Abstract
Poland is one of Europe’s leading producers and exporters of beer. The study, herein, describes the measurement of ochratoxin A, deoxynivalenol, nivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol, and zearalenone levels in 69 Polish beers. Analytical methodologies based on high performance liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) and fluorescence detection were developed, validated, and used to perform the above determinations. The most prevalent mycotoxins were deoxynivalenol (96%), ochratoxin A (93%), and HT-2 toxin (74%), respectively. Three quarters of the samples contained at least three analytes. The mean ochratoxin A concentration was 0.057 (SD 0.065) ng/mL, and in four beer samples its level exceeded 0.2 ng/mL, a value postulated in the literature to be the maximum limit. Deoxynivalenol was found at a maximum level of 56.2 ng/mL, and its mean concentration was 17.1 (SD 9.0) ng/mL. An evaluation of the estimated daily intake (EDI) of mycotoxins from beer in different European populations was made using food-consumption data prepared by WHO. Based on the mean ochratoxin A concentration in beers, the EDI represented 0.8–1.1% of the tolerable daily intake (TDI), while in a worst-case scenario (maximum concentration) it reached 5.0–7.5% of TDI. For deoxynivalenol, the EDI was in the range of 4.1–6.0% of TDI, whereas, based on maximum values, it reached the level of 14–21% of TDI. There were no significant differences between “scenarios” in the HT-2 case (mean—5.0–7.5% of TDI; maximum—6.5–9.7% of TDI) due to the fact that its concentration was near the limit of quantification (LOQ) value taken for calculation. The significance of these results are discussed, herein.
Keywords: beer, mycotoxins, ochratoxin A, deoxynivalenol, HT-2 toxin, occurrence, risk assessment
1. Introduction
Beer is one of the most popular alcoholic beverages in the world, and it is consumed in large amounts in almost every country. It originates from prehistoric ages. Beer-like beverages were known in China, as long as 70 centuries before the current era. One of the beer precursor could be “braga” or “bosa”, relatively low alcoholic drinks, which were made over a large area of Europe, stretching from Poland to the Balkans [1]. Whatever its origins, the popularity of beer continues; it is one of the most consumed beverage in the world. Hence beer plays an important role in the human diet, and any foodstuff consumed in such large quantities is a potential path for ingestion of harmful substances.
Mycotoxins are a group of around 500 toxic secondary metabolites produced mainly by the fungi of the Claviceps, Alternaria, Fusarium, Penicillium, and Aspergillus genera. These fungi grow on a variety of foodstuffs of both animal and plant origin. Maximum permitted levels of mycotoxins in food products are regulated by the EU [2,3,4,5,6,7] and other regional agencies. However, beer is one of a number of foodstuffs where maximum permitted levels of mycotoxins have yet to be firmly established. Poland is currently the third-ranking beer producer in Europe (Table 1) and so was the focus of this study. In 2016, Germany had the highest consumption of beer in Europe with 85.5 million hectoliters of beer consumed (104 L per person), followed by the United Kingdom with 43.7 million hectoliters (67 L per person), Spain with 38.6 million hectoliters (46 L per person), Poland with 37.9 million hectoliters (98 L per person), and France with 21.3 million hectoliters (33 L per person). The Czech Republic had the highest per capita consumption of beer in Europe, with 143 L of beer consumed, followed by Germany and Austria, with 103 L per person [8].
Table 1.
Top beer producing countries in Europe [8].
| Beer Production (105 L) | |||||
|---|---|---|---|---|---|
| Country | 2012 | 2013 | 2014 | 2015 | 2016 |
| Germany | 94.6 | 94.4 | 95.3 | 95.6 | 95.0 |
| United Kingdom | 44.2 | 44.2 | 44.3 | 44.0 | 43.7 |
| Poland | 39.3 | 40.0 | 40.1 | 40.9 | 41.4 |
| Spain | 33.0 | 32.7 | 33.6 | 35.0 | 36.5 |
| The Netherlands | 24.3 | 23.6 | 23.7 | 24.0 | 24.6 |
| France | 17.6 | 18.3 | 19.9 | 20.3 | 20.7 |
| Belgium | 18.8 | 18.1 | 18.2 | 19.8 | 20.7 |
| Czech Republic | 18.7 | 18.7 | 19.1 | 19.5 | 20.5 |
| Romania | 18.0 | 16.1 | 14.8 | 16.0 | 15.8 |
The possibility of mycotoxins finding their way into beer was always considered likely; species of Fusarium, as well as Aspergillus ochraceous and Penicillium verrucosum grow in poorly stored grain, and also contaminate growing cereal plants [9]. The latter two fungi produce ochratoxin A (OTA), which is nephrotoxic, teratogenic, immunotoxic, and a possible neurotoxin. OTA is also suspected to be the cause of chronic diseases including, Balkan Endemic Nephropathy (BEN); Chronic Interstitial Nephropathy (in North Africa); and urinary tract tumors in humans. OTA was classified by The International Agency for Research on Cancer as a possible human carcinogen (group 2B) [10]. The possibility of OTA getting into beer from contaminated grains used in brewing has been pointed out in literature. On the other hand, it is considered that OTA can persist the fermentation processes, but normally it can be reduced (up to 89%) during the malting process used in beer production [11]. Other mycotoxins likely to be found in beer are the trichothecenes (mainly deoxynivalenol (DON)), and zearalenone (ZEN) produced by the Fusarium species. Levels of DON might decrease or increase, depending on the process stage and the parameters of beer production [12,13]. The main source of mycotoxins in beer seems to derive from contaminated barley and malt feedstocks. The presence of OTA and Fusarium toxins in raw materials intended for malting and brewing, has previously been examined worldwide [14,15,16]. Similarly, beer contamination with mycotoxins has been the subject of numerous studies. OTA levels in beer might vary from pg/mL [17,18] to µg/mL in traditional African beers [19]. Fusarium derived mycotoxins do not usually exceed a value of several dozen ng/mL [12,20,21,22]. Higher values for mycotoxins have been recorded for craft beer samples, where processing is less controlled [23].
