Abstract
Disease, drugs and the placebos used as comparators are inextricably linked in the methodology of the double-blind, randomized controlled trial. Nonetheless, pharmacogenomics, the study of how individuals respond to drugs based on genetic substrate, focuses primarily on the link between genes and drugs, while the link between genes and disease is often overlooked and the link between genes and placebos is largely ignored. Herein, we use the example of the enzyme catechol-O-methyltransferase to examine the hypothesis that genes can function as pharmacogenomic hubs across system-wide regulatory processes that, if perturbed in randomized controlled trials, can have primary and combinatorial effects on drug and placebo responses.
We begin by discussing how genetic variation in catechol-O-methyltransferase (COMT) influences a broad spectrum of diseases from psychiatric and neurological disorders, to cardiometabolic disease and cancer. We then examine how some drugs and supplements used to prevent and treat these conditions are influenced by COMT in these four disease domains. Next, we discuss how COMT genetic variation influences the placebo response. We continue by discussing the possibility that genetic interactions may lead to different responses in the drug and placebo arms of randomized controlled trials (RCTs), and thereby distort or confound the outcomes. We argue that including the disease and placebo axes with the gene–drug axis in pharmacogenomics has the potential to advance drug development and clinical care.
Catechol-O-methyltransferase in form & function
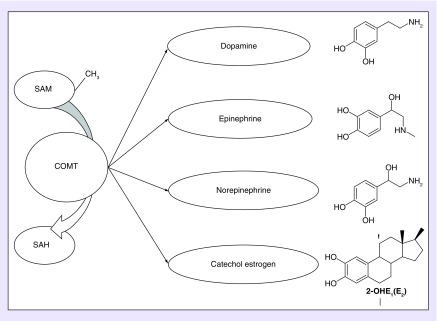
COMT is a Phase II enzyme (EC2.1.1.6) which, in the presence of magnesium ions, transfers a methyl group from S-adenosylmethionine (SAM) to a hydroxyl group on the catechol ring of endogenous and xenobiotic catechol substrates (Figure 1) [1]. During COMT-catalyzed O-methylation, SAM is converted to a competitive inhibitor, S-adenosylhomocysteine (SAH), resulting in a negative feedback regulatory loop. The endogenous substrates of COMT include the catecholamine neurotransmitters and the hormones dopamine, norepinephrine, and epinephrine (Table 1) [2]. In the absence of methylation, these catecholamines can accumulate and generate semiquinone and quinone free radicals, which promote DNA and lipid damage [3]. Hence, COMT is an important detoxifier of reactive molecules and can protect cells from oxidative stress known to influence neurodegenerative and cardiometabolic disease and cancer (Figure 1).
Figure 1. Catechol-O-methyltransferase enzymatic functions.
COMT is a Phase II enzyme that, in the presence of magnesium ions, transfers a methyl group (CH3) from SAM to the hydroxyl group of catechol-containing COMT substrates. SAM is thus converted to SAH, a competitive inhibitor of COMT. Endogenous substrates of COMT include the catecholamines, dopamine, epinephrine, and norepinephrine and the catechol-containing metabolic product of estrogen, catechol estrogen.
COMT: Catechol-O-methyltransferase; SAH: S-adenosylhomocysteine; SAM: S-adenosylmethionine.
Table 1. Catechol-O-methyltransferase endogenous catechol substrates, their receptors and function.
| Endogenous substrates | Receptor | Biological function |
|---|---|---|
| Dopamine | Dopamine family of receptors (DRD1–5) | Neurotransmission of reward and incentive salience, motor control. Dopamine inhibits norepinephrine release, acts as a vasodilator, and can increase sodium excretion and urine output. In the pancreas, dopamine reduces insulin production and reduces gastrointestinal motility in the digestive system; in the immune system, it reduces the activity of lymphocytes. Dopamine is converted to norepinephrine by DBH |
| Norepinephrine | α and β-adrenergic receptors (ADRα and β) | Norepinephrine is a key neurotransmitter in the sympathetic nervous system and is important in the fight-or-flight response. It acts by increasing heart rate, blood pressure and glucose levels, while also increasing blood availability in skeletal muscle and reducing it in the GI tract. Norepinephrine is converted to epinephrine by PNMT |
| Epinephrine | α and β-adrenergic receptors (ADRα and β) | Like norepinephrine, epinephrine is important in the fight-or-flight response, increasing heart and respiratory rate, muscle contraction, vasomotor tone, blood glucose and lipolysis. Epinephrine is derived from norepinephrine by PNMT |
| Catechol estrogen | Membrane (mER) and nuclear estrogen receptors (ERα and ERβ) | Estrogen is converted to catechol estrogens by cytochrome P450 enzymes. Catechol estrogens have procarcinogenic, proproliferative, and anti-apoptotic activities. COMT converts catechol estrogens to methoxyestradiols that are protective against carcinogenesis |
COMT: Catechol-O-methyltransferase; DBH: dopamine β-hydroxylase; GI: Gastro-intestinal; PNMT: Phenylethanolamine N-methyltransferase
Catechol estrogens, the product of estradiol hydroxylation by cytochrome P450 Phase I enzymes, represent another class of COMT substrates. Like catecholamines, catechol estrogens undergo oxidation to produce reactive semiquinones and quinones (Table 1) [4]. Beyond the potential for free radical DNA and lipid damage, catechol estrogens have estrogenic properties and are able to promote proliferation of hormone-sensitive tumors [5]. Methylation by COMT, therefore, not only limits the carcinogenicity of catechol estrogens, but also produces 2- and 4-methoxy estradiol, which have antiproliferative, pro-apoptotic, anticarcinogenic, and anti-inflammatory properties [4]. These methoxy-derivatives also appear to exhibit beneficial cardiometabolic effects [6].
The COMT gene
The COMT gene is located on chromosome 22q11.2 and contains six exons that encode membrane and soluble forms of the enzyme. COMT is ubiquitously expressed with the highest levels in the adrenal gland, liver, lung, ovary, urinary bladder, and placenta [7]. Whereas the soluble form is dominant in most tissues, the membrane form is dominant in the brain. Sexual dimorphism in COMT expression has been attributed to its regulation by estrogen and its role in estrogen metabolism [8,9]. COMT expression also varies with age, increasing in the liver tenfold from infancy to adulthood and then decreasing with age [10]. A three megabase deletion in chromosome 22q11.2, which includes the COMT gene, results in DiGeorge/velocardiofacial syndrome [11]. The manifestations of this syndrome, including higher rates of schizophrenia, and susceptibility to cardiovascular disease and cancer, cross many organ systems, and are thought to arise in part because of loss of COMT and its role in catecholamine metabolism and detoxification of reactive oxygen species.
The most widely studied COMT polymorphism, rs4680 (val158/108met), encodes a G (valine) to A (methionine) transition in exon 4 at codon 158 in the membrane, and 108 in the soluble form [12]. This polymorphism results in a three- to fourfold reduction in thermostability and enzymatic activity, and a commensurate increase in circulating catecholamines in individuals homozygous for the methionine (met/met) versus valine (val/val) form of the enzyme [13]. Rs4680 is a commonly occurring variant, with minor allele frequencies that vary by population ancestry but allow for powerful genetic analysis even in small studies. For example, the frequencies of the val-allele among samples of people of European, African, and Asian ancestry are 0.48, 0.69, and 0.62, respectively [14]. Although most studies focus on rs4680 owing to its functional consequences, the linked synonymous polymorphism rs4818 has also been shown to have clinical phenotypes [15,16], and COMT haplotypes have been studied in schizophrenia [17] and pain [15,16].
COMT & disease
COMT effects on executive function & neuropsychiatric symptoms
COMT accounts for most of the dopamine clearance in the prefrontal cortex, where monoamine oxidases and dopamine transporters are poorly expressed [18]. Hence, higher order cognitive functions and behavioral endophenotypes modulated in the prefrontal cortex are more directly influenced by variations in the levels of COMT activity than other regions of the brain. The prefrontal cortex is responsible for a set of cognitive processes that mediate executive function. Although these processes, which include cognitive flexibility, working memory, and attention, have been associated with COMT, the magnitude and direction of the effects differ by health status and disease type. For example, among healthy volunteers, meta-analysis of COMT association with cognitive flexibility revealed that the met/met genotype was associated with better performance on the Wisconsin Card Sorting Test, compared with those with the val/val genotype (Table 2) [19]. However, this difference was not detected among patients with schizophrenia. Interestingly, during manic episodes, when dopamine levels are thought to be increased in the prefrontal cortex, val/val patients with bipolar disorder performed better on the Wisconsin Card Sorting Test [20]. In general, val/val adults do not perform as well as met/met adults on tests of memory and attention [21,22]. Like humans, transgenic mice humanized with the met allele (met/met) had a 30% reduction in COMT activity and performed better on tests of spatial working memory compared with transgenic val/val mice [23]. Treatment of these val/val mice with the COMT inhibitor tolcapone restored the deficit in spatial working memory.
