Abstract
Obesity and type 2 diabetes are a veritable global pandemic. There is an imperative to develop new therapies for these conditions that can be delivered at scale to patients, which deliver effective and titratable weight loss, amelioration of diabetes, prevention of diabetic complications and improvements in cardiovascular health. Although agents based on glucagon‐like peptide‐1 (GLP‐1) are now in routine use for diabetes and obesity, the limited efficacy of such drugs means that newer agents are required. By combining the effects of GLP‐1 with other gut and metabolic hormones such as glucagon (GCG), oxyntomodulin, glucose‐dependent insulinotropic peptide (GIP) and peptide YY (PYY), we may obtain improved weight loss, increased energy expenditure and improved metabolic profiles. Drugs based on dual agonism of GLP1R/GCGR and GLP1R/GIPR are being actively developed in clinical trials. Triple agonism, for example with GLPR1/GCGR/GIPR unimolecular agonists or using GLP‐1/oxyntomodulin/PYY, is also being explored. Multi‐agonist drugs seem set to deliver the next generation of therapies for diabetes and obesity soon.
1. THE PROBLEM OF OBESITY AND DIABETES
The prevalence of overweight and obesity has dramatically increased over the last decades posing a significant health problem with associated complications and major socioeconomic impact. According to the WHO, worldwide obesity has nearly tripled since 1975. In 2016, more than 1.9 billion adults were overweight of which 650 million were obese. These numbers correspond respectively to 39% and 13% of the adult population.1 Obesity drives the pathogenesis of other diseases such as type 2 diabetes, cardiovascular disease, related musculoskeletal disorders such as osteoarthritis and some cancers (endometrial, breast, ovarian, prostate, liver, gallbladder, kidney and colon).2 Obesity is therefore responsible for a great deal of mortality throughout the world.3 The aetiology of obesity lies fundamentally in an imbalance of energy homeostasis from the interaction of three factors: (a) increased energy intake through the supply of unlimited quantities of energy dense food and drink which are tuned to appeal to our appetites; (b) decreased energy expenditure as result of modern lifestyles (a more sedentary environment, contemporary modes of transportation, urbanization, reduction in sleep quality); (c) genetic and ethnic predispositions to obesity.
Although bariatric surgery is clearly an effective treatment for obesity with long‐term follow‐up data proving its efficacy and success in improving lifespan, enforcing a remission of diabetes in some patients and ameliorating other obesity‐associated co‐morbidities,4 it can never be the sole solution. Firstly, given the numbers of people with obesity, bariatric surgery can never be implemented at the scale necessary without immense expense for healthcare systems. Secondly, bariatric surgery can be unpredictable in its effects, leading to varying levels of weight loss and diabetes remission from standardized “one size” procedures which do not necessarily fit all. Thirdly, complications such as post‐prandial hypoglycaemia and nutritional deficiencies cause long‐term morbidity and require long‐term follow‐up. Furthermore, a large part of the morbidity associated with overweight and obesity is found in overweight people,3 and the magnitudes of weight loss from bariatric surgery may be inappropriate for this population. Therefore, there is an imperative to develop new therapies which deliver key benefits such as titratable and effective weight loss, amelioration of diabetes, prevention of diabetic complications and improvements in cardiovascular health. This review will look at the latest developments in the sphere of gut hormone treatments for obesity and diabetes and consider whether combination gut hormone therapies may prove to be the next big thing in obesity treatment.
2. GLP‐1 ACTION, CLINICAL APPLICATIONS AND LIMITATIONS
Glucagon‐like peptide‐1 (GLP‐1) has been the forerunner of the gut hormone‐based therapies. GLP‐17‐37 and GLP‐17‐36amide are secreted from the neuroendocrine L‐cells of the small intestine. GLP‐1 together with glucose‐dependent insulinotropic peptide (GIP) are responsible for the incretin effect, ie, augmented insulinotropy in response to an ingested glucose load as opposed to an isoglycaemic intravenous glucose challenge.5 The incretin effect is reduced in type 2 diabetes.6 GLP‐1 is derived from the post‐translational processing of proglucagon in L‐cells and some brainstem neurones by prohormone convertase 1 (PC1). Classically, proglucagon processing by PC2 in pancreatic α cells releases glucagon itself,7, 8 but a subset of α cells has been shown to process proglucagon to GLP‐1.9 Proglucagon is additionally processed in L‐cells to the products glicentin, oxyntomodulin (identical to glucagon with a C‐terminal octapeptide extension) and GLP‐2. After secretion, GLP‐1 has a short half‐life of 1‐2 minutes as it is rapidly degraded and inactivated by the endopeptidase dipeptidyl peptidase‐4 (DPP‐IV), resulting in the formation of the inactive metabolite GLP‐19‐37 and GLP‐19‐36amide. GLP‐1 exerts its effects