Abstract
As antiretroviral therapy has become more accessible across the world and coformulations have improved patient compliance; the morbidity and mortality of HIV/AIDS has decreased. However, there is still a substantial gap in knowledge regarding the impact of genetic variation on the metabolism of and response to some of the most commonly prescribed antiretrovirals, including the nucleotide reverse transcriptase inhibitor tenofovir. While it has been scientifically established that tenofovir must be activated to be efficacious against HIV, the enzymes responsible for this activation have not been well characterized. The purpose of this review is to summarize and clarify the scientific knowledge regarding the enzymes that phosphorylate and activate this clinically important drug.
Keywords: : antiretroviral therapy, drug metabolism, genetic variants, HIV, precision medicine
Introduction to tenofovir metabolism
The development and implementation of antiretroviral therapy (ART) has led to a substantial decrease in HIV-related mortality [1]. Research into the drug combinations that constitute ART has aimed to decrease both the number of adverse events and incidents of clinical inefficacy through the lens of pharmacokinetics and pharmacodynamics. Although there are several drug combinations used in ART regimens, one drug that has emerged as a preferential component is tenofovir.
Tenofovir disoproxil fumarate was approved by the US FDA in 2001 [2,3]. Tenofovir disoproxil fumarate is a prodrug that upon hydrolysis is transformed to tenofovir, which is an adenosine monophosphate analog. As a monophosphate analog, tenofovir requires two sequential phosphorylation steps (carried out by kinases within host cells) to become pharmacologically active by forming the nucleotide triphosphate analog that competitively inhibits HIV reverse transcriptase. Thus, tenofovir is first phosphorylated to tenofovir-monophosphate and then is subsequently phosphorylated to tenofovir-diphosphate. As such, tenofovir belongs to the nucleoside/nucleotide reverse transcriptase inhibitor class of drugs. Clinical trials demonstrated that suppression of HIV-1 by a combination of tenofovir disoproxil fumarate and emtricitabine (also a nucleoside/nucleotide reverse transcriptase inhibitor) with efavirenz, a non-nucleoside reverse transcriptase inhibitor, was superior when compared with zidovudine/lamivudine with efavirenz [4]. Furthermore, in the CASTLE clinical trials conducted over 96 weeks, 93% of HIV-1-infected individuals maintained efficacious viral suppression when combining protease inhibitors with tenofovir disoproxil fumarate/emtricitabine [5]. The combination of tenofovir disoproxil fumarate and emtricitabine is also the only US FDA approved drug for HIV pre-exposure prophylaxis, which is a pharmacologic approach to prevent infection in HIV-naive individuals [6]. Tenofovir is well tolerated by most patients and the majority of adverse events are reported as mild or moderate in severity [7]. In the context of HIV pre-exposure prophylaxis, tenofovir has been the focus of several clinical trials designed to allow on-demand usage and prevent lapses in adherence. These trials include the formulation and development of drug-containing vaginal rings, long-acting subdermal implants and tenofovir-containing enemas [8–10].
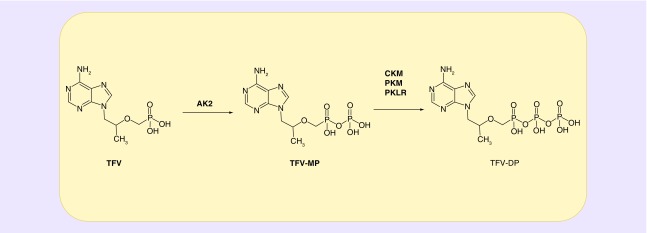
While the pharmacology and pharmacokinetics of tenofovir have been well characterized, there are gaps in knowledge that persist. For instance, the mechanisms underlying instances of tenofovir inefficacy have not been fully explored. Occurrences of HIV seroconversion during clinical trials investigating tenofovir-containing formulations for use in HIV pre-exposure prophylaxis have been largely attributed to nonadherence to medication [11–13]. However, patient noncompliance may not be the cause of all instances of drug failure [14]. Additionally, cases of off-target toxicity are rare, but consistently reported in the HIV treatment setting. Nephrotoxicity in the form of Fanconi syndrome, acute renal failure and nephrogenic diabetes insipidus attributed to tenofovir has been noted [15,16]. It has also been suggested that common ART regimens may be associated with adverse bone events, characterized by increased risk of fractures and osteoporosis [17,18]. Taken together, gaining an understanding of the enzymes that activate tenofovir may provide insight into both the failure of tenofovir to prevent HIV