Summary
The outbreak of bluetongue virus (BTV) serotype 8 (BTV‐8) during 2006–2009 in Europe was the most costly epidemic of the virus in recorded history. In 2015, a BTV‐8 strain re‐emerged in France which has continued to circulate since then. To examine anecdotal reports of reduced pathogenicity and transmission efficiency, we investigated the infection kinetics of a 2007 UK BTV‐8 strain alongside the re‐emerging BTV‐8 strain isolated from France in 2017. Two groups of eight BTV‐naïve British mule sheep were inoculated with 5.75 log10TCID50/ml of either BTV‐8 strain. BTV RNA was detected by 2 dpi in both groups with peak viraemia occurring between 5–9 dpi. A significantly greater amount of BTV RNA was detected in sheep infected with the 2007 strain (6.0–8.8 log10 genome copies/ml) than the re‐emerging BTV‐8 strain (2.9–7.9 log10 genome copies/ml). All infected sheep developed BTV‐specific antibodies by 9 dpi. BTV was isolated from 2 dpi to 12 dpi for 2007 BTV‐8‐inoculated sheep and from 5 to 10 dpi for sheep inoculated with the remerging BTV‐8. In Culicoides sonorensis feeding on the sheep over the period 7–12 dpi, vector competence was significantly higher for the 2007 strain than the re‐emerging strain. Both the proportion of animals showing moderate (as opposed to mild or no) clinical disease (6/8 vs. 1/8) and the overall clinical scores (median 5.25 vs. 3) were significantly higher in sheep infected with the 2007 strain, compared to those infected with the re‐emerging strain. However, one sheep infected with the re‐emerging strain was euthanized at 16 dpi having developed severe lameness. This highlights the potential of the re‐emerging BTV‐8 to still cause illness in naïve ruminants with concurrent costs to the livestock industry.
Keywords: arboviruses, bluetongue virus, European serotypes, infection kinetics, virus isolation RT‐qPCR
1. INTRODUCTION
Bluetongue (BT) is an infectious haemorrhagic disease of ruminants caused by the bluetongue virus (BTV) which is transmitted via Culicoides biting midges (Carpenter, Groschup, Garros, Felippe‐Bauer, & Purse, 2013). BTV is the type species of the genus Orbivirus within the family Reoviridae and is a serologically and genetically diverse virus. The BTV genome is comprised of 10 double‐stranded RNA segments encoding several structural and non‐structural proteins. BTV segment‐2 encodes the most variable BTV protein (VP2) which contains the majority of epitopes that interact with neutralising antibodies (Maclachlan, Henderson, Schwartz‐Cornil, & Zientara, 2014). Since 1998, a number of BTV serotypes have caused both sporadic and widespread incursions into the EU (Belbis et al., 2017).
In August 2006, a BTV serotype 8 (BTV‐8) strain of sub‐Saharan origin (Maan et al., 2008) was detected within animal holdings in The Netherlands, the first time that the virus had been identified in northern Europe. This BTV‐8 strain successfully re‐emerged in 2007 and subsequently spread throughout most northern European countries causing widespread clinical disease and major economic damage to the farming sector (Wilson & Mellor, 2009). However, in conjunction with animal movement restrictions, the implementation of vaccination campaigns by the EU member states was effective in reducing and eventually preventing further transmission of BTV‐8 and only sporadic outbreaks of the disease occurred in the EU by 2009.
In August 2015, BTV‐8 re‐emerged in France and subsequently spread throughout the entire country (Sailleau et al., 2017). How BTV‐8 persisted in areas that were thought to have been free of virus transmission remains unknown, although it has been recently proposed that low‐level circulation of BTV‐8 occurred in France prior to the detection in 2015 (Courtejoie et al., 2018). Importantly however, anecdotal observation suggested that changes had occurred in the epidemiology of the re‐emerging strain. Whereas the 2007–2009 BTV‐8 strain caused widespread clinical signs in cattle and sheep (Elbers, Spek, & Rijn, 2009; Zanella et al., 2013), the re‐emerging BTV‐8 strain has thus far only caused mild clinical illness (Sailleau et al., 2017). In addition, the rate of spread of the virus in France appeared slower than the original BTV‐8 strain. At the time of the BTV‐8 re‐emergence in 2015, it had been estimated that the ruminant herd immunity in France was 18% (Bournez et al., 2018).
Genetically, the re‐emerging BTV‐8 strain (GenBank accession numbers: KP56990–KP56999) differs from a 2007 UK BTV‐8 strain (accession numbers: KP820957, KP821077, KP821199, KP821319, KP821439, KP7821559, KP821681, KP821801, KP821921 and KP822042) in just 11 amino acids occurring in segments 1 (3), 2 (1), 3 (1), 4 (1), 8 (1), 9 (3) and 10 (1). The high degree of amino acid similarity between the 2007 and 2015 strains underpins the hypothesis that this is not a new introduction of BTV‐8 (Sailleau et al., 2017).