Table 2. COMT rs4680† (val158met) association with disease in selected studies.
| Condition | Findings | Ref. |
|---|---|---|
| Executive function | ||
| Cognitive flexibility | The met/met genotype was associated with better performance on the WCST, compared with val/val genotype. However, this difference was not detected among patients with schizophrenia. During manic episodes, when dopamine levels are thought to be increased in the prefrontal cortex, val/val patients with BPD performed better on the WCST | [19,20] |
| Memory and attention | The met-allele was associated with enhanced working memory and better performance on attention-related tasks. Aging also influences cognition and among a biracial cohort of elders, COMT was associated with rate of cognitive decline, with the val-allele having a protective effect | [21,24] |
| Aggression | Although there was no main effect of COMT on aggression, in nonhuman primates, val/val macaques of higher rank exhibited higher rates of aggression. Conversely, rates of aggression decreased with rank among met/met and val/met animals | [25] |
| Stress response | Infants carrying the met allele had significantly higher negative emotionality and a greater cortisol response with repeated exposure to stress. The met-allele was associated with sensitivity to stressful events in childhood and adolescence and higher cortisol response to stress. Met-allele homozygotes were more susceptible to stress mindset interventions | [26–27] |
| Harm avoidance and novelty seeking | The val-allele was associated with more effective processing of aversive emotional stimuli, extraversion, novelty seeking. The met-allele was associated with harm avoidance which has an inverse relationship with novelty seeking | [21,28,29] |
| Psychiatric disease | ||
| Attention-deficit hyperactivity disorder | In a recent comprehensive meta-analysis of rs4680 genetic association studies across 15 psychiatric disorders and 363 datasets, the val-allele was more prevalent among ADHD cases versus controls | [30] |
| Substance-use disorder | In the same meta-analysis as above, the val-allele was more prevalent among SUD cases versus controls | [30] |
| Panic disorder | Among individuals of Asian ancestry, in the same meta-analysis as above, the val-allele was more prevalent among panic disorder cases versus controls | [30] |
| Bipolar disorder | BPD tended to be associated with the met-allele among individuals of Asian ancestry | [30] |
| Obsessive–compulsive disorder | OCD tended to be associated with the met-allele among males, in the same meta-analysis as above | [30] |
| Suicide | Although there is inconsistency across the many studies looking at COMT and suicide, a recent meta-analysis of 17 studies (3282 cases and 3774 controls) found the val-allele was a risk factor in males but protective in females | [31] |
| Major depressive disorder | The val/val genotype was significantly associated with reduced sadness scores and reduced disposition to depression. Although studies on COMT association with MDD are mixed, a recent large meta-analysis found no association overall or in gender subgroups | [32,33] |
| Neurological disease | ||
| Delirium | Perturbations in executive function can result in delirium which affects mainly attention, and dementia which affects mainly memory. Although COMT does not appear to directly influence delirium, it modified the known association between C-reactive protein (CRP), an acute phase reactant, and postoperative delirium | [34,35] |
| Functional pain disorders | A recent meta-analyses of COMT and functional pain disorders found that the low-activity met-allele was associated with fibromyalgia. An earlier meta-analysis also found that the met-allele was associated with fibromyalgia and widespread chronic pain, but not migraine headache or chronic musculoskeletal pain conditions | [36,37] |
| Chronic fatigue syndrome | Chronic fatigue syndrome patients were more likely to be homozygous for the met-allele compared with val-allele carriers | [38,39] |
| Pain | COMT low-activity haplotypes were associated with greater sensitivity to nociceptive, but lower sensitivity to neuropathic pain. Conversely, high-activity haplotypes were associated with lower sensitivity to nociceptive but greater sensitivity to neuropathic pain. In breast cancer survivors, pain sensitivity and fatigue was greater among met-allele homozygotes | [40,41] |
| Parkinson disease | COMT inhibitor drugs are used as adjunctive treatment for patients with Parkinson disease. Researchers have failed to find a clear association between COMT and Parkinson disease, but several studies have demonstrated COMT genotype modulation of prefrontal cortex dysfunction early in the course of Parkinson disease. Specifically, increasing numbers of met-alleles was associated with diminished performance on tasks that tested prefrontal cortex functions | [42–44] |
| Alzheimer disease | Similarly, although metabolism of dopamine and other catecholamines can influence Alzheimer disease, results of COMT association with the disease have yielded directionally inconsistent findings. Still, recent meta-analyses reported a COMT association with AD among Asians but not Caucasians | [45] |
| Autoimmune disease | COMT’s role in limiting the damaging effects of oxidative and emotional stress make its potential role in the etiology of auto-immune diseases plausible. Studies in psoriasis, narcolepsy and systemic lupus erythematosus suggest a link between COMT and autoimmune diseases | [46–47] |
| Cardiometabolic disease | ||
| Cardiometabolic disease risk factors | The COMT rs4680 high-activity val-allele, which is associated with lower levels of catecholamines, is consistently associated with lower cardiometabolic risk factors, including triglycerides, systolic blood pressure and hemoglobin A1c | [48–49] |
| Diabetes | The COMT val-allele was modestly protective from risk of Type II diabetes | [[50–51] |
| Cardiovascular disease | The val-allele was protective from risk of cardiovascular disease in several studies | [48,52,53] |
| Pre-eclampsia | The met-allele and a low-COMT activity haplotype were associated with recurrent pre-eclampsia. In murine models of pre-eclampsia, pre-eclampsia-like phenotypes in COMT-deficient pregnant mice were corrected with 2-methoxyestradiol treatment | [6] |
| Cancer | ||
| Invasive cancers | Although the met-allele is frequently associated with increased risk of cancers, there are as many studies with null or opposite effects. Consistent with this hypothesis, we recently demonstrated that COMT was associated with rates of invasive cancer in two large RCTs of vitamin E for cancer prevention | [4,54] |
The COMT rs4680 val-allele encodes a form of the enzyme which is 3–4× more active than the met-allele form.
ADHD: Attention-deficit hyperactivity disorder; BPD: Bipolar disorder; COMT: Catechol-O-methyltransferase; OCD: Obsessive-compulsive disorder; MDD: Major depressive disorder; RCT: Randomized controlled trial; SUD: Substance-use disorder; WCST: Wisconsin Card Sorting Test.
Given the link between executive function and aggressive behavior, it is not surprising that COMT has been shown to influence aggression, as well. In nonhuman primates, val/val macaques of higher rank exhibited higher rates of aggression, but rates of aggression decreased with rank among met/met and val/met animals [25]. Notably, there was no main effect of COMT on aggression. COMT gene expression was significantly higher in patients with Alzheimer disease (AD) who also display aggressive behavior, and this effect was more pronounced in the later stages of the disease [55].
Executive function is influenced by stress [56], and low-COMT activity seems to predispose individuals across the age spectrum to be more susceptible to stress. In met/met infants, repeated exposure to stress induced higher levels of cortisol and emotionality [26]. Similarly, the met-allele was associated with greater sensitivity to stress-inducing events in childhood and adolescence, again with higher cortisol responses to stress [57,58]. Met/met adults were also found to be more susceptible to stress mindset interventions [27], and practiced greater harm avoidance [29]. In contrast, the val-allele was associated with positive emotionality, extraversion [28], novelty seeking [59], a greater ability to handle stress, and reduced scores evaluating sadness [32]. These differential stress-related phenotypes have also been observed in mice, where the COMT gene is highly conserved (almost 80% homology to the human gene), and manipulation of the COMT gene in murine models has yielded similar cognitive, behavioral, and emotional phenotypes as those observed in humans [60]. For example, COMT knockout mice had better working memory compared with wild-type and transgenic mice expressing high levels of COMT. Furthermore, anxiety-like behaviors were also reduced in wild-type mice and transgenic mice expressing high levels of COMT, whereas knockout mice exhibited increased anxiety in stress tests. Sexual dimorphism in COMT effects has also been observed in mice. Male transgenic val/val mice displayed lower prepulse inhibition (a reflex behavior induced by presentation of a weak stimulus to prepare for a subsequent stronger stimulus) compared with their met/met counterparts. This effect was reversed among female mice.