infection in certain individuals and the development of idiosyncratic tenofovir-induced toxicities. With this in mind, studies have been performed to investigate the phosphorylation of tenofovir in cells and tissue susceptible to HIV infection [19]. Through this work, it has been demonstrated that adenylate kinase (AK) 2 can phosphorylate tenofovir to tenofovir-monophosphate in peripheral blood mononuclear cells, vaginal tissue and colorectal tissue. Pyruvate kinase muscle (PKM) and pyruvate kinase liver, red blood cell (PKLR) can both catalyze the formation of the pharmacologically active tenofovir-diphosphate from tenofovir-monophosphate in peripheral blood mononuclear cells and vaginal tissue. In contrast, in colorectal tissue creatine kinase muscle (CKM) has been implicated in carrying out this step. Figure 1 outlines the current hypothesis regarding the phosphorylation of tenofovir in peripheral blood mononuclear cells, vaginal tissue and colorectal tissue. Since two phosphorylation steps are required for the activation of tenofovir, each of the enzymes noted may play a crucial role in tenofovir efficacy. The goal of this review is to contribute toward a better understanding of genetic variations that exist in the kinases responsible for the activation of tenofovir.
Figure 1. . Schematic of the intracellular phosphorylation of TFV.
TFV requires two sequential phosphorylation steps to become a pharmacologically active triphosphate analogue, denoted as TFV-DP. Lade et al. [19] discovered that AK2 can catalyze the phosphorylation of TFV to TFV-MP in peripheral blood mononuclear cells, vaginal, and colorectal tissues. In addition, PKM and PKLR were found to catalyze the phosphorylation of TFV-MP to TFV-DP in peripheral blood mononuclear cells and vaginal tissue, while CKM phosphorylated TFV-MP to TFV-DP in colorectal tissue.
AK2: Adenylate kinase 2; CKM: Creatine kinase muscle; DP: Diphosphate; MP: Monophosphate; PKM: Pyruvate kinase muscle; PKLR: Pyruvate kinase liver and red blood cell; TFV: Tenofovir.
The AK family
The AK are abundant enzymes that play a crucial role in regulating energy metabolism and maintenance of cellular adenine nucleotide ratios. Specifically, the adenylate kinase family enzymes regulate adenine nucleotide ratios by catalyzing the reversible transfer of the γ-phosphate group from ATP to AMP [20]. There are nine isoforms that make up the AK family, with homology between the enzymes being 15–60%, and all prefer AMP as the primary substrate [21]. The main phosphate donor is ATP, though several AK enzymes have the ability to utilize GTP or NTP [22]. The AKs have three functional domains, including the core, a phosphate donor-binding glycine rich region known as the P-loop, the lid domain and a substrate binding site [22]. Although there are nine known AK enzymes present in human tissue, only AK2 has been associated with tenofovir metabolism in cells/tissues relevant to HIV infection thus far [19,23]. Since AK2 has been implicated in performing the first phosphorylation step required for tenofovir activation in all cells and tissue examined to date, this enzyme may be a key participant in governing the response to tenofovir. Of note, AK2 is located within the mitochondria suggesting that this enzyme plays a crucial role in cellular energy homeostasis. Interestingly, individuals with AK2 genetic mutations display reticular dysgenesis, which is the absence of granulocytes and lymphocytes [24]. With this in mind, it can be envisioned that tenofovir activation could be impacted in individuals carrying AK2 genetic variants. In line with this, in a recent study of 505 participants from the USA, Thailand and South Africa; 17 single nucleotide variants (detected across 16 individuals) predicted to result in a mutation at the amino acid level were identified [23]. Using in silico tools, several of these variants were predicted to impact the activity of AK2 toward tenofovir, and indeed five variants, namely V19G, K28R, A55V, K62E and T194I, were found to exhibit decreased ability to phosphorylate tenofovir in vitro [23]. Two additional studies that genotyped individuals from the USA, Uganda and South Africa similarly identified variants predicted in silico to modulate AK2-mediated phosphorylation of tenofovir [19,25] It should be noted that since tenofovir is an adenine nucleotide analog other enzymes beyond AK2 in the AK family of enzymes could potentially phosphorylate tenofovir and this remains to be investigated.