via the GLP‐1 receptor (GLP1R) which belongs to the family of G protein‐coupled receptors. GLP1R is abundantly expressed in the pancreas, gut and central nervous system but also in the heart, lungs, vasculature and peripheral nervous system.10 As noted above, GLP1R activation on β cells causes enhanced glucose dependent insulin secretion11; at the same time it suppresses glucagon secretion from α cells. The effects of GLP‐1 on β cells extend beyond the insulinotropic effect on glucose homeostasis including inhibition of β cell apoptosis, induction of their proliferation and expansion of their mass in rodents.9 GLP1R agonism reduces food intake in animals12, 13 and humans14, 15, 16 acting on regions of the hypothalamus and hindbrain as evidenced in animal studies.17, 18 GLP‐1 also acts in the gastrointestinal tract through inhibition of gastric secretion and deceleration of gastric emptying19 attenuating the postprandial rise in glucose levels. It also reduces hepatic steatosis, liver inflammation and hepatocyte injury; these effects could be either direct20 or indirect through weight loss.9, 21 Amongst the multifaceted mechanisms of action of GLP‐1 is also its ability to activate invariant natural killer cells (i‐NKT) which triggers the production of fibroblast growth factor 21 leading to weight loss in mice.22 GLP‐1 may also possess neurotropic effects improving learning in rats and exerting neuroprotective effects.23, 24 The presence of GLP‐1 receptors in the heart suggests a physiological role of GLP‐1 in cardiac function.25 Mice lacking the GLP1R have reduced resting heart rate, increased left ventricular end‐diastolic pressure and increased left ventricular wall thickness.26 GLP1R agonists have a characteristic positive chronotropic effect which is reduced but not completely abrogated in mice with cardiomyocyte‐selective knockouts for GLP1R, suggesting that the effect is partially mediated by a direct effect on cardiomyocytes and partially via the autonomic nervous system.27
Such diversity of actions of GLP‐1 has led to the development of GLP‐1 based therapies for improving glycaemia in type 2 diabetes, for weight loss, and more recently for improving cardiovascular outcomes in patients with diabetes and established cardiovascular disease. The first GLP1R agonist approved for clinical use was exenatide (synthetic exendin‐4), a peptide originally isolated from Heloderma suspectum lizard venom by John Eng in 1992.28 Pivotal studies in patients with type 2 diabetes led to the approval of twice daily exenatide which was the first GLP1R agonist in the market in 2005. Since then, other GLP1R agonists have been marketed including Lixisenatide, Liraglutide, Dulaglutide, Albiglutide and Semaglutide. Exenatide LAR, Dulaglutide and Semaglutide are notable in that they are long‐lasting preparations, enabling effective treatment of type 2 diabetes with one injection a week, an attractive proposition for patients.29 One of the key properties of GLP1R agonists that sets them apart from other diabetes treatments is the weight loss associated with treatment, which varies in trials from 1.01 to 1.62 kg mean weight loss at the doses utilised for diabetes.30 As a result, the wider use of GLP1R agonists in overweight/obesity has been explored, and Liraglutide was the first GLP1R agonist to be approved for the treatment of obesity as a once daily injection in 2014, based on studies utilising higher doses of 3 mg daily in overweight and obese patients with and without diabetes. At 3 mg daily, Liraglutide can reduce weight by a mean of 8%.31 The GLP‐1 analogues Liraglutide,32 Semaglutide,33 Exenatide LAR,34 Albiglutide,35 and Dulaglutide36 have recently been shown to reduce cardiovascular events (notably non‐fatal myocardial infarctions) in diabetic patients at high risk for cardiovascular disease, an effect that has been attributed to reduction in cardiovascular inflammation, although the exact mechanisms remain obscure.29
The efficacy of GLP1R agonists is partially limited by the adverse effects which are mainly gastrointestinal in nature (nausea, vomiting, loose stools or constipation), although these adverse effects are subject to tachyphylaxis and can be mostly avoided with a slow up‐titration in dose. Another limitation is that GLP1R agonist treatment does not significantly increase energy expenditure.37 Patients can vary widely in their weight loss responses to Liraglutide 3 mg: despite the abovementioned mean weight loss, the SCALE trial also demonstrated that 37% of patients given liraglutide lost less than the minimally acceptable weight loss of 5% (some even gained weight).31 Even for those that respond to treatment, a mean weight loss of 8% is not enough to address higher grades of obesity, and is not competitive with the typical weight loss from bariatric surgery of 20% or so.38 Newer GLP1R agonists such as Semaglutide possess better efficacy in terms of glycaemic improvement39 and weight loss (with mean reductions in weight of 6%‐14%).40 Nevertheless, to achieve better and titratable outcomes, for example 15%‐20% weight loss, it will be necessary to exploit the power of combination therapy with other gut hormones.