COMT & psychiatric disease
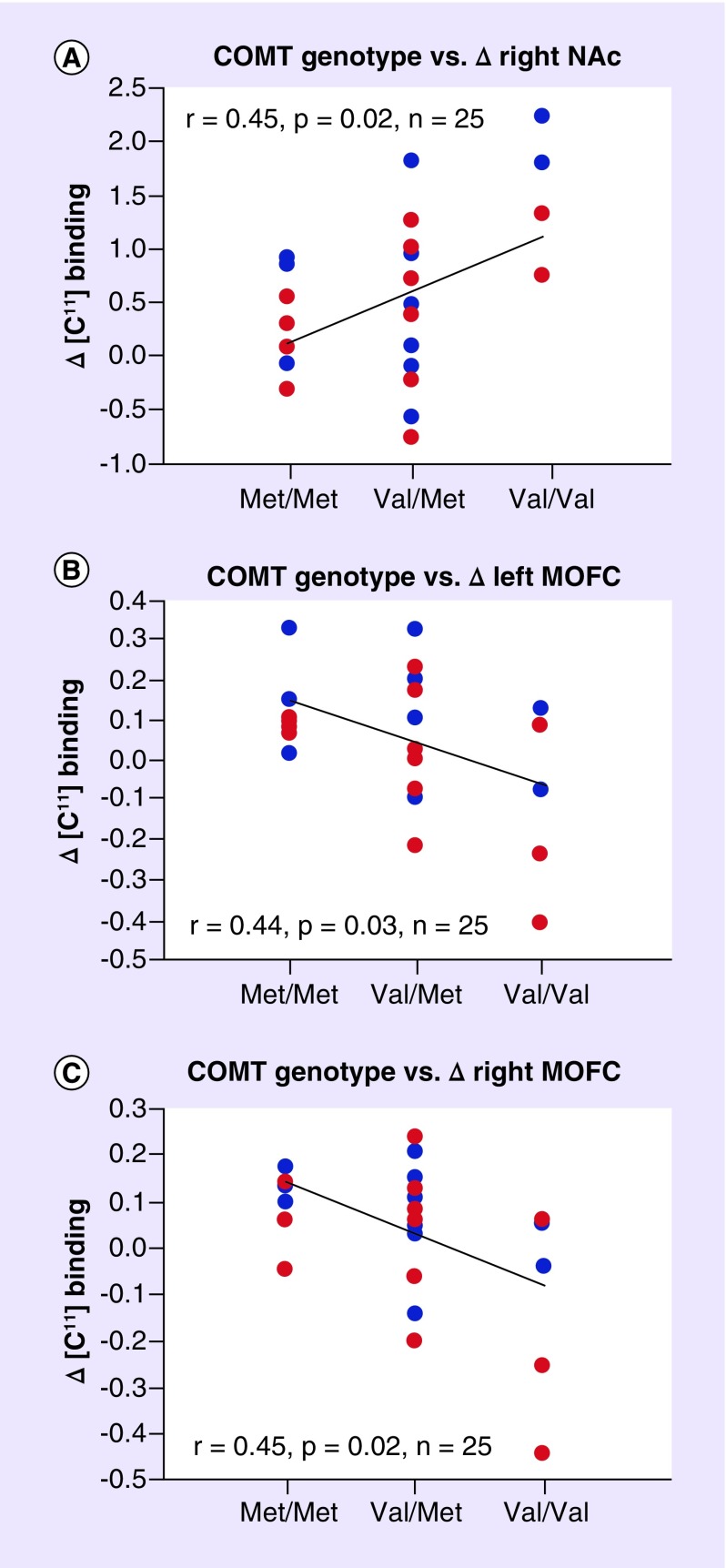
COMT rs4680 is one of a set of polymorphisms related to catecholaminergic neurotransmission that have been extensively studied in psychiatry and human neurosciences. However, COMT effects on executive function do not translate to consistent associations with psychiatric or neurological disease. This inconsistency could be due to the greater complexity of these conditions, as well as the variability in study size, population structure, sexual dimorphism, history of stress, and trauma, pharmacogenomic effects and the influence of other genes [30]. Still, with increasing numbers of studies and several recent meta-analyses, some significant trends are beginning to emerge. In a recent comprehensive meta-analysis of rs4680 genetic association studies across 15 psychiatric disorders and 363 datasets, statistically significant associations were reported in attention-deficit hyperactivity disorder (ADHD) and substance abuse disorder (SUD), where the high-activity val-allele was more prevalent among cases versus controls [30]. The link between COMT val-allele and increased alcohol consumption was further corroborated in neuroimaging studies demonstrating significant differential opioid release following alcohol consumption by COMT genotype in regions of the brain associated with reward (Figure 2) [61]. These results suggested that val/val individuals might gain greater hedonistic benefit from alcohol use.
Figure 2. Relationship of the COMT rs4680 genotype to endogenous opioid release following alcohol consumption.
Endorphin release is significantly greater in the right NAc (A: t = 3.25, p = 0.009) and significantly lower in the left mOFC (B: t = 2.34, p = 0.041) and right mOFC (C: t = 2.60, p = 0.027) of individuals with the val/val genotype. Blue circles indicate control subjects, red circles indicate heavy social drinkers.
Reproduced with permission from [61] under the terms of the Creative Commons Attribution License (CC BY) https://creativecommons.org/licenses/by/4.0/
COMT: Catechol-O-methyltransferase; mOFC: Medial orbito-frontal cortex; NAc: Nucleus accumbens.
In subpopulation analyses, panic disorder was significantly associated with the val-allele among individuals of Caucasian ancestry, and bipolar disorder was significantly associated with the met-allele among individuals of Asian ancestry. Obsessive–compulsive disorder was also associated with the met-allele among males [30]. In a separate meta-analysis of 17 studies on COMT and suicide, the val-allele was a risk factor in males but protective in females [31]. Finally, although the val/val genotype was significantly associated with reduced sadness scores and reduced disposition to depression [32], overall COMT association studies with major depressive disorder (MDD) are mixed. A recent large meta-analysis of 21 case–control studies found no association overall, or in subgroups stratified by sex [33].
Neurological conditions
Delirium
Perturbations in executive function can result in delirium, which affects mainly attention, and dementia, which affects mainly memory. Although COMT does not appear to influence delirium directly [34], it modified the known association between C-reactive protein (CRP), an acute phase reactant, and postoperative delirium [35]. Aging also influences cognition and among a biracial cohort of elders, COMT was associated with rate of cognitive decline with the val-allele having a protective effect [24].
Pain & fatigue
COMT influences pain perception through catecholamines, which have differential effects based on the regional specificities of α and β adrenergic receptors [40]. One of the first studies on COMT and pain perception, published in 2003, found that individuals homozygous for the rs4680 low-activity met-allele were more sensitive to pain induced by hypertonic saline infusion into the masseter muscle than those with the high-activity val-allele [62]. Since that time, our understanding of COMT effects on pain sensitivity has broadened to include effects of haplotypes in nociceptive and neuropathic pain. Generally, individuals with low-activity haplotypes tended to be more sensitive to nociceptive pain but less sensitive to neuropathic pain [40]. Conversely, individuals with high-activity haplotypes tended to report lower sensitivity to nociceptive pain but greater sensitivity to neuropathic pain. A recent meta-analyses of functional pain disorders found that the met-allele was associated with fibromyalgia and widespread chronic pain [36]. An earlier meta-analysis also found that the met-allele was associated with fibromyalgia, but not migraine headache or chronic musculoskeletal pain [37]. Consistent with the hypothesis that chronic fatigue syndrome (CFS) results from an overactive autonomic nervous system, CFS patients were more likely to be homozygous for the met-allele compared with val-allele carriers [38,39].
Pain sensitivity and fatigue symptoms co-occur in cancer and have been associated with COMT. In several studies, postsurgical cancer patients who were met-allele homozygotes had a lower pain threshold and higher levels of fatigue [41]. Consistent with these profiles, in wild-type mice, sustained delivery of the COMT inhibitor OR486 increased sensitivity to mechanically induced pain [63]. COMT knockout mice have enhanced sensitivity to thermal pain, and transgenic mice that overexpress COMT are more resistant to thermal pain [60].
Parkinson disease, AD & other diseases
Despite the link between dopamine induced oxidative stress and neuronal damage in the substantia nigra, researchers have failed to establish a clear link between genetic variation in COMT and the risk of Parkinson disease [64]. However, several studies have demonstrated COMT genotype modulation of prefrontal cortex dysfunction early in the course of Parkinson disease. Specifically, increasing numbers of met-alleles was associated with diminished performance on tasks that tested prefrontal cortex functions [43,44,65]. Furthermore, this reduced function was more pronounced among patients on dopaminergic treatment [66], leading to the hypothesis that impaired function results from an ‘overload’ of prefrontal cortex dopamine, which is worsened by levodopa.
