Abstract
Apicomplexan parasites including Toxoplasma gondii and Plasmodium spp. manufacture a complex arsenal of secreted proteins used to interact with and manipulate their host environment. These proteins are organised into three principle exocytotic compartment types according to their functions: micronemes for extracellular attachment and motility, rhoptries for host cell penetration, and dense granules for subsequent manipulation of the host intracellular environment. The order and timing of these events during the parasite's invasion cycle dictates when exocytosis from each compartment occurs. Tight control of compartment secretion is, therefore, an integral part of apicomplexan biology. Control of microneme exocytosis is best understood, where cytosolic intermediate molecular messengers cGMP and Ca2+ act as positive signals. The mechanisms for controlling secretion from rhoptries and dense granules, however, are virtually unknown. Here, we present evidence that dense granule exocytosis is negatively regulated by cytosolic Ca2+, and we show that this Ca2+‐mediated response is contingent on the function of calcium‐dependent protein kinases TgCDPK1 and TgCDPK3. Reciprocal control of micronemes and dense granules provides an elegant solution to the mutually exclusive functions of these exocytotic compartments in parasite invasion cycles and further demonstrates the central role that Ca2+ signalling plays in the invasion biology of apicomplexan parasites.
Keywords: Apicomplexa, calcium, dense granules, Protozoa, signalling, Toxoplasma
1. INTRODUCTION
Apicomplexan parasites comprise a large phylum of primarily obligate intracellular parasites of humans and animals that have a significant impact on human health and livestock production. Notable apicomplexan genera include blood parasites Plasmodium (causative agents of malaria), Babesia and Theileria (common cattle parasites), enteric epithelial parasites Cryptosporidium and Eimeria, and systemic parasites Toxoplasma and Neospora. The phylum embraces at least 6,000 species with global distribution infecting animals and even other protists (Adl et al., 2012). Moreover, metagenomic environmental sampling shows that apicomplexans can be dominant components of natural communities, indicating significant roles in ecosystems and the evolutionary success of this group (de Vargas et al., 2015; Mahé et al., 2017). One key to the success of apicomplexans is their efficient infection cycles in which they select their host cell, penetrate it nondestructively, feed and multiply within this cell while subduing or deflecting host organism defences, and finally escape from the host cell, releasing multiple progeny (Blader, Coleman, Chen, & Gubbels, 2015). This cycle is largely mediated by co‐ordinated release of a number of different exocytotic compartments delivering cargo to a range of extracellular destinations.
Toxoplasma gondii has served as a model for apicomplexan infection cycle events with three categories of secretory compartments identified—micronemes, rhoptries, and dense granules—that facilitate the major events of the invasion cycle (Carruthers & Sibley, 1997). Micronemes are exocytosed when the parasite is searching for a host cell, and secreted microneme proteins (MICs) decorate the parasite cell surface to act as attachment ligands and enable the characteristic gliding motility of the group (Frénal, Dubremetz, Lebrun, & Soldati‐Favre, 2017). Upon selection of a cell to invade, proteins from rhoptry organelles are then secreted into the host, forming a “moving junction” entry structure through which the parasite penetrates the host (Guérin et al., 2017). As the parasite enters, a host plasma membrane‐derived parasitophorous vacuole (PV) invaginates and surrounds the parasite. PV formation is accompanied by secretion of further rhoptry proteins into the host, some of which actively block host attack of this new internal foreign body (Etheridge et al., 2014; Håkansson, Charron, & Sibley, 2001). Completion of invasion isolates the PV from the plasma membrane, and a third wave of secretion from the dense granules now occurs (Carruthers & Sibley, 1997; Dubremetz, Achbarou, Bermudes, & Joiner, 1993; Mercier & Cesbron‐Delauw, 2015; Sibley, Niesman, Parmley, & Cesbron‐Delauw, 1995). Dense granule proteins (GRAs) populate and modify the PV membrane for nutrient uptake and help create an elaborate PV‐contained membranous nanotubular network (Mercier, Adjogble, Däubener, & Delauw, 2005; Sibley et al., 1995). Other GRAs target the host cytoplasm and nucleus and actively reprogram host cell regulatory pathways and functions to facilitate parasite survival and growth (Hakimi, Olias, & Sibley, 2017). After multiple rounds of parasite division, a new infection cycle begins with the secretion of MICs that disrupt host membranes and reactivate gliding motility for escape, dissemination, and targeting of new host cells (Kafsack et al., 2009). Broadly, control of secretion from micronemes is critical for the extracellular stages of the Toxoplasma infection cycle, control of rhoptry release for the invasion events, and control of dense granule release for the establishment and maintenance of the host cell environment for the parasite. The coordination of organelle‐specific exocytosis is, therefore, a central feature of the parasite's biology.
Only the control of microneme exocytosis has been studied and illuminated in any detail. The elevation of cytosolic calcium ion (Ca2+) levels by release from intracellular stores signals release of MICs to the extracellular environment (Carruthers, Giddings, & Sibley, 1999; Sidik et al., 2016). Ca2+ also stimulates other processes, including extrusion of the conoid and activation of motility, so Ca2+ signalling is clearly part of a broader signalling network of the extracellular events of the invasion cycle (Billker, Lourido, & Sibley, 2009; Borges‐Pereira et al., 2015; Graindorge et al., 2016; Stewart et al., 2017; Tang et al., 2014; Wetzel, Chen, Ruiz, Moreno, & Sibley, 2004). Two Ca2+‐dependent protein kinases, TgCDPK1 and TgCDPK3, are major controllers of Ca2+‐dependent extracellular processes, including MIC secretion (Lourido et al., 2010; Lourido, Jeschke, Turk, & Sibley, 2013; Lourido, Tang, & Sibley, 2012; McCoy, Whitehead, van Dooren, & Tonkin, 2012; Treeck et al., 2014). Loss of function of either results in changes to Ca2+‐induced microneme exocytosis, although changes are not identical, suggesting some level of specialisation and/or cooperativity of these kinases (Lourido et al., 2012). Numerous protein substrates have been identified for both TgCDPK1 and TgCDPK3, further evidence for an elaborate signalling network that they control (Lourido et al., 2013; Treeck et al., 2014). Ca2+ also has a downstream and direct role for MIC release with exocytosis of micronemes at the apical plasma membrane facilitated by DOC2.1 that recruits the membrane fusion machinery in a Ca2+‐dependent manner (Farrell et al., 2012).
Other signalling molecules and stimuli occur upstream of Ca2+ and illustrate an even broader network of control processes for parasite sensing of cues for its invasion cycle (Carruthers, Moreno, & Sibley, 1999). Cyclic guanosine monophosphate (cGMP) activates protein kinase G (PKG), and PKG in turn triggers cytosolic Ca2+ flux in Toxoplasma and Plasmodium (Brochet et al., 2014; Sidik et al., 2016; Stewart et al., 2017). In Plasmodium berghei, PKG acts upon phosphoinositide metabolism that ultimately releases inositol (1,4,5)‐trisphosphate (IP3) from diacylglycerol (DAG), and IP3 releases Ca2+ stores in many systems (Brochet et al., 2014; Schlossmann et al., 2000). This phospholipid metabolism, notably DAG to phosphatidic acid (PA) interchange, has also been shown to contribute directly to microneme docking at the plasma membrane in Toxoplasma, further implicating cGMP‐controlled events in MIC release (Bullen et al., 2016). The ultimate stimulation mechanism(s) for these cGMP‐ and Ca2+‐dependent events has not been identified; however, in Toxoplasma, both a reduction in extracellular pH and potassium ion levels appear to have important roles as a cues for these processes (Endo, Tokuda, Yagita, & Koyama, 1987; Roiko, Svezhova, & Carruthers, 2014). Upon successful entry of parasites into their host cell, all of this activation for MIC release and motility must then be supressed in order for the rhoptry‐ and dense granule‐mediated intracellular events to progress. Recent work suggests that cAMP‐signalling that activates protein kinase A (PKA) is involved in reducing cytosolic Ca2+ levels upon host cell entry and, in turn, reversing the processes that led to MIC secretion (Jia et al., 2017; Uboldi et al., 2018).