3. WHAT OTHER HORMONE ACTIONS CAN BE COMBINED WITH GLP‐1's ACTIONS?
3.1. Glucagon
Glucagon is a 29‐amino acid peptide hormone that is produced by the α cells of the pancreatic islets41 as an alternative product of post‐translational proglucagon processing by PC2. Glucagon activates the G‐protein coupled glucagon receptor (GCGR) which is expressed most abundantly in the liver and kidney but to a lesser extent in cardiac, adrenal, gut and adipose tissues.42 Historically, glucagon was characterised as a hyperglycaemic hormone, increasing hepatic glucose production via glycogenolysis and gluconeogenesis.43 Its secretion is stimulated by low blood glucose levels or fasting, and glucagon classically acts as a counter‐regulatory hormone to insulin. In addition, glucagon stimulates lipolysis from adipose tissue44, 45; decreases muscle protein synthesis46; increases hepatic amino acid uptake, amino acid catabolism, and ureagenesis47; and acts as an insulinotropic hormone.48 Further studies of glucagon have demonstrated a range of other metabolic effects. It has been consistently demonstrated to increase energy expenditure through a mechanism that appears to be independent of brown adipose tissue.49, 50, 51 Furthermore, glucagon directly affects satiety and its infusion has been shown to reduce food intake,52 seemingly via both central and peripheral pathways.53, 54 Two clinical studies conducted by our group have demonstrated the potential beneficial effects of GLP‐1/glucagon combination in humans. Ten non‐diabetic overweight/obese individuals were co‐infused with glucagon and GLP‐1 for 45 minutes at doses of 50 ng/kg/min and 0.8 pmol/kg/min respectively.55 On different occasions, single hormone infusions were also administered (at the same doses), as well as a placebo infusion. Resting energy expenditure—as determined by indirect calorimetry—increased significantly in both the glucagon and combined infusion groups, but not in the GLP‐1 infusion group. Whilst the glucagon infusion caused a rise in plasma glucose as expected, this rise was blunted in the combined infusion, owing to a significant synergistic effect between GLP‐1 and glucagon on insulin secretion.55 A subsequent study by Cegla et al56 confirmed that the addition of GLP‐1 to glucagon infusion protected from glucagon induced hyperglycaemia, and that co‐infusion of GLP‐1 and glucagon at sub‐anorectic doses led to a significant and synergistic reduction in food intake of 13%. Hence, the GLP‐1/glucagon combination possesses three highly favourable features over and above the individual hormones, ie, (a) synergistic reduction in food intake; (b) an increase in energy expenditure that would counteract any tendency to reduce resting energy expenditure with weight loss; (c) gluconeutrality, with the possibility of improved glycaemia in the long term with weight loss.
3.2. Oxyntomodulin
Oxyntomodulin is co‐secreted by the L cells of the small intestine with GLP‐1. As noted above, it is an additional posttranslational product of proglucagon in the gut. In addition to the glucagon sequence, oxyntomodulin has a C‐terminal eight amino acid octapeptide.57 Oxyntomodulin is an agonist for both GLP1R and GCGR (albeit with less affinity than the cognate peptide for each receptor), making it the prototypical unimolecular GLP‐1/glucagon dual agonist.58 Indeed, studies have shown that the effect of oxyntomodulin via the GLP1R is anorectic whereas the observed increase in energy expenditure is mediated via the glucagon receptor.59, 60, 61 Pocai et al characterised the preclinical effects of a GLP1R/GCGR dual agonist based on oxyntomodulin. They demonstrated a significant reduction in body weight, food intake and fat mass of a greater magnitude compared to a pure GLP1R agonist. In addition, they again demonstrated improvements in fasting glucose and glycaemic profiles post‐glucose tolerance test which were comparable to the GLP1R agonist.58 The effects of a short IV infusion of the combination of GLP‐1 and OXM on food intake were examined a study performed by Field et al62 in overweight subjects, where a synergistic effect from combining GLP‐1 and OXM resulted in a 42% reduction in energy intake.
3.3. Glucose‐dependent insulinotropic peptide
Glucose‐dependent insulinotropic peptide is a peptide secreted by the neuroendocrine K cells of the small intestine; its principal physiological roles, mediated by the GIP receptor GIPR, are as an incretin (with GLP‐1), potentiation of glucagon secretion, and regulation of adipogenesis, notably increasing fat deposition in adipose tissue. The insulinotropic properties of GIP make it an attractive prospect for treating type 2 diabetes. Although the beneficial effects of GIPR agonism appear to be attenuated in the hyperglycaemic conditions seen in patients with type 2 diabetes,63 even when administered at supraphysiological levels,64, 65 the impaired insulinotropic effect of GIP seems to be fully recoverable following a period of normalised plasma glucose levels.66 This suggests a role for co‐agonism with GLP‐1, utilising its glucose lowering effects to induce improvement in glycaemia that is enough to enable GIP to exert its insulinotropic effects and synergistically improve glucose levels further. The initial preclinical study by Irwin et al67 demonstrated improvement in glucose tolerance, as well as in food intake and weight reduction with a combination of the GLP1R agonist exendin‐4 and the GIPR agonist N‐AcGIP. These findings were corroborated in a subsequent preclinical study which demonstrated a synergistic effect of the combination on improved glucose levels, reduced food intake and weight loss.68 The data from clinical studies into the acute effects of combined GLP‐1/GIP in patients with type 2 diabetes have not been as promising however. In a study by Daousi et al,69 GLP‐1 and GIP were infused individually and in combination into six healthy lean participants and six overweight diabetic participants. Whilst the combined infusion led to a greater potentiation of insulin secretion in the lean group, this effect was not observed in the overweight diabetic group where insulin levels matched those from the GLP‐1 only infusion. The excursion of glucose in response to intravenous insulin, as measured by the AUC for glucose, was similar between GLP‐1 and GLP‐1/GIP, as was the food intake during an ad libitum meal test. There was a reduction in resting energy expenditure with GIP which was not replicated during combined infusion in both groups. The lack of synergistic effect in type 2 diabetic patients is consistent with the established evidence that GIP‐mediated insulin secretion is less effective in the hyperglycaemic state. Nevertheless, GLP‐1/GIP based dual agonists have continued into clinical development (see below). Other groups have explored GIPR antagonism, which might be therapeutically attractive as a means of suppressing the hyperglucagonaemia and hence hyperglycaemia of type 2 diabetes, as well as reducing fat deposition.70 GIPR antagonism with (Pro3)GIP was shown to ameliorate weight gain, insulin resistance and normalise glucose tolerance in high‐fat diet fed mice.71 Some exploratory work looking at the physiological effects of GIPR antagonism in human volunteers has recently been published which demonstrates that the antagonist GIP3‐30amide is capable of suppressing the incretin effect of GIP,72 but it is too early to tell yet whether GIPR antagonism may be a valid therapeutic strategy.