Similarly, although metabolism of dopamine and other catecholamines can influence AD, results of COMT association with the disease have yielded directionally inconsistent findings. Still, recent meta-analyses reported a COMT association with AD among Asians but not among Caucasians [45].
COMT’s role in limiting the damaging effects of oxidative and emotional stress also make its potential role in the etiology of auto-immune diseases plausible [46]. Studies in psoriasis [67], narcolepsy [68], and systemic lupus erythematosus [47,69] support this hypothesis, but more work needs to be done in this area.
COMT & cardiometabolic disease
Catecholamines influence cardiometabolic functioning through manifold effects on insulin secretion, glucose metabolism, natriuresis, vascular tone, and heart rate. Given COMT’s role in catecholamine metabolism, it is not surprising that the COMT rs4680 high-activity val-allele, which is associated with lower levels of catecholamines, is consistently associated with lower cardiometabolic risk factors, including triglycerides, systolic blood pressure [48,70,71], and hemoglobin A1c [50].
In terms of disease, a consistent protective association of the val-allele was also observed with Type II diabetes [50,72] with only one exception, men with obesity [51]. COMT deficiency in mouse models resulted in glucose intolerance which was rescued by 2-methoxyestradiol (2-ME) [49]. The COMT association extends to cardiovascular disease, specifically to the risk of myocardial infarction and stroke where the rate of incident events is lower among val-allele than met-allele homozygotes [52,53,73]. Interestingly, among women diagnosed with depression, the val-allele was associated with increased risk of cardiovascular disease [74].
Epidemiological and animal studies have demonstrated COMTs role in pre-eclampsia, a condition in pregnancy characterized by high blood pressure and proteinuria [6]. The finding that the met-allele and a low-COMT activity haplotype are associated with recurrent pre-eclampsia was supported by murine models in which pre-eclampsia-like phenotypes in COMT-deficient pregnant mice were corrected with 2-methoxyestradiol treatment, the product of COMT catechol estrogen metabolism [6].
COMT & cancer
Multiple pathways link COMT to cancer. Catecholamines have varied effects on tumor angiogenesis, a process that is necessary for tumor growth and progression [75]. Whereas epinephrine and norepinephrine promote tumor angiogenesis by upregulating the synthesis of proangiogenic factors IL-6 and VEGF via β-adrenergic signaling, dopamine inhibits the formation of new blood vessels by suppressing vascular permeability factor/VEGF receptor-A (VPF/VEGFR-2) [76]. In vitro and animal studies support COMT’s role in transforming and eliminating the carcinogenic threat of catechol estrogens and other catecholamines which are prone to breaking down into free radicals [77]. High COMT expression was associated with survival in colorectal and pancreatic cancer patients [78] and overexpression of the COMT gene in colorectal and pancreatic cancer cell lines resulted in reduced proliferation and invasive ability [78,79]. Furthermore, catecholamines are produced in response to stress, and these angiogenic and pro-oxidative stress links provide a potential mechanism through which undue stress might support carcinogenesis [80].
Despite several positive findings on COMT and cancer, its association with the risk of hormonal and other cancers remains equivocal. Although the met-allele is frequently associated with increased risk of cancers, there are as many studies with null or opposite effects. There are several potential factors that contribute to this inconsistency, including heterogeneity in race/ethnicity, sex, age, disease, and potential gene–drug interactions (discussed below). For a recent and detailed discussion of the variability in the COMT-cancer relationship, we refer the reader to a recent comprehensive review by Sak [4].
COMT, drugs & supplements
The presence of a catechol moiety makes many drugs, supplements, and their metabolites substrates for COMT. Additionally, drugs that target pathways involved in catecholamine anabolism, signaling or metabolism can also interact with COMT. Hence, given the multiple overlapping functions between COMT and pathways targeted by drugs, COMT gene–drug interaction effects are prevalent in randomized controlled trial (RCTs) of disease domains influenced by COMT (Table 3).
Table 3. Selected drugs that modify catechol-O-methyltransferase effects.
| Condition | Drug/treatment | Findings | Ref. |
|---|---|---|---|
| Psychiatric disorders | |||
| Attention deficit hyperactivity disorder | Methylphenidate is a norepinephrine–dopamine reuptake inhibitor | A recent review of COMT-stimulant pharmacogenetic effects suggested the strongest benefit of methylphenidate for ADHD was observed among val/val patients | [81] |
| Substance-use disorder | Nicotine replacement therapy and bupropion a norepinephrine–dopamine reuptake inhibitor | In a large RCT in smokers of European ancestry, compared with placebo, met/met homozygotes were more likely to abstain from smoking than val-allele carriers. In a trial of bupropion, COMT haplotypes predicted the response to drug versus placebo | [82,83] |
| Major depressive disorder | Antidepressants milnacipran, fluoxetine, paroxetine, fluvoxamine, mirtazapine, venlafaxine, duloxetine and electroconvulsive therapy | Many studies report significant COMT rs4680 associations with antidepressant treatment response, for example, milnacipran, fluoxetine, paroxetine, fluvoxamine, mirtazapine, venlafaxine and duloxetine. Notably, many of these studies did not include a placebo control. An RCT of duloxetine for MDD did not find a genome-wide association between COMT in placebo or drug treatment arms. The val-allele was also found to be influence treatment response to electroconvulsive therapy | [84–85] |
| Generalized Anxiety disorder | Venlafaxine, a serotonin-norepinephrine reuptake inhibitor | A nominally significant association with response to venlafaxine was reported for the secondary treatment outcome | [86] |
| Bipolar disorder | Lamotrigine (an anticonvulsant) plus folic acid (vitamin B9) | There was a negative impact of randomized lamotrigine plus folic acid on met-allele carrier | [87] |
| Schizophrenia | Antipsychotic medications including olanzapine, clozapine and risperidone | In a recent meta-analysis (N = 10 studies), response to antipsychotic treatment was significantly associated with the COMT met/met genotype | [88] |
| Chronic fatigue syndrome | Clonidine, an α-2 adrenergic agonist | In an RCT of clonidine for treating chronic fatigue syndrome, COMT modified effects of clonidine in the primary outcome, steps taken per day | [89] |
| Working memory | Tetrahydrocannabinol (THC) a cannabinoid receptor agonist | Val/Val individuals in two studies were impaired on cognitive tests when randomized to THC compared with placebo | [90,91] |
| Neurological disease and conditions | |||
| Parkinson disease | Vitamin C (ascorbic acid) is an anti-oxidant | Vitamin C was one of the first drugs evaluated as a possible adjunct therapy to levodopa because of its COMT inhibitory effects. More recently, in the human blood metabolite GWAS, O-methylascorbate a metabolite of vitamin C was significantly associated with rs4680 (p = 5e-178) | [92,93] |
| Nitrocatechols for example, entacapone and tolcapone are selective and reversible inhibitors of COMT [94] | Entacapone acts peripherally and is only slightly able to penetrate the blood brain barrier. Tolcapone can penetrate the blood brain barrier and an RCT is underway in patients with relapsed or refractory neuroblastoma | [45,95] | |
| Pain | Morphine and fentanyl are exogenous opioids | COMT genetic variation has been associated with variability in opioid requirement for postoperative and cancer pain | [96,97] |
| Cardiometabolic disease | |||
| Bradycardia | Isoproterenol is an analog of epinephrine and a β adrenoreceptor agonist | An epinephrine analog is used to treat bradycardia | [98] |
| Diabetes | Insulin detemir | In an RCT of recombinant insulin, reduction of HbA1c was significantly better among met-allele carriers compared with val-allele homozygotes | [99] |
| Pre-eclampsia | Hydralazine and verapamil | Hydralazine, a widely accepted drug for the treatment of pre-eclampsia, was shown to inhibit placental COMT activity. Verapamil was explored as a potential drug to treat pre-eclampsia. COMT activity was suppressed in term placental explants after 6-hour exposure to verapamil or hydralazine | [100] |
| Hypertension | Calcium-channel blockers, angiotensin-receptor blockers and diuretics | The val-allele was associated with lower systolic blood pressure in participants treated with Calcium-channel blockers. This effect was even stronger among elderly individuals. Similar effects were seen among participants treated with angiotensin-receptor blockers and there was a significant association with another COMT SNP rs737865 and diuretics | [101] |
| Cardiovascular disease prevention | Aspirin, an antiplatelet, analgesic and anti-inflammatory drug and vitamin E an anti-oxidant | Significant COMT-by-aspirin and COMT-by-vitamin E effect modification were reported in a randomized trial of cardiovascular disease prevention | [52] |
| Cancer prevention | |||
| Prostate cancer prevention | Finasteride, inhibits dihydrotestosterone production | In the PCPT RCT, the val-allele was associated with increased risk of prostate cancer among patients randomized to finasteride | [102] |
| Prevention of all invasive cancers | Vitamin E (α-tocopherol) is an anti-oxidant | In two large RCTs, COMT-by-vitamin E effect modification was reported in cancer prevention such that met-allele homozygotes benefited from vitamin E therapy but val-allele homozygotes did not | [52,103] |
| Prevention of chronic disease | Quercetin and fisetin are flavonoids used as supplements in chronic disease prevention | These phytochemicals are found in onions, apples and red wine. They have antioxidant and anti-inflammatory effects and inhibit COMT function. They are sold as supplements to prevent cancer and other chronic diseases | |
| Cancer prevention | Green tea catechins include catechin, epicatechin and epigallocatechin-3-O-gallate [104] | In a population-based case–control study, risk of breast cancer was significantly lower among tea drinkers who were met-allele carriers. The combination of quercetin and green tea was found to enhance inhibition of prostate cancer xenograft tumor growth. Higher energy expenditure and fat oxidation were observed among met/met participants randomized to placebo in an RCT of the cardiometabolic effects of green tea. Differential effects were also observed in the green tea treatment arm | [105–106] |
ADHD: Attention-deficit hyperactivity disorder; MDD: Major depressive disorder; RCT: Randomized controlled trial.