Whereas Ca2+ and cGMP have emerged as central signals for positive control of microneme exocytosis, almost nothing is known about how secretion from rhoptries and dense granules is controlled. Dense granules present a particular conundrum, for although some secretion through these organelles has been characterised as unregulated or constitutive, a strong burst of GRA secretion occurs as a postinvasion event, implying some mechanism for its control (Carruthers & Sibley, 1997; Chaturvedi et al., 1999; Coppens, Andries, Liu, & Cesbron‐Delauw, 1999; Dubremetz et al., 1993; Mercier & Cesbron‐Delauw, 2015; Sibley et al., 1995). Nevertheless, GRA secretion from extracellular parasites is detectable and has often been used as a presumed invariant secretion control in assays of regulated MIC release. This assumption of GRA behaviour, however, has never been thoroughly tested, and, in fact, a decrease in GRA secretion has been seen (but rarely commented upon) when extracellular parasites are treated with some Ca2+ agonists (Carruthers, Moreno, & Sibley, 1999; Farrell et al., 2012; Kafsack et al., 2009; Paul et al., 2015).
Here, we have examined the role of Ca2+ and cGMP in the regulation of GRA secretion in extracellular tachyzoites using a range of modulators of both Ca2+ and cGMP levels. We have also tested for GRA secretion behaviour in mutant cell lines of TgCDPK1, TgCDPK3, and TgRNG2, and using kinase inhibitors, all of which have known defects in Ca2+‐ or cGMP‐dependent secretion (Katris et al., 2014; Lourido et al., 2012; McCoy et al., 2012). Our data consistently indicate that Ca2+ has a role in negatively controlling GRA secretion, providing a reciprocal control mechanism to that of MIC secretion in extracellular parasites.
2. RESULTS
2.1. Agonists and antagonists of cytosolic Ca2+ inversely modulated microneme and dense granule exocytotis
To test for any Ca2+‐dependent responses in dense granule exocytosis from extracellular tachyzoites, we applied a range of concentrations of two commonly used Ca2+ ionophores, ionomycin and A23187, which induce a range in levels of MIC secretion response. A gradual increase in MIC2 secretion (above constitutive levels) was observed with increasing concentrations of both ionomycin and A23187 from 1 to 5μM (Figure 1a). We simultaneously assayed for secretion of the dense GRAs GRA1, GRA2, and GRA5. In all cases, we saw an inverse response to the ionophore treatment, with decreased secretion of dense GRAs observed with increasing ionophore concentration (Figure 1a). We also assayed for MIC5 secretion, a protein that is proteolytically processed before sorting to the micronemes. The shorter, processed form is secreted from the micronemes, whereas the longer pro‐form of the protein (proMIC5) is believed to take an alternative route, and its secretion has been described as constitutive (Brydges, Harper, Parussini, Coppens, & Carruthers, 2008). We saw reciprocal responses of MIC5 and proMIC5 secretion with Ca2+ ionophore treatment: MIC5 release responded positively to Ca2+, as for MIC2; whereas proMIC5 showed reduced secretion with Ca2+, similar to the GRA protein responses (Figure 1a).
Figure 1.
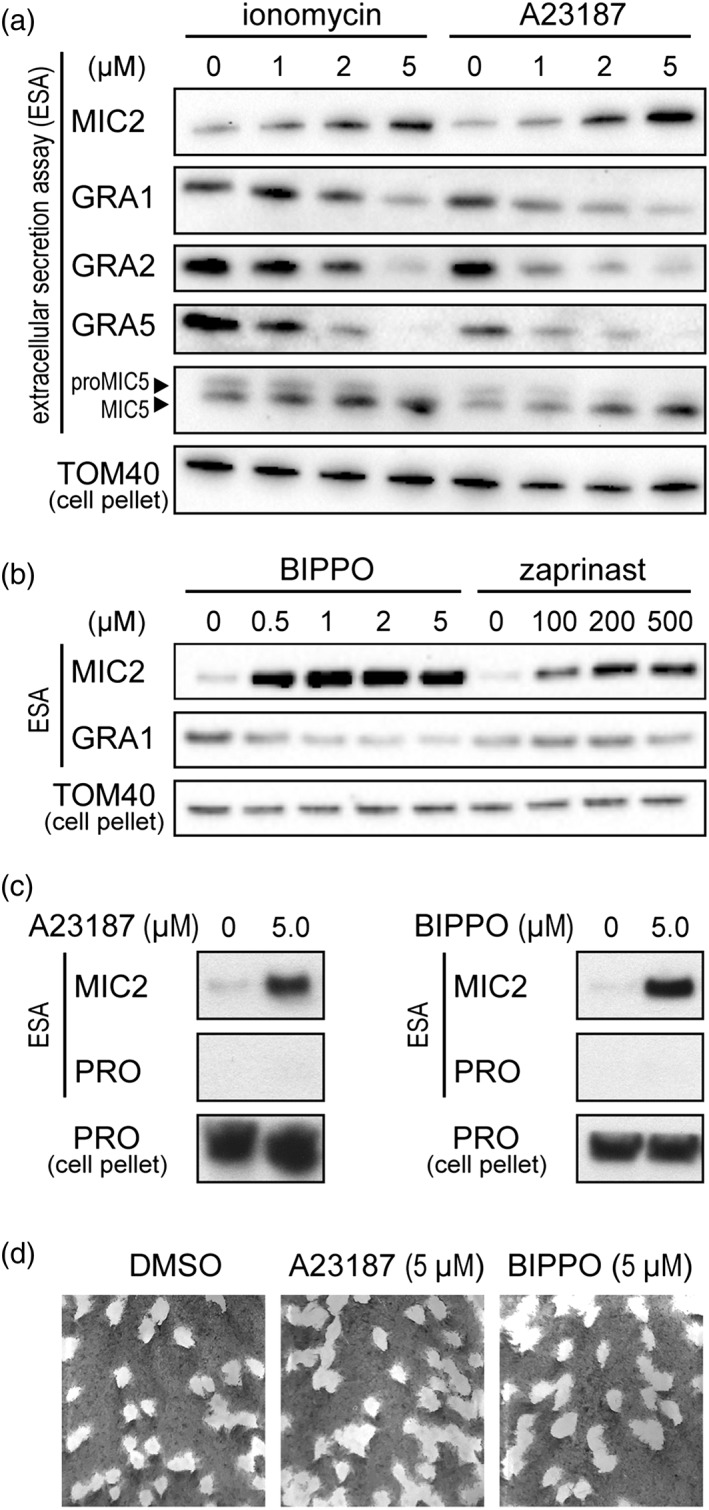
Microneme and dense granule secretion responses to Ca2+‐ and cGMP‐based stimulation. Secreted proteins assayed by western blot of select microneme proteins (MICs) and granule proteins (GRAs) in response to Ca2+ ionophores ionomycin and A23187 (a) and phosphodiesterase inhibitors BIPPO and zaprinast (b). Mitochondrial protein TOM40 in parasite pellets serve as parasite equivalent loading controls for the extracellular secretion assays (ESA). (c) Profilin (PRO) release to the extracellular environment was used to test for cell lysis during the secretion assay. (d) Plaque assays were performed with tachyzoites after the secretion assay and tested for maintenance of cell viability after this procedure. The vehicle DMSO was used in the no‐stimulation controls
Cytosolic Ca2+ levels can also be indirectly elevated by activating the PKG signalling pathway with cGMP (Sidik et al., 2016; Stewart et al., 2017). Two phosphodiesterase inhibitors have been widely used to increase cGMP levels: zaprinast, and a more potent analogue 5‐benzyl‐3‐iso‐propyl‐1H‐pyrazolo[4.3‐d]pyrimindin‐7(6H)‐one (BIPPO; Howard et al., 2015). Increasing concentrations of BIPPO and zaprinast over a range shown to stimulate MIC secretion (0–5μM and 0–500μM, respectively) were applied to tachyzoites. MIC2 secretion was far more responsive to BIPPO than zaprinast, with approximately equivalent MIC2 secretion seen with 0.5μM BIPPO and 200–500μM zaprinast (Figure 1b). At these concentrations, only minor reduction in GRA1 secretion was seen; however, as BIPPO concentrations were further increased through 1–5μM, a clear decrease in secreted GRA1 was observed (Figure 1b). We note that phosphodiesterase inhibitors have been reported to elevate cAMP in addition to cGMP in Plasmodium (Howard et al., 2015). In Toxoplasma, cAMP is implicated in reducing cytosolic Ca2+ and, although we do not know if any similar elevation of cAMP occurs with BIPPO/zaprinast treatment, the observed increase in cytosolic Ca2+ in Toxoplasma with these agents suggest that cAMP is unlikely to be a major contributor to this response (Jia et al., 2017; Stewart et al., 2017; Uboldi et al., 2018).