The objective of this study was to determine ochratoxin A, as well as trichothecenes group B (deoxynivalenol and nivalenol) and group A (T-2 toxin, HT-2 toxin, and diacetoxyscirpenol), and zearalenone, in beer produced by the main Polish breweries, and to evaluate the exposure of European populations to those compounds, considering the high position of Poland in the beer export, and taking into account the European consumption data. For this purpose, two analytical methods based on HPLC were validated.
2. Results and Discussion
2.1. Method Validation
Six-point calibration curves were constructed, based on standard solutions, with the concentation ranges of: DON: 12.5–503 ng/mL; NIV: 13.1–525 ng/mL; T2: 4.7–188 ng/mL; HT-2: 4.7–188 ng/mL; DAS: 3.6–145 ng/mL; and ZEN: 1.3–55.6 ng/mL, containing fixed amounts of internal standards ((ISs); except for the OTA) analysis. The correlation coefficients (r) of all analytes determined by the sum of least squares regression analyses, were higher than 0.995. To evaluate the experimental accuracy and precision, recovery experiments were carried out, using a blank lager beer (a sample with the concentration of the mycotoxins of interest lower than the detection limits) as a representative sample matrix. The recovery was calculated using the blank beer samples spiked with seven mycotoxins, at three different levels, with triplicate analyses conducted for each level. The limit of detection (LOD) and the limit of quantification (LOQ) were fixed at the mean concentration, at which the signal to noise ratio (S/N) equaled 3 and 10, respectively. For quality control, both a blank sample and positive samples (n = 3, ~every 20 samples) were applied in control spiking experiments, giving the recovery rate >70%. Validation data are summarized in Table 2.
Table 2.
Validation data of mycotoxins from artificially contaminated beer samples.
| Mycotoxin | Spiking Level [ng/mL] | Calculated Concentration [ng/mL] | Recovery [%] | Precision [%] | LOD [ng/mL] |
LOQ [ng/mL] |
|---|---|---|---|---|---|---|
| OTA | 0.05 | 0.0408 | 81.6 | 2.5 | 0.003 | 0.011 |
| 0.25 | 0.199 | 79.6 | 6.1 | |||
| 1.0 | 0.858 | 85.8 | 4.2 | |||
| DON | 40 | 38.16 | 95.4 | 2.9 | 3.50 | 11.6 |
| 200 | 190.6 | 95.3 | 1.2 | |||
| 400 | 378.2 | 94.5 | 3.9 | |||
| NIV | 40 | 40.84 | 102.1 | 5.2 | 4.30 | 14.3 |
| 200 | 202.2 | 101.1 | 1.7 | |||
| 400 | 407.2 | 101.8 | 1.3 | |||
| T-2 | 8 | 9.36 | 117.0 | 3.9 | 0.31 | 1.03 |
| 40 | 44.64 | 111.6 | 3.0 | |||
| 80 | 92.8 | 116.0 | 1.3 | |||
| HT-2 | 8 | 9.10 | 113.8 | 5.2 | 0.36 | 1.21 |
| 40 | 47.64 | 119.1 | 3.0 | |||
| 80 | 93.04 | 116.3 | 1.2 | |||
| DAS | 8 | 8.72 | 109.0 | 4.0 | 0.28 | 0.92 |
| 40 | 43.92 | 109.8 | 2.6 | |||
| 80 | 84.64 | 105.8 | 4.1 | |||
| ZEN | 4 | 4.14 | 103.5 | 3.4 | 0.07 | 0.23 |
| 20 | 20.74 | 103.7 | 2.5 | |||
| 40 | 42.56 | 106.4 | 3.0 |
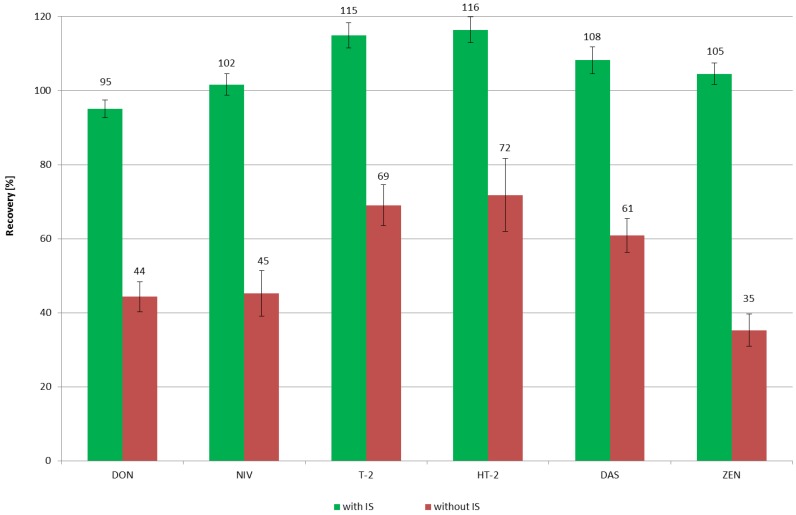
For Fusarium mycotoxins, determined by the HPLC-MS/MS technique, the recovery experiments were performed both with and without the internal standards. The results showed significant differences in recovery values (Figure 1), so that only the samples with the added ISs, met the performance criteria for the analytical methods, laid down by the UE, for the official control of the levels of mycotoxins in foodstuffs [24,25]. The recovery levels, therein, were given as follows: OTA 50–120%; DON 60–110%, T-2 60–130%, HT-2 60–130%, and ZEN 60–120%. The significant differences in the recovery values, with and without ISs, confirmed the complexity of the beer matrix, resulting in significant matrix effects, especially when the applied sample clean-up procedure was not of high selectivity. The acetonitrile-based extraction caused a precipitation of polar matrix components and might also have resulted in precipitation of polar analytes, and thus decreased the recovery. Low recovery values observed in the case of ZEN, might be attributed to strong suppression caused by the co-eluting non-polar matrix [26].