3.4. Peptide YY
Peptide YY (PYY) is another peptide hormone secreted by the L‐cells of the intestine in response to eating.73 The PYY3‐36 peptide, which is derived from the full‐length PYY1‐36 peptide by DPP‐IV processing, binds to the neuropeptide Y2 (Y2R) and Y5 (Y5R) receptors, and has a well‐characterised appetite‐suppressive effect which is importantly preserved in obesity.74 Neary et al studied the coadministration of PYY3‐36 with GLP‐17‐36amide in 10 healthy volunteers and found that it was associated with a 27% reduction in energy intake from a buffet meal. The combination was more effective in inhibiting appetite than either peptide alone.75 This result was replicated in a later study by De Silva et al76 where the combination of PYY3‐36 and GLP‐17‐36amide resulted in a reduction in food intake which was similar to the summed effects of the single hormones (PYY3‐36 or GLP‐17‐36amide), and this was reflected by a reduced activation, as assessed by BOLD fMRI, of areas of the brain implicated in appetite and interest in food. Similarly, Schmidt et al77 in 2014 showed that the co‐infusion of GLP‐1 and PYY3‐36 reduced energy intake compared with placebo and more than the sum of the individual infusions, demonstrating a synergistic effect. Therefore, the combination of GLP‐1 and PYY3‐36 bears some promise of augmented weight loss compared to GLP‐1 alone.
4. DEVELOPMENT OF DUAL AGONISTS
4.1. GLP1R/GCGR dual agonists
As noted above, there are positive therapeutic effects of GLP‐1/glucagon combinations in terms of increasing energy expenditure and reducing food intake; GLP‐1 can counterbalance the hyperglycaemia induced by glucagon. As a result, there has been considerable interest in developing GLP‐1/glucagon dual agonists. In early work from 2009, Day et al78 developed a range of unimolecular co‐agonists and tested their binding affinities to both glucagon and GLP‐1 receptors. Following this, they tested two of the novel peptides in mice and showed reductions in body weight, fat mass and most pertinently, reduction in blood glucose. In addition, despite the lack of hyperglycaemia induced by the co‐agonist, the glucagon‐associated effects of increased energy expenditure and improved lipid profile were preserved.
A recently published Phase 2A, randomized, double‐blind, placebo controlled trial assessed the efficacy, safety and tolerability of MEDI0382 (AstraZeneca/Medimmune), a GLP1R/GCGR dual agonist in overweight and obese patients with type 2 diabetes.79 The volunteers given MEDI0382 once a day for 41 days were shown to have a better glucose tolerance in response to a mixed meal test compared to placebo, as well as a reduction in body weight: the mean fall in body weight between baseline and day 41 was 3.84 kg compared to 1.70 kg for placebo. HbA1c fell by 0.9% with MEDI0382 compared to 0.6% for placebo. Twenty patients experienced treatment‐emergent adverse events (reduced appetite, vomiting, headache) with MEDI0382, compared to 15 for placebo.79 Exploratory analyses presented in the American Diabetes Association 2018 meeting also suggest that there was a significant higher relative reduction in liver fat content when patients with fatty liver disease given MEDI0382 vs placebo, a potentially important effect.80
Another GLP1R/GCGR dual agonist, SAR425899 (Sanofi, Frankfurt, Germany) has recently been evaluated in single‐ascending dose and multiple‐ascending dose Phase 1 trials when given once a day over 28 days.81 At the highest maintenance doses tested, there was a reduction of HbA1c by 0.54%‐0.59% when given to overweight/obese diabetic patients, and mean weight losses of 2.37‐5.46 kg over the 28 days. SAR425899 was generally well tolerated, with treatment‐emergent adverse effects of reduced appetite and nausea. It should be noted that two patients in the study were withdrawn due to increases in serum lipase activity. These promising trial results therefore suggest that GLP1R/GCGR dual agonism is generally safe and efficacious, although head‐to‐head comparisons with GLP1R agonists will be required to evaluate the comparative advantages of the GLP1R/GCGR dual agonists.