We tested that the changes to MIC and GRA secretion observed with these Ca2+ and cGMP agonists were not due to adverse secondary effects on the cell, including cell lysis or premature cell death during the secretion assay. To test for cell lysis or loss of plasma membrane integrity, we assayed for the release of the soluble cytosolic protein profilin under secretion assay conditions using 5μM of either A23187 or BIPPO. No release of this marker was seen with either treatment (Figure 1c). To test for cell death, cells were pelleted after the secretion assays, washed in growth medium, and equal volumes used to inoculate host cell monolayers for each of the 5μM A23187, 5μM BIPPO, or vehicle (DMSO) control treatments. After 8 days of growth, plaque density in the host monolayer reported the relative number of cells that were invasion‐competent after the secretion assay and able to generate an ongoing lytic infection cycle. No difference was seen between agonist treatments and the control (Figure 1d). Thus, tachyzoites evidently remain intact and viable throughout the secretion assay.
We further tested for the effect on GRA secretion of modulators of cytosolic Ca2+ by treating cells with either BAPTA‐AM or thapsigargin. BAPTA‐AM is a membrane‐permeable Ca2+‐chelator so treatment reduces available Ca2+, whereas thapsigargin is an inhibitor of the sarco/endoplasmic reticulum Ca2+ATPase (SERCA), which is believed responsible for recharging sequestered Ca2+ pools. Thapsigargin treatment, thus, leads to cytosolic accumulation of Ca2+. BAPTA‐AM treatment resulted in loss of constitutive secretion from micronemes, and no change to GRA secretion, consistent with a role of elevated Ca2+ in both of these processes (Figure 2a). Thapsigargin treatment resulted in elevated MIC secretion, but no change in GRA secretion when applied at 10μM (Figure 2a).
Figure 2.
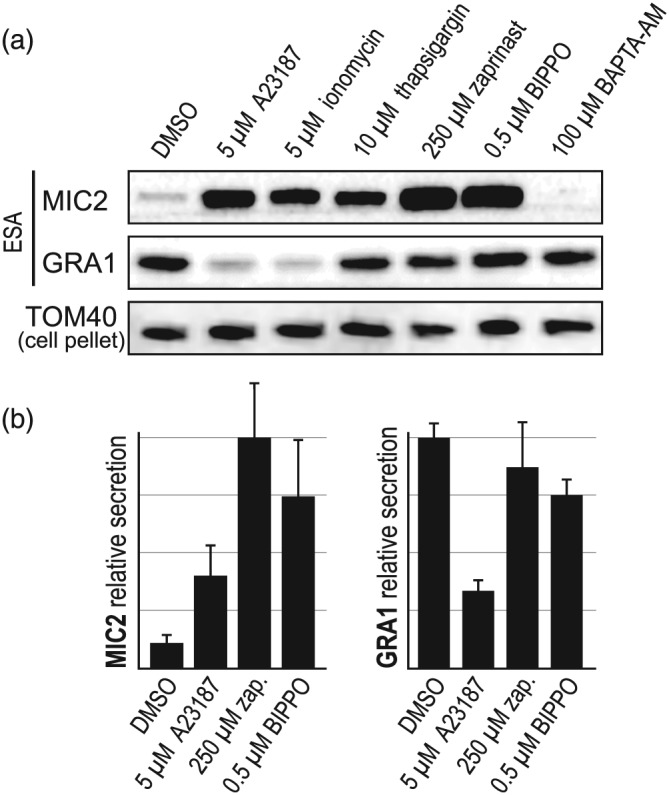
Relative effects of modulators of cytosolic Ca2+ on microneme protein (MIC) and granule protein (GRA) secretion. (a) Ca2+ ionophores A23187 and ionomycin; Ca2+ sequestration inhibitor thapsigargin; cGMP agonists zaprinast (zap.) and BIPPO; and Ca2+ chelator BAPTA‐AM; all affect MIC secretion and have varying effects on GRA secretion. (b) Relative secretion of MIC2 and GRA1 shown over eight biological replicates. ESA; extracellular secretion assay: TOM40 used as a cell equivalents loading control. Error bars = SEM
In summary, we observe an inverse correlation between MIC and GRA secretion in response to changes in cytosolic Ca2+. Further, there is evidence of separation between the manner of eliciting Ca2+ signalling and the proportional responses of MIC and GRA secretion. Ionophore treatment to directly release Ca2+ stores result in marked increase in MIC secretion and concomitant decrease in GRA secretion (Figures 1 and 2). Conversely, indirect methods for elevating cytosolic Ca2+—moderate cGMP stimulus and thapsigargin—result in strong MIC secretion, but proportionately less inhibition of GRA secretion (Figures 1 and 2).
2.2. Mutants in Ca2+ signalling disrupt both MIC and GRA secretion responses
Ca2+‐dependent protein kinases (TgCDPKs) 1 and 3 in Toxoplasma are involved in controlling cell processes relevant to invasion and egress, including microneme exocytosis, where elevated Ca2+ triggers activation of these processes (Lourido et al., 2010; Lourido et al., 2012; McCoy et al., 2012). Given our data that GRA secretion negatively correlates with cytosolic Ca2+ level increase, we tested if TgCDPK1 and/or 3 might be involved in regulating dense granule exocytosis. To test for a role of TgCDPK1, we used an inducible knockdown cell line (iΔHA‐TgCDPK1) (Lourido et al., 2010) in which TgCDPK1 levels were strongly depleted after 72 hr of anhydrotetracycline (ATc) treatment (Figure 3a). Untreated (−ATc) iΔHA‐TgCDPK1 cells showed typical constitutive MIC and GRA secretion, and both A23187‐responsive MIC secretion and coincident inhibition of GRA secretion (Figure 3a–c), consistent with wildtype cells (Figures 1 and 2). When TgCDPK1 was depleted (+ATc), levels of MIC secretion were strongly reduced in both constitutive and A23187‐treated states compared with the −ATc controls. The suppression of GRA secretion with A23187 treatment was also reduced in the TgCDPK1‐depleted cells (Figure 3a,c). Therefore, depletion of TgCDPK1 simultaneously results in reductions in both the Ca2+‐induced increase in microneme exocytosis and inhibition of dense granule exocytosis.
Figure 3.