Figure 1.
Impact of addition of internal standard (IS) on mycotoxin recovery rates.
2.2. Mycotoxins Occurrence
The results of the analyses of 69 beer samples are summarized in Table 3. OTA was detected in 93% of the samples and 78% of them contained OTA at a concentration higher than the LOQ—with the highest value at 0.347 ng/mL. In 4 beer samples (1 lager, 1 unpasteurized, and 2 porters), OTA concentrations were higher than 0.2 ng/mL; the proposed maximum acceptable level for this toxin [11].
Table 3.
Concentration of ochratoxin A (OTA) and Fusarium mycotoxins in the analyzed beer samples.
| Toxin | Positive/Samples | Mean [ng/mL] |
Median [ng/mL] |
Positive Samples [ng/mL] | ||
|---|---|---|---|---|---|---|
| Min | Max | Mean; SD | ||||
| OTA | 64/69 | 0.053 | 0.032 | <LOQ | 0.347 | 0.057; 0.065 |
| DON | 66/69 | 16.3 | <LOQ | <LOQ | 56.2 | 17.1; 9.0 |
| NIV | 1/69 | <LOD | <LOD | - | <LOQ | - |
| T-2 | 11/69 | <LOD | <LOD | <LOQ | <LOQ | <LOQ |
| HT-2 | 51/69 | <LOQ | <LOQ | <LOQ | 1.57 | 1.23; 0.08 |
| DAS | 0/69 | - | - | - | <LOD | - |
| ZEN | 4/69 | <LOD | <LOD | <LOQ | 0.413 | 0.304; 0.073 |
The mean and median OTA concentrations were in the range of tens of pg/mL; these and the contamination rates are in accordance with previous European results [17,18,27,28,29]. In some traditional beers coming from Africa, however, the level of OTA might have exceeded values of 2 µg/mL [19]. DAS was not detected in any of the samples, but at least one of the Fusarium mycotoxins was present in 97% of samples. NIV was detected only in one sample (<LOQ), four of the samples contained ZEN (3 samples below LOQ value and 0.413 ng/mL) and the levels of T-2 and HT-2 were close to the LOQs. The mean and highest concentration of DON (detected in 96% of the samples and in 45% at the level >LOQ) were 17.1 ng/mL and 56.2 ng/mL, respectively. OTA, DON, and HT-2 were the only compounds detected in more than 75% of the samples. The results sorted by beer type are presented in Table 4.
Table 4.
Occurrence of OTA, DON, and HT-2 in different beer type samples.
| Beer Type | Positive/Samples | Mean [ng/mL] | Median [ng/mL] | Positive Samples [ng/mL] | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | |||||
| OTA | Normal lager | 23/23 | 0.051 | 0.034 | <LOQ | 0.347 | 0.051 |
| Strong lager | 19/19 | 0.062 | 0.056 | 0.012 | 0.114 | 0.062 | |
| Porter | 5/5 | 0.111 | 0.068 | <LOQ | 0.262 | 0.112 | |
| Unpasteurized | 8/9 | 0.059 | 0.023 | <LOQ | 0.269 | 0.066 | |
| Flavored | 5/8 | 0.017 | 0.014 | <LOQ | 0.046 | 0.027 | |
| Non-alcoholic | 4/5 | 0.013 | <LOQ | <LOQ | 0.030 | 0.016 | |
| DON | Normal lager | 22/23 | 17.9 | <LOQ | <LOQ | 56.2 | 18.7 |
| Strong lager | 19/19 | 19.2 | 18.8 | <LOQ | 34.7 | 19.2 | |
| Porter | 4/5 | 16.5 | <LOQ | <LOQ | 33.8 | 20.7 | |
| Unpasteurized | 9/9 | 13.4 | <LOQ | <LOQ | 16.3 | 13.4 | |
| Flavored | 8/8 | 12.3 | <LOQ | <LOQ | 16.7 | 12.3 | |
| Non-alcoholic | 4/5 | <LOQ | <LOQ | <LOQ | 12.7 | 11.9 | |
| HT-2 | Normal lager | 17/23 | <LOQ | <LOQ | <LOQ | 1.57 | 1.23 |
| Strong lager | 19/19 | 1.23 | <LOQ | <LOQ | 1.52 | 1.23 | |
| Porter | 4/5 | <LOQ | <LOQ | <LOQ | 1.52 | 1.29 | |
| Unpasteurized | 6/9 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| Flavored | 3/8 | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | |
| Non-alcoholic | 2/5 | <LOQ | <LOD | <LOQ | <LOQ | <LOQ | |
Widespread beer contamination with DON were found, herein; confirming the results of other studies conducted in Poland and Europe [22,30,31,32]. However, differences could be observed in the prevalence of DON in beer samples. Pascari et al. [33] analyzed 64 commercially available European beers but found DON only in 4 samples (6%), whereas ZEN was present in 65% of the samples they examined; with concentrations ranging between 8.24 and 62.98 ng/mL. This is in contrast to our study where ZEN was present in only 6% of the samples, and at levels below 0.5 ng/mL. Low ZEN levels (maximum 2.0 ng/mL), similar to those herein, were also reported by Bauer et al. [31]. However, the authors there detected mycotoxin in all their samples. A selected overview of mycotoxins surveys in beer is presented in Table 5.