4.2. GLP1R/GIPR dual agonists
Notwithstanding the equivocal evidence for benefit from GLP‐1/GIP co‐infusion studies, unimolecular GLP1R/GIPR dual agonists have been developed with promising pre‐clinical results (enhanced weight loss, improved glycaemia, reduced hepatosteatosis) in animal models.82 One recent study has reported the Phase 2A clinical trial results of one such dual agonist, NNC0090‐2746, which possesses balanced affinities for the GIPR and the GLP1R.83 A 1.8 mg once‐daily dose was administered to 96 overweight/obese patients with type 2 diabetes. There was a significant reduction in HbA1c of 0.63% and 0.96% at 8 and 12 weeks respectively when compared to placebo. In addition, body weight was significantly reduced by 1.8% after 8 weeks (when compared to placebo), although the reduction in body weight was not significant at 12 weeks. Post‐hoc analysis found that the best weight reduction and improvement in HbA1c was demonstrated in the group of patients with HbA1c <8.5%. This finding was again in keeping with the previous observations that GIP is less effective in more hyperglycaemic conditions.
More impressive, however, are the data from Frias et al84 who studied the effects of LY3298176, a once‐weekly GLP1R/GIPR dual agonist, in a Phase 2 trial in type 2 diabetic patients. LY3298176 differs from NNC0090‐2746 in that it possesses greater affinity for GIPR vs GLP1R. LY3298176, when given for 26 weeks, delivered mean reductions in HbA1c ranging from 1.06% to 1.94% in comparison to a group taking Dulaglutide, who had a mean reduction of HbA1c of 1.21%. Even more impressively, the LY3298176‐treated patients had mean weight reductions ranging between 0.9 and 11.3 kg, compared to 2.7 kg for Dulaglutide. Surprisingly, however, there was a 30% non‐response rate (ie, weight loss <5% of baseline) even when given the higher doses of the drug. LY3298176 was associated with nausea, diarrhoea and vomiting in up to 60% of the patients given the highest dose (35% for Dulaglutide). Nevertheless, these early results do suggest that LY3298176 possesses enhanced efficacy compared to a benchmark GLP‐1 analogue in terms of weight loss and improvements in glycaemia, and augurs well for this class of dual agonists.
4.3. GLP1R/Y2R dual agonism
Peptide YY analogues are currently under development for the treatment of obesity (ClinicalTrials.Org NCT01515319 and 85). Novo Nordisk has a peptide YY analogue (PYY‐1562 or NN‐9748) in Phase I trials; this is likely to be combined with GLP1R agonists such as Semaglutide to achieve enhanced effects on food intake suppression,86 following on from the proof‐of‐concept studies noted above.
5. TRIPLE AGONISM—MORE IS BETTER?
5.1. GLP1R/GIPR/GCGR triple agonism
Complex unimolecular triple agonists combining GLP‐1/GIP/glucagon activity such as MAR423, which are based on modification of a pre‐existing GLP1R/GIPR dual agonist to incorporate GCGR agonist activity have been designed by Richard DiMarchi and Matthias Tschöp,87 and pre‐clinical studies again suggest promising metabolic benefits in animal models of obesity.88, 89 At the same time, Hanmi Pharmaceutical has developed an independent triple agonist, HM15211, which also shows promising pre‐clinical benefits in a model of non‐alcoholic steatohepatitis.90 At present, no clinical trial or clinical study results are available for this class of triple agonists.
5.2. GLP‐1, Oxyntomodulin & PYY triple agonism—replicating bariatric surgery
As noted, Roux‐en‐Y Gastric Bypass (RYGB) surgery has been proven in multiple studies to be an effective treatment for obesity and diabetes.4 Dramatic changes in gut hormones, namely the rise in postprandial levels of GLP‐1, OXM and PYY even days after the operation, account for the improvement in glycaemic control which can reach diabetes remission as well as for the weight loss and other beneficial metabolic effects such as enhancement of insulin sensitivity and improved lipid profile.91, 92 We asked whether it might be possible to replicate the benefits of RYGB using a triple hormone infusion of GLP‐1, oxyntomodulin & PYY (GOP for short). In a proof of concept study, we demonstrated that a subcutaneous infusion of GOP hormones in ten obese healthy subjects for 10.5 hours can replicate the post‐prandial gut hormone levels seen after RYGB.93 Importantly, the GOP infusion can induce a mean reduction of food intake by 32%. Additionally, glucose and insulin levels after lunch and dinner were significantly lower on the GOP infusion compared to placebo. Resting energy expenditure showed a non‐significant elevation from baseline on the GOP infusion. Finally, the GOP infusion induced nausea in a minority of participants, which settled within the first 4 hours without any vomiting. Continuing studies are underway to determine the effects of the GOP infusion when given for extended periods of time.
6. CONCLUSIONS
Decades of animal and human research in the field of gut hormones have produced effective and safe therapies for the treatment of diabetes and obesity with the GLP1R agonists now accepted as routine treatments for diabetes and obesity. Furthermore, we now have clinical evidence for the cardiovascular benefits of GLP1R agonists. To go beyond the modest effects of GLP1R agonism, we now need to understand how to combine the benefits of GLP‐1 with the complementary properties of its cousin gut hormones to achieve desired therapeutic goals (Figure 1). Dual and triple gut hormone receptor agonists are now placed to deliver the next generation of therapies for diabetes and obesity.