In this study we directly compare the infection of sheep with the original and re‐emerging European BTV‐8 strains by assessing viraemia, clinical signs, antibody production and transmission to Culicoides biting midges. By using British mule sheep, representative of the UK flock from a region not originally exposed to BTV‐8 infection, the aim is to understand if the changes in epidemiology and pathogenicity observed in France will also occur in regions beyond those affected by outbreaks from 2006 to 2009. The findings from this study will inform response in both the UK and other countries previously unaffected by BTV‐8 and allow for evidence‐based surveillance and control measures to be implemented.
2. MATERIALS AND METHODS
2.1. Ethical statement for animal studies
All animal experiments were carried out in accordance with the UK Animal Scientific Procedure Act (ASPA) 1986 which transposes the European Directive 2010/63/EU into UK national law. The animal studies were approved by the UK Home Office in granting Project licence 70/7819 under the ASPA and all protocols underwent appropriate local ethical review procedures by the Animal Welfare and Ethics Review Board of the Pirbright Institute.
2.2. Preparation of inocula
A 2017 French BTV‐8 strain (FRA2017) which had been isolated from Culicoides sonorensis (KC) cells through a single passage was provided by the French BTV NRL (Animal Health Laboratory, ANSES, Maisons‐Alfort). BTV‐8 isolate UKG2007/03 (UKG2007), isolated through a single passage from the 2007 UK index case was obtained from the Orbivirus Reference Collection, (Pirbright Institute, UK). Both the UKG2007 and FRA2017 strains were re‐passaged once on KC cells. Strains can be accessed via the European Virus Archive (https://www.european-virus-archive.com/evag-portal). Following harvest, viral inocula were titrated on KC cells using a 96‐well titration assay in combination with immunofluorescence microscopy. Briefly, 10‐fold serial dilutions of the virus were added to KC cells immediately following addition of the cells to 96 well tissue culture plates. After 5 days incubation at 26°C, the cells were fixed using 4% paraformaldehyde for 45 min and following multiple washes with PBS were permeabilized with 0.2% Triton X‐100 for 20 min following. Viral proteins were labelled using an anti‐BTV polyclonal guinea‐pig sera (ORAB279) and anti‐guinea‐pig‐Alexa488 and immunofluorescence was visualized on a Nikon Eclipse TE300 microscope to calculate viral titres according to Spearman‐Kärber. Subsequently, the inocula were adjusted using Schneiders media to yield a final titre of 5.75 log10 TCID50/ml.
2.3. Study design
Eighteen adult female British mule sheep, representing the most common British mixed breed of >7 years of age were chosen for use in this study. All sheep were previously tested as BTV‐antibody negative using C‐ELISA. The sheep were randomly assigned (see Supporting Information) into two groups of nine individuals. The two groups were housed in separate rooms of the Pirbright Institute's high containment animal facility. Eight sheep per group were inoculated with 1 ml subcutaneously in the left neck and 0.5 ml intradermally distributed over five inoculation sites into the inner left thigh of either UKG2007 or FRA2017. One sheep in each respective group was kept uninfected as a contact transmission control (Sheep 2 and Sheep 18). Blood samples (EDTA and whole blood) were taken from the jugular vein at—1, 2, 3, 5, 6, 7, 8, 9, 10, 12, 14, 16, 19, and 20/21 dpi. Sheep body temperatures were recorded daily and clinical scoring was performed throughout as described previously (Darpel et al., 2007).
2.4. Vector competence
Culicoides sonorensis from a colony maintained at The Pirbright Institute (PIRB‐s‐3 strain) were fed on two sheep from each group at 6 dpi (UKG2007) or 7 dpi (FRA2017) to coincide with determined peak viraemia as described previously (Baylis, O'Connell, & Mellor, 2008). Engorged individuals were incubated at 25°C for 8 days and surviving individuals were homogenized in GMEM using a tissue lyser (Qiagen) and made up to a final volume of 1 ml per sample (Veronesi et al., 2013). Culicoides samples were analyzed in pools of eight individuals for BTV genome detection by using RT‐qPCR as described below. Pools that reported a cycle threshold (C T value) less than that of the original blood meal in midges harvested on the day of feeding were considered to contain one or more individuals competent for BTV transmission.
2.5. Molecular analyses
2.5.1. Preparation of plasmid for quantitation in real‐time RT‐PCR assay
Plasmid pGEM‐3Zf(+) carrying a 97 bp sequence from BTV segment 10 (derived from the assay described by, Hofmann, Griot, Chaignat, Perler, & Thur, 2008) was transformed into JM109 competent cells (Promega), purified and then quantified using a nanodrop to create working stocks of 106 BTV genome copies/μl.
2.5.2. RNA extraction and RT‐qPCR analysis
BTV RNA was extracted from 100 μl of EDTA blood and eluted into 80 μl buffer using the KingFisher Flex automated extraction platform and the MagVet Universal nucleic acid extraction kit (ThermoFisher Scientific, Paisley, UK). Ten microliters of sample RNA was analyzed as per the assay described by (Hofmann et al., 2008) with modifications (Flannery et al., 2018) using the Express One‐Step qRT‐PCR kit (ThermoFisher) on an Applied Biosystems 7500 Fast instrument (ThermoFisher). A log‐dilution series of the plasmid (1 × 100–1 × 106 copies per μl) was included in triplicate on each RT‐qPCR run. BTV RNA copies were determined by comparing sample C T values to the standard curves and expressed as log10 genome copies/ml.