Table 5.
Overview of mycotoxin occurrence in beer samples.
| Mycotoxin | Number of Analyzed Beer Samples | Number of Positive Samples | Mycotoxin Concentration Range (ng/mL) | Reference |
|---|---|---|---|---|
| OTA | 69 | 69 | 0.008–0.498 | [18] |
| 19 | 10 | 1.5–2340 | [19] | |
| 318 | 233 | 0.01–0.293 | [27] | |
| 88 | 73 | 0.007–0.204 | [29] | |
| 1000 | 6 | <0.3–0.6 | [34] | |
| DON | 154 | 60 | 24.5–47.7 | [22] |
| 374 | 204 | 3.2–89.3 | [30] | |
| 44 | 33 | 2.2–20.0 | [31] | |
| 100 | 83 | 1.0–73.6 | [32] | |
| 64 | 4 | 20.97–46.74 | [33] | |
| 1000 | 64 | <10–412 | [34] | |
| NIV | 100 | 56 | 0.5–7.6 | [32] |
| 1000 | 4 | 8–21 | [34] | |
| T-2 | 1000 | 3 | <0.5–2.3 | [34] |
| HT-2 | 154 | 14 | 24.2–38.2 | [22] |
| 64 | 1 | 23.72 | [33] | |
| 1000 | 1 | 3.4 | [34] | |
| ZEN | 35 | 7 | 2.6–426 | [19] |
| 64 | 12 | 8.24–62.96 | [33] | |
| 44 | 44 | 0.35–2.0 | [31] | |
| 1000 | 6 | <0.3–0.3 | [34] |
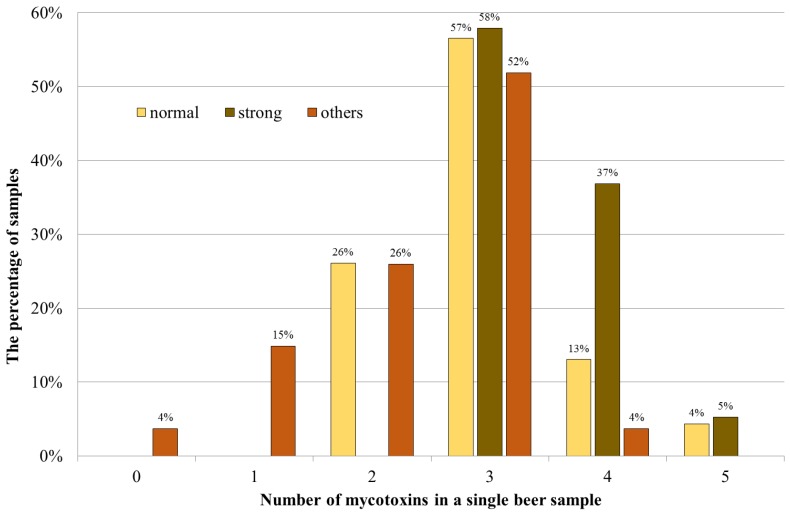
Grain and, thus, their products might be contaminated with various species of molds. Furthermore, each species can produce several mycotoxins, therefore, it is important to evaluate their co-occurrence. The association of mycotoxins might enhance the adverse health effects when compared to individual compounds [35]. More than 93% of the samples contained at least two mycotoxins, i.e., OTA and DON; and at least three compounds were present in 74% of the analyzed beers. The highest number of mycotoxins detected in one sample was 5 (3% of the samples). The distribution of mycotoxins in particular beer samples has revealed that the highest percentage, up to 60% of the samples of each group, contained 3 mycotoxins (Figure 2).
Figure 2.
Distribution of mycotoxins in particular beer samples.
2.3. Dietary Exposure Assessment
Since very few of our samples were positive for mycotoxins, other than OTA, DON, and HT-2, the calculations below were only performed for those three compounds. Polish foreign trade data on beer export were used for these evaluations [36]. The Global Environmental Monitoring System (GEMS)/Food cluster diets data on mean beer consumption were as follows: Germany and Poland - 259.5mL/day; The Netherlands and Belgium—234.4; United Kingdom—180.2; USA, Canada, and Italy—174.3; Czech Republic, Hungary, Ireland, Romania, and Slovakia—225.2; and Cyprus—174.3. The mean body weight values of 70.8 kg for Europe, and 80.7 kg for Northern America were adopted for these calculations [37]. Estimated daily intake ((EDI): Table 5) was presented as a percentage of TDI (tolerable daily intake) namely: 17.14 ng/kg body-weight (b.w.) for OTA (derived from the tolerable weekly intake (TWI) = 120 ng/kg b.w.); [38], 1000 ng/kg b.w. for DON, and 60 ng/kg b.w. for HT-2 [2].