Figure 1.
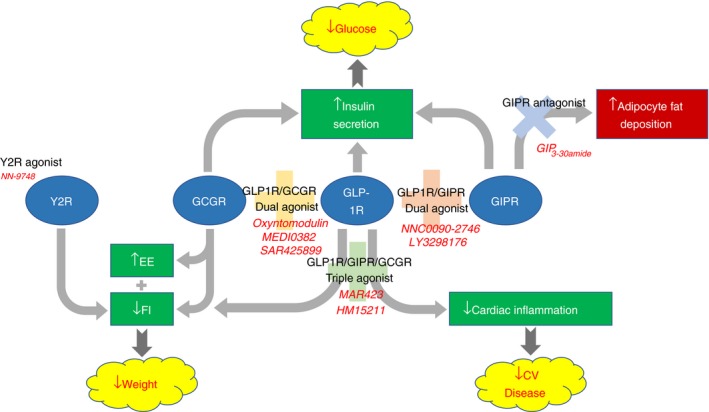
Complementary actions of gut hormone receptors combine to achieve desirable therapeutic outcomes
ACKNOWLEDGEMENTS
This article presents independent research supported by the NIHR CRF and BRC at Imperial College Healthcare NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
The Division of Diabetes, Endocrinology and Metabolism is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7‐ HEALTH‐2009‐ 241592 EuroCHIP grant and is supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Alexiadou K, Anyiam O, Tan T. Cracking the combination: Gut hormones for the treatment of obesity and diabetes. J Neuroendocrinol. 2019;31:e12664 10.1111/jne.12664
REFERENCES
- 1. World Health Organisation . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i‐xii, 1‐253. [PubMed] [Google Scholar]
- 2. Nyberg ST, Batty GD, Pentti J, et al. Obesity and loss of disease‐free years owing to major non‐communicable diseases: a multicohort study. Lancet Public Health. 2018;3(10):e490‐e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C‐peptide responses. J Clin Endocrinol Metab. 1986;63(2):492‐498. [DOI] [PubMed] [Google Scholar]
- 6. Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non‐insulin‐dependent) diabetes. Diabetologia. 1986;29(1):46‐52. [DOI] [PubMed] [Google Scholar]
- 7. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post‐translational processing. J Biol Chem. 1986;261(25):11880‐11889. [PubMed] [Google Scholar]
- 8. Novak U, Wilks A, Buell G, McEwen S. Identical mRNA for preproglucagon in pancreas and gut. Eur J Biochem. 1987;164(3):553‐558. [DOI] [PubMed] [Google Scholar]
- 9. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819‐837. [DOI] [PubMed] [Google Scholar]
- 10. Mayo KE, Miller LJ, Bataille D, et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55(1):167‐194. [DOI] [PubMed] [Google Scholar]
- 11. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet. 1987;2(8571):1300‐1304. [DOI] [PubMed] [Google Scholar]
- 12. Goke R, Fehmann HC, Linn T, et al. Exendin‐4 is a high potency agonist and truncated exendin‐(9‐39)‐amide an antagonist at the glucagon‐like peptide 1‐(7‐36)‐amide receptor of insulin‐secreting beta‐cells. J Biol Chem. 1993;268(26):19650‐19655. [PubMed] [Google Scholar]
- 13. Tang‐Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP‐1‐(7‐36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 Pt 2):R848‐R856. [DOI] [PubMed] [Google Scholar]
- 14. Turton MD, O'Shea D, Gunn I, et al. A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature. 1996;379(6560):69‐72. [DOI] [PubMed] [Google Scholar]
- 15. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verdich C, Flint A, Gutzwiller JP, et al. A meta‐analysis of the effect of glucagon‐like peptide‐1 (7‐36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86(9):4382‐4389. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Conde K, Zhang P, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon‐like peptide‐1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897‐909.e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez‐Ferreras L, Richard JE, Noble EE, et al. Lateral hypothalamic GLP‐1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry. 2018;23(5):1157‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP‐1 (proglucagon 78‐107‐amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38(4):665‐673. [DOI] [PubMed] [Google Scholar]
- 20. Jin T, Weng J. Hepatic functions of GLP‐1 and its based drugs: current disputes and perspectives. Am J Physiol Endocrinol Metab. 2016;311(3):E620‐E627. [DOI] [PubMed] [Google Scholar]
- 21. Drucker DJ. Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab. 2016;101(3):778‐786. [DOI] [PubMed] [Google Scholar]
- 22. Lynch L, Hogan AE, Duquette D, et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 2016;24(3):510‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. During MJ, Cao L, Zuzga DS, et al. Glucagon‐like peptide‐1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173‐1179. [DOI] [PubMed] [Google Scholar]
- 24. Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon‐like peptide‐1 and exendin‐4. J Pharmacol Exp Ther. 2002;302(3):881‐888. [DOI] [PubMed] [Google Scholar]
- 25. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon‐like peptide‐1 receptor. Endocrinology. 1996;137(7):2968‐2978. [DOI] [PubMed] [Google Scholar]
- 26. Gros R, You X, Baggio LL, et al. Cardiac function in mice lacking the glucagon‐like peptide‐1 receptor. Endocrinology. 2003;144(6):2242‐2252. [DOI] [PubMed] [Google Scholar]
- 27. Baggio LL, Ussher JR, McLean BA, et al. The autonomic nervous system and cardiac GLP‐1 receptors control heart rate in mice. Mol Metab. 2017;6(11):1339‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin‐4, an exendin‐3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402‐7405. [PubMed] [Google Scholar]
- 29. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metab. 2018;27(4):740‐756. [DOI] [PubMed] [Google Scholar]
- 30. Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon‐like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta‐analysis. PLoS One. 2015;10(6):e0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 32. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 34. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 36. Eli Lilly and Co . Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. 2018. https://investor.lilly.com/news-releases/news-release-details/trulicityr-dulaglutide-demonstrates-superiority-reduction. Accessed October, 2018.