Drugs used in treating psychiatric disorders
Based on its role in neurotransmitter signaling, executive function, and its association with psychiatric endophenotypes and disease, COMT has been explored as a candidate gene to explain the variability in treatment response in several neuropsychiatric disorders. Methylphenidate increases catecholamine signaling in the prefrontal cortex, has the potential to improve executive function in patients with ADHD. Not surprisingly, with the higher frequency of the val/val genotype among ADHD patients, the greatest benefit of methylphenidate therapy for ADHD was observed among val/val patients [81].
COMT effects in substance abuse treatment, specifically with regard to nicotine dependence, and alcohol use have been extensively studied. The results, however, have been inconsistent. Hence, early studies that suggested COMT may be a predictor of bupropion [83] or nicotine replacement therapy response in the treatment of tobacco dependence, have not been borne out [82].
In MDD, although numerous studies reported significant COMT rs4680 associations with antidepressant treatment response (i.e., milnacipran [84], fluoxetine [107], paroxetine [108], fluvoxamine [109], mirtazapine [110], venlafaxine [111], and duloxetine [112]), many of these studies were small and did not include a placebo control. Given the relationship between COMT and psychiatric disease and placebo response (discussed below), the absence of a placebo control in these studies, makes it almost impossible to ascribe these effects solely to gene–drug interactions.
COMT effect modification was also observed for venlafaxine for generalized anxiety disorder [86] and several antipsychotic medications used to treat schizophrenia [88,113]. Interestingly, a subset of these drugs form metabolites that are substrates of COMT, for example, paroxetine-catechol and duloxetine-catechol, suggesting other possible mechanisms for the COMT–drug interactions reported in the literature [114].
Drugs used to treat neurological disease
Interest in COMT as a druggable target was motivated by the need to inhibit peripheral COMT in patients with Parkinson disease who were treated with the dopamine precursor levodopa. Levodopa is the gold standard treatment for patients with Parkinson disease because unlike dopamine, it is able to cross the blood–brain barrier. Ascorbic acid (vitamin C), a weak competitive inhibitor of COMT [115], was one of the early molecules evaluated, albeit unsuccessfully, as a potential adjunctive therapy to preserve levodopa in the periphery [92]. Although the weak COMT-vitamin C interaction has been largely ignored, in the recent genome-wide association study of human blood metabolites, vitamin C metabolites were found to be strikingly significantly associated with COMT [93].
Once the crystal structure of COMT was solved, rational design was used to create nitrocatechol inhibitors, such as entacapone and tolcapone, which contain a catechol with a nitro group at position 4 on the benzene ring [94,116]. These drugs are potent reversible inhibitors of COMT, and although their primary clinical application is as an adjunct to levodopa in Parkinson disease, RCTs have explored their effects in attention [117], cognitive deficits in schizophrenia [118], and based on tolcapone’s apoptotic effects, as a chemotherapeutic in neuroblastoma [95,119].
Consistent with the differential pain sensitivity [110], rs4680 and COMT haplotypes were associated with opioid requirement for postoperative [96,120] and cancer [97] pain. CFS is thought to be a result of an overactive sympathetic nervous system and in a randomized controlled clinical trial (RCT) of the antihypertensive clonidine, designed to reduce catecholamine tone in CFS, individuals homozygous for the val-allele had poorer outcomes when randomized to clonidine compared with placebo, whereas neither a drug nor placebo effect was observed in the met-allele homozygotes [89]. Finally, val/val individuals in two studies were impaired on cognitive tests when randomized to tetrahydrocannabinol compared with placebo [90,91].
Drugs used to treat cardiometabolic disease
Several drugs used to treat various conditions related to cardiometabolic disease physically or pharmacogenomically interact with COMT. For example, isoproterenol is an analog of epinephrine, and, as such, a substrate for COMT. In pharmacogenetic studies of hypertensive medications, the val-allele of rs4680 was associated with lower systolic blood pressure in participants treated with calcium-channel blockers, and this effect was even stronger among elderly individuals [101]. Similar effects were seen among participants treated with angiotensin-receptor blockers; a significant association with another COMT SNP, rs737865, and diuretics were also reported. Hydralazine, a widely used drug for the treatment of high blood pressure in pregnancy and pre-eclampsia, was shown to inhibit placental COMT activity [100]. Verapamil, a drug that was also explored as a potential therapy for pre-eclampsia, also suppressed COMT activity in term placental explants [100]. The antiplatelet agent aspirin was found to interact significantly with COMT in the Women’s Health Study, a large RCT of aspirin for cardiovascular disease prevention, such that met-allele homozygotes had a lower rate of cardiovascular disease when randomized to aspirin treatment compared with placebo [52]. The inverse relationship was observed for val-allele homozygotes who had higher rates of cardiovascular disease with randomized aspirin treatment compared with placebo. Strikingly, a similar directional and statistically significant COMT gene–drug effect was observed with vitamin E [52].
Drugs & supplements used to prevent cancer & other chronic diseases
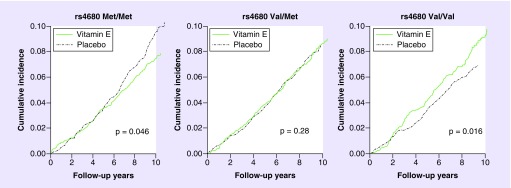
Despite considerable historic controversy over the risks and benefits of vitamin C, there is renewed interest in its potential as an anticancer agent [121]. Vitamin C has several links to COMT. As discussed above, it was one of the first drugs tested as a potential COMT inhibitor to protect levodopa from COMT metabolism. In the human blood metabolite genome-wide association study, O-methylascorbate and other metabolites of vitamin C were strikingly associated with COMT rs4680 (p = 5e–178) [93]. Vitamin C is a water-soluble anti-oxidant that scavenges oxygen radicals in the aqueous phase and interacts with the lipid-soluble antioxidant vitamin E, regenerating it once it has scavenged oxygen radicals [122]. Effects of vitamin E were also found to be modified by genetic variation in COMT in two large RCTs of vitamin E for cancer prevention, The Women’s Health Study (Figure 3) and the α-Tocopherol, β-Carotene Cancer Prevention Study [123]. Met/met homozygotes randomized to vitamin E had lower rates of all invasive cancers compared with placebo, while the opposite effect was observed for val/val homozygotes. Vitamin E had no effect among heterozygous participants in either treatment arm. Additionally, COMT effect modification of vitamin E as well as aspirin was observed in cardiovascular disease prevention in the WHS [52]. Interestingly, the original findings of the overall vitamin E and aspirin effects on cancer and cardiovascular disease in these RCTs were null [124–126]. Hence, accounting for COMT effects in post hoc analyses of these RCTs allowed for reinterpretation of the benefits and harms of this anti-oxidant supplement. In the Prostate Cancer Prevention Trial, an RCT of finasteride treatment for prostate cancer prevention, the COMT high-activity val-allele was associated with greater prostate cancer risk among men with high levels of estrone and cholesterol and lower levels of serum estradiol [102]. In the Prostate Cancer Prevention Trial, the val-allele was also associated with increased risk of prostate cancer among patients randomized to finasteride, a drug that inhibits dihydrotestosterone production. These findings suggest COMT’s effects on drug treatment response may also be influenced by its role in hormone metabolism.
