: percutaneous transhepatic biliary drainage (PTBD) and endoscopic biliary drainage (EBD). Two recent meta‐analyses, found a higher procedure conversion, cholangitis and pancreatitis rate in the EBD group without showing differences in post‐operative complications and survival.25, 26 A recent RCT comparing PTBD to EBD was stopped for excess mortality in the PTBD group (41% vs 11%), whereas severe preoperative drainage‐related complications were comparable in the two populations. However, the sample size of this study was small (27 patients per arm).27
To overcome the morbidity of EBD, endoscopic nasobiliary drainage (ENBD) has been proposed in Japan. They report a lower rate of conversion to PTBD.28 Others failed to show differences when comparing ENBD with EBD and PTBD.29 Among the drawbacks of ENBD are the patient discomfort and the possibility of accidental dislodgment. Because cholangitis is the main source of morbidity of PBD, the placement of a PTBD above the ampulla has been advocated to reduce bacterial contamination.30 Dislocation of the tip of the PTBD proximal to the tumour is a challenging complication of this strategy.
Based on the current evidence, a reasonable approach would be to perform drainage of the FLR only in patients presenting with cholangitis as well as in patients with both a bilirubin level exceeding 4 mL/dL and an FLR below 40%. Drainage of the contralateral liver is reserved for those patients with persistent jaundice and/or sepsis. In the absence of large RCTs, there is no definitive evidence to recommend PTBD, EBD or ENBD.
2.4. Portal vein embolization and ALPPS
An inadequate FLR volume and function poses the patient at risk to post‐hepatectomy liver failure (PHLF). PVE aims to decrease the risk of PHLF by occluding the portal vein to the side of the liver that is resected, causing hypertrophy of the FLR.31, 32 In two systematic reviews, preoperative PVE was associated with a relative increase in FLR of about 40% and a 2.5% adverse events rate.31, 32 A more recent review details the four largest studies on PVE for pCCA, conducted in a total of 586 patients.33, 34, 35, 36, 37 Only one patient died because of PHLF.
No consensus on the optimal FLR cut‐off to perform PVE exists.33 In most studies, cutoffs of the FLR range from 20%‐40%, sometimes in combination with assessment of indocyanine green clearance.33 A study in 217 Dutch and US patients detailed a model to predict PHLF, consisting of FLR volume, jaundice, cholangitis and preoperative serum bilirubin. The model had a good predictive accuracy with a c‐index of 0.79.38 One of the most experienced centres in the world recommends PVE for an FLR below 40%.6 This approach resulted in a PHLF (grade B/C) rate of 3.2% and a post‐operative mortality rate of 1.4%. Several studies investigated the predictive value of liver function tests. 99mTc‐mebrofenin scintigraphy was evaluated in a cohort of 216 pCCA patients. The authors concluded that scintigraphy helped predicting PHLF.39 The challenge of liver function tests is that studies have not shown how they can avoid unnecessary PVE in patients with a small FLR volume or avoid PHLF by recommending PVE in patients with a large FLR volume.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is another option for patients with a small FLR. During the first procedure of ALPPS, the liver parenchyma is (partially) transected with portal vein ligation of the liver with the tumour. The second stage, after the FLR has become hypertrophic, involves the resection.40, 41 In a recent study, conducted with data from the international ALPPS registry, mortality was 44% for ALPPS patients, vs 24% for matched non‐ALPPS patients who had a similar FLR. The authors concluded that PVE remains the standard approach.42 Meanwhile, techniques for ALPPS have become more refined, but ALPPS should not be the initial approach for patients with pCCA.
2.5. Approach to resection
The majority of patients with pCCA require an (extended) hemi‐hepatectomy with resection of the extrahepatic bile duct (Figure 1). Right trisectionectomy has the advantage of a greater length of the left hepatic duct (2‐3 cm) as opposed to the right duct (<1 cm).43 En‐bloc resection of the caudate lobe is recommended because the tumour typically extends into the caudate lobe via small branches draining into the right or left hepatic ducts or the biliary confluence.44 For Bismuth IIIB tumours, a left hepatectomy or trisectionectomy extended to second‐order biliary radicals is needed, often requiring reconstruction of multiple right‐sided ducts.45 dCCA (ie, located in the intrapancreatic bile duct), is treated with a pancreatoduodenectomy. Resection of only the extrahepatic bile duct may be considered for Bismuth I pCCA, especially in frail patients. However, in a study of patients with Bismuth I or II tumours, 5‐year survival was 30% with extrahepatic bile duct resection alone vs 50% with en‐bloc liver resection.46 Lymphadenectomy of locoregional lymph nodes in the hepatoduodenal ligament is recommended, but has a bigger impact on staging than on improving survival.
Figure 1.