Pyruvate kinase – liver/erythrocyte & muscle
The pyruvate kinases (PK) are primarily responsible for catalyzing the final rate limiting step of glycolysis, which is the conversion of phosphoenolpyruvate to pyruvate and ATP [26]. Pyruvate kinase can be found in many tissue and cell types, including the liver and red blood cells (PKLR) and the muscle tissue (PKM). PKM is responsible for catalyzing the final rate-limiting step of glycolysis [27]. PKM has two isoforms that are denoted as PKM-1 and PKM-2. PKM-1 is present in differentiated adult tissues like heart and brain, while PKM-2 is present primarily in rapidly dividing cells, such as embryonic and tumor cells [28]. Active PKM-2 occurs in both tetrameric and dimeric forms [29], but the tetrameric form is characterized by high affinity for the substrate phosphoenolpyruvate [30]. PKM-1 is constitutively active, while PKM-2 is tightly regulated due to its critical role in development [31]. Cancer cells have been found to preferentially express PKM-2 and mutations found in critical domains of PKM-2 result in B-lymphoblastoid cells in Bloom syndrome patients [32,33]. PKM has been demonstrated to phosphorylate tenofovir-monophosphate to tenofovir-diphosphate, including in peripheral blood mononuclear cells and vaginal tissue [19,34]. Several variants of PKM have been identified including one denoted as rs778625515, which is found in the ATP binding site and has been predicted to disrupt kinase function [23,35]. Thus, it is possible that individuals carrying this variant or similar variants could exhibit a decrease in their ability to active tenofovir.
Active PKLR is present as a tetrameric quaternary structure formed by four 62-kDa subunits [36]. PKLR shares approximately 70% amino acid sequence homology with the enzyme guanylate kinase 1 (GUK1), which is known to phosphorylate other antiretroviral drugs structurally similar to tenofovir [37,38]. Deficiencies in PKLR are some of the most frequent and well-studied enzymatic abnormalities on the Embden–Meyerhof pathway, resulting in hereditary nonspherocytic hemolytic anemia [39]. It has been projected that 0.15% of the general population may have some form of PKLR deficiency, based on estimations from neonatal studies involving blood cord samples [40]. Serious detrimental pyruvate kinase deficiencies have also been identified in other disease contexts [41]. With regard to nucleoside/nucleotide reverse transcriptase inhibitor metabolism, PKLR phosphorylates tenofovir-monophosphate into tenofovir-diphosphate in both peripheral blood mononuclear cells and vaginal tissue [19]. It has been predicted that PKLR missense variants may result in decreased tenofovir activation [19]. In the above-mentioned study in which 505 individuals from the USA, Thailand and South Africa were genotyped, 39 total PKLR single nucleotide variants and deletions predicted to be nonsynonymous were detected. This included SNV rs147689373, which has previously been associated with pyruvate kinase deficiency [36]. Additionally, in a study of 142 women from the USA, Uganda and South Africa, 3% of these individuals carried PKLR variants predicted in silico to be deleterious or damaging to PKLR protein function [19].
Creatine kinase
In colorectal tissue, creatine kinase muscle (CKM) has been demonstrated to carry out phosphorylation of tenofovir-monophosphate into the pharmacologically active tenofovir-diphosphate [19]. While several different isoforms of CK are expressed in humans, all creatine kinases catalyze the reversible transfer of the γ-phosphate from ATP to the guanidine group of creatine to generate phosphor-creatine and ADP [42]. CKM mediates cytosolic storage of phosphates for rapid replenishment of ATP, which is crucial for cellular energy homeostasis [43]. The CKM protein consists of independent subunits that are often arranged as dimers in the cytosol [44]. Genetic variations in the CKM gene result in altered serum CK levels, which is a biomarker for statin-induced myotoxicity [45].
Dozens of single nucleotide variants or deletions have been detected in CKM suggesting that tenofovir phosphorylation by CKM may be impacted by genetics [19,23,25]. For instance, a CKM variant denoted as rs11559024, which results in the change of glutamic acid to glycine, is predicted based on in silico analysis to result in a decrease or loss of kinase activity. Interestingly, while a majority of individuals carrying CKM variants were wild-type heterozygous, an individual carrying rs11559024 was identified as homozygous suggesting that tenofovir phosphorylation could be markedly impacted in certain cells/tissues in this individual [23].