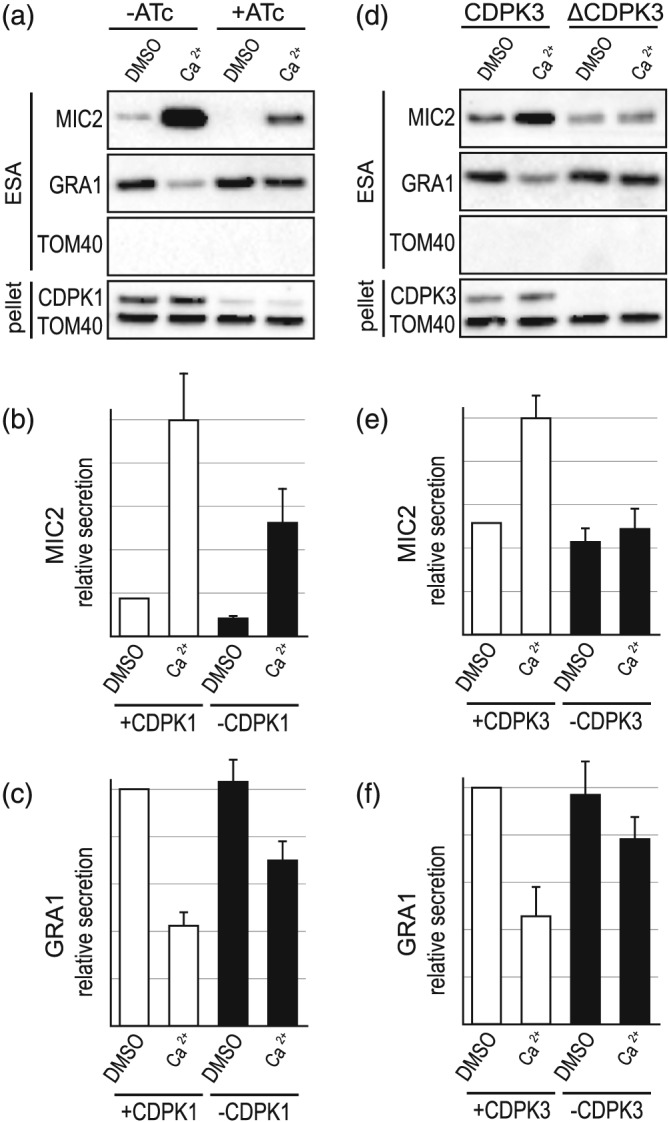
Effect of loss of CDPK1 or CDPK3 on Ca2+‐induced microneme protein (MIC) and granule protein (GRA) secretion. (a–c) Extracellular secretion assays (ESA) with and without ATc‐induced CDPK1 depletion in iΔHA‐CDPK1 cells. Depletion of HACDPK1 is seen by immuno‐detection of HA in the cell pellet. A23187 (5μM) is used for Ca2+ stimulation. Relative secretion of MIC2 (b) and GRA1 (c) is shown (n = 8). (d–f) ESA and relative secretion (n = 7) measurements for wildtype (+CDPK3) versus CDPK3 knockout (−CDPK3) cells. Absence of CDPK3 is seen by CDPK3 immuno‐detection in the cell pellet (d). TOM40 in ESA serves a control for cell lysis and controls for cell equivalent loading in the pellet. Error bars = SEM
We also used a chemical inhibition strategy to test for the role of TgCDPK1 in the control of secretion from dense granules. The kinase inhibitor 3‐methyl‐benzyl pyrazolo [3,4‐d] pyrimidine (3‐MB‐PP1) is specific to TgCDPK1 in T. gondii (Lourido et al., 2010; Lourido et al., 2012), and cells treated with this inhibitor were concurrently assayed for changes of microneme and dense granule exocytosis. Extracellular parasites were treated with 3‐MB‐PP1 for 5 min post harvesting, and then assayed for protein secretion. Ca2+‐induced (A23187 treatment) MIC secretion was lost with 3‐MB‐PP1 treatment, consistent with inhibition of TgCDPK1 (Figure 4a(i)). Interestingly, constitutive levels of microneme secretion were unaffected, unlike the TgCDPK1 KD. In these experimental conditions, dense granule exocytosis was also found to be unaffected in that increasing [Ca2+] could inhibit levels of GRA secretion, equivalent to the 3‐MB‐PP1‐untreated cells (Figure 4a(i),b(ii)). Therefore, extracellular parasites showed some behaviours similar to the TgCDPK1 knockdown, but not all when treated with 3‐MB‐PP1 after cell egress. It is possible that TgCDPK1 had phosphorylated targets upon egress, but before 3‐MB‐PP1 treatment, which might be responsible for the Ca2+‐inducible dense granule control. To test this, we pretreated intracellular parasites with the 3‐MB‐PP1 kinase inhibitor for 5 min before mechanical egress and secretion assays. With this preharvest treatment, all microneme secretion was lost: both constitutive and Ca2+‐induced MIC secretion (Figure 4a(ii)). Furthermore, Ca2+‐induced inhibition of dense GRA secretion was also substantially lost (Figure 4a(ii),b(iii)). These results mimic the effects of TgCDPK1 depletion and suggest that in the first 3‐MB‐PP1 experiment, some TgCDPK1 targets were phosphorylated after egress and persisted in this state despite subsequent 3‐MB‐PP1 inhibition.
Figure 4.
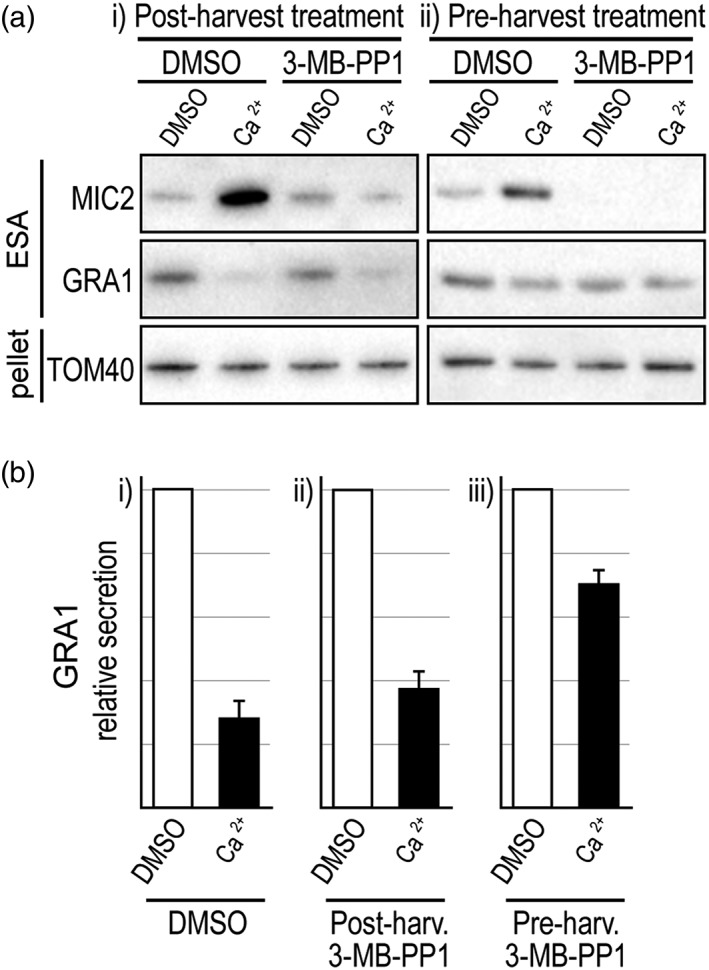
Effect of CDPK1‐inhibitor 3‐MB‐PP1 on Ca2+‐induced microneme protein (MIC) and granule protein (GRA) secretion. (a) Parasites were treated with 3‐MB‐PP1 either (i) after mechanical egress from host cells, or (ii) before egress, and then extracellular secretion assays (ESA) of MIC2 and GRA1 without or with A23187 (5μM)‐induced Ca2+ release. (b) Relative GRA1 secretion was measured for DMSO controls (n = 4), postharvest 3‐MB‐PP1 (n = 4) and preharvest 3‐MB‐PP1 (n = 3). Error bars = SEM
We also tested for a role of TgCDPK3 in GRA secretion regulation using a cell line with the cdpk3 gene knocked out (ΔTgCDPK3; McCoy et al., 2012). Unlike the TgCDPK1 KD, TgCDPK3 absence did not affect constitutive MIC secretion (Figure 3d,e). When treated with A23187, Δ TgCDPK3 cells showed neither an increase in MIC secretion nor a decrease in GRA secretion, suggesting that TgCDPK3 is required for Ca2+‐mediated control of both of these processes (Figure 3d–f).