Figure 3. Kaplan–Meier curves demonstrating differential effects of vitamin E versus placebo by COMT rs4680 genotype strata over the 10 years of the Women’s Health Study, a randomized placebo controlled clinical trial.
(A) met/met women; (B) val/met women; (C) val/val women.
Catechol-containing catechins found in teas are strong inhibitors of COMT activity in the liver [104]. In a population-based case–control study, the risk of breast cancer was significantly lower among tea drinkers who were met-allele carriers [105]. The flavonoids quercetin and fisetin can also modify COMT function by both competing with endogenous catechols and by the production of S-adenosylhomocysteine, a competitive COMT inhibitor generated with SAM demethylation (Figure 1) [104,127]. The combination of quercetin and green tea was found to enhance inhibition of prostate cancer xenograft tumor growth in one study [128]. In another study of vitamin C and quercetin as micronutrients consumed in the form of blueberry/apple juice, vitamin E levels increased but so did the level of adduct formation in met/met lymphocytes examined ex vivo [129]. In a recent comprehensive review of COMT and cancer, Sak also proposed that use of flavonoids through supplements or diet could further interact with COMT masking its effects on cancer [4]. Although these supplements are marketed as preventive for cancer and cardiovascular disease, there is evidence in animals that they can increase tumors and metastases [104].
COMT & placebo (inert treatments)
Before we begin our discussion of COMT and the placebo response, it is helpful to distinguish between ‘placebo response’ and ‘placebo effect’. A placebo response is defined as an improvement in target outcome detected in the placebo arm of RCTs. Placebo responses include spontaneous remission, normal fluctuations, and regression to the mean [130]. In preventive RCTs, they may reflect an inherent risk of disease. A placebo response can at times also include effects related to participation in the therapeutic encounter, characterized by verbal and nonverbal cues embedded in rituals, symbols, and behaviors [131–133]. These latter effects are placebo effects, and they rely on complex cognitive and neural processing mechanisms, including involvement with catecholaminergic and opioid signaling and activation of specific, quantifiable, and relevant regions of the brain, including the prefrontal cortex [134].
While great strides have been made in establishing the veracity and neurobiological basis of the placebo effect, the field is challenged with many methodological hurdles [135]. In order to separate spontaneous improvement from genuine placebo effects in pharmaceutical and procedural RCTs, an additional no-treatment control (NTC) arm is required to capture and control for regression to the mean, and the natural history of the disease, in studies of the placebo response. Given that these distinctions are not viewed to be directly relevant to drug discovery, an NTC arm is rarely included in RCTs; this limits discussion of placebo effects based on routine RCTs. In the last decade, however, there have been a significant number of RCTs that deliberately included NTC arms demonstrating that there is, indeed, a genuine, statistically meaningful and clinically relevant placebo effect embedded in the placebo arms of RCTs for multiple diseases [136–139]. Placebo research conducted in a laboratory setting (often on healthy volunteers), is more likely to utilize a NTC arm to measure accurately the placebo effect, but the results are often not generalizable to RCTs or the clinical setting. A further limitation of gaining information for placebo (and drug) effects in typical routine RCTs, is that at times placebo effects can be different in the drug and placebo arm of an RCT; that is, the drug and placebo responses are not directionally additive [130,140–143]. Genetic interactions in disease, drug and placebo effects may play a significant role in this phenomenon. Given the overlap between catecholaminergic and opioid signaling and placebo response pathways, COMT is a strong candidate among a growing number of ‘placebome’ genes/proteins implicated in modifying the placebo response [144–147]. It is, therefore, important to account for main and interaction effects of genes like COMT in both the drug and placebo treatment arms of RCTs.
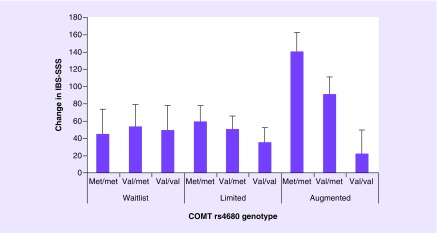
As with drug response, the directionality of COMT associations with outcomes in placebo control arms of RCTs are variable (Table 4). The most consistent associations of COMT with placebo responses are in pain-related studies. In these studies, the COMT rs4680 low-activity met-allele alone or with opioid receptor polymorphisms was associated with placebo analgesia and with placebo-induced fear of pain [148]. Similarly, in an RCT of placebo response in irritable bowel syndrome (IBS), a functional pain syndrome, the strongest effects were observed among met-allele homozygotes in response to placebo treatment augmented with a purposefully positive clinical interaction, suggesting that variability in placebo response may also be influenced by the nature of the placebo treatment (Figure 4) [149].
Table 4. COMT and placebo response.
| Condition | Study design, N | Outcome | Results | Ref. |
|---|---|---|---|---|
| Psychiatric studies | ||||
| Working memory | RCT of tolcapone in healthy normal volunteers N = 68 | N-back | In the placebo group, met-allele homozygotes outperformed val-allele homozygotes on tests of memory and were more risk averse. Tolcapone reversed these effects | [150] |
| MDD | RCT in MDD patients, N = 132 | HAM-D17 and MADRS | Met-alleles showed a statistical trend toward a lower magnitude of placebo response. But in a recent GWAS of a placebo controlled RCT of duloxetine for MDD, COMT association did not reach genome-wide significance | [144,151] |
| Pain and neurological studies | ||||
| Pain | Experimental pain in healthy normal volunteers. Repeated measures, N = 296 | VAS | The COMT met allele plus OPRM1 rs1799971 Asn/Asn associated with placebo analgesic response | [152] |
| Pain | Experimental Pain in healthy normal volunteers. N = 223 | VAS and FOP | Met allele was associated with increased fear of pain but not placebo analgesia | [148] |
| Pain | Experimental Pain in healthy normal volunteers, N = 48 | fMRI measures | Met alleles were linearly associated with stronger experimental placebo analgesia | [153] |
| IBS | RCT in IBS patients with NTC, N = 104 | IBSSS | Met/met had greater placebo response in placebo arm augmented with positive clinical engagement | [149] |
| Cardiometabolic disease studies | ||||
| CVD | RCT in healthy normal women, N = 104 | CVD events | Val allele associated with lower rates of CVD in placebo treatment arm | [52] |
| Energy expenditure and fat oxidation | Green tea (capsules) versus placebo in randomized cross-over study among healthy normals, N = 14 | Energy expenditure and fat oxidation | Differential COMT genetic green tea effects were observed with higher energy expenditure and fat oxidation among met/met participants with placebo compared with val/val participants | [106] |
| Other conditions | ||||
| Inflammatory disease | Meta-analysis of RCTs in asthma and other inflammatory disease | Quality of Life (QOL) | No effect was observed for COMT | [154] |
| Nocebo effects in immunosuppression | RCT of immunosuppression | General side effects | The val-allele was found to be positively associated the perception and reporting of side effects in a study of placebo in immunosuppression | [155] |
Asn/Asn: Asparagine polymorphism in OPRM1; CVD: Cardiovascular disease; FOP: Fear of pain; HAM-D17: Hamilton Depression Rating Scale score; IBS: Irritable bowel syndrome; IBSSS: IBS symptom severity score; MADRS: Montgomery-Asberg and Depression Rating Scales; MDD: Major depressive disorder; met: Methionine; NTC: No-treatment control; RCT: Randomized clinical trial; rs-fMRI: Resting-state functional MRI; val: Valine; VAS: Visual analog pain scale.
Figure 4. Interaction effect of catechol-O-methyltransferase genotype and treatment arm on change in IBS-SSS.
The interaction between COMT genotype (number of met alleles) and treatment arm was statistically significant (β = 0.17; p = 0.035). Regression model included the following parameters: COMT genotype (number of met alleles), treatment arm, baseline IBS-SSS and their interaction (COMT genotype x treatment arm). Error bars indicate the standard error of the mean. n = 104.
IBS-SSS: Irritable Bowel syndrome – Symptom Severity Scale.