2.5.3. Serological analyses
Whole blood samples were centrifuged at 3,000 g for 5 min and the serum was decanted and stored at +4°C until analysis. BTV antibodies were detected using the ID Screen® Bluetongue Competition kit (ID Vet, Grabels, France) in accordance with the manufacturer's instructions. The serum neutralization test (SNT) against BTV‐8 was performed as described previously (Batten et al., 2012).
2.5.4. Virus isolation
EDTA blood cells were washed 3× with PBS and sonicated as described in the World Organisation for Animal Health (OIE) manual (Savini, 2014). KC cells were inoculated with 100 μl of washed blood and incubated at 26°C in Schneiders media (1% Amphotericin B, 1% Penicillin/Streptomycin and 10% FBS). Media was replenished 24 hr post infection and cells were incubated for a further 6 days. Following the 7‐day incubation, cells were harvested, centrifuged at 3,000 g for 5 min and the supernatant tested for BTV using RT‐qPCR as described above. Virus was considered to have been isolated if the BTV C T value of the harvested material was 3‐C T values less than that of the original EDTA‐blood inoculum.
2.6. Statistical analysis
Body temperatures and clinical scores were analyzed by comparing the maximum values and the dpi at which they occurred for each strain using the Wilcoxon rank‐sum (also known as Mann–Whitney U) tests. The proportion of sheep showing moderate (as opposed to mild or no) clinical disease in each group was compared using a Fisher exact test. Viraemia for sheep infected with each strain was analyzed by comparing the maximum titer, the dpi at which it occurred and the area under the curve (a measure of total virus production; computed using the trapezium rule) using Wilcoxon rank‐sum tests. Non‐parametric tests were preferred because of the small group sizes and potential non‐normality of the data.
The vector competence for each BTV‐8 strain was calculated from the number of positive pools and the number of pools tested using a binomial likelihood and noting that the probability of a positive pool is equal to 1 − (1 − b)n, where b is the vector competence (i.e., the probability of an individual midge becoming infected) and n is the number of midges in the pool. A second model was also considered in which vector competence was independent of strain, but depended on the viral titre (log10 genome copies/ml) in the sheep on which each pool of midges fed, so that b = 1/(1 + exp(−a 0 − a 1 × titer)).
3. RESULTS
3.1. Clinical observations
Typical clinical signs of BTV infection (fever, depression, facial oedema, reddening of the mucosal membrane and coronary bands) were seen more frequently and were more pronounced in the UKG2007‐inoculated sheep compared to the FRA2017‐inoculated sheep (Figure 1). Overall, 6/8 UKG2007‐inoculated sheep were classed as moderately ill, while two sheep were classed as mildly ill. By contrast, only one sheep was classed as moderately affected in the FRA2017‐inoculated sheep, with the remaining 7/8 being classed as mildly affected. The proportion of sheep showing moderate (as opposed to mild) clinical disease was significantly (p = 0.04) higher in sheep infected with UKG2007 compared with those infected with FRA2017. In addition, the maximum clinical score was significantly (p = 0.014) higher in sheep infected with UKG2007 (median score 5.25, range 2–6) compared with those infected with FRA2017 (median score 3, range 1.5–3.5), but the time at which the maximum score occurred did not differ significantly (p = 1.0) between the two groups of BTV‐8 inoculated sheep.
Figure 1.
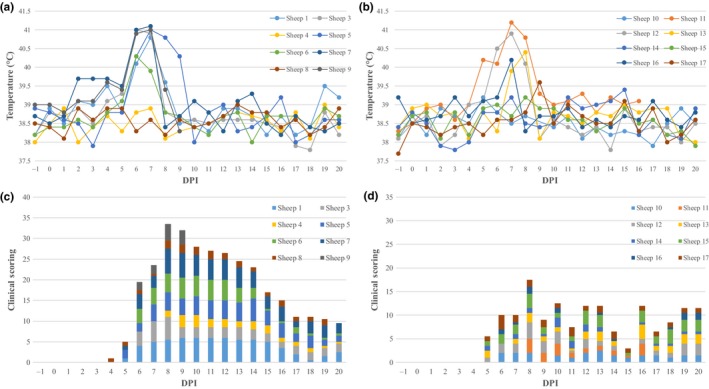
Daily temperatures of sheep inoculated with UKG2007 (a) and FRA2017 (c) and combined clinical scores of sheep inoculated with UKG2007 (b) and FRA2017 (d) [Colour figure can be viewed at wileyonlinelibrary.com]
Five of eight UKG2007‐inoculated sheep showed pyrexia (>40.5°C), in contrast with 2/8 FRA2017‐inoculated sheep (Figure 1). However, the difference between the groups was not statistically significant for either maximum temperature (p = 0.49) or the dpi at which this occurred (p = 0.76).
Two sheep were euthanized on welfare grounds during the study, one in each group. The sheep from the UKG2007‐inoculated group was euthanized at 9 dpi for reaching the humane endpoint (at the end of the moderate clinical spectrum) of the moderate study protocol. In this sheep, typical haemorrhagic lesions on mucosal membranes, coronary band, lymph nodes, pulmonary artery as well as the epithelium of the reticulum and omasum were observed during necropsy. The sheep from the FRA2017‐inoculated group developed acute lameness on all four feet late in infection and was euthanized on humane grounds at 17 dpi.