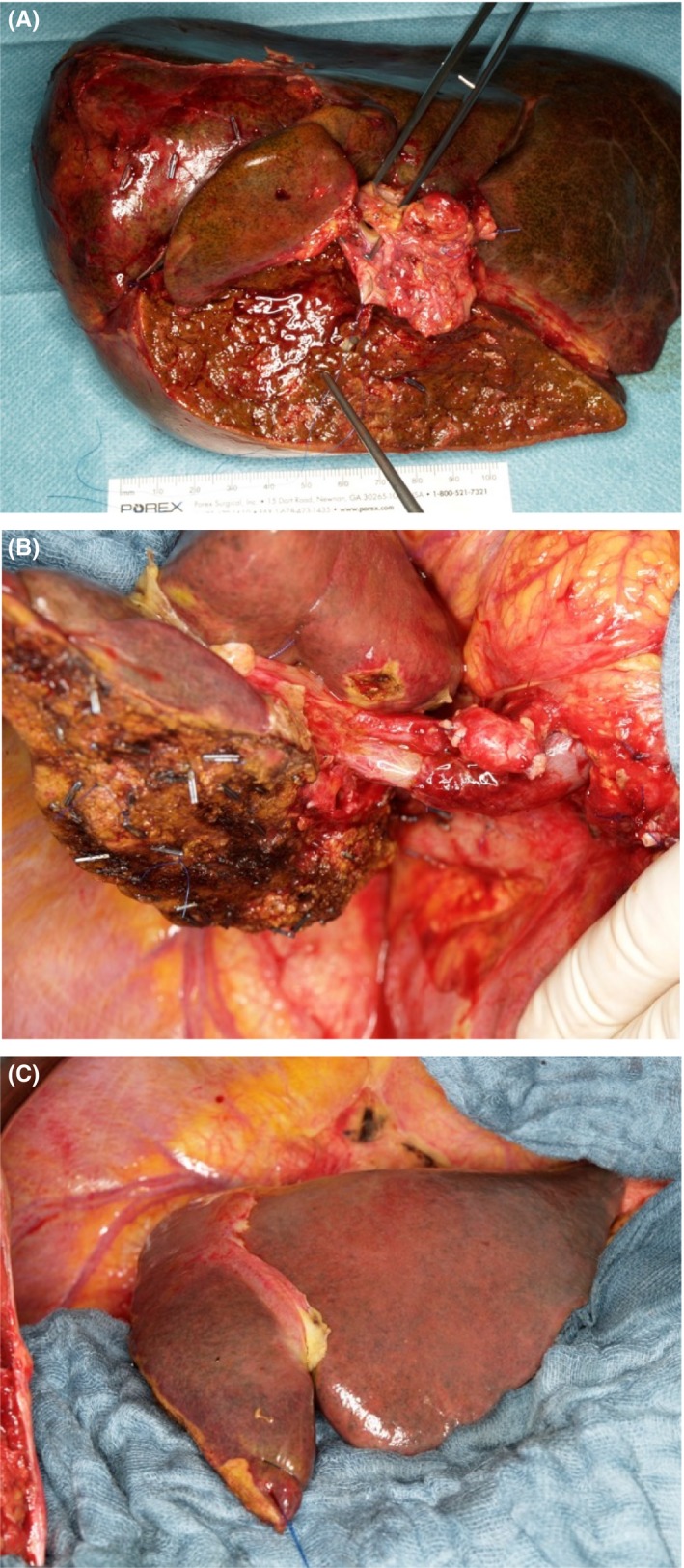
(A) Resected specimen: extended right hemihepatectomy including segment I, extrahepatic bile duct, portal vein bifurcation and hilar tissue. Long suture at proximal cut end of left bile duct and forceps in resected portal vein bifurcation. (B) Lateral view of liver remnant (segments II, III and part of IV) after extended right hemihepatectomy with end‐to‐end anastomosis of the portal vein and transected left bile duct visible below left portal vein, prior to hepaticojejunostomy. (C) Anterior view of liver remnant (segments II, III and part of IV) after extended right hemihepatectomy
Portal vein resection and reconstruction may be required and may improve resection rates, R0 resection rates and survival.6, 47 Hepatic artery resection and reconstruction has also been reported, though morbidity and mortality rates can be high and survival with main or unilateral hepatic artery involvement is poor.48, 49 A staging laparoscopy to rule out undetected liver or peritoneal metastases is recommended with a yield above 20% in many studies.50 Results of minimal‐invasive resection for pCCA have been mostly disappointing.51
2.6. Perioperative systemic chemotherapy
Beside the role of chemoradiotherapy (CRT) in liver transplantation (discussed below), the benefit of preoperative therapy has not been established. Nelson et al reported R0 resection of 91% in twelve patients treated preoperatively either for borderline resectable or unresectable extrahepatic cholangiocarcinoma; these patients also showed a better 5‐year survival rate compared to the 33 patients who did not receive neoadjuvant treatment (53% vs 23%).52 More recently, a retrospective study by a South Korean group focused on patients with locally advanced pCCA.53 The twelve patients who received preoperative CRT, compared to the 45 who did not, showed a higher rate of R0 resections that was not statistically different (83% vs 64%, P = 0.32) and no difference in disease‐free survival (DFS) and overall survival (OS). The phase II trial of the NACRAC study is currently in progress: twenty‐four patients with cholangiocarcinoma have been recruited and R0 resection has been obtained in 71%.54 With all the limitations of the studies above, such as small samples and different therapeutic regimens, preoperative CRT has proved to be safe and seems to enhance R0 resection rate for (borderline) unresectable pCCA. Definitive data from the NACRAC study are awaited to determine whether neoadjuvant CRT also improves survival (UMIN000000992 and UMIN000001754).
With regards to the role of adjuvant treatment for pCCA, more studies are available. Firstly, a large Japanese RCT, including 118 patients with cholangiocarcinoma, had failed to demonstrate a better OS for patients (n = 58) treated with mitomycin and 5‐FU after surgery, compared to the controls (n = 60) who had not received any adjuvant treatment.55 Later studies found an improvement in OS after adjuvant treatment, especially for patients with positive lymph nodes.56, 57 In particular, in a cohort of 260 patients who underwent R0/R1 resection for pCCA, Kang reported a statistically significant survival benefit with 5‐FU‐based adjuvant regimen for patients with positive lymph nodes.58 This result has recently been confirmed on multivariate analysis by a multicentre retrospective study of 249 patients after curative resection for pCCA.59 Most adjuvant RCTs include all patients with biliary tumours. These RCT show conflicting results as discussed below in the section on iCCA.
2.7. Outcomes after resection
Outcomes for patients with resectable pCCA depend on three factors:
Tumour characteristics: local extent of the tumour, tumour differentiation, lymphovascular, perineural and/or microvascular invasion, lymph node involvement and the presence of distant metastasis.
Whether or not a patient is considered for resection at a multidisciplinary meeting including experienced hepatobiliary surgeons.
The surgical approach aiming at a radical (R0) resection, and perioperative morbidity and mortality.
The reported 3‐ and 5‐year survival rates after resection for pCCA are around 45% (35 to 60%) and 30% (15 to 40%).6, 36, 60, 61 Most patients are not cured after resection for pCCA; about 80% will develop recurrent disease mostly within two years after surgery.62, 63 R0‐resection rates range from 50% to 90%. About 35% of patients have positive lymph nodes (pN1). These two parameters are the main features determining survival. While an R0‐resection is associated with a 5‐year survival of up to 60%, it is less than 10% for R1‐resections. In case of negative lymph nodes, 5‐year survival is reported up to 55% vs less than 20% in case of positive lymph nodes – regardless of an R0‐resection.
Another important tumour characteristic is tumour differentiation (well vs moderate/poor), although not as strong as R‐status and nodal involvement. Other poor prognostic factors are lymphovascular invasion, microvascular invasion and perineural invasion. Their impact, however, mostly disappears in multivariable analysis, adjusting for R status, nodal involvement and tumour differentiation. Several nomograms predicting prognosis have been devised.64, 65
The early post‐operative (procedure‐related) mortality is around 10% in large Western centres, but may vary considerably from 2% to 15%.4, 5, 24 Italian and French multicentre studies both found a post‐operative mortality of about 10%. Nagino reports a mortality of 11% before 1990 and 1.4% after 2005.6 Common serious complications are PHLF, biliary complications, infectious complications and vascular complications.