Using mean values for OTA in beer samples, the daily intake of OTA from beer approximates to 0.8–1.1% of TDI. Even a ‘worst-case’ scenario (i.e., using the maximum concentration observed, herein) only equates to 5.0–7.5% of TDI. These values are in general agreement with previous estimates from the SCOOP Task 3.2.7 [39], in which, the contribution of dietary beer to OTA intake was 0.01–0.14 ng/kg b.w. This ranks beer as a relatively small source of OTA; the contribution of other food groups was estimated as follows—cereals (44%); others (15%); wine (10%); coffee (9%); beer (7%); cocoa and derived products (5%); dried fruits (4%); meat (3%); and spices (3%). Similarly, previous studies of OTA in beer revealed that OTA intake does not exceed the TDI value [34,40].
In the case of HT-2, there were no significant differences between ‘scenarios’ (Table 6; mean—5.0–7.5% of TDI; maximum—6.5–9.7% of TDI). This was due to the fact that although HT-2 was present in 74% of the samples, its concentration was near the LOQ value of the method described herein. Rodríguez-Carrasco et al. [22] analyzed 154 beer samples for Fusarium mycotoxins and evaluated the average intake level of HT-2 toxin from beer to be ~7–12% of TDI, set by the Scientific Committee on Food in 2002 (0.1 μg/kg b.w.), which corresponded to ~11–20% of the TDI applied in this study.
Table 6.
Estimated daily intake (EDI) of OTA, DON, and HT-2 derived from the determined mycotoxin level, beer consumption in various European countries [41], mean body weight [37], and tolerable daily intake (TDIs) [2,38].
| Cluster | Country | Toxin Level | EDI [ng/kg b.w.] | % TDI | ||||
|---|---|---|---|---|---|---|---|---|
| OTA | DON | HT-2 | OTA | DON | HT-2 | |||
| G07 | UK | mean | 0.14 | 42.0 | 3.11 | 0.80 | 4.20 | 5.19 |
| maximum | 0.89 | 145 | 4.00 | 5.21 | 14.5 | 6.74 | ||
| G08 | Germany Poland |
mean | 0.20 | 60.4 | 4.48 | 1.14 | 6.04 | 7.47 |
| maximum | 1.28 | 208 | 5.80 | 7.50 | 20.8 | 9.69 | ||
| G10 | Canada Italy USA |
mean | 0.13 | 40.6 | 3.01 | 0.77 | 4.06 | 5.02 |
| maximum | 0.86 | 140 | 3.90 | 5.04 | 14.0 | 6.52 | ||
| G15 | Czech Republic Hungary Ireland Romania Slovakia |
mean | 0.17 | 52.4 | 3.89 | 0.99 | 5.24 | 6.49 |
| maximum | 1.12 | 181 | 5.10 | 6.51 | 18.1 | 8.42 | ||
If the mean concentration of DON from this study was used, the calculated daily exposure to this mycotoxin was in the range of 4.1–60% of TDI, whereas, based on maximum values, it reached the level of 14–21% of TDI (Table 6). This married well with previous reports, which estimated exposure to DON from beer at the level of a few percent of the TDI, for either DON [22,31] or DON with its conjugates [30,33]. Higher levels of possible exposure, close to 20% of TDI, have also been previously found [23].
3. Conclusions
Herein, a set of 69 beer samples was analyzed for seven mycotoxins using validated HPLC methods, relying on either fluorescence (OTA) or MS/MS (trichothecenes and ZEN) detection. Our results indicate that DON was the most frequently occurring mycotoxin, followed by the OTA and the HT-2 toxin. Moreover >93% of the beer samples examined, contained detectable levels of at least 2 analytes; and ~74% of the samples contained 3 or more mycotoxins.
Using the above data, the potential exposure of various European populations to mycotoxins from beer produced in Poland was calculated. The evidence from this analysis demonstrated that mycotoxin intake from beers made by major breweries was unlikely to exceed 20% of TDI, for any mycotoxin examined herein, and was more likely to be 5%. On one hand, we might conclude that beer did not seem to be a major source of the mycotoxins examined in this study. However, the occurrence of multiple mycotoxins in many samples and the possibility of undocumented synergies between them suggests that a cumulative mycotoxin limit might be required for beer products in the EU. In addition, the generally higher levels of mycotoxins found in craft beers suggests that these types of products should be tested more often, to protect ‘beer enthusiasts’. We also share the opinion of Varga et al. [30] and Peters et al. [34], that the maximum levels of the most frequently occurring mycotoxins found in beers should be set by the EU, in order to ensure consumer safety.
4. Materials and Methods
4.1. Sampling
Sixty-nine beer samples (from four major Polish brewing companies) were purchased randomly between January and June 2018, from retail trade in Poland, as follows—42 lager (23 normal < 6.2 abv; 19 strong > 6.2 abv), 5 porter, 9 unpasteurized, 8 flavored, and 5 non-alcoholic beers. All samples were stored at 4 °C and degassed the day before analysis.
4.2. Standards and Chemicals
Ochratoxin A, deoxynivalenol, nivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS), zearalenone (ZEN), 13C-DON, 13C-T-2, 13C-HT-2 toxins, and 13C-ZEN were purchased from Biopure (Tulln, Austria). Ammonium acetate, Celite® 545, acetic acid, acetonitrile (ACN; gradient grade), and methanol (MeOH; MS grade) were supplied by Merck-Millipore (Darmstadt, Germany). The Simplicity 185 (Millipore, Bedford, MA, USA) water purification system was used for the deionized water production.