The two transmission control sheep remained clinically unaffected other than exhibiting a mild nasal discharge over a 2‐day period during the experiment.
3.2. Molecular analyses
Figure 2 shows the BTV concentrations in EDTA blood over the study period. In five UKG2007‐inoculated sheep and in three FRA2017‐inoculated sheep BTV RNA was detected by using RT‐qPCR at 2 dpi. BTV RNA was detected in all other inoculated sheep at 5 dpi with the exception of Sheep 17 (that had been inoculated with FRA2017).
Figure 2.
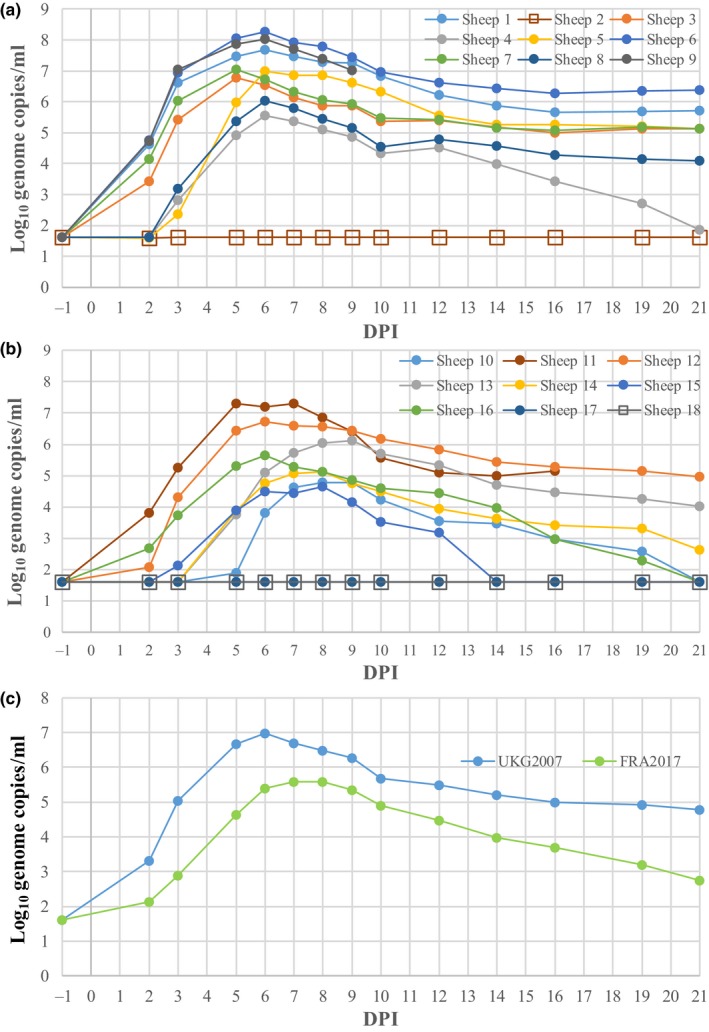
Bluetongue virus genome concentrations in sheep inoculated with UKG2007 (a) and FRA2017 (b) and mean genome concentrations for both groups (c). Sheep 2 and Sheep 18 are the control animals [Colour figure can be viewed at wileyonlinelibrary.com]
Peak viraemia occurred earlier for UKG2007‐inoculated sheep (between 5 and 6 dpi) than for FRA2017‐inoculated sheep (5–9 dpi), but this difference was not statistically significant (p = 0.11). At peak viraemia, the BTV concentrations in blood (log10 genome copies/ml) were significantly (p = 0.021) higher for UKG2007‐inoculated sheep (median 7.0; range: 5.5–8.3) compared with FRA2017‐inoculated sheep (median 5.4; range: 1.6–7.3). Furthermore, the area under the curve (a measure of total virus production) was significantly (p = 0.028) greater for UKG2007‐inoculated sheep (median 7.5; range: 6.0–8.8) compared with FRA2017‐inoculated sheep (median 5.9; range: 2.9–7.9).
At 21 dpi in the remaining animals, three sheep inoculated with the FRA2017 strain had cleared the infection. BTV remained detectable in all seven sheep and in three sheep inoculated with UKG2007 and FRA2017 strains, respectively. At the end of the experiment (21 dpi), the mean BTV concentrations were 5.26 (range: 4.08–5.70) and 3.87 (range: 2.63 and 4.97) log10 genome copies/ml for UKG2007 and FRA2017‐inoculated sheep respectively. BTV RNA was not detected in the transmission controls or in Sheep 17 throughout the experiment.
3.3. Vector competence
A total of 272 individual C. sonorensis fed on Sheep 6 and Sheep 9 that were infected with the UKG2007 strain survived incubation and this equated to 34 pools of midges for testing. A total of 280 individuals fed on Sheep 11 and Sheep 12 that were infected with FRA2017 were tested in 36 pools. Three midge pools from the UKG2007‐inoculated sheep had lower C T values than the mean value obtained in C. sonorensis tested on the day of feeding (C T 30.9). C. sonorensis tested on the day of feeding on FRA2017‐infected sheep had a mean C T value of 34.7. BTV RNA was not detected in any midge pools from the FRA2017‐inoculated sheep.