In an attempt to improve the R0 resection rate, more extensive resections have been proposed. One type of extended resection for pCCA is combining liver resection with a pancreatoduodenectomy, particularly in patients in whom the tumour extends towards the distal bile duct. Post‐operative mortality and long‐term survival are favourable in high‐volume centres, but poor in less experienced centres.
Many publications have shown that both the R0‐resection rates and post‐operative mortality rates depend strongly on the experience of the centre.66, 67 These results clearly argue for performing surgery in patients with pCCA only in highly experienced centres. However, about 25% of patients will have an R1 resection, even in experienced centres. Only a liver transplantation (LT) could reassure an R0 resection in these patients.
2.8. Liver transplantation
The rationale of LT in patients with pCCA is to avoid two unfavourable outcomes of surgical resection; a positive margin and an inadequate FLR with PHLF. Furthermore, LT is effective in removing the underlying liver disease, such as PSC.68 Early experience with LT for pCCA was poor. The 207 LT patients reported by the Cincinnati Transplant Tumour Register (from 1968 to 1997) had a 5‐year survival of 23%.69 In Spain, France and Germany, a similar series reported a 3‐year survival rate of 30%‐38% with very high rate of recurrent disease.70, 71
The Mayo Clinic protocol (Table 1) for patients with unresectable pCCA, introduced in the 1993 (based on a previous University of Nebraska study), represented a breakthrough.72 The protocol consists of external beam radiation (40‐45 Gy) followed by transcatheter radiation (20‐30 Gy) with iridium wires, intravenous 5‐flurouracil administered for chemosensitization during radiation therapy, and capecitabine administered afterward while waiting LT.73, 74 Staging surgery with lymph node biopsies is performed after brachytherapy. The reported intention‐to‐treat survival rates at 1, 3 and 5 years were 82%, 62% and 56% respectively. The overall survival after LT at 1, 3 and 5 years was 91%, 81% and 74% respectively.75 These favourable data were subsequently reproduced in a multicentre study including 12 high‐volume centres in the US.76 The 5‐years intention‐to‐treat survival was similar (53%). Notably, more than two‐thirds of the patients had PSC as underlying disease, compared to about 5% in other pCCA cohorts.
Table 1.
Mayo clinic protocol
| Mayo clinic protocol | External beam radiation therapy (45 Gy in 30 fractions, 1.5 Gy twice daily) |
| Brachytherapy (20 Gy at 1 cm in approximately 20‐25 h) – administered 2 wks following completion of external beam radiation therapy | |
| Capecitabine – administered until the time of transplantation, held during perioperative period for staging | |
| Abdominal exploration for staging – as time nears for deceased donor transplantation or day prior to living donor transplantation | |
| Liver transplantation | |
| Inclusion criteria | Diagnosis of pCCA (transcatheter biopsy or brush cytology, CA 19‐9 > 100 mg/mL and/or a mass on cross‐sectional imaging with a malignant appearing stricture on cholangiography) |
| Unresectable tumour above cystic duct (pancreatoduodenectomy for microscopic involvement of CBD, resectable pCCA arising in PSC) | |
| Radial tumour diameter 3 cm | |
| Absence of intrahepatic and extrahepatic metastases | |
| Candidate for liver transplantation | |
| Exclusion criteria | Intrahepatic cholangiocarcinoma |
| Uncontrolled infection | |
| Prior radiation or chemotherapy | |
| Prior biliary resection or attempt resection | |
| Intrahepatic metastases | |
| Evidence of extrahepatic disease | |
| History of other malignancy within 5 years | |
| Transperitoneal biopsy (including percutaneous and EUS‐guided FNA) |
Whether the results of the Mayo Clinic series are because of careful selection of the patients enrolled or to the efficacy of the pretransplant therapy is still debated. In a retrospective study, Mantel identified 28 patients with pCCA who met the strict selection criteria for the Mayo Clinic protocol but had not undergone neo‐adjuvant chemoradiation therapy.77 Five‐year survival in this subgroup was 59%, which is comparable to the results obtained in the Mayo series.
A further criticism to the results of Mayo protocol is that in the case of a patient with a negative cytology, negative FISH, and no residual tumour in the specimen after transplant the question remains whether the patient ever had a cancer. The authors estimate this possibility to be about 15% of patients.78 However, the high risk of recurrence after transperitoneal biopsy justifies this approach at least until new and more accurate diagnostic techniques are developed.
Ethun et al reported that patients resected for pCCA and meeting transplantation criteria had a significantly worse 5‐year survival compared to patients who received LT (18% vs 64%).68 Among patients who underwent resection for tumours smaller than 3 cm with node‐negative disease, and after excluding PSC patients, transplant was still associated with improved OS (5‐year: 54% vs 29%; P = 0.03).
Prioritization to LT for pCCA patients is one of the most debated issues. In 2009, the UNOS Board of Directors voted to implement the allocation changes adopting the Mayo Clinic criteria for a MELD score exception adjustment. How the introduction of “Share 35” policy will affect the results of LT in pCCA is not known yet. In Italy, a new allocation policy was proposed allowing the use of 5% of donors for patients with non‐hepatocellular carcinoma (HCC) oncological indications including pCCA.79
2.9. Locoregional treatments
When a patient is not eligible for surgery, systemic therapy is usually recommended. The current guidelines of the European Society of Medical Oncology and the National Comprehensive Cancer Network recommend the use of cisplatin and gemcitabine alone or in combination.7, 80 In the absence of distant metastatic disease, several locoregional treatment options can be considered.
Hepatic arterial infusion pump (HAIP) chemotherapy, has several theoretical advantages in pCCA: the tumour is small and the arterial supply is the prominent component. Since HAIP is expected to increase the local concentration of drugs, also the effectiveness is expected to be superior in comparison to systemic therapy. HAIP was found to be more effective in unresectable iCCA (section below) in comparison with other arterial‐directed therapies.81 However, limited data are available for pCCA. Wang et al reported a phase II study on 37 patients that were treated with HAIP with oxaliplatin (40 mg/m2 for 2 hours) plus 5‐fluorouracil (800 mg/m2 for 22 hours on days 1‐3) every 3‐4 weeks for six courses followed by oral capecitabine until progression.82 They reported prolonged survival in those patients with periductal infiltrating pCCA rather than in those with mass‐forming pCCA. The overall toxicity was mild. In this study, there was no a control group, thus limiting the generalizability of the conclusions.