4.3. Sample Preparation
Before the extraction, the pH of all samples was adjusted to 7.2, by addition of 1 M NaOH. OTA was isolated using immunoaffinity columns (IAC) Ochraprep® (R-Biopharm Rhône Ltd., Glasgow, UK)—30 mL of beer was passed through the column at a flow rate of 2–3 mL/min. The column was washed with 20 mL of H2O and dried by passing air, throughout. OTA was then eluted using 2.0 mL of MeOH:CH3COOH (98:2). The eluate was evaporated to dryness under nitrogen and then dissolved in 1.0 mL of H2O:MeOH:CH3COOH (50:49:1).
To prepare the samples for trichothecene and ZEN determination, 4 mL of each beer was shaken with 16 mL of ACN, 0.5 g of Celite® 545, and 20 µL of 13C-ZEN (an internal standard for ZEN) solution for 20 min. After centrifugation (4000× g, 10 min), 50 µL of isotopically labelled analogues (ISs: 13C-DON, 13C-T-2, 13C-HT-2) were added to 5 mL of the supernatant. Samples were then evaporated to dryness under nitrogen and dissolved in 0.5 mL of MeOH:H2O (1:4).
4.4. Instrumental Analysis
OTA evaluation was performed using high-performance liquid chromatography (HPLC) with fluorescence detection (Ex: 330 nm, Em: 460 nm), using the following materials, HPLC—LaChrom ELITE HPLC system (Merck-Hitachi, Darmstadt, Germany), chromatographic column—LiChrospher 100 RP-18 (250 × 4.0 mm, 5 μm), oven temperature—25 °C, mobile phase—ACN:2%CH3COOH (70:30), flow rate—1 mL/min, injection volume—50 μL.
Trichothecenes and ZEN were determined using HPLC with MS/MS detection. Analytes were separated and determined using HPLC Shimadzu Nexera (Shimadzu, Tokyo, Japan), API 4000 mass spectrometer (Sciex, Foster City, CA, USA), the Gemini–NX C18 (150 × 4.6 mm, 5 µm) (Phenomenex Inc., Torrance, CA, USA) chromatographic column, and an oven temperature of 27 °C; mobile phase of A was H2O + 5 mM CH3COONH4 + 1% CH3COOH, B: MeOH + 5 mM CH3COONH4 + 1% CH3COOH, flow rate was 0.7 mL/min, and the injection volume was 20 µL. HPLC-MS/MS method was performed in multiple reaction monitoring (MRM) mode in negative and positive modes, with following conditions—collision gas 6 psi, curtain gas 25 psi, ion source gas1 50 psi, ion source gas2 50 psi, ion spray voltage 5000 V (positive mode) and −4000 V (negative mode), and source temperature 500 °C. MRM analysis had two transitions per compound. Optimized analyte-dependent MS/MS parameters are given in Table 7.
Table 7.
Optimized mass spectrometry conditions.
| Compound | Precursor Ion (m/z) | Declustering Potential (V) | Product Ions (m/z) a | Collision Energy (V) | Cell Exit Potential (V) |
|---|---|---|---|---|---|
| Nivalenol | 371.1 [M + CH3COO]− | −40 | 281.0/59.0 | −22/−40 | −14/−5 |
| Deoxyniwalenol | 355.1 [M + CH3COO]− | −35 | 264.8/58.9 | −20/−38 | −17/−1 |
| 13C-Deoxyniwalenol | 370.2 [M + CH3COO]− | −50 | 310.0 | −14 | −7 |
| Diacetoxyscirpenol | 384.1 [M + NH4]+ | 51 | 307.0/247.0 | 17/19 | 20/16 |
| T-2 toxin | 484.1 [M + NH4]+ | 61 | 215.0/185.0 | 29/25 | 12/14 |
| 13C-T-2 toxin | 508.3 [M + NH4]+ | 61 | 322.1 | 19 | 8 |
| HT-2 toxin | 442.2 [M + NH4]+ | 51 | 215.0/263.0 | 19/17 | 14/18 |
| 13C-HT-2 toxin | 464.1 [M + NH4]+ | 51 | 278.1 | 17 | 18 |
| Zearalenone | 317.1 [M − H]− | −85 | 130.8/174.9 | −40/−32 | −7/−9 |
| 13C-Zearalenone | 355.1 [M − H]− | −100 | 139.9 | −42 | −7 |
a quantifier ions are given in bold.
The analyte identification was done, based on the retention time of the calibration solutions, as well as the spiked samples, and the signal value (area) increment, with reference to the spiking amount. For MS/MS detection, two characteristic MRM transitions for each analyte were applied as well (Table 7).
4.5. Statistical Analysis
To evaluate the significant differences in mycotoxin concentration among the beer types, the Kruskal-Wallis ANOVA test was applied (α = 0.05). The average values of the analytes recovered were compared, using the t-Student test (α = 0.05). For the calculation, the results below the LOD value were set as 0.5 LOD, whereas the results comprising between the LOD and the LOQ were set as the LOQ value for each mycotoxin. A positive sample refers to a sample with a result above the limit of detection. All statistical analyses were applied using the Statistica 10.0 software package (StatSoft, Krakow, Poland).
4.6. Dietary Exposure Assessment
Calculations of the estimated daily intake (EDI) of mycotoxins in different European populations were prepared, using food-consumption data derived from the GEMS/Food cluster diets 2012, prepared by WHO [41]. All contamination data (mean and median concentrations) were taken from the results of analyses conducted herein. Since beer is an alcoholic beverage, the calculation was performed for adults only; using mean body weight values adjusted for different world regions, as previously described [37]. The EDI was calculated as follows:
| (1) |
where C is the concentration of mycotoxin in contaminated beer; Cons stands for the average daily consumption of beer in the study region; and b.w. represents the body weight.