TgRNG2 is an apical complex protein involved in relaying a cGMP signal through to MIC secretion (Katris et al., 2014). Depletion of TgRNG2 interrupts the relay of this signal, although microneme secretion can be rescued with direct Ca2+ stimulation by A23187 (Figure 5a(i),b(i)). These data suggest that TgRNG2 operates between cGMP sensing and cytosolic Ca2+ elevation. We therefore used a TgRNG2 inducible knockdown cell line (iΔHA‐TgRNG2) to independently test if dense granule exocytosis control is regulated directly by Ca2+ rather than cGMP. TgRNG2‐depleted cells showed normal levels of Ca2+‐induced dense granule exocytosis inhibition and concurrent elevation of microneme secretion (Figure 5a(i),b(i),c(i)). When TgRNG2‐depleted cells were activated via cGMP (2.5μM BIPPO), no change in dense granule secretion was seen compared with untreated cells (Figure 5a(ii),c(ii)). These data are consistent with dense granule regulation responding directly to Ca2+ and not cGMP.
Figure 5.
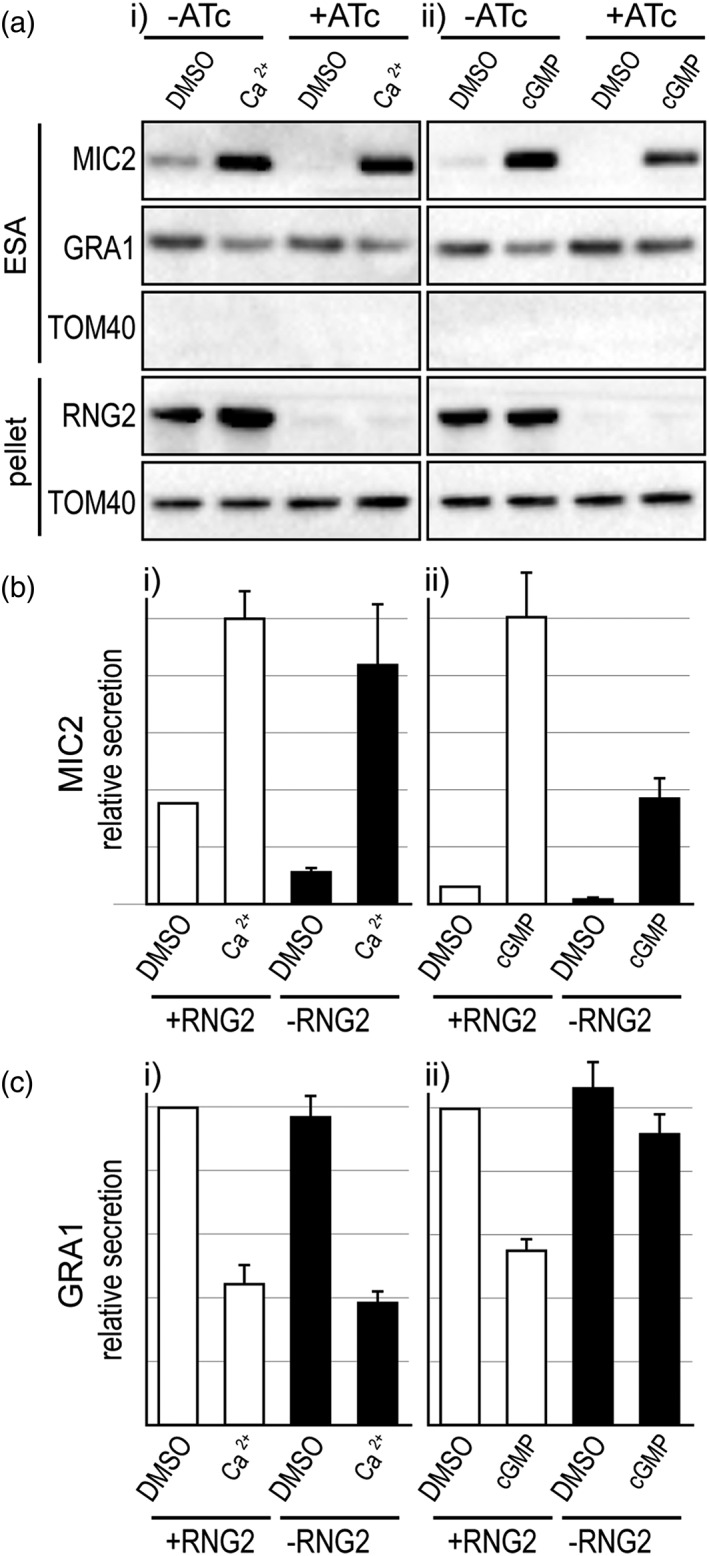
Effect of loss of RNG2 on Ca2+‐ and cGMP‐induced microneme protein (MIC) and granule protein (GRA) secretion. (a–c) Extracellular secretion assays (ESA) with and without ATc‐induced RNG2 depletion in iΔHA‐RNG2 cells. Depletion of HARNG2 is seen by immuno‐detection of HA in the cell pellet. A23187 (5μM) is used for Ca2+ stimulation (i), and BIPPO (2.5μM) is used for cGMP stimulation (ii). Relative MIC2 (b) and GRA1 (c) is shown for eight (Ca2+, b(i), c(i)) and 10 (cGMP, b(ii), c(ii)) biological replicates. TOM40 in ESA serves a control for cell lysis and controls for cell‐equivalents loading in the pellet. Error bars = SEM
3. DISCUSSION
We have tested for changes to rates of dense granule protein secretion from extracellular tachyzoites in response to stimuli known to elicit changes in microneme exocytosis, namely, treatments that elevate cytosolic Ca2+ (summarised in Figure 6). We consistently see evidence of reduced GRA secretion in conditions that raise cytosolic Ca2+, including both ionophore treatment that allows discharge of Ca2+ stores into the cytoplasm, and cGMP treatment that indirectly raises cytosolic Ca2+ through activation of PKG (Brochet et al., 2014; Sidik et al., 2016; Stewart et al., 2017). Both treatment types increased MIC secretion. Mutant cell lines for TgCDPK1, TgCDPK3, and TgRNG2 with known phenotypes in microneme secretion control (Katris et al., 2014; Lourido et al., 2010; McCoy et al., 2012) all similarly displayed reciprocal GRA control phenotypes, further supporting a role for cytosolic Ca2+ levels in downregulation of GRA secretion. We also note that several published reports show evidence of Ca2+‐mediated suppression of secretion of dense GRAs, although in these cases, the effects seen were either not commented on or explored (Carruthers, Moreno, & Sibley, 1999; Farrell et al., 2012; Kafsack et al., 2009; Paul et al., 2015). Chaturvedi et al. (1999) explicitly tested for increase of GRA secretion with Ca2+ stimulation by supplying exogenous Ca2+ to streptolysin O‐permeabilized cells. They saw no change in GRA secretion, but it is not known what other cell processes might be perturbed by this permeabilization treatment, and a range of Ca2+ concentrations was not tested.
Figure 6.