Based on these results for pooled midges, the estimated vector competence (i.e., probability of an individual midge becoming infected) was 1.2% (95% CI: 0.3, 3.0) for UKG2007 and 0% (95% CI: 0, 0.7) for FRA2017, which are significantly different. Viral titres (log10 genome copies/ml) in the sheep infected with UKG2007 were higher on the day of Culicoides feeding than for those infected with FRA2017 (8.3 and 8.0 compared with 7.3 and 6.6, respectively). An increase in competence was associated with an increase in titre, but this was not statistically significant (estimate for coefficient, a 1: 2.6; 95% CI: −0.1, 3.6).
3.4. Serological analyses
Figure 3 shows the BTV antibody response using C‐ELISA. In the sheep inoculated with UKG2007, five of the eight seroconverted by 7 dpi, and by 8 dpi all inoculated sheep had seroconverted (defined as <40% inhibition by the manufacturer). In the group inoculated with FRA2017, none of the sheep seroconverted at 7 dpi yet all had seroconverted by 9 dpi. Based on the ELISA results, SNTs were performed on serum samples obtained at 7, 9, 12, 14 and 21 dpi. Neutralizing antibodies were first detected at 7 and 9 dpi for UKG2007 and FRA2017‐inoculated sheep, respectively. By 14 dpi, all sheep had neutralizing antibodies with titres (log10) between 1.18 and 2.68, rising to between 1.60 and 2.81 at 21 dpi. The mean antibody titres at 21 dpi were 2.01 and 1.54 for the UKG2007 and FRA2017‐inoculated sheep, respectively. The transmission control sheep in both groups did not seroconvert.
Figure 3.
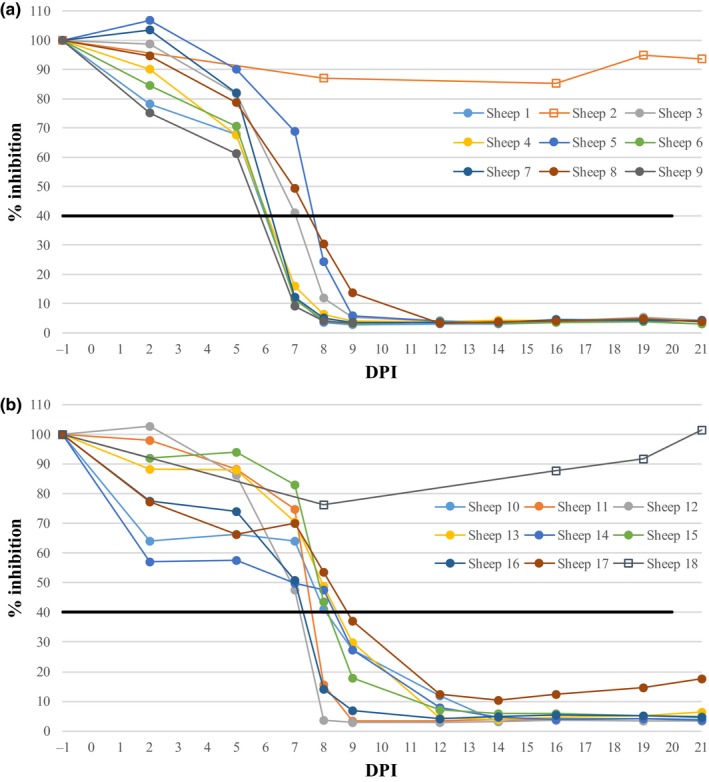
Antibody response of sheep inoculated with UKG2007 (a) and FRA2017 (b) as determined by C‐ELISA. The dashed line represents the positive/negative cut off of the ELISA whereby values <40% inhibition are considered positive. Sheep 2 and Sheep 18 are the control animals [Colour figure can be viewed at wileyonlinelibrary.com]
3.5. Virus isolation
Virus isolation from EDTA blood was performed for all inoculated sheep at 2, 5, 7, 10, 12, 14, 16 and 21 dpi. For UKG2007‐inoculated sheep, virus was isolated at 2 dpi (3/8 sheep), 5 dpi (7/8 sheep), 7–10 dpi (8/8 sheep) until 12 dpi (1 sheep). For FRA2017‐inoculated sheep, virus was isolated at 5–7 dpi (6/8 sheep) until 10 dpi (4/8 sheep).
4. DISCUSSION
Constant and largely unpredictable outbreaks of BTV present a significant global challenge in terms of proportionate response. This is exacerbated by our inability to accurately define the pathogenicity of emerging and re‐emerging BTV strains, which is the main determining factor in vaccine production by industry and subsequent uptake by farmers. The aim of this study was to compare the infection kinetics and clinical severity of a re‐emerging BTV‐8 strain in sheep to understand the impact of this strain in areas not affected by BTV‐8 during 2006–2009. The study confirms anecdotal evidence from the field that viraemia and clinical signs are reduced in the re‐emerging strain. However, this strain may significantly impact upon naïve sheep populations as evidenced by one sheep developing acute lameness during the convalescence phase of infection.