Conclusion
While conventionally thought of as enzymes that solely perform important endogenous functions, AK, CK, PKLR and PKM can also participate in pharmacology through the phosphorylation of clinically important drugs. As such, investigation of the kinases that phosphorylate drugs such as tenofovir as well as other nucleoside/nucleotide reverse transcriptase inhibitors is an emerging area of research with the potential to have a translational impact. Several genetic variants in these kinases have been identified that could affect not only their endogenous activity but also their activities toward therapeutics. Thus, genetic variation may underlie certain clinically observed interindividual differences in response to tenofovir and NRTIs more broadly since these compounds require phosphorylation in order to become pharmacologically active. Increased understanding of the contribution of individual enzymes to tenofovir disposition, as well as the impact of genetic variation on kinase function has the potential to lead to the ability to better predict drug outcomes.
Future perspective
This review presents an updated view on the metabolism of the antiretroviral drug tenofovir, with an emphasis on the various kinases involved in its activation. Future studies that measure the levels of tenofovir, tenofovir-monophosphate and tenofovir-diphosphate, following observed dosing, in cells/tissues of individuals that carry AK2, CKM, PKLR and PKM genetic variants are required to move from in silico predictions to definitively establishing the connection between genotype and drug response. Further, it would be of particular interest to genotype HIV seroconverters in tenofovir-based HIV pre-exposure prophylaxis clinical trials in order to probe whether differential kinase activity as a result of genetic variation could underlie tenofovir inefficacy. In addition, the potential impact of transporters on modulating tenofovir and tenofovir-diphosphate levels, particularly within the context of interindividual variability in response to tenofovir requires attention. For instance, tenofovir has been demonstrated to be a substrate for efflux transporters multidrug resistance protein type 4 and 2 [46,47]. Further, tenofovir has been suggested to be a substrate for drug uptake transporters human organic anion transporter 1 and 3 [48,49]. Studies to determine the relative contributions of drug transporters versus kinases to variability in response to tenofovir are an important future direction. As such, the studies cited in this review regarding AK2, CKM, PKLR and PKM genotyping provide a foundation for such work.
Executive summary.
Tenofovir metabolism
Tenofovir is a common component of the most frequently prescribed antiretroviral drug combinations to treat HIV/AIDS.
Tenofovir is a component of the only approved regimen for HIV pre-exposure prophylaxis.
Tenofovir is an acyclic nucleoside phosphonate diester analog that works by competition with deoxyadenosine 5′-triphosphate for incorporation into the HIV-1 DNA.
Tenofovir must be phosphorylated in a stepwise fashion by endogenous kinases in order to become an active drug.
Adenylate kinase family
There are nine adenylate kinase isozymes that are found in different tissues in humans.
Adenylate kinase (AK) 2 has been demonstrated to phosphorylate tenofovir.
Deficiencies in AK2 expression have been identified as a causal factor in serious diseases.
Genetic variation in AK2 may contribute to interindividual variability in tenofovir disposition.
Pyruvate kinase family
Pyruvate kinases in the liver and red blood cells are enzymes essential for executing the final step of glycolysis.
The pyruvate kinase muscle and pyruvate kinase liver, red blood cell also play a role in phosphorylating tenofovir and genetic variation in these enzymes may alter tenofovir metabolism.
Creatine kinase
Creatine kinase is primarily found in muscle tissue and responsible for maintenance of the cytosolic stores of phosphates for production of ATP and therefore may play a role in tenofovir phosphorylation.