Summary of known regulatory events that contribute to the control of microneme and dense granule exocytosis. Chemical agonists (green) of secondary messengers cGMP and Ca2+ and antagonists (red) of these messengers or kinases used in this study are shown. Microneme interaction and fusion at the plasma membrane via APH‐PA interactions and Ca2+‐dependent DOC2.1 activity is also shown. APH: acylated pleckstrin‐homology; (PH) domain‐containing protein; CDPK: Ca2+‐dependent protein kinase; DAG: diacylglycerol; PA: phosphatidic acid; PKG: protein kinase G
Whereas a reciprocal response of MIC and GRA secretion to Ca2+‐based signalling is consistently evident, differences in their relative responses depends on the type of treatment, suggesting that different Ca2+‐signalling events might drive these processes, as is also the case for other infection‐cycle events (Lourido et al., 2012). For instance, thapsigargin elevates cytosolic Ca2+, but although this does lead to microneme secretion, it does not lead to increased motility or conoid extrusion without increased extracellular Ca2+ (Pace, Mcknight, Liu, Jimenez, & Moreno, 2014). We also observed no change in GRA secretion with thapsigargin, although MIC secretion does increase. Similarly, cGMP agonists BIPPO and zaprinast both resulted in strong MIC secretion increase, but much more subdued GRA secretion inhibition compared with the Ca2+ ionophores. These data indicate that the regulation of microneme exocytosis is more sensitive to Ca2+ than the regulation of dense granule exocyotsis. Alternatively, cGMP‐based signalling might contribute to some MIC secretion that is independent of Ca2+, such as lipid‐mediated control of microneme exocytosis (Figure 6; Bullen et al., 2016).
The comparison of the TgCDPK1 and TgCDPK3 mutants provides further evidence of a separation between the mechanisms of Ca2+‐mediated control of microneme versus dense granule secretion. Depletion of either of these kinases results in loss of Ca2+‐dependent inhibition of GRA secretion, suggesting that substrates of both are required for this process. However, the MIC secretion response is quite different between TgCDPK1 and TgCDPK3 depletion: TgCDPK1 loss maintains a Ca2+‐dependent MIC response although greatly reduced in magnitude, whereas TgCDPK3 loss results in Ca2+ insensitivity. This is consistent with some cooperativity between these two kinases where TgCDPK3 might activate the responsiveness of TgCDPK1 for MIC secretion, as others have suggested (Treeck et al., 2014). The complexity of Ca2+ signalling targets and their dynamics is further indicated by 3‐MB‐PP1 treatment either before or after egress, which also result in differences to constitutive MIC secretion as well as GRA control. Post‐egress 3‐MB‐PP1 treatment leaves GRA secretion Ca2+‐responsive, yet pre‐egress treatment blocks this. This suggests that some stable, necessary TgCDPK1 phosphorylation of substrates occurs at the point of egress, and that TgCDPK3 substrates are then sufficient for the responsiveness of dense granules to Ca2+.
The location of GRA secretion from Toxoplasma tachyzoites has not been unambiguously determined; however, it has been suggested that dense granules fuse laterally at the parasite cell surface, rather than apically as micronemes and rhoptries do (Dubremetz et al., 1993). In any case, it is unlikely that suppression of dense granule release is a direct effect of increased microneme secretion and competition for space at the site of secretion. Even if they were to share the same exit point, our data show instances of strongly elevated microneme secretion with no change to dense granule secretion (e.g., zaprinast 250μM and thapsigargin 10μM).
The mechanism for Ca2+‐mediated control of GRA secretion is currently unclear. Some GRA proteins that bear transmembrane domains and are membrane‐associated after release into the host cell environment are known be maintained as soluble high‐molecular mass protein aggregates within the dense granules prior to release (Labruyere, Lingnau, Mercier, & Sibley, 1999; Lecordier, Mercier, Sibley, & Cesbron‐Delauw, 1999; Sibley et al., 1995). GRA1, a highly abundant GRA with two Ca2+ binding domains, has been speculated to potentially play a role in control of this aggregated state (Lebrun, Carruthers, & Cesbron‐Delauw, 2014). It is conceivable that switching from aggregated to disaggregated state of GRAs has a role in secretion regulation and that Ca2+ could modulate this. If so, dense granule luminal Ca2+ levels would be relevant and must also be controlled. Irrespective of such an internal control process, cytosolic factors are likely to be implicated given evidence of both TgCDPK1 and TgCDPK3 substrates participating in GRA regulation. These substrates might control trafficking of the dense granules or derived vesicles to the relevant location for exocytosis, and/or mediate fusion with the plasma membrane. Several proteins implicated in vesicular trafficking have been identified as CDPK targets in Plasmodium (Brochet et al., 2014). If a lateral site of dense granule fusion occurs, some reorganisation of the IMC including membrane cisternae and subpellicular filamentous network would likely be required for vesicular contact with the plasma membrane, and this might require further direction from CDPK1/3‐mediated processes.
Although the mechanism for Ca2+‐controlled secretion of dense granules is currently unknown, reciprocal control of micronemes and dense granules using the same signals has a clear biological logic. The protein cargos are mutually exclusive in terms of function—one for the extracellular processes, the other for the intracellular processes. After successful parasite invasion of a host cell, the suppression of MIC secretion by cAMP‐mediated depletion of cytosolic Ca2+ would concomitantly allow for relaxation of GRA secretion suppression. Furthermore, the coupling of these signalling networks reinforces the potential for signal disruption as a druggable therapeutic strategy. Dysregulation of both MIC and GRA secretion could both interrupt control of the lytic invasion cycle and promote immune‐recognition of a wide suite of GRA proteins that might otherwise only be released in the intracellular context.
4. EXPERIMENTAL PROCEDURES
4.1. Parasites cultures
T. gondii tachyzoites were grown by serial passage in human foreskin fibroblast (HFF) cells as previously described (Jacot, Meissner, Sheiner, Soldati‐Favre, & Striepen, 2014). Briefly, Toxoplasma RH strain parasites were serially passaged in confluent HFF cells containing in ED1 media (Dulbecco's modified Eagle's medium [DMEM] supplemented with 1% foetal bovine serum [FBS], 0.2mM additional L‐Glut, 50 Units/ml Penicillin/Streptomycin, and 0.25 μg/ml of amphotericin‐B).
4.2. Secretion assays
Parasite cultures were preincubated for at least 48 hr with or without ATc. Parasites were harvested after multiple rounds of parasite replication within the PV and with approximately 50–80% of vacuoles intact prior to natural egress. Cultures were scraped, host cells disrupted by syringe passing through a 26 gauge needle, filtered through a 3‐μm polycarbonate filter to remove host debris, and parasites pelleted at 1000x g at 15°C for 10 minutes. Pellets were aspirated and washed with 3 ml of invasion buffer (DMEM with 3% FBS and 10mM HEPES, pH 7.4) and pelleted as before. Supernatants were aspirated and pellets resuspended in invasion buffer at 2.5 × 108 cells.ml−1.Fifty microlitres of parasite suspension was then mixed with an equal volume of invasion buffer containing 2× the final concentration of agonist or vehicle (DMSO) equivalent. Parasite samples were incubated at 37°C for 20 min to allow secretion and quenched on ice for 2 min to stop secretion. Cells were then separated from supernatants by centrifugation (8,000 rpm, 2 min, 4°C), 85 μl of supernatant removed, and this was centrifuged a second time to remove any remaining cells, with a final volume of 75 μl carefully aspirated. The cell pellets were washed with 1× PBS, repelleted as before, and the supernatant was aspirated. Supernatant and cell pellet proteins were solubilised in SDS sample buffer, separated by SDS‐PAGE, transferred to nitrocellulose membranes, and GRA, MIC, or mitochondrial proteins immuno‐detected. Western blot detection was performed with horseradish peroxidase conjugated secondary antibodies detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce). Signal strength was quantified using a BioRad Chemidoc imager and ImageLab software, and replicate assays signals were normalised to their respective cell pellet TOM40 signal to control for any minor cell number variation. Standard error of the means (SEM) were calculated for replicate data as a measure of experimental consistency, however, due to the non‐linear nature of chemiluminescent detection, statistical analyses of these data are not appropriate. Plaque assays were performed on cells recovered after the 2‐min ice quenching. Cells were pelleted, washed, and then resuspended in 1 ml of growth medium, and finally inoculated into HFFs at equal parasite densities.