Reproduced with permission from [149], under the terms of under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/
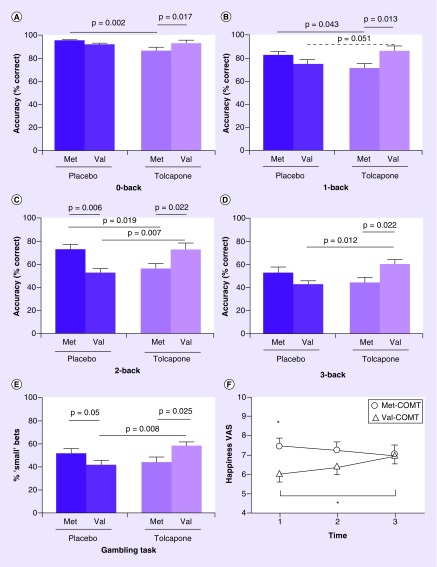
There are several studies in which significant COMT association with outcomes in the placebo arm is directionally reversed in the drug treatment arm. For example, in an RCT of tolcapone and working memory and behavioral effects, the higher response observed among met/met participants in the placebo arm was reduced with tolcapone treatment and vice versa (Figure 5). In an RCT of CFS, where higher levels of catecholamines were associated with disease [53,74], the positive response in the placebo arm among val-allele homozygotes was abrogated with randomized clonidine treatment [89]. The potential for differential responses in the placebo and drug treatment arm, underscore the importance of including and analysing genetic effects in both active and control arms in clinical trials. This problem is evident in several single-arm trials of MDD, see Table 3. Still there is considerable variability in trials that did include placebo controls. For example, whereas in one trial there was a trend for higher placebo response with the val-allele [151], in a genome-wide association study from an RCT for duloxetine versus placebo in MDD no genome-wide significant effects of COMT were reported in either arm [144].
Figure 5. Interactive behavioral effects of catechol-O-methyltransferase genotype and catechol-O-methyltransferase inhibition.
(A) 0-back: main effect of drug (F[1,56] = 4.14; p = 0.047) and genotype × drug interaction (F[1,56] = 6.75; p = 0.012). Met-COMT subjects given tolcapone perform worse than those given placebo. (B) 1-back: genotype × drug interaction (F[1,56] = 9.26; p = 0.006). Tolcapone impairs performance in Met-COMT subjects and, as a trend, improves it in Val-COMT subjects, compared with their respective placebo groups. (C) 2-back: genotype × drug interaction (F[1,56] = 13.62; p = 0.001). Met-COMT subjects perform better than Val-COMT subjects on placebo. Tolcapone reverses this difference, impairing Met-COMT subjects and enhancing Val-COMT subjects, compared with their respective placebo groups. (D) 3-back. Genotype × drug interaction (F[1,56] = 8.03; p = 0.006). Val-COMT subjects given tolcapone perform better than those given placebo. (E) Gambling task, showing percentage of times when 5 not 25 was gambled. Genotype × drug interaction (F[1,61] = 7.91; p = 0.007). On placebo, Met-COMT subjects are more likely than Val-COMT subjects to make a small rather than a large bet. Tolcapone reverses this difference, making Val-COMT subjects significantly more risk averse, compared with those given placebo. (F) Happiness VAS ratings for tolcapone-treated subjects. Data are adjusted means with standard errors. Time 1: immediately before drug administration. Time 2: T1 + 90 min. 3: T1 + 210 min.
*p = 0.026.
VAS: visual analogue scale.
Reproduced with permission from [150] under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/
These differential placebo and drug treatment arm effects are not limited to subjective outcomes. Higher energy expenditure and fat oxidation were observed among met/met participants randomized to placebo in an RCT of the cardiometabolic effects of green tea [106]. Differential COMT effects were also observed in the green tea treatment arm. In a large RCT of cardiovascular disease prevention, the high-activity val-allele was associated with better outcomes with randomized placebo treatment, but increased risk of cardiovascular disease with randomization to aspirin or vitamin E [52]. Although a meta-analysis of RCTs in inflammatory disease found no association of COMT with placebo response [154], the val-allele was found to be positively associated the perception and reporting of adverse effects in a study of placebo in immunosuppression [155].
One hypothesis for the differential drug and placebo responses in some of these trials is that the drug is perturbing the placebo response/placebo effect. That drugs can disrupt the placebo response is not a novel concept. Early studies on placebo effects (that included NTCs) in molar tooth extractions demonstrated that naloxone could block a robust placebo effect [156,157]. A series of interesting studies followed which used drugs that targeted neurotransmission [158,159], and one even earlier study which included aspirin [160], provided evidence of different placebo effect pathways. Although the findings from these studies laid the groundwork for our understanding of the biochemical pathways induced by placebo treatment and led to the assertion that there are multiple placebo response pathways, little is known about the influence of genetic variation in COMT and other genes on these effects.
Although COMT association with outcomes among individuals treated with placebo seem to vary by health status of the patient (healthy normal versus patient), condition (pain, IBS, anxiety), and study design (RCT versus laboratory experiment), there are enough statistically significant findings to warrant consideration and further investigation. A deeper understanding of potential, different placebo effects in the placebo arm of RCTs may help elucidate unexpected absences or variability in detecting drug–placebo differences that is not uncommon to find in drug development.
COMT is a member of the placebome, a network of proteins implicated in the placebo response [161]. Mapping the placebome to the larger network of human protein–protein interactions, the interactome, indicated that the placebome was also enriched for proteins implicated in disease and targeted by a wide cross-section of drugs, for example, analgesics, antidepressive agents, antioxidants, and adrenergic agonists [146]. Hence, there are likely to be several other genes/proteins involved in the drug–disease–placebo axis, and the understanding of how genetics can perturb potential drug–placebo interactions will require much additional research.
Conclusion
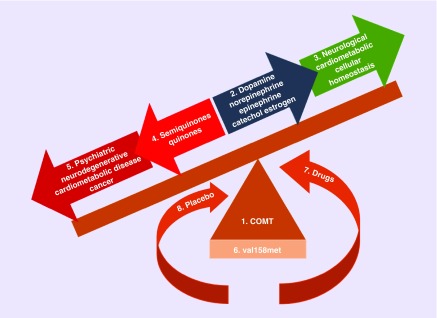
Genetic variation in COMT can have main and combinatorial effects on disease, drug response, and placebo effects/response, and thereby influence outcomes in RCTs. Hence, COMT is an example of a pharmacogenomic hub that has not previously been synthesized into a systems regulatory model as a means to integrate the evidence (Figure 6). This is a first attempt to examine this hypothesis using COMT as a model. COMT effects, as reviewed here, are many and varied in direction and magnitude, and may sometimes (or frequently) be masked by the many interacting and overlapping pathways within which it functions.
Figure 6. The COMT dynamic disease-placebo-pharmacogenomic hub.
1. COMT metabolizes catecholamine substrates. 2. COMT substrates include the catecholamine neurotransmitters and hormones – dopamine, norepinephrine, epinephrine and catechol estrogen. 3. COMT substrates are important in maintaining neurological, cardiometabolic and cellular homeostasis. 4. When COMT enzymatic activity is perturbed, the catecholamines form chemically reactive semiquinones and quinones that can damage DNA and lipids. 5. Perturbation in the COMT gene is associated with psychiatric disorders, neurodegenerative disease, cardiometabolic diseases and cancer. 6. COMT can be perturbed by genetic variation at loci like rs4680 (val158met). 7. Drugs can also modify COMT activity. 8. Genetic variation in COMT can also modify placebo effects/response.
This potential for genetic variation to modify disease as well as drug and placebo effects/response differentially poses a challenge to the RCT, a cornerstone of drug development. The RCT was designed to determine the efficacy of novel pharmaceutical treatments above any placebo response. However, the current approach to analyzing efficacy does not account for differences in disease progression or susceptibility and treatment or placebo response as a function of genetic variation. This limitation, in part, stems from the fact that we do not have many strong candidate genes known to perturb clinical outcomes predictably. Further, for the handful of candidate genes we do have, the mechanisms and sources of variability in treatment response are not fully understood. Given the potential for system-wide effects, we propose that pharmacogenomics broaden its scope to include investigation of genes like COMT that might not only influence drug response, but disease and placebo responses, as well.
No doubt, physicians increasingly bombarded by questions derived from personal genotyping are frustrated by how clinical utility of pharmacogenomics has lagged behind the sharp rise in online accessibility to genotyping data. Although, this review raises several interesting and potential points of intervention, given the considerable variability in outcomes, it is still too soon for clinical recommendations based on COMT genotype. Still, with the recent increase in big clinical data and developments in systems biology, network medicine, and machine learning/artificial intelligence, the promise of precision medicine to improve medicine has been revitalized and we anticipate, that considerable progress can now be made to actualize this area of research.