British mule sheep were used in this study as representative of the UK flock and seem slightly more resistant to the UKG2007 strain than pure‐bred Dorset Poll sheep when infected with the UK BTV‐8 strain (Moulin et al., 2012). Indeed, one sheep inoculated with the 2007 strain had almost cleared the infection by the end of the experiment (21 dpi). This variability in the severity of BTV infection has been attributed to numerous host factors, such as the breed, health and age of the ruminant (Caporale et al., 2014; Maclachlan, Drew, Darpel, & Worwa, 2009). It has been previously reported that in field settings, environmental factors, such as exposure to sunlight, elevated temperatures, stress and bacterial or viral co‐infections can exacerbate BT clinical signs in sheep (Kyriakis et al., 2015). Therefore, the clinical presentation of infection with the BTV‐8 strains in the field is likely to be more severe than what we have reported in this study where animals were housed within the high‐sanitary and controlled‐husbandry conditions of the containment facilities. This highlights that infection with the re‐emerging BTV‐8 strain may still have a considerable economic impact in the field.
Interestingly, one of the sheep inoculated with the re‐emerging BTV‐8 strain did not develop detectable viraemia throughout the entire study, yet seroconverted and developed a neutralizing antibody response. This suggests that the sheep did receive the inoculum. Historically studies have previously reported that ruminants experimentally‐inoculated with BTV did not develop detectable viraemia (in the presence or absence of seroconversion), although viraemia detection was based on virus isolation rather than the more sensitive use of real‐time PCR (Baylis et al., 2008; Flanagan, Wilson, Trueman, & Shepherd, 1982; Parsonson et al., 1987). Other studies have further noted seroconversion of individual BTV‐inoculated sheep or cattle either in the presence of very low detectable RNA levels by RT‐qPCR (van der Sluijs et al., 2013) or in the absence of detectable viraemia, however, the latter was associated with either a low‐titre (Baylis et al., 2008; Di Gialleonardo, Migliaccio, Teodori, & Savini, 2011) or a high‐passage inoculum (Janowicz et al., 2015). Interestingly, a recent study also highlighted that a highly‐passaged BTV‐8 strain infected and was detectable in the skin and draining lymph nodes of inoculated sheep however did not result in detectable RNA levels in the systemic blood (Melzi et al., 2016). Overall all these studies, including the data presented here, demonstrate that manifestation of clinical disease and viraemia varies in individual animals even when infected by the same route and with the same dosage of virus, highlighting differences in individual susceptibility to BTV infection. Alternatively, it is possible that this sheep had residual immunological memory from previous vaccination which could provide some protection from infection even in the absence of detectable antibodies. BTV antibodies can be detected in sheep up to 2.5 years following vaccination (Batten, Edwards, & Oura, 2013) and it has been estimated that they can persist for 5–6 years following infection or vaccination (Bournez et al., 2018). The sheep used in our study were at an advanced age (>7 years old), as reported by the farmer and by assessing the general condition of the sheep.
Significantly greater RNA copy numbers were detected in sheep inoculated with the 2007 strain than the re‐emerging strain. In addition, BTV was isolated more frequently from sheep inoculated with the 2007 strain. By the end of the experiment, three sheep inoculated with the re‐emerging strain had also cleared infection (BTV RNA was not detected). This reduced viraemia was reflected in the quantity of BTV RNA imbibed by C. sonorensis implying that monitoring through the venous route was representative of the quantity of viral RNA in the skin. Furthermore, C. sonorensis demonstrated a higher vector competence (1.2%) towards the 2007 strain than the re‐emerging strain. The lower vector competence observed using FRA2017 is possibly related to lower viral titres in the blood of sheep infected with this strain, though the small number of sheep used for the vector infection studies preclude drawing robust conclusions about the relationship between competence and viraemia. Overall, these results suggest that the re‐emerging strain elicits a lower viraemia, for a shorter duration in sheep and is less efficient at infecting Culicoides biting midges. These findings are consistent with the relatively slow spread of the re‐emerged BTV‐8 strain which has remained largely within the French borders since circulation was detected in August 2015.
The infection kinetics of the two BTV‐8 strains were similar to each other and were in‐line with those reported for other BTV serotypes in sheep (Schulz et al., 2018; van der Sluijs et al., 2013). However, the re‐emerging strain was found to be less virulent than the 2007 strain. It has been postulated that the re‐emerging strain has been circulating asymptomatically in wildlife since the end of the 2006–2009 BTV‐8 epizootic (Sailleau et al., 2017). The reason behind the attenuation of BTV strains over a period of time has been attributed to selective pressures placed on circulating BTV serotypes during replication within their mammalian hosts and vectors (Caporale et al., 2014). The respective lineages of the two BTV‐8 strains used in this study differ by a few amino acids, thus, the impact of future genomic mutations in the re‐emerging BTV‐8 strain is uncertain. Nonetheless, detailed genomic comparison between different BTV strains used in experimental infection studies may increase our overall understanding of the molecular determinants of BTV virulence.
We have performed this experimental infection study in sheep; therefore, the impact of the re‐emerged BTV‐8 strain in cattle is yet to be determined experimentally. Previous experimental infection studies of other European BTV‐8 strains in cattle did not result in overt disease or caused mild clinical signs which were in contrast with the clinical illness reported in sheep (Darpel et al., 2007; Di Gialleonardo et al., 2011; Martinelle et al., 2011). An experimental comparison of BTV‐8 strain‐specific virulence (similar to our study here) is likely to be more challenging in cattle.