Abbreviations
| Abv | alcohol by volume |
| ACN | acetonitrile |
| b.w. | body-weight |
| DAS | diacetoxyscirpenol |
| DON | deoxynivalenol |
| EDI | estimated daily intake |
| HPLC | high performance liquid chromatography |
| ISs | internal standards |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| NIV | nivalenol |
| OTA | ochratoxin A |
| TDI | tolerable daily intake |
| ZEN | zearalenone |
Author Contributions
Conceptualization, J.G. and A.B.-K.; methodology, A.B.-K., R.K.; validation, A.B.-K., R.K.; formal analysis, R.K., A.B.-K.; investigation, R.K., A.B.-K.; resources, J.G., R.K., A.B.-K., M.T.; writing—original draft preparation, J.G., A.B.-K.; writing—review and editing, R.K., M.T.; supervision, J.G.; project administration, J.G.; funding acquisition, J.G., M.T., A.B.-K.
Funding
This study was supported by the Polish Minister of Science and Higher Education, under the program “Regional Initiative of Excellence” in 2019–2022 (Grant No. 008/RID/2018/19).
Conflicts of Interest
The authors declare no conflicts of interest.
Key Contribution
The study focuses on the co-occurrence of 7 mycotoxins in Polish beer, as well as the evaluation of their intake, taking into account a significant role of Poland in beer production and exportation.
References
- 1.Hornsey I. A History of Beer and Brewing. The Royal Society of Chemistry; Cambridge, UK: 2003. [Google Scholar]
- 2.European Commission Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006;L364:5–24. [Google Scholar]
- 3.European Commission Commission regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union. 2007;L255:14–17. [Google Scholar]
- 4.European Commission Commission regulation (EU) No 105/2010 of 5 February 2010 amending regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off. J. Eur. Union. 2010;L35:7–8. [Google Scholar]
- 5.European Commission Commission regulation (EU) No 165/2010 of 26 February 2010 amending Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union. 2010;L50:8–12. [Google Scholar]
- 6.European Commission Commission regulation (EU) No 594/2012 of 5 July 2012 amending Regulation (EC) 1881/2006 as regards the maximum levels of the contaminants ochratoxin A, non dioxin-like PCBs and melamine in foodstuffs. Off. J. Eur. Union. 2012;L176:43–45. [Google Scholar]
- 7.European Commission Commission recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union. 2013;L91:12–15. [Google Scholar]
- 8.Beer Statistics 2017. The Brewers of Europe; Brussels, Belgium: 2017. Beer Production 2010–2016. [Google Scholar]
- 9.Milićević D.R., Škrinjar M., Baltić T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins. 2010;2:572–592. doi: 10.3390/toxins2040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayman P., Baker J.L. Ochratoxins: A global perspective. Mycopathologia. 2006;162:215–223. doi: 10.1007/s11046-006-0055-4. [DOI] [PubMed] [Google Scholar]
- 11.Anli E., Mert Alkis I. Ochratoxin A and brewing technology: A review. J. Inst. Brew. 2010;116:23–32. doi: 10.1002/j.2050-0416.2010.tb00394.x. [DOI] [Google Scholar]
- 12.Lancova K., Hajslova J., Poustka J., Krplova A., Zachariasova M., Dostalek P., Sachambula L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. 2008;25:732–744. doi: 10.1080/02652030701779625. [DOI] [PubMed] [Google Scholar]
- 13.Kostelanska M., Zachariasova M., Lacina O., Fenclova M., Kollos A.L., Hajslova J. The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC-TOFMS method. Food Chem. 2011;126:1870–1876. doi: 10.1016/j.foodchem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Wolf-Hall C.E. Mold and mycotoxin problems encountered during malting and brewing. Int. J. Food Microbiol. 2007;119:89–94. doi: 10.1016/j.ijfoodmicro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Habschied K., Šarkanj B., Klapec T., Krstanović V. Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chem. 2011;129:329–332. doi: 10.1016/j.foodchem.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 16.Bĕláková S., Benešová K., Čáslavský J., Svoboda Z. The occurrence of the selected Fusarium mycotoxins in Czech malting barley. Food Control. 2014;37:93–99. doi: 10.1016/j.foodcont.2013.09.033. [DOI] [Google Scholar]
- 17.Jørgensen K. Survey of pork, poultry, coffee, beer and pulses for ochratoxin A. Food Addit. Contam. 1998;15:550–554. doi: 10.1080/02652039809374680. [DOI] [PubMed] [Google Scholar]
- 18.Medina A., Valle-Algarra F.M., Gimeno-Adelantado J.V., Mateo R., Mateo F., Jiménez M. New method for determination of ochratoxin A in beer using zinc acetate and solid-phase extraction silica cartridges. J. Chromatogr. A. 2006;1121:178–183. doi: 10.1016/j.chroma.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Odhav B., Naicker V. Mycotoxins in South African traditionally brewed beers. Food Addit. Contam. 2002;19:55–61. doi: 10.1080/02652030110053426. [DOI] [PubMed] [Google Scholar]
- 20.Zöllner P., Berner D., Jodlbauer J., Lindner W. Determination of zearalenone and its metabolites alpha- and beta-zearalenol in beer samples by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000;738:233–241. doi: 10.1016/S0378-4347(99)00521-6. [DOI] [PubMed] [Google Scholar]