Genetic variation in creatine kinase muscle may modulate tenofovir activation in certain individuals.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH (grant numbers R01 AI128781, UM1 AI068613 and UM1 AI106707). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J. Acquir. Immune Defic. Syndr. 2006;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 2.Jenh AM, Thio CL, Pham PA. Tenofovir for the treatment of hepatitis B virus. Pharmacotherapy. 2009;29(10):1212–1227. doi: 10.1592/phco.29.10.1212. [DOI] [PubMed] [Google Scholar]
- 3.Masho SW, Wang CL, Nixon DE. Review of tenofovir–emtricitabine. Ther. Clin. Risk Manag. 2007;3(6):1097–1104. [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant JE, Dejesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N. Engl. J. Med. 2006;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 5.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J. Acquir. Immune Defic. Syndr. 2010;53(3):323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 6.Plosker GL. Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis. Drugs. 2013;73(3):279–291. doi: 10.1007/s40265-013-0024-4. [DOI] [PubMed] [Google Scholar]
- 7.Dejesus E, Ramgopal M, Crofoot G, et al. Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, Phase 3b, non-inferiority study. Lancet HIV. 2017;4(5):e205–e213. doi: 10.1016/S2352-3018(17)30032-2. [DOI] [PubMed] [Google Scholar]
- 8.Seneviratne HK, Hendrix CW, Fuchs EJ, Bumpus NN. MALDI mass spectrometry imaging reveals heterogeneous distribution of tenofovir and tenofovir diphosphate in colorectal tissue of subjects receiving a tenofovir-containing enema. J. Pharmacol. Exp. Ther. 2018;367(1):40–48. doi: 10.1124/jpet.118.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunawardana M, Remedios-Chan M, Miller CS, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015;59(7):3913–3919. doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman AR, Schwartz JL, Brache V, et al. Randomized, placebo controlled Phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PLoS ONE. 2018;13(6):e0199778. doi: 10.1371/journal.pone.0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson-Rose C, Gutin SA, Cummings B, Jaiantilal P, Johnson K, Mbofana F. ART adherence as a key component of prevention with persons living with HIV in mozambique. J. Assoc. Nurses AIDS Care. 2016;27(1):44–56. doi: 10.1016/j.jana.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr. Opin. Infect. Dis. 2012;25(1):51–57. doi: 10.1097/QCO.0b013e32834ef5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am. J. Kidney Dis. 2011;57(5):773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Calza L, Trapani F, Salvadori C, et al. Incidence of renal toxicity in HIV-infected, antiretroviral-naive patients starting tenofovir/emtricitabine associated with efavirenz, atazanavir/ritonavir, or lopinavir/ritonavir. Scand. J. Infect. Dis. 2013;45(2):147–154. doi: 10.3109/00365548.2012.712213. [DOI] [PubMed] [Google Scholar]
- 17.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 18.Young B, Dao CN, Buchacz K, Baker R, Brooks JT Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin. Infect. Dis. 2011;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 19.Lade JM, To EE, Hendrix CW, Bumpus NN. Discovery of genetic variants of the kinases that activate tenofovir in a compartment-specific manner. EBioMedicine. 2015;2(9):1145–1152. doi: 10.1016/j.ebiom.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerns SJ, Agafonov RV, Cho YJ, et al. The energy landscape of adenylate kinase during catalysis. Nat. Struct. Mol. Biol. 2015;22(2):124–131. doi: 10.1038/nsmb.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzeja P, Terzic A. Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 2009;10(4):1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panayiotou C, Solaroli N, Karlsson A. The many isoforms of human adenylate kinases. Int. J. Biochem. Cell Biol. 2014;49:75–83. doi: 10.1016/j.biocel.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa DB, Tillotson J, Li M, et al. Discovery of genetic variants of the kinases that activate tenofovir among individuals in the United States, Thailand, and South Africa: HPTN067. PLoS ONE. 2018;13(4):e0195764. doi: 10.1371/journal.pone.0195764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenig M, Pannicke U, Gaspar HB, Schwarz K. Recent advances in understanding the pathogenesis and management of reticular dysgenesis. Br. J. Haematol. 2018;180(5):644–653. doi: 10.1111/bjh.15045. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa DB, Madeen EP, Tillotson J, et al. Genetic variation of the kinases that phosphorylate tenofovir and emtricitabine in peripheral blood mononuclear cells. AIDS Res. Hum. Retroviruses. 2018;34(5):421–429. doi: 10.1089/aid.2017.