ACKNOWLEDGEMENTS
We would like to thank Philip Thompson for BIPPO, Chris Tonkin for technical advice and gifting the 3‐MB‐PP1 inhibitor and TgCDPK3 KO cell line, Sebastian Lourido for gifting the TgCDPK1 iHA KD cell line, David Sibley for gifting the MIC2 antibody, Vern Carruthers for the MIC5 antibody, Dominique Soldati‐Favre for profilin antibody and Corinne Mercier for the GRA1, GRA2 and GRA5 antibodies. This work was supported by the Australian Research Council (DP120100599) and the Medical Research Council (UK: MR/M011690/1).
Katris NJ, Ke H, McFadden GI, van Dooren GG, Waller RF. Calcium negatively regulates secretion from dense granules in Toxoplasma gondii . Cellular Microbiology. 2019;21:e13011 10.1111/cmi.13011
REFERENCES
- Adl, S. M. , Simpson, A. G. B. , Lane, C. E. , Lukes, J. , Bass, D. , Bowser, S. S. , … Heiss, A. (2012). The revised classification of eukaryotes. Journal of Eukaryotic Microbiology, 59(5), 429–493. 10.1111/j.1550-7408.2012.00644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker, O. , Lourido, S. , & Sibley, L. D. (2009). Calcium‐dependent signaling and kinases in apicomplexan parasites. Cell Host & Microbe, 5(6), 612–622. 10.1016/j.chom.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader, I. J. , Coleman, B. I. , Chen, C.‐T. , & Gubbels, M.‐J. (2015). Lytic cycle of Toxoplasma gondii: 15 years later. Annual Review of Microbiology, 69, 463–485. 10.1146/annurev-micro-091014-104100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges‐Pereira, L. , Budu, A. , Mcknight, C. A. , Moore, C. A. , Vella, S. A. , Hortua Triana, M. A. , … Moreno, S. N. (2015). Calcium signaling throughout the Toxoplasma gondii lytic cycle: A study using genetically encoded calcium indicators. Journal of Biological Chemistry, 290(45), 26914–26926. 10.1074/jbc.M115.652511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet, M. , Collins, M. O. , Smith, T. K. , Thompson, E. , Sebastian, S. , Volkmann, K. , … Billker, O. (2014). Phosphoinositide metabolism links cGMP‐dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLoS Biology, 12(3), e1001806 10.1371/journal.pbio.1001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges, S. D. , Harper, J. M. , Parussini, F. , Coppens, I. , & Carruthers, V. B. (2008). A transient forward‐targeting element for microneme‐regulated secretion in Toxoplasma gondii . Biology of the Cell, 100(4), 253–264. 10.1042/BC20070076 10.1042/BC20070076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen, H. E. , Jia, Y. , Yamaryo‐Botté, Y. , Bisio, H. , Zhang, O. , Jemelin, N. K. , … Soldati‐Favre, D. (2016). Phosphatidic acid‐mediated signaling regulates microneme secretion in Toxoplasma . Cell Host & Microbe, 19(3), 349–360. 10.1016/j.chom.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Carruthers, V. , Moreno, S. , & Sibley, L. (1999). Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii . The Biochemical Journal, 342, 379–386. 10.1042/bj3420379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers, V. B. , Giddings, O. K. , & Sibley, L. D. (1999). Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cellular Microbiology, 1(3), 225–235. 10.1046/j.1462-5822.1999.00023.x [DOI] [PubMed] [Google Scholar]
- Carruthers, V. B. , & Sibley, L. D. (1997). Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. European Journal of Cell Biology, 73(2), 114–123. [PubMed] [Google Scholar]
- Chaturvedi, S. , Qi, H. , Coleman, D. , Rodriguez, A. , Hanson, P. I. , Striepen, B. , … Joiner, K. A. (1999). Constitutive calcium‐independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. The Journal of Biological Chemistry, 274(4), 2424–2431. 10.1074/jbc.274.4.2424 [DOI] [PubMed] [Google Scholar]
- Coppens, I. , Andries, M. , Liu, J. L. , & Cesbron‐Delauw, M.‐F. (1999). Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. European Journal of Cell Biology, 78, 463–472. 10.1016/S0171-9335(99)80073-9 [DOI] [PubMed] [Google Scholar]
- de Vargas, C. , Audic, S. , Henry, N. , Decelle, J. , Mahé, F. , Logares, R. , … Carmichael, M. (2015). Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science, 348(6237). 1261605. 10.1126/science.1261605 [DOI] [PubMed] [Google Scholar]
- Dubremetz, J. F. , Achbarou, A. , Bermudes, D. , & Joiner, K. A. (1993). Kinetics and pattern of organelle exocytosis during Toxoplasma gondii host‐cell interaction. Parasitology Research, 79(5), 402–408. 10.1007/BF00931830 [DOI] [PubMed] [Google Scholar]
- Endo, T. , Tokuda, H. , Yagita, K. , & Koyama, T. (1987). Effects of extracellular potassium on acid release and motility initiation in Toxoplasma gondii . The Journal of Protozoology, 34(3), 291–295. 10.1111/j.1550-7408.1987.tb03177.x [DOI] [PubMed] [Google Scholar]
- Etheridge, R. D. , Alaganan, A. , Tang, K. , Lou, H. J. , Turk, B. E. , & Sibley, L. D. (2014). The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host & Microbe, 15(5), 537–550. 10.1016/j.chom.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, A. , Thirugnanam, S. , Lorestani, A. , Dvorin, J. D. , Eidell, K. P. , Ferguson, D. J. P. , … Gubbels, M. J. (2012). A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science, 335(6065), 218–221. 10.1126/science.1210829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frénal, K. , Dubremetz, J.‐F. , Lebrun, M. , & Soldati‐Favre, D. (2017). Gliding motility powers invasion and egress in Apicomplexa. Nature Reviews Microbiology, 15(11), 645–660. 10.1038/nrmicro.2017.86 [DOI] [PubMed] [Google Scholar]
- Graindorge, A. , Frénal, K. , Jacot, D. , Salamun, J. , Marq, J.‐B. , & Soldati‐Favre, D. (2016). The conoid associated motor MyoH is indispensable for Toxoplasma gondii entry and exit from host cells. PLoS Pathogens, 12(1), e1005388 10.1371/journal.ppat.1005388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin, A. , Corrales, R. M. , Parker, M. L. , Lamarque, M. H. , Jacot, D. , El Hajj, H. , … Lebrun, M. (2017). Efficient invasion by Toxoplasma depends on the subversion of host protein networks. Nature Microbiology, 2(10), 1358–1366. 10.1038/s41564-017-0018-1 [DOI] [PubMed] [Google Scholar]
- Håkansson, S. , Charron, A. J. , & Sibley, L. D. (2001). Toxoplasma evacuoles: A two‐step process of secretion and fusion forms the parasitophorous vacuole. The EMBO Journal, 20(12), 3132–3144. 10.1093/emboj/20.12.3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi, M.‐A. , Olias, P. , & Sibley, L. D. (2017). Toxoplasma effectors targeting host signaling and transcription. Clinical Microbiology Reviews, 30(3), 615–645. 10.1128/CMR.00005-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, B. L. , Harvey, K. L. , Stewart, R. J. , Azevedo, M. F. , Crabb, B. S. , Jennings, I. G. , … Gilson, P. R. (2015). Identification of potent phosphodiesterase inhibitors that demonstrate cyclic nucleotide‐dependent functions in apicomplexan parasites. ACS Chemical Biology, 10, 1145–1154. 10.1021/cb501004q [DOI] [PubMed] [Google Scholar]