- 37. Horowitz M, Flint A, Jones KL, et al. Effect of the once‐daily human GLP‐1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract. 2012;97(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 38. Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery or intensive medical therapy for diabetes after 5 years. N Engl J Med. 2017;376(20):1997. [DOI] [PubMed] [Google Scholar]
- 39. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 40. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet. 2018;392(10148):637‐649. [DOI] [PubMed] [Google Scholar]
- 41. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol Cell Endocrinol. 1994;105(2):131‐137. [DOI] [PubMed] [Google Scholar]
- 43. Knop FK. EJE PRIZE 2018: a gut feeling about glucagon. Eur J Endocrinol. 2018;178(6):R267‐R280. [DOI] [PubMed] [Google Scholar]
- 44. Schade DS, Woodside W, Eaton RP. The role of glucagon in the regulation of plasma lipids. Metabolism. 1979;28(8):874‐886. [DOI] [PubMed] [Google Scholar]
- 45. Richter WO, Robl H, Schwandt P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides. 1989;10(2):333‐335. [DOI] [PubMed] [Google Scholar]
- 46. Preedy VR, Garlick PJ. The effect of glucagon administration on protein synthesis in skeletal muscles, heart and liver in vivo. Biochem J. 1985;228(3):575‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fitzpatrick GF, Meguid MM, Gitilitz PH, Brennan MF. Glucagon infusion in normal man: effects on 3‐methylhistidine excretion and plasma amino acids. Metabolism. 1977;26(5):477‐485. [DOI] [PubMed] [Google Scholar]
- 48. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43(8):1012‐1019. [DOI] [PubMed] [Google Scholar]
- 49. Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab. 1987;64(5):896‐901. [DOI] [PubMed] [Google Scholar]
- 50. Dicker A, Zhao J, Cannon B, Nedergaard J. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am J Physiol. 1998;275(5 Pt 2):R1674‐R1682. [DOI] [PubMed] [Google Scholar]
- 51. Salem V, Izzi‐Engbeaya C, Coello C, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Geary N, Kissileff HR, Pi‐Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol. 1992;262(6 Pt 2):R975‐R980. [DOI] [PubMed] [Google Scholar]
- 53. Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264(1 Pt 2):R116‐R122. [DOI] [PubMed] [Google Scholar]
- 54. Kurose Y, Kamisoyama H, Honda K, et al. Effects of central administration of glucagon on feed intake and endocrine responses in sheep. Anim Sci J. 2009;80(6):686‐690. [DOI] [PubMed] [Google Scholar]
- 55. Tan TM, Field BC, McCullough KA, et al. Coadministration of glucagon‐like peptide‐1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cegla J, Troke RC, Jones B, et al. Coinfusion of low‐dose GLP‐1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63(11):3711‐3720. [DOI] [PubMed] [Google Scholar]
- 57. Bataille D, Tatemoto K, Coudray AM, Rosselin G, Mutt V. Bioactive “enteroglucagon” (oxyntomodulin): evidence for a C‐terminal extension of the glucagon molecule. C R Seances Acad Sci III. 1981;293(6):323‐328. [PubMed] [Google Scholar]
- 58. Pocai A, Carrington PE, Adams JR, et al. Glucagon‐like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258‐2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon‐like peptide‐1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546‐558. [DOI] [PubMed] [Google Scholar]
- 60. Kosinski JR, Hubert J, Carrington PE, et al. The glucagon receptor is involved in mediating the body weight‐lowering effects of oxyntomodulin. Obesity (Silver Spring). 2012;20(8):1566‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696‐4701. [DOI] [PubMed] [Google Scholar]
- 62. Field BC, Wren AM, Peters V, et al. PYY3‐36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes. 2010;59(7):1635‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. J Clin Invest. 1993;91(1):301‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elahi D, McAloon‐Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (7‐37) in normal and diabetic subjects. Regul Pept. 1994;51(1):63‐74. [DOI] [PubMed] [Google Scholar]
- 65. Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45(8):1111‐1119. [DOI] [PubMed] [Google Scholar]
- 66. Hojberg PV, Vilsboll T, Rabol R, et al. Four weeks of near‐normalisation of blood glucose improves the insulin response to glucagon‐like peptide‐1 and glucose‐dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199‐207. [DOI] [PubMed] [Google Scholar]
- 67. Irwin N, Hunter K, Frizzell N, Flatt PR. Antidiabetic effects of sub‐chronic activation of the GIP receptor alone and in combination with background exendin‐4 therapy in high fat fed mice. Regul Pept. 2009;153(1–3):70‐76. [DOI] [PubMed] [Google Scholar]