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Bruggen R, Gualtieri C, Iliescu A, et al. Modulation of malaria phenotypes by pyruvate kinase (PKLR) variants in a Thai population. PLoS ONE. 2015;10(12):e0144555. doi: 10.1371/journal.pone.0144555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi K, Sugito N, Shinohara H, et al. Organ-specific microRNAs (MIR122, 137, and 206) contribute to tissue characteristics and carcinogenesis by regulating pyruvate kinase M1/2 (PKM) expression. Int. J. Mol. Sci. 2018;19(5) doi: 10.3390/ijms19051276. pii:E1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 2011;43(7):969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Weernink PA, Rijksen G, Mascini EM, Staal GE. Phosphorylation of pyruvate kinase type K is restricted to the dimeric form. Biochim. Biophys. Acta. 1992;1121(1–2):61–68. doi: 10.1016/0167-4838(92)90337-d. [DOI] [PubMed] [Google Scholar]
- 30.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005;15(4):300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Gui DY, Lewis CA, Vander Heiden MG. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci. Signal. 2013;6(263):pe7. doi: 10.1126/scisignal.2003925. [DOI] [PubMed] [Google Scholar]
- 32.Chaneton B, Gottlieb E. Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem. Sci. 2012;37(8):309–316. doi: 10.1016/j.tibs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Anitha M, Kaur G, Baquer NZ, Bamezai R. Dominant negative effect of novel mutations in pyruvate kinase-M2. DNA Cell Biol. 2004;23(7):442–449. doi: 10.1089/1044549041474797. [DOI] [PubMed] [Google Scholar]
- 34.Varga A, Graczer E, Chaloin L, et al. Selectivity of kinases on the activation of tenofovir, an anti-HIV agent. Eur. J. Pharm. Sci. 2013;48(1–2):307–315. doi: 10.1016/j.ejps.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Anastasiou D, Yu Y, Israelsen WJ, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012;8(10):839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Wijk R, Huizinga EG, Van Wesel AC, Van Oirschot BA, Hadders MA, Van Solinge WW. Fifteen novel mutations in PKLR associated with pyruvate kinase (PK) deficiency: structural implications of amino acid substitutions in PK. Hum. Mutat. 2009;30(3):446–453. doi: 10.1002/humu.20915. [DOI] [PubMed] [Google Scholar]
- 37.Gentry BG, Gentry SN, Jackson TL, Zemlicka J, Drach JC. Phosphorylation of antiviral and endogenous nucleotides to di- and triphosphates by guanosine monophosphate kinase. Biochem. Pharmacol. 2011;81(1):43–49. doi: 10.1016/j.bcp.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgibbon J, Katsanis N, Wells D, Delhanty J, Vallins W, Hunt DM. Human guanylate kinase (GUK1): cDNA sequence, expression and chromosomal localisation. FEBS Lett. 1996;385(3):185–188. doi: 10.1016/0014-5793(96)00365-1. [DOI] [PubMed] [Google Scholar]
- 39.Zanella A, Bianchi P. Red cell pyruvate kinase deficiency: from genetics to clinical manifestations. Baillieres Best Pract. Res. Clin. Haematol. 2000;13(1):57–81. doi: 10.1053/beha.1999.0057. [DOI] [PubMed] [Google Scholar]
- 40.Mohrenweiser HW. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proc. Natl Acad. Sci. USA. 1981;78(8):5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ning X, Qi H, Li R, et al. Discovery of novel naphthoquinone derivatives as inhibitors of the tumor cell specific M2 isoform of pyruvate kinase. Eur. J. Med. Chem. 2017;138:343–352. doi: 10.1016/j.ejmech.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 42.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40(5):1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitzenberg D, Colgan SP, Glover LE. Creatine kinase in ischemic and inflammatory disorders. Clin. Transl. Med. 2016;5(1):31. doi: 10.1186/s40169-016-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLeish MJ, Kenyon GL. Relating structure to mechanism in creatine kinase. Crit. Rev. Biochem. Mol. Biol. 2005;40(1):1–20. doi: 10.1080/10409230590918577. [DOI] [PubMed] [Google Scholar]
- 45.Dube MP, Zetler R, Barhdadi A, et al. CKM and LILRB5 are associated with serum levels of creatine kinase. Circ. Cardiovasc. Genet. 2014;7(6):880–886. doi: 10.1161/CIRCGENETICS.113.000395. [DOI] [PubMed] [Google Scholar]
- 46.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallants R, Van Oosterwyck K, Van Vaeck L, Mols R, De Clercq E, Augustijns P. Multidrug resistance-associated protein 2 (MRP2) affects hepatobiliary elimination but not the intestinal disposition of tenofovir disoproxil fumarate and its metabolites. Xenobiotica. 2005;35(10–11):1055–1066. doi: 10.1080/00498250500354493. [DOI] [PubMed] [Google Scholar]
- 48.Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6) J. Pharmacol. Exp. Ther. 2005;314(2):923–931. doi: 10.1124/jpet.105.084301. [DOI] [PubMed] [Google Scholar]
- 49.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2008;47(3):298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]