- Jacot, D. , Meissner, M. , Sheiner, L. , Soldati-Favre, D. , & Striepen, B. (2014). Toxoplasma gondii: The model Apicomplexan—Perspectives and methods In Weiss L. M., & Kim K. (Eds.), Genetic Manipulation of Toxoplasma gondii (2nd ed.) (pp. 577–611). Academic Press; 10.1016/B978-0-12-396481-6.00017-9 [DOI] [Google Scholar]
- Jia, Y. , Marq, J.‐B. , Bisio, H. , Jacot, D. , Mueller, C. , Yu, L. , … Soldati‐Favre, D. (2017). Crosstalk between PKA and PKG controls pH‐dependent host cell egress of Toxoplasma gondii . EMBO Journal, 36(21), 3250–3267. 10.15252/embj.201796794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack, B. F. C. , Pena, J. D. O. , Coppens, I. , Ravindran, S. , Boothroyd, J. C. , & Carruthers, V. B. (2009). Rapid membrane disruption by a perforin‐like protein facilitates parasite exit from host cells. Science, 323(5913), 530–533. 10.1126/science.1165740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katris, N. J. , van Dooren, G. G. , McMillan, P. J. , Hanssen, E. , Tilley, L. , & Waller, R. F. (2014). The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma . PLoS Pathogens, 10(4), e1004074 10.1371/journal.ppat.1004074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruyere, E. , Lingnau, M. , Mercier, C. , & Sibley, L. D. (1999). Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii . Molecular and Biochemical Parasitology, 102(2), 311–324. 10.1016/S0166-6851(99)00092-4 [DOI] [PubMed] [Google Scholar]
- Lebrun, M. , Carruthers, V. B. , & Cesbron‐Delauw, M.‐F. (2014). Toxoplasma gondii: The model Apicomplexan—Perspectives and methods In Weiss L. M., & Kim K. (Eds.), Toxoplasma secretory proteins and their roles in cell invasion and intracellular survival (2nd ed.) (pp. 389–453). Academic Press; 10.1016/B978-0-12-396481-6.00012-X [DOI] [Google Scholar]
- Lecordier, L. , Mercier, C. , Sibley, L. D. , & Cesbron‐Delauw, M. F. (1999). Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Molecular Biology of the Cell, 10(4), 1277–1287. 10.1091/mbc.10.4.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido, S. , Jeschke, G. R. , Turk, B. E. , & Sibley, L. D. (2013). Exploiting the unique ATP‐binding pocket of Toxoplasma calcium‐dependent protein kinase 1 to identify its substrates. ACS Chemical Biology, 8(6), 1155–1162. 10.1021/cb400115y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido, S. , Shuman, J. , Zhang, C. , Shokat, K. M. , Hui, R. , & Sibley, L. D. (2010). Calcium‐dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma . Nature, 465(7296), 359–362. 10.1038/nature09022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido, S. , Tang, K. , & Sibley, L. D. (2012). Distinct signalling pathways control Toxoplasma egress and host‐cell invasion. The EMBO Journal, 31(24), 4524–4534. 10.1038/emboj.2012.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahé, F. , de Vargas, C. , Bass, D. , Czech, L. , Stamatakis, A. , Lara, E. , … Dunthorn, M. (2017). Parasites dominate hyperdiverse soil protist communities in Neotropical rainforests. Nature Ecology & Evolution, 1(4), 91 10.1038/s41559-017-0091 [DOI] [PubMed] [Google Scholar]
- McCoy, J. M. , Whitehead, L. , van Dooren, G. G. , & Tonkin, C. J. (2012). TgCDPK3 regulates calcium‐dependent egress of Toxoplasma gondii from host cells. PLoS Pathogens, 8(12), e1003066 10.1371/journal.ppat.1003066.s010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier, C. , Adjogble, K. D. Z. , Däubener, W. , & Delauw, M.‐F.‐C. (2005). Dense granules: Are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? International Journal for Parasitology, 35(8), 829–849. 10.1016/j.ijpara.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Mercier, C. , & Cesbron‐Delauw, M.‐F. (2015). Toxoplasma secretory granules: One population or more? Trends in Parasitology, 31(2), 60–71. 10.1016/j.pt.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Pace, D. A. , Mcknight, C. A. , Liu, J. , Jimenez, V. , & Moreno, S. N. J. (2014). Calcium entry in Toxoplasma gondii and its enhancing effect of invasion‐linked traits. Journal of Biological Chemistry, 289, 19637–19647. 10.1074/jbc.M114.565390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, A. S. , Saha, S. , Engelberg, K. , Jiang, R. H. Y. , Coleman, B. I. , Kosber, A. L. , … Duraisingh, M. T. (2015). Parasite calcineurin regulates host cell recognition and attachment by apicomplexans. Cell Host & Microbe, 18, 1–12. 10.1016/j.chom.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko, M. S. , Svezhova, N. , & Carruthers, V. B. (2014). Acidification activates Toxoplasma gondii motility and egress by enhancing protein secretion and cytolytic activity. PLoS Pathogens, 10, e1004488 10.1371/journal.ppat.1004488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmann, J. , Ammendola, A. , Ashman, K. , Zong, X. , Huber, A. , Neubauer, G. , … Ruth, P. (2000). Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature, 404(6774), 197–201. 10.1038/35004606 [DOI] [PubMed] [Google Scholar]
- Sibley, L. D. , Niesman, I. R. , Parmley, S. F. , & Cesbron‐Delauw, M. F. (1995). Regulated secretion of multi‐lamellar vesicles leads to formation of a tubulo‐vesicular network in host‐cell vacuoles occupied by Toxoplasma gondii . Journal of Cell Science, 108(Pt 4), 1669–1677. [DOI] [PubMed] [Google Scholar]
- Sidik, S. M. , Hortua Triana, M. A. , Paul, A. S. , El Bakkouri, M. , Hackett, C. G. , Tran, F. , … Moreno, S. N. (2016). Using a genetically encoded sensor to identify inhibitors of Toxoplasma gondii Ca2+ signaling. Journal of Biological Chemistry, 291(18), 9566–9580. 10.1074/jbc.M115.703546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, R. J. , Whitehead, L. , Nijagal, B. , Sleebs, B. E. , Lessene, G. , McConville, M. J. , … Tonkin, C. J. (2017). Analysis of Ca2+ mediated signaling regulating Toxoplasma infectivity reveals complex relationships between key molecules. Cellular Microbiology, 19(4). 10.1111/cmi.12685 [DOI] [PubMed] [Google Scholar]
- Tang, Q. , Andenmatten, N. , Hortua Triana, M. A. , Deng, B. , Meissner, M. , Moreno, S. N. J. , … Ward, G. E. (2014). Calcium‐dependent phosphorylation alters class XIVa myosin function in the protozoan parasite Toxoplasma gondii . Molecular Biology of the Cell, 25(17), 2579–2591. 10.1091/mbc.E13-11-0648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck, M. , Sanders, J. L. , Gaji, R. Y. , LaFavers, K. A. , Child, M. A. , Arrizabalaga, G. , … Boothroyd, J. C. (2014). The calcium‐dependent protein kinase 3 of Toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis. PLoS Pathogens, 10(6), e1004197 10.1371/journal.ppat.1004197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uboldi, A. D. , Wilde, M.‐L. , McRae, E. A. , Steward, R. J. , Dagley, L. F. , Yang, L. , … Botte, C. Y. (2018). Protein Kinase A negatively regulates Ca2+ signalling in Toxoplasma gondii . PLoS Biology (in press), 16(9), e2005642 10.1371/journal.pbio.2005642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel, D. M. , Chen, L. A. , Ruiz, F. A. , Moreno, S. N. J. , & Sibley, L. D. (2004). Calcium‐mediated protein secretion potentiates motility in Toxoplasma gondii . Journal of Cell Science, 117(Pt 24), 5739–5748. 10.1242/jcs.01495 [DOI] [PubMed] [Google Scholar]