- 68. Gault VA, Kerr BD, Harriott P, Flatt PR. Administration of an acylated GLP‐1 and GIP preparation provides added beneficial glucose‐lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clin Sci (Lond). 2011;121(3):107‐117. [DOI] [PubMed] [Google Scholar]
- 69. Daousi C, Wilding JP, Aditya S, et al. Effects of peripheral administration of synthetic human glucose‐dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin Endocrinol (Oxf). 2009;71(2):195‐201. [DOI] [PubMed] [Google Scholar]
- 70. Gasbjerg LS, Gabe MBN, Hartmann B, et al. Glucose‐dependent insulinotropic polypeptide (GIP) receptor antagonists as anti‐diabetic agents. Peptides. 2018;100:173‐181. [DOI] [PubMed] [Google Scholar]
- 71. McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high‐fat diet. Am J Physiol Endocrinol Metab. 2007;293(6):E1746‐E1755. [DOI] [PubMed] [Google Scholar]
- 72. Gasbjerg LS, Christensen MB, Hartmann B, et al. GIP(3‐30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double‐blinded, placebo‐controlled, crossover study. Diabetologia. 2018;61(2):413‐423. [DOI] [PubMed] [Google Scholar]
- 73. Adrian TE, Ferri GL, Bacarese‐Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070‐1077. [DOI] [PubMed] [Google Scholar]
- 74. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3‐36. N Engl J Med. 2003;349(10):941‐948. [DOI] [PubMed] [Google Scholar]
- 75. Neary NM, Small CJ, Druce MR, et al. Peptide YY3‐36 and glucagon‐like peptide‐17‐36 inhibit food intake additively. Endocrinology. 2005;146(12):5120‐5127. [DOI] [PubMed] [Google Scholar]
- 76. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3‐36 and GLP‐1 7‐36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schmidt JB, Gregersen NT, Pedersen SD, et al. Effects of PYY3‐36 and GLP‐1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab. 2014;306(11):E1248‐E1256. [DOI] [PubMed] [Google Scholar]
- 78. Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP‐1 co‐agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5(10):749‐757. [DOI] [PubMed] [Google Scholar]
- 79. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP‐1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double‐blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607‐2618. [DOI] [PubMed] [Google Scholar]
- 80. Jain M, Tsai L‐F, Robertson D, et al. MEDI0382, a GLP/glucagon receptor dual agonist, significantly reduces hepatic fat content in subjects with type 2 diabetes mellitus. Diabetes. 2018;67(Supplement 1):78‐OR.29079704 [Google Scholar]
- 81. Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon‐like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo‐controlled first‐in‐human and first‐in‐patient trials. Diabetes Obes Metab 2018;21(1):120‐128. [DOI] [PubMed] [Google Scholar]
- 82. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209):209ra151. [DOI] [PubMed] [Google Scholar]
- 83. Frias JP, Bastyr EJ 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP‐1 receptor agonist, NNC0090‐2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):343‐352.e342. [DOI] [PubMed] [Google Scholar]
- 84. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet. 2018;392:2180‐2193. [DOI] [PubMed] [Google Scholar]
- 85. Albertsen L, Andersen JJ, Paulsson JF, Thomsen JK, Norrild JC, Stromgaard K. Design and synthesis of peptide YY analogues with c‐terminal backbone amide‐to‐ester modifications. ACS Med Chem Lett. 2013;4(12):1228‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Novo Nordisk . R&D pipeline. 2018. http://www.wip.novonordisk.com/investors/rd_pipeline.html. Accessed October, 2018.
- 87. Tschop MH, Finan B, Clemmensen C, et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24(1):51‐62. [DOI] [PubMed] [Google Scholar]
- 88. Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 89. Jall S, Sachs S, Clemmensen C, et al. Monomeric GLP‐1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol Metab. 2017;6(5):440‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Choi IY, Kim JK, Lee JS, et al. Effect of a novel long‐acting GLP‐1/GIP/glucagon triple agonist (HM15211) in a NASH and fibrosis animal model. Diabetes 2018;67(Supplement 1):1106‐P. [Google Scholar]
- 91. le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux‐en‐Y gastric bypass. Ann Surg. 2007;246(5):780‐785. [DOI] [PubMed] [Google Scholar]
- 92. Laferrere B. Bariatric surgery and obesity: influence on the incretins. Int J Obes Suppl. 2016;6(Suppl 1):S32‐S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tan T, Behary P, Tharakan G, et al. The effect of a subcutaneous infusion of GLP‐1, OXM, and PYY on energy intake and expenditure in obese volunteers. J Clin Endocrinol Metab. 2017;102(7):2364‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]