Irreversible electroporation (IRE) is a promising image‐guided ablation technique based on short‐pulsed high‐voltage current fields that destroy the cell membrane, then altering the intracellular compartment leading finally to cell apoptosis.83 The non‐thermal physical mechanism implies the absence of the heat/cold‐sink effect and of the damage to adjacent structures (ie, portal vein and hepatic artery). This is the main advantage of IRE over other ablative procedures, such as microwave and radiofrequency ablations. IRE should be limited to those pCCA patients that are ineligible to curative surgery. IRE aims to avoid or delay local progression of disease with progressive isolation of sectoral and segmental bile ducts. Martin et al showed the results of a single‐centre experience on the local control of the biliary obstructions in 26 patients with unresectable pCCA.84 They reported that IRE achieved biliary decompression with a decreased median time to biliary drain removal and an extended median time of biliary drain absence in comparison to a control group. Coelen et al published the protocol of an ongoing multicentre phase I/II trial of IRE in unresectable pCCA patients.85
Photodynamic therapy (PDT) is another ablative method used in patients with unresectable pCCA. After administration of a photo‐sensitizer with selective uptake by cancer cells, PDT activates the photo‐sensitizer. This results in the generation of oxygen radicals that lead to cancer cell death. Typically, 5‐aminolevulinic acid is used as photo‐sensitizing, and the percutaneous route is preferred over the endoscopic route because of lower risks, increased feasibility, and easy repeatability.86 Some authors reported promising results of PDT for bile duct tumours, with respect to relief of jaundice, improvement in quality of life and improved survival.87, 88, 89 A main drawback of PDT is the photosensitization of the skin, which lasts up to 8 weeks and exposes the patient to skin infections and complications.
2.10. Future research
Future research should aim to increase the R0 resection rate. This can be achieved by more extensive resection, particularly of the proximal bile ducts. The best option to achieve that aim is liver transplantation. To evaluate LT for pCCA, a prospective study has just been initiated in Germany (pro‐duct002 trial, DRKS00013276). Downstaging with preoperative CRT may also increase the R0 resection rate. In this respect, the results of standard chemotherapy have not been really promising, but a combination of targeted therapies with local irradiation treatment might offer potential – once appropriate targets for targeted therapies for this tumour have been identified.
Another option to prevent recurrence after resection could be adjuvant treatment. So far, adjuvant chemotherapy appears to be of limited value. New targeted therapies or immunotherapies are needed.
Finally, reducing perioperative morbidity and mortality is an important goal to improve outcome of surgery. To achieve that, pCCA patients should be treated only in experienced centres after referral prior to drainage.
3. INTRAHEPATIC CHOLANGIOCARCINOMA
3.1. Diagnostic work‐up
Intrahepatic cholangiocarcinoma (iCCA) arises in the biliary tree, proximal to the second‐order bile ducts. Most iCCA patients are diagnosed with a large mass on imaging after presenting with unspecific complaints including abdominal pain, weight loss and fatigue. About 15% of patients present with jaundice caused by biliary obstruction. Imaging in iCCA patients presenting with jaundice shows a large mass (ie, >3 cm) that has grown towards the liver hilum. This should be distinguished from pCCA patients showing a smaller mass arising from the biliary confluence or main hepatic ducts. iCCA can also be diagnosed in an asymptomatic patient who underwent imaging for elevated liver enzymes or a reason unrelated to iCCA. The majority of iCCA patients have no underlying liver disease. Underlying liver disease, however, is a risk factor for iCCA. Patients with cirrhosis in a screening programme for early detection of HCC are sometimes found to have iCCA. Especially small iCCA may be difficult to distinguish from HCC on imaging. Sometimes, the final diagnosis is only made at pathological examination after surgical resection or liver transplantation.
Serum tumour markers play a modest role in the diagnostic work‐up. CA19‐9 is elevated in most patients with iCCA. About 10% of patients cannot synthesize CA 19‐9, and CA 19‐9 elevation can also be attributed to biliary obstruction. Elevated alpha‐foetoprotein (AFP) is more likely in patients with HCC. Serum IgG4 is elevated in patients with auto‐immune cholangitis, which may sometimes present as an intrahepatic mass.90 However, not all patients with elevated IgG4 have auto‐immune cholangitis and patients with elevated IgG4 may also have cancer. Sensitivity and specificity are improved when measuring the IgG4/IgG RNA ratio.91 Many of these patients had long‐term exposure to solvents, oil products and other organic agents. A biopsy or a course of steroids should be considered in these patients. Some patients have a patient history or imaging consistent with another autoimmune disease such as autoimmune pancreatitis.
A high quality tri‐phasic CT is the standard imaging for iCCA for both diagnosis and surgical planning. iCCA typically shows early arterial peripheral enhancement with gradual filling towards the centre of the lesion. Sometimes multiple lesions are present across the liver or small satellites surrounding a large tumour. An additional delayed phase CT shows progressive enhancement of iCCA lesions compared to the surrounding normal liver tissue. A CT of the chest is recommended to rule out pulmonary metastases. In most patients, a CT is sufficient for diagnosis and treatment plan. MRI can be performed instead of or in addition to CT, when the diagnosis is uncertain. A recent study compared contrast‐enhanced MRI for HCC and iCCA. None of the imaging features was unique to iCCA. However, HCC was more likely to show wash‐out, capsule and intra‐lesional fat; iCCA was more likely to show peripheral arterial phase enhancement and progressive central enhancement.92 Moreover, MRCP is superior in delineation of the biliary extent of tumours growing towards the liver hilum and causing biliary obstruction. PET‐CT may find lesions suspicious for metastastic disease that were not visible on CT. Unfortunately, PET‐CT has a low yield of about 10% for finding occult metastases and a considerable risk of false‐negative findings requiring additional invasive diagnostic tests and delay of surgery.9, 10 We recommend FDG‐PET only in patients with very extensive disease (eg, multiple lesions) or a high surgical risk.
A percutaneous biopsy for suspected iCCA is rarely indicated if a patient is eligible for complete resection. Moreover, it is typically avoided because of the risk of recurrent disease along the biopsy tract. Imaging mostly provides a high degree of certainty about the diagnosis. If imaging is not specific for iCCA and CA 19‐9 is normal, one should rule out that the liver tumour(s) represent metastatic disease of another malignancy, in particular colorectal, gastric or breast cancer. Work‐up should then include a colonoscopy, upper endoscopy and mammography. Pathological evaluation including immunohistochemistry may not be able to distinguish iCCA from metastatic disease.
3.2. Indication for resection
The decision for surgical resection of iCCA requires a trade‐off between anticipated oncological benefit (ie, superior OS and quality of life) vs surgical risk (ie, post‐operative mortality and morbidity). This trade‐off is easy in a healthy young patient with a solitary 3 cm lesion in segment 3 of the liver. At the other end of the spectrum is a frail octogenerian with a 10‐cm lesion with several satellites and suspicious hilar lymph nodes who requires an extended right hemihepatectomy. Shared decision making requires the combination of patient preference with oncological benefit and surgical risk.