Considering that morbidity rates in field conditions are likely to be higher than what we have reported here and coupled with animal movement restrictions, it is probable that future incursions of the re‐emerged BTV‐8 will have an economic impact on the livestock industry. Therefore, appropriate surveillance and vaccination strategies should be considered in advance of the likely incursion of this re‐emerged BTV‐8 strain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGEMENTS
This study was funded by the European Commission and the Department for Environment, Food and Rural Affairs (Defra), grant number: SE2621 and Biotechnology and Biological Sciences Research Council (BBSRC) through projects BBS/E/I/00007030, BBS/E/I/00007033, BBS/E/I/00007036 and BBS/E/I/00007037. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Flannery J, Sanz‐Bernardo B, Ashby M, et al. Evidence of reduced viremia, pathogenicity and vector competence in a re‐emerging European strain of bluetongue virus serotype 8 in sheep. Transbound Emerg Dis. 2019;66:1177–1185. 10.1111/tbed.13131
REFERENCES
- Batten, C. A. , Edwards, L. , & Oura, C. A. L. (2013). Evaluation of the humoral immune responses in adult cattle and sheep, 4 and 2.5 years post‐vaccination with a bluetongue serotype 8 inactivated vaccine. Vaccine, 31, 3783–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten, C. A. , Henstock, M. R. , Bin‐Tarif, A. , Steedman, H. M. , Waddington, S. , Edwards, L. , & Oura, C. A. L. (2012). Bluetongue virus serotype 26: Infection kinetics and pathogenesis in Dorset Poll sheep. Veterinary Microbiology, 157, 119–124. [DOI] [PubMed] [Google Scholar]
- Baylis, M. , O'Connell, L. , & Mellor, P. S. (2008). Rates of bluetongue virus transmission between Culicoides sonorensis and sheep. Medical and Veterinary Entomology, 22, 228–237. [DOI] [PubMed] [Google Scholar]
- Belbis, G. , Zientara, S. , Breard, E. , Sailleau, C. , Caignard, G. , Vitour, D. , & Attoui, H. (2017). Bluetongue virus: From BTV‐1 to BTV‐27. Advances in Virus Research, 99, 161–197. [DOI] [PubMed] [Google Scholar]
- Bournez, L. , Cavalerie, L. , Sailleau, C. , Breard, E. , Zanella, G. , de Almeida, R. S. , … Calavas, D. (2018). Estimation of French cattle herd immunity against bluetongue serotype 8 at the time of its re‐emergence in 2015. BMC Veterinary Research, 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale, M. , Di Gialleonorado, L. , Janowicz, A. , Wilkie, G. , Shaw, A. , Savini, G. , … Palmarini, M. (2014). Virus and host factors affecting the clinical outcome of bluetongue virus infection. Journal of Virology, 88, 10399–10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, S. , Groschup, M. H. , Garros, C. , Felippe‐Bauer, M. L. , & Purse, B. V. (2013). Culicoides biting midges, arboviruses and public health in Europe. Antiviral Research, 100, 102–113. [DOI] [PubMed] [Google Scholar]
- Courtejoie, N. , Durand, B. , Bournez, L. , Gorlier, A. , Breard, E. , Sailleau, C. , … Zanella, G. (2018). Circulation of bluetongue virus 8 in French cattle, before and after the re‐emergence in 2015. Transboundary and Emerging Diseases, 65, 281–284. [DOI] [PubMed] [Google Scholar]
- Darpel, K. E. , Batten, C. A. , Veronesi, E. , Shaw, A. E. , Anthony, S. , Bachanek‐Bankowska, K. , … Oura, C. A. L. (2007). Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Veterinary Record, 161, 253–261. [DOI] [PubMed] [Google Scholar]
- Di Gialleonardo, L. , Migliaccio, P. , Teodori, L. , & Savini, G. (2011). The length of BTV‐8 viraemia in cattle according to infection doses and diagnostic techniques. Research in Veterinary Science, 91, 316–320. [DOI] [PubMed] [Google Scholar]
- Elbers, A. R. W. , Spek, A. N. , & Rijn, P. A. (2009). Epidemiologic characteristics of bluetongue virus serotype 8 laboratory‐confirmed outbreaks in The Netherlands in 2007 and a comparison with the situation in 2006. Preventive Veterinary Medicine, 92, 1–8. [DOI] [PubMed] [Google Scholar]
- Flanagan, M. , Wilson, A. J. , Trueman, K. F. , & Shepherd, M. A. (1982). Bluetongue virus serotype‐20 infection in pregnant merino sheep. Australian Veterinary Journal, 59, 18–20. [DOI] [PubMed] [Google Scholar]
- Flannery, J. , Rajko‐Nenow, P. , Hicks, H. , Hill, H. , Gubbins, S. , & Batten, C. (2018). Evaluating the most appropriate pooling ratio for EDTA blood samples to detect bluetongue virus using real‐time RT‐PCR. Veterinary Microbiology, 217, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, M. , Griot, C. , Chaignat, V. , Perler, L. , & Thur, B. (2008). Blauzungenkrankheit erreicht die Schweiz. Schweizer Archiv für Tierheilkunde, 150, 49–56. [DOI] [PubMed] [Google Scholar]