- 21.Schothorst R.C., Jekel A.A. Determination of trichothecenes in beer by capillary gas chromatography with flame ionisation detection. Food Chem. 2003;82:475–479. [Google Scholar]
- 22.Rodríguez-Carrasco Y., Fattore M., Albrizio S., Berrada H., Mañes J. Occurrence of Fusarium mycotoxins and their dietary intake through beer consumption by the European population. Food Chem. 2015;178:149–155. doi: 10.1016/j.foodchem.2015.01.092. [DOI] [PubMed] [Google Scholar]
- 23.Piacentini K.C., Savi G.D., Olivo G., Scussel V.M. Quality and occurrence of deoxynivalenol and fumonisins in craft beer. Food Control. 2015;50:925–929. doi: 10.1016/j.foodcont.2014.10.038. [DOI] [Google Scholar]
- 24.European Commission Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union. 2006;L70:12–34. [Google Scholar]
- 25.European Commission Commission regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union. 2014;L147:29–43. [Google Scholar]
- 26.Habler K., Gotthardt M., Schüler J., Rychlik M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in beer. Food Chem. 2017;218:447–454. doi: 10.1016/j.foodchem.2016.09.100. [DOI] [PubMed] [Google Scholar]
- 27.Bresch H., Urbanek M., Hell K. Ochratoxin A in coffee, tea and beer. Arch. Fur Lebensm. 2000;51:89–94. [Google Scholar]
- 28.Tangni E.K., Ponchaut S., Maudoux M., Rozenberg R., Larondelle Y. Ochratoxin A in domestic and imported beers in Belgium: Occurrence and exposure assessment. Food Addit. Contam. 2002;19:1169–1179. doi: 10.1080/02652030210007859. [DOI] [PubMed] [Google Scholar]
- 29.Medina A., Jiménez M., Gimeno-Adelantado J.V., Valle-Algarra F.M., Mateo R. Determination of ochratoxin A in beer marketed in Spain by liquid chromatography with fluorescence detection using lead hydroxyacetate as a clean-up agent. J. Chromatogr. A. 2005;1083:7–13. doi: 10.1016/j.chroma.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 30.Varga E., Malachowa A., Schwartz H., Krska R., Berthiller F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyl-deoxynivalenol on 374 beer samples. Food Addit. Contam. 2013;30:137–146. doi: 10.1080/19440049.2012.726745. [DOI] [PubMed] [Google Scholar]
- 31.Bauer J.I., Gross M., Gottschalk C., Usleber E. Investigations on the occurrence of mycotoxins in beer. Food Control. 2016;63:135–139. doi: 10.1016/j.foodcont.2015.11.040. [DOI] [Google Scholar]
- 32.Bryła M., Ksieniewicz-Woźniak E., Waśkiewicz A., Szymczyk K., Jędrzejczak R. Co-occurrence of nivalenol, deoxynivalenol and deoxynivalenol-3-glucoside in beer samples. Food Control. 2018;92:319–324. doi: 10.1016/j.foodcont.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascari X., Ortiz-Solá J., Marín S., Ramos A.J., Sanchis V. Survey of mycotoxins in beer and exposure assessment through the consumption of commercially available beer in Lleida, Spain. LWT Food Sci. Technol. 2018;92:87–91. doi: 10.1016/j.lwt.2018.02.021. [DOI] [Google Scholar]
- 34.Peters J., van Dam R., van Doorn R., Katerere D., Berthiller F., Haasnoot W., Nielen M.W.F. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE. 2017;12:e0185887. doi: 10.1371/journal.pone.0185887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenier B., Oswald I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011;4:285–313. doi: 10.3920/WMJ2011.1281. [DOI] [Google Scholar]
- 36.The Yearbook of Foreign Trade Statistics of the Polish Central Statistical Office, Warsaw. [(accessed on 4 April 2019)];2017 Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-handlu-zagranicznego-2017,9,11.html.
- 37.Walpole S.C., Prieto-Merino D., Edwards P., Cleland J., Stevens G., Roberts I. The weight of nations: An estimation of adult human biomass. BMC Public Health. 2012;12:439. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EFSA (European Food Safety Authority) Statement on recent scientific information on the toxicity of Ochratoxin A. EFSA J. 2010;8:1626. doi: 10.2903/j.efsa.2010.1626. [DOI] [Google Scholar]
- 39.European Commission Task 3.2.7 Assessment of dietary intake of Ochratoxin A by the population of EU Member States. [(accessed on 4 April 2019)];2002 Available online: https://ec.europa.eu/food/safety/chemical_safety/contaminants/catalogue/ochratoxin_en.
- 40.Coronel M.B., Marín S., Cano-Sancho G., Ramos A.J., Sanchis V. Exposure assessment to ochratoxin A in Catalonia (Spain) based on the consumption of cereals, nuts, coffee, wine, and beer. Food Addit. Contam. Part A. 2012;29:979–993. doi: 10.1080/19440049.2012.660708. [DOI] [PubMed] [Google Scholar]
- 41.GEMS/Food Consumption Cluster Diets . Department of Food Safety and Zoonoses, World Health Organization; Geneva: 2012. [(accessed on 4 April 2019)]. Global Environment Monitoring System Food Contamination Monitoring and Assessment Programme. Available online: https://extranet.who.int/sree/Reports?op=vs&path=/WHO_HQ_Reports/G7/PROD/EXT/GEMS_cluster_diets_2012&userid=G7_ro&password=inetsoft123. [Google Scholar]