Resection in the setting of distant (extrahepatic) metastatic disease results in median OS similar to palliative chemotherapy. Patients with nodal involvement beyond the hepatoduodenal and gastrohepatic ligament are also less likely to benefit from a resection. Even patients with locoregional nodal metastases have a recurrence rate approaching 100%.93 Nevertheless, most iCCA patients will die from liver disease. Therefore, controlling disease in the liver with surgery can improve survival, even when cure is not likely. A SEER database study of 169 node‐positive iCCA patients, however, found a median OS of only 19 months after resection, vs 20 months with systemic chemotherapy alone (P = 0.32).94 iCCA patients with more than one lesion also have a worse OS and are less likely to benefit from resection. Several studies found a 5‐year OS of about 10% with resection in patients with multiple tumours.95, 96 Resection could be considered in selected iCCA patients with two to three lesions. A large multicentre study reported similar OS for patients who did and did not undergo vascular reconstruction of the hepatic artery, portal vein, vena cava or hepatic veins.97
3.3. Approaches to resection
The aim of resection for iCCA is similar to any oncological liver resection: a complete (R0) resection with an adequate liver remnant. Most patients have a single large tumour requiring an (extended) hemihepatectomy. PVE is recommended if the future liver remnant volume is below 30%‐40% (see section on PVE in first part on pCCA). ALPPS can be considered if the remnant volume remains inadequate after PVE or if intraoperatively a larger resection is needed than anticipated on imaging.98
About 15% of iCCA patients who undergo a resection presented with biliary obstruction. Most of these patients will require preoperative biliary drainage, in particular in the setting of cholangitis or a small FLR (see also section on biliary drainage in first part on pCCA). Resection without biliary drainage should be considered if the future liver remnant exceeds 50%. Resection of the biliary confluens is typically needed in patients with biliary obstruction, followed by a roux‐Y hepaticojejunostomy.
Staging laparoscopy should be considered in all patients with iCCA to rule out occult metastastic disease. This risk is particularly high in patients with high CA 19‐9, major vascular invasion and suspicious lymph nodes.99 Exploratory laparotomy without resection should be avoided because it delays palliative systemic chemotherapy.
Suspicious lymph nodes beyond the hepatoduodenal ligament should be sent for frozen section. However, preoperative assessment of suspicious aortocaval and truncal lymph nodes with endoscopic ultrasound and fine needle aspiration is preferred. Guidelines recommend to perform a lymphadenectomy in all patients with resectable iCCA.99 For optimal staging, the 8th edition of the AJCC staging systems recommends to harvest at least six locoregional lymph nodes.1 It appears that lymphadenectomy is mainly a staging procedure with little effect on OS.
3.4. Perioperative systemic chemotherapy
Systemic chemotherapy with gemcitabine and cisplatin is the standard of care in patients with advanced biliary cancer.100 The ABC‐02 RCT found a superior median OS of 11.7 months with gemcitabine and cisplatin, compared to 8.2 months with gemcitabine alone. The hazard ratio (HR) for the subgroup of 80 patients with iCCA was 0.57 (95% CI: 0.34‐0.94).
Several RCTs investigated systemic chemotherapy in the adjuvant setting. The BILCAP trial compared adjuvant capecitabine with observation. The study included 447 patients with biliary cancer, of whom 84 had iCCA. The median OS was 51 months with capecitabine vs 36 months with observation (HR 0.80; 95% CI 0.63‐1.04: P = 0.097).101 The BCAT trial found no survival difference between gemcitabine with observation (HR 1.01; 95% CI 0.70‐1.45; P = 0.97).102 However, the BCAT trial only included patients with extrahepatic cholangiocarcinoma. Finally, the Prodige‐11 trial compared adjuvant gemcitabine plus oxaliplatin vs observation after resection of biliary cancer and also found no survival benefit (HR 1.08; 95% CI 0.70‐1.66).103 These three trials have insufficient power for a definitive recommendation of adjuvant chemotherapy. Meanwhile, the ACTICCA −1 trial (sample size 781) is still recruiting patients to compare adjuvant gemcitabine with cisplatin vs capecitabine alone (NCT02170090).
Preoperative systemic chemotherapy for patients with resectable or unresectable iCCA has not been evaluated in an RCT. A multinational study included 62 patients with resectable or unresectable iCCA who received preoperative systemic chemotherapy.104 They found a median OS of 47 months for patients who underwent a complete resection after preoperative chemotherapy. In a recent study of 74 patients with unresectable iCCA, 39 patients (53%) underwent a resection after a median of six cycles of systemic chemotherapy. The median OS was 24 months after completion of both induction chemotherapy and surgical resection, which was similar to a separate cohort of patients who underwent upfront surgery for resectable iCCA with a median OS of 26 months.105 This outcome is superior to OS with palliative systemic chemotherapy without resection in the ABC‐02 trial.100 Preoperative systemic chemotherapy can be considered as a “test of time”. Downstaging from unresectable to resectable with systemic chemotherapy may be succesful in rare cases.
3.5. Outcomes after resection
Post‐operative mortality after resection for iCCA depends on both patient and tumour characteristics. A minor liver resection1, 2, 3 should have a mortality rate of about 1% in high‐volume centres. Most patients with iCCA (75%) will require an (extended) hemihepatectomy with higher mortality.106 Post‐operative mortality is further increased in patients requiring vascular (portal vein, hepatic artery or caval vein) reconstructions, patients with cirrhosis and patients requiring preoperative biliary drainage.
Most patients will develop recurrent disease after resection of iCCA. The median recurrence‐free survival in a large study of 301 patients was 20 months. Most patients developed an initial intrahepatic recurrence (61%). An initial extrahepatic recurrence was found in 21% and 19% had a simultaneous intra‐ and extrahepatic recurrence. 107 Another study of 189 patients found that the initial recurrence within 24 months was only intrahepatic in 54% of patients with a recurrence, compared to 33% after 24 months.93 Small studies have reported favourable outcomes after resection of recurrence in selected patients.108
The median OS after curative‐intent resection is about 30 months with a 5‐year OS of about 30% based on several large series.109 Most patients died from recurrent disease. Poor prognostic factors for OS include the presence of multiple tumours, large tumour size, vascular invasion, lymph node involvement, poor tumour differentiation and a positive surgical resection margin. Several prognostic models were developed for survival after resection of iCCA. Most models used a combination of known poor prognostic factors. The model of Wang et al also included serum tumour markers (CA 19‐9 and CEA) and had the best c‐statistic of 0.74.110
3.6. Liver transplantation
Historical series of liver transplantation for unresectable iCCA showed a poor 5‐year OS below 25%.111 Considering these poor outcomes and the scarcity of organs, iCCA has been a contraindication for liver transplantation. A recent series found that cirrhotic patients with uninodular iCCA of less than 2 cm had a similar OS after liver transplantation as matched HCC patients.112 More recently, highly selected patients with well‐differentiated iCCA of less than 2 cm (ie, very early iCCA) had a 5‐year OS of 65%.113 However, both studies apply only to a small proportion of patients with iCCA.
Liver transplantation has also been performed for unresectable iCCA in 12 patients with stable disease on neoadjuvant systemic chemotherapy.46 Three patients (25%) developed recurrent disease within one year. Living donor liver transplantation may expand the role of transplantation for iCCA. Careful patient selection is needed of patients who are not expected to develop extrahepatic disease within a few years after transplantation.
3.7. Locoregional treatments
Surgical resection is the preferred treatment for resectable iCCA. However, a lesion of less than 2 or 3 cm located centrally in the liver could be considered for thermal ablation (eg, RFA or MWA) in patients with a high surgical risk (eg, cirrhosis). The main drawback of thermal ablation is an increased risk of local recurrence. Omission of lymphadenectomy is less concerning: nodal metastasis is unlikely in small lesions, and removing positive lymph nodes has not been shown to improve survival.
Locoregional treatments are mainly applied in patients with unresectable disease. The median OS with systemic chemotherapy (gemcitabine with cisplatin) for patients with unresectable iCCA is <1 year with very few survivors beyond 2 years.100, 114 Most patients with iCCA eventually die from tumour burden limited to the liver. Progressive disease in the liver eventually causes biliary obstruction and liver failure. Locoregional treatments aim to improve survival by controlling disease in the liver as long as possible. If local control is succesful, most patients will eventually die from distant metastases.
Unresectable iCCA is too large for percutaneous ablation. Therefore, various transarterial treatments have been investigated. Transarterial chemo‐embolization (TACE) causes ischaemia and delivers cytotoxic agents. Two small studies reported a median OS of 12 and 18 months.115, 116 Radioembolization with 90Y has shown promising results in patients with unresectable HCC and colorectal liver metastases. Hepatic arterial infusion pump (HAIP) chemotherapy with floxuridine has been investigated in a study of 104 patients.117 Because floxuridine, which is similar to 5‐FU, has a 95% first‐pass effect, it can be delivered in the hepatic artery at a very high dose. The partial response rate was about 60%. Median OS was 31 months compared to 18 months in patients who received systemic chemotherapy alone. Five‐year OS was 20% in patients who received HAIP chemotherapy. Although no direct comparison has been performed, the results of HAIP chemotherapy are promising.
3.8. Future research
Future research should improve patient selection for resection of iCCA. Resection is technically feasible even in patients with multiple lesions or requiring vascular reconstruction. However, a small survival benefit may not justify considerable post‐operative mortality, particularly in frail patients. Predictive models for survival with and without resection vs the risk of post‐operative complications could aid shared decision‐making.
Most patients will develop recurrent disease after resection of iCCA. Several RCTs investigating adjuvant systemic chemotherapy for biliary cancer found conflicting results. Better adjuvant treatments are needed to address occult metastatic disease. Induction HAIP chemotherapy demonstrated a high response rate in unresectable iCCA and could also reduce the high intra‐hepatic recurrence rate in the adjuvant setting. Personalized systemic treatments in the preoperative and adjuvant setting based on molecular profiling of tumours may further improve outcomes.
Finally, a minimal‐invasive (laparoscopic or robotic) approach may have a modest impact on post‐operative complications and recovery, but probably not on survival outcomes.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
ACKNOWLEDGEMENT
The authors of this review article are members of the European Network for the Study of Cholangiocarcinoma (ENSCCA) and participate in the initiative COST Action EURO‐CHOLANGIO‐NET granted by the COST Association (CA18122).
Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl. 1):143–155. 10.1111/liv.14089
REFERENCES
- 1. AJCC Cancer Staging Manual ‐, 8th edn Springer; 2017:1032 pp. [Google Scholar]
- 2. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farges O, Regimbeau Jm, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274‐283. [DOI] [PubMed] [Google Scholar]
- 5. Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long‐term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26‐34. [DOI] [PubMed] [Google Scholar]
- 6. Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single‐center 34‐year review of 574 consecutive resections. Ann Surg. 2013;258:129‐140. [DOI] [PubMed] [Google Scholar]
- 7. Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v28‐v37. [DOI] [PubMed] [Google Scholar]
- 8. Zaydfudim VM, Rosen CB, Nagorney DM. Hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:247‐263. [DOI] [PubMed] [Google Scholar]
- 9. Petrowsky H, Wildbrett P, Husarik DB, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43‐50. [DOI] [PubMed] [Google Scholar]
- 10. Corvera CU, Blumgart LH, Akhurst T, et al. 18F‐fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57‐65. [DOI] [PubMed] [Google Scholar]
- 11. Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta‐analysis. Br J Radiol. 2012;85:1255‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660‐678. [DOI] [PubMed] [Google Scholar]
- 13. Corvera CU, Blumgart LH, Darvishian F, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201:862‐869. [DOI] [PubMed] [Google Scholar]
- 14. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170‐178. [PubMed] [Google Scholar]
- 15. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507‐517; discussion 517‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision‐making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176‐1181. [DOI] [PubMed] [Google Scholar]
- 17. Shoup M, Gonen M, D'Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325‐330. [DOI] [PubMed] [Google Scholar]
- 18. de Graaf W, van Lienden KP, van Gulik TM, Bennink RJ. (99m)Tc‐mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51:229‐236. [DOI] [PubMed] [Google Scholar]
- 19. Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Gouma DJ. Inflammatory and immunologic effects of obstructive jaundice: pathogenesis and treatment. J Am Coll Surg. 1995;181:567‐581. [PubMed] [Google Scholar]
- 20. Nakayama T, Ikeda A, Okuda K. Percutaneous transhepatic drainage of the biliary tract. Gastroenterology. 1978;74:554‐559. [PubMed] [Google Scholar]
- 21. Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Gaag NA, Rauws E, van Eijck C, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129‐137. [DOI] [PubMed] [Google Scholar]
- 23. Hochwald SN, Burke EC, Jarnagin WR, Fong Y, Blumgart LH. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261‐266. [DOI] [PubMed] [Google Scholar]
- 24. Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg. 2016;223(321–331):e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hameed A, Pang T, Chiou J, et al. Percutaneous vs. endoscopic pre‐operative biliary drainage in hilar cholangiocarcinoma ‐ a systematic review and meta‐analysis. HPB (Oxford). 2016;18:400‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al Mahjoub A, Menahem B, Fohlen A, et al. Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? A meta‐analysis. J Vasc Interv Radiol. 2017;28:576‐582. [DOI] [PubMed] [Google Scholar]
- 27. Coelen R, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3:681‐690. [DOI] [PubMed] [Google Scholar]
- 28. Kawashima H, Itoh A, Ohno E, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg. 2013;257:121‐127. [DOI] [PubMed] [Google Scholar]
- 29. Jo JH, Chung MJ, Han DH, et al. Best options for preoperative biliary drainage in patients with Klatskin tumors. Surg Endosc. 2017;31:422‐429. [DOI] [PubMed] [Google Scholar]
- 30. Kubota K, Hasegawa S, Iwasaki A, et al. Stent placement above the sphincter of Oddi permits implementation of neoadjuvant chemotherapy in patients with initially unresectable Klatskin tumor. Endosc Int Open. 2016;4:E427‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta‐analysis. Ann Surg. 2008;247:49‐57. [DOI] [PubMed] [Google Scholar]
- 32. Van Lienden KP, Van Den Esschert JW, De Graaf W, et al. Portal vein embolization before liver resection: A systematic review. Cardiovas Interv Radiol. 2013;36:25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glantzounis Gk, Tokidis E, Basourakos S‐P, Ntzani Ee, Lianos Gd, Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. a systematic review. Eur J Surg Oncol. 2017;43:32‐41. [DOI] [PubMed] [Google Scholar]
- 34. Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepato‐Biliary‐Pancreat Sci. 2010;17:476‐489. [DOI] [PubMed] [Google Scholar]
- 35. Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg. 2012;29:23‐29. [DOI] [PubMed] [Google Scholar]
- 36. Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa SI. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surgery. 2006;244:240‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olthof PB, Wiggers JK, Groot Koerkamp B, et al. Postoperative liver failure risk score: identifying patients with resectable perihilar cholangiocarcinoma who can benefit from portal vein embolization. J Am Coll Surg. 2017;225:387‐394. [DOI] [PubMed] [Google Scholar]
- 39. Olthof PB, Coelen R, Bennink RJ, et al. 99mTc‐mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB. 2017;19:850‐858. [DOI] [PubMed] [Google Scholar]
- 40. Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498‐1503. [DOI] [PubMed] [Google Scholar]
- 41. Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2‐staged extended right hepatic resection in small‐for‐size settings. Ann Surg. 2012;255:405‐414. [DOI] [PubMed] [Google Scholar]
- 42. Olthof PB, Coelen R, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case‐control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017;19:381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19:1602‐1608. [DOI] [PubMed] [Google Scholar]
- 44. Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535‐543; discussion 544. [DOI] [PubMed] [Google Scholar]
- 45. Uesaka K. Left hepatectomy or left trisectionectomy with resection of the caudate lobe and extrahepatic bile duct for hilar cholangiocarcinoma (with video). J Hepatobiliary Pancreat Sci. 2012;19:195‐202. [DOI] [PubMed] [Google Scholar]
- 46. Lim JH, Choi GH, Choi SH, Kim KS, Choi JS, Lee WJ. Liver resection for Bismuth type I and type II hilar cholangiocarcinoma. World J Surg. 2013;37:829‐837. [DOI] [PubMed] [Google Scholar]
- 47. de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi‐institutional analysis of 305 cases. Cancer. 2012;118:4737‐4747. [DOI] [PubMed] [Google Scholar]
- 48. van Vugt J, Gaspersz MP, Coelen R, et al. The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB (Oxford). 2018;20:83‐92. [DOI] [PubMed] [Google Scholar]
- 49. Abbas S, Sandroussi C. Systematic review and meta‐analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford). 2013;15:492‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bird N, Elmasry M, Jones R, et al. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg. 2017;104:418‐425. [DOI] [PubMed] [Google Scholar]
- 51. Levi Sandri GB, Spoletini G, Mascianà G, et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur J Surg Oncol. 2017;43:1617‐1621. [DOI] [PubMed] [Google Scholar]
- 52. Nelson JW, Ghafoori AP, Willett CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73:148‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jung JH, Lee HJ, Lee HS, et al. Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma. World J Gastroenterol. 2017;23:3301‐3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katayose Y, Rikiyama T, Motoi F, et al. Phase I trial of neoadjuvant chemoradiation with gemcitabine and surgical resection for cholangiocarcinoma patients (NACRAC study). Hepatogastroenterology. 2011;58:1866‐1872. [DOI] [PubMed] [Google Scholar]
- 55. Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685‐1695. [DOI] [PubMed] [Google Scholar]
- 56. Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651‐658. [DOI] [PubMed] [Google Scholar]
- 57. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta‐analysis. J Clin Oncol. 2012;30:1934‐1940. [DOI] [PubMed] [Google Scholar]
- 58. Kang MJ, Jang JY, Chang J, et al. Actual long‐term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg. 2016;40:2451‐2459. [DOI] [PubMed] [Google Scholar]
- 59. Krasnick BA, Jin LX, Davidson JT, et al. Adjuvant therapy is associated with improved survival after curative resection for hilar cholangiocarcinoma: a multi‐institution analysis from the U.S. extrahepatic biliary malignancy consortium. J Surg Oncol. 2018;117:363‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476‐489. [DOI] [PubMed] [Google Scholar]
- 61. Bhardwaj N, Garcea G, Dennison AR, Maddern GJ. The surgical management of klatskin tumours: has anything changed in the last decade? World J Surg. 2015;39:2748‐2756. [DOI] [PubMed] [Google Scholar]
- 62. Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221:1041‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang X‐F, Beal EW, Chakedis J, et al. Defining early recurrence of hilar cholangiocarcinoma after curative‐intent surgery: a multi‐institutional study from the US extrahepatic biliary malignancy consortium. World J Surg. 2018;42:2919‐2929. [DOI] [PubMed] [Google Scholar]
- 64. Chen P, Li B, Zhu Y, et al. Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget. 2016;7:37319‐37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Groot Koerkamp B, Wiggers Jk, Gonen M, et al. Survival after resection of perihilar cholangiocarcinoma‐development and external validation of a prognostic nomogram. Ann Oncol. 2015;26:1930‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB (Oxford). 2012;14:142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ratti F, Fiorentini G, Cipriani F, Paganelli M, Catena M, Aldrighetti L. Perioperative and long‐term outcomes of laparoscopic versus open lymphadenectomy for biliary tumors: a propensity‐score‐based, case‐matched analysis. Ann Surg Oncol. 2018;26:564‐575. [DOI] [PubMed] [Google Scholar]
- 68. Ethun CG, Lopez‐Aguiar AG, Anderson DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 2018;267:797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633‐1637. [DOI] [PubMed] [Google Scholar]
- 70. Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seehofer D, Thelen A, Neumann UP, et al. Extended bile duct resection and [corrected] liver and transplantation in patients with hilar cholangiocarcinoma: long‐term results. Liver Transpl. 2009;15:1499‐1507. [DOI] [PubMed] [Google Scholar]
- 72. Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201‐207. [DOI] [PubMed] [Google Scholar]
- 73. Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929‐935. [DOI] [PubMed] [Google Scholar]
- 74. Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long‐term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774‐779. [DOI] [PubMed] [Google Scholar]
- 75. Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23:692‐697. [DOI] [PubMed] [Google Scholar]
- 76. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98 e83; quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mantel HT, Westerkamp AC, Adam R, et al. Strict selection alone of patients undergoing liver transplantation for hilar cholangiocarcinoma is associated with improved survival. PLoS One. 2016;11:e0156127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rosen CB, Darwish Murad S, Heimbach JK, Nyberg SL, Nagorney DM, Gores GJ. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg. 2012;215:31‐38; discussion 38‐40. [DOI] [PubMed] [Google Scholar]
- 79. Cillo U, Burra P, Mazzaferro V, et al. A multistep, consensus‐based approach to organ allocation in liver transplantation: toward a "Blended Principle Model". Am J Transplant. 2015;15:2552‐2561. [DOI] [PubMed] [Google Scholar]
- 80. Benson AB, D'Angelica MI, Abbott DE, et al. Guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111:213–220. [DOI] [PubMed] [Google Scholar]
- 82. Wang X, Hu J, Cao G, et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5‐fluorouracil for advanced perihilar cholangiocarcinoma. Radiology. 2017;283:580–589. [DOI] [PubMed] [Google Scholar]
- 83. Tarek M. Membrane electroporation: a molecular dynamics simulation. Biophys J. 2005;88:4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martin EK, Bhutiani N, Egger ME, et al. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB (Oxford). 2018;20:1092–1097. [DOI] [PubMed] [Google Scholar]
- 85. Coelen R, Vogel JA, Vroomen L, et al. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): a multicentre phase I/II feasibility study protocol. BMJ Open. 2017;7:e015810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ortner M, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. [DOI] [PubMed] [Google Scholar]
- 88. Cheon YK, Cho YD, Baek SH, et al. Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage. Korean J Gastroenterol. 2004;44:280–287. [PubMed] [Google Scholar]
- 89. Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426–2430. [DOI] [PubMed] [Google Scholar]
- 90. Roos E, Hubers LM, Coelen R, et al. IgG4‐associated cholangitis in patients resected for presumed perihilar cholangiocarcinoma: a 30‐year tertiary care experience. Am J Gastroenterol. 2018;113:765–772. [DOI] [PubMed] [Google Scholar]
- 91. Doorenspleet ME, Hubers LM, Culver EL, et al. Immunoglobulin G4(+) B‐cell receptor clones distinguish immunoglobulin G 4‐related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology. 2016;64:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92. Horvat N, Nikolovski I, Long N, et al. Imaging features of hepatocellular carcinoma compared to intrahepatic cholangiocarcinoma and combined tumor on MRI using liver imaging and data system (LI‐RADS) version 2014. Abdom Radiol (NY). 2018;43:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doussot A, Gonen M, Wiggers JK, et al. Recurrence patterns and disease‐free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223(493–505):e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kizy S, Altman AM, Marmor S, et al. Surgical resection of lymph node positive intrahepatic cholangiocarcinoma may not improve survival. HPB (Oxford). 2018;21:235‐241. [DOI] [PubMed] [Google Scholar]
- 95. Addeo P, Jedidi I, Locicero A, et al. Prognostic impact of tumor multinodularity in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 96. Conci S, Ruzzenente A, Viganò L, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25:3719–3727. [DOI] [PubMed] [Google Scholar]
- 97. Reames BN, Ejaz A, Koerkamp BG, et al. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi‐institutional analysis. J Surg Oncol. 2017;116:133–139. [DOI] [PubMed] [Google Scholar]
- 98. Lang H, de Santibañes E, Schlitt HJ, et al. 10th anniversary of ALPPS‐lessons learned and quo Vadis. Ann Surg. 2019;269:114–119. [DOI] [PubMed] [Google Scholar]
- 99. Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 101. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol. 2017;35:4006‐4006. [Google Scholar]
- 102. Ebata T, Mizuno T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg. 2018;105:829–838. [DOI] [PubMed] [Google Scholar]
- 103. Edeline J, Bonnetain F, Phelip JM, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12‐ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol. 2017;35:225‐225. [Google Scholar]
- 104. Buettner S, Koerkamp BG, Ejaz A, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients‐A multi‐institutional analysis. J Surg Oncol. 2017;115:312–318. [DOI] [PubMed] [Google Scholar]
- 105. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. [DOI] [PubMed] [Google Scholar]
- 106. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi‐institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. [DOI] [PubMed] [Google Scholar]
- 107. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bartsch F, Paschold M, Baumgart J, Hoppe‐Lotichius M, Heinrich S, Lang H. Surgical resection for recurrent intrahepatic cholangiocarcinoma. World J Surg. 2018;43:1105‐1116. [DOI] [PubMed] [Google Scholar]
- 109. Groot Koerkamp B, Fong Y. Outcomes in biliary malignancy. J Surg Oncol. 2014;110:585–591. [DOI] [PubMed] [Google Scholar]
- 110. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. [DOI] [PubMed] [Google Scholar]
- 111. Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg. 1995;19:807–813. [DOI] [PubMed] [Google Scholar]
- 112. Sapisochin G, Fernandez de Sevilla E, Echeverri J, Charco R . Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol. 2015;7:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sapisochin G, Facciuto M, Rubbia‐Brandt L, et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178–1188. [DOI] [PubMed] [Google Scholar]
- 114. Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta‐analysis of two randomised trials. Ann Oncol. 2014;25:391–398. [DOI] [PubMed] [Google Scholar]
- 115. Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead‐transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–443. [DOI] [PubMed] [Google Scholar]
- 116. Schiffman SC, Metzger T, Dubel G, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18:431–438. [DOI] [PubMed] [Google Scholar]
- 117. Konstantinidis IT, Koerkamp BG, Do R, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]