- Janowicz, A. , Caporale, M. , Shaw, A. , Gulletta, S. , Di Gialleonardo, L. , Ratinier, M. , & Palmarini, M. (2015). Multiple genome segments determine virulence of bluetongue virus serotype 8. Journal of Virology, 89, 5238–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis, C. S. , Billinis, C. , Papadopoulos, E. , Vasileiou, N. G. C. , Athanasiou, L. V. , & Fthenakis, G. C. (2015). Bluetongue in small ruminants: An opinionated review, with a brief appraisal of the 2014 outbreak of the disease in Greece and the south‐east Europe. Veterinary Microbiology, 181, 66–74. [DOI] [PubMed] [Google Scholar]
- Maan, S. , Maan, N. S. , Ross‐Smith, N. , Batten, C. A. , Shaw, A. E. , Anthony, S. J. , … Mertens, P. P. C. (2008). Sequence analysis of bluetongue virus serotype 8 from the Netherlands 2006 and comparison to other European strains. Virology, 377, 308–318. [DOI] [PubMed] [Google Scholar]
- Maclachlan, N. J. , Drew, C. P. , Darpel, K. E. , & Worwa, G. (2009). The pathology and pathogenesis of Bluetongue. Journal of Comparative Pathology, 141, 1–16. [DOI] [PubMed] [Google Scholar]
- Maclachlan, N. J. , Henderson, C. , Schwartz‐Cornil, I. , & Zientara, S. (2014). The immune response of ruminant livestock to bluetongue virus: From type I interferon to antibody. Virus Research, 182, 71–77. [DOI] [PubMed] [Google Scholar]
- Martinelle, L. , Dal Pozzo, F. , Sarradin, P. , De Leeuw, I. , De Clercq, K. , Thys, C. , … Saegerman, C. (2011). Two alternative inocula to reproduce bluetongue virus serotype 8 disease in calves. Vaccine, 29, 3600–3609. [DOI] [PubMed] [Google Scholar]
- Melzi, E. , Caporale, M. , Rocchi, M. , Martin, V. , Gamino, V. , di Provvido, A. , … Palmarini, M. (2016). Follicular dendritic cell disruption as a novel mechanism of virus‐induced immunosuppression. Proceedings of the National Academy of Sciences of the United States of America, 113, E6238–E6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin, V. , Noordegraaf, C. V. , Makoschey, B. , van der Sluijs, M. , Veronesi, E. , Darpel, K. , … de Smit, H. (2012). Clinical disease in sheep caused by bluetongue virus serotype 8, and prevention by an inactivated vaccine. Vaccine, 30, 2228–2235. [DOI] [PubMed] [Google Scholar]
- Parsonson, I. M. , Dellaporta, A. J. , McPhee, D. A. , Cybinski, D. H. , Squire, K. R. E. , & Uren, M. F. (1987). Experimental infection of bulls and cows with bluetongue virus serotype‐20. Australian Veterinary Journal, 64, 10–13. [DOI] [PubMed] [Google Scholar]
- Sailleau, C. , Breard, E. , Viarouge, C. , Vitour, D. , Romey, A. , Garnier, A. , … Zientara, S. (2017). Re‐emergence of bluetongue virus serotype 8 in France, 2015. Transboundary and Emerging Diseases, 64, 998–1000. [DOI] [PubMed] [Google Scholar]
- Savini, G. (2014). Bluetongue (infection with bluetongue virus). Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: Office of International Des Epizooties. [Google Scholar]
- Schulz, C. , Sailleau, C. , Breard, E. , Flannery, J. , Viarouge, C. , Zientara, S. , … Hoffmann, B. (2018). Experimental infection of sheep, goats and cattle with a bluetongue virus serotype 4 field strain from Bulgaria, 2014. Transboundary and Emerging Diseases, 65, e243–e250. [DOI] [PubMed] [Google Scholar]
- van der Sluijs, M. T. W. , Schroer‐Joosten, D. P. H. , Fid‐Fourkour, A. , Smit, M. , Vrijenhoek, M. P. , Moulin, V. , … Moormann, R. J. M. (2013). Transplacental transmission of BTV‐8 in sheep: BTV viraemia, antibody responses and vaccine efficacy in lambs infected in utero. Vaccine, 31, 3726–3731. [DOI] [PubMed] [Google Scholar]
- Veronesi, E. , Antony, F. , Gubbins, S. , Golding, N. , Blackwell, A. , Mertens, P. P. , … Carpenter, S. (2013). Measurement of the infection and dissemination of bluetongue virus in culicoides biting midges using a semi‐quantitative rt‐PCR assay and isolation of infectious virus. PLoS ONE, 8, e70800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A. J. , & Mellor, P. S. (2009). Bluetongue in Europe: Past, present and future. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 2669–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella, G. , Martinelle, L. , Guyot, H. , Mauroy, A. , Clercq, K. , & Saegerman, C. (2013). Clinical pattern characterization of cattle naturally infected by BTV‐8. Transboundary and Emerging Diseases, 60, 231–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