Our goal here has been to provide the reader with a sense of the network of influence a potential pharmacogenomic mediator like COMT can have. To simplify our exploration of this network, we focused on just one locus in COMT. With almost 5000 entries in PubMed, this review is by no means an exhaustive exploration of the associations between COMT and disease, drugs, or placebos. It is a reasonable attempt at a comprehensive review. Furthermore, there are likely many more genes like COMT that affect disease, as well as drug and placebo responses, differently. Owing to this complexity, systems pharmacogenomics approaches [162] will be needed to harness the potential to perturb these networks for clinical benefit and to optimize our ability to discern the efficacy and safety of drugs being tested.
Executive summary.
Catechol-O-methyltransferase in form & function
COMT endogenous substrates include catechol estrogen a key metabolite in estrogen metabolism and the catecholamines dopamine, epinephrine and norepinephrine.
In the presence of magnesium ions, COMT transfers a methyl group from S-adenosylmethionine (SAM) to a hydroxyl group on the catechol ring of endogenous and xenobiotic catechol substrates. SAM is then converted to S-adenosylhomocysteine (SAH) a competitive inhibitor of COMT.
The most widely studied polymorphism in COMT is rs4680 (val158met). It encodes a valine to methionine change that results in a 3-4-fold reduction in COMT enzymatic activity.
COMT & disease
COMT influences diseases in at least four domains – psychiatric, neurological, and cardiometabolic diseases and cancer.
COMT effects in psychiatric (ADD and bipolar disorder) and neurological (pain) diseases likely result from perturbations in dopamine and norepinephrine signalling in the brain.
COMT effects in cardiometabolic (preeclampsia) and cancer likely result from perturbations in catechol estrogen and epinephrine.
COMT, drugs & supplements
Genetic variation in COMT can modify thye effects of both active and pharmacologically inactive (placebo) treatments.
COMT is modified by drugs and supplements containing a catechol ring. COMT effects can also be modified by drugs and supplements that modify the action or availability of COMT enymatic substrates.
Genetic variation in COMT may differentially influence placebo and drug treatment responses and thus mask drug efficacy in clinical trials.
Acknowledgements
The authors thank Kenneth Mukamal and Daniel Chasman for insightful discussions.
Footnotes
Financial & competing interests disclosure
The authors have no competing interests to declare. KH is funded by NIH grant # 1K01HL130625 and TJK is funded by # NIH/NCCIH grant R01AT008573 and grant #R33AT009306. JL is supported by AHA grant D700382 and NIH grants HG006790, HL061795, HL119145 and GM107618. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Axelrod J, Senoh S, Witkop B. O-methylation of catechol amines in vivo. J. Biol. Chem. 233(3), 697–701 (1958). [PubMed] [Google Scholar]
- 2.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J. Biol. Chem. 233(3), 702–705 (1958). [PubMed] [Google Scholar]
- 3.Chiueh CC, Wu RM, Mohanakumar KP. et al. In vivo generation of hydroxyl radicals and MPTP-induced dopaminergic toxicity in the basal ganglia. Ann. NY Acad. Sci. 738, 25–36 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Sak K. The val158met polymorphism in COMT gene and cancer risk: role of endogenous and exogenous catechols. Drug Metab. Rev. 49(1), 56–83 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Zhu BT. On the mechanism of homocysteine pathophysiology and pathogenesis: a unifying hypothesis. Histol. Histopathol. 17(4), 1283–1291 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Kanasaki K, Palmsten K, Sugimoto H. et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453(7198), 1117–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Fagerberg L, Hallstrom BM, Oksvold P. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 13(2), 397–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunbridge EM, Harrison PJ. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Curr. Top. Behav. Neurosci. 8, 119–140 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Yager JD. Catechol-O-methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug Discov. Today Dis. Mech. 9(1–2), e41–e46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol. Rev. 27(2), 135–206 (1975). [PubMed] [Google Scholar]
- 11.Lambert MP, Arulselvan A, Schott A. et al. The 22q11.2 deletion syndrome: cancer predisposition, platelet abnormalities and cytopenias. Am. J. Med. Genet. A 176( 10), 2121– 2127 ( 2017). [DOI] [PubMed] [Google Scholar]
- 12.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6(3), 243–250 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Lotta T, Vidgren J, Tilgmann C. et al. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34(13), 4202–4210 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Lek M, Karczewski KJ, Minikel EV. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nackley AG, Shabalina SA, Lambert JE. et al. Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: enrichment for marker SNPs. PLoS ONE 4(4), e5237 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nackley AG, Shabalina SA, Tchivileva IE. et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 314(5807), 1930–1933 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Zumarraga M, Arrue A, Basterreche N. et al. COMT haplotypes, catecholamine metabolites in plasma and clinical response in schizophrenic and bipolar patients. Pharmacogenomics 17(8), 837–851 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Kaenmaki M, Tammimaki A, Myohanen T. et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J. Neurochem. 114(6), 1745–1755 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase val158met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol. Psychiatry 12(5), 502–509 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Soeiro-de-Souza MG, Machado-Vieira R, Soares Bio D, Do Prado CM, Moreno RA. COMT polymorphisms as predictors of cognitive dysfunction during manic and mixed episodes in bipolar I disorder. Bipolar Disord. 14(5), 554–564 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev. Neurosci. 17(3), 359–367 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Egan MF, Goldberg TE, Kolachana BS. et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl Acad. Sci. USA 98(12), 6917–6922 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risbrough V, Ji B, Hauger R, Zhou X. Generation and characterization of humanized mice carrying COMT 158 Met/Val alleles. Neuropsychopharmacology 39(8), 1823–1832 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiocco AJ, Lindquist K, Ferrell R. et al. COMT genotype and cognitive function: an 8-year longitudinal study in white and black elders. Neurology 74(16), 1296–1302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutleb DR, Roos C, Noll A, Ostner J, Schulke O. COMT Val(158) Met moderates the link between rank and aggression in a non-human primate. Genes Brain Behav. 17(4), e12443 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Montirosso R, Provenzi L, Tavian D, Missaglia S, Raggi ME, Borgatti R. COMTval158met polymorphism is associated with behavioral response and physiologic reactivity to socio-emotional stress in 4-month-old infants. Infant Behav. Dev. 45(Pt A), 71–82 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Crum AJ, Akinola M, Turnwald BP, Kaptchuk TJ, Hall KT. Catechol-O-methyltransferase moderates effect of stress mindset on affect and cognition. PLoS ONE 13(4), e0195883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase val158met polymorphism with the personality trait of extraversion. Neuroreport 16(10), 1135–1138 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto R, Noguchi H, Hori H, Ohi K, Yasuda Y, Takeda M. et al. A possible association between the val158met polymorphism of the catechol-O-methyl transferase gene and the personality trait of harm avoidance in Japanese healthy subjects. Neurosci. Lett. 428(1), 17–20 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Taylor S. Association between COMT val158met and psychiatric disorders: a comprehensive meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177(2), 199–210 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Castro TB, Hernandez-Diaz Y, Juarez-Rojop IE. et al. The role of COMT gene Val108/158Met polymorphism in suicidal behavior: systematic review and updated meta-analysis. Neuropsychiatr. Dis. Treat. 14, 2485–2496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 1(2), 109–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein M, Schmoeger M, Kasper S, Schosser A. Meta-analysis of the COMT val158met polymorphism in major depressive disorder: the role of gender. World J. Biol. Psychiatry 17(2), 147–158 (2016). [DOI] [PubMed] [Google Scholar]
- 34.van Munster BC, Baas F, Tanck MW, de Rooij SE. Polymorphisms in the catechol-O-methyltransferase gene and delirium in the elderly. Dement. Geriatr. Cogn. Disord. 31(5), 358–362 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Vasunilashorn SM, Ngo LH, Jones RN. et al. The association between c-reactive protein and postoperative delirium differs by catechol-O-methyltransferase genotype. Am. J. Geriatr. Psychiatry 27(1), 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YH, Kim JH, Song GG. Association between the COMT val158met polymorphism and fibromyalgia susceptibility and fibromyalgia impact questionnaire score: a meta-analysis. Rheumatol. Int. 35(1), 159–166 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet. Genomics 22(9), 673–691 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Goertzel BN, Pennachin C, de Souza Coelho L, Gurbaxani B, Maloney EM, Jones JF. Combinations of single nucleotide polymorphisms in neuroendocrine effector and receptor genes predict chronic fatigue syndrome. Pharmacogenomics 7(3), 475–483 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Sommerfeldt L, Portilla H, Jacobsen L, Gjerstad J, Wyller VB. Polymorphisms of adrenergic cardiovascular control genes are associated with adolescent chronic fatigue syndrome. Acta Paediatrica 100(2), 293–298 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I: dichotomous effects of COMT in neuropathic vs nociceptive pain modalities. CNS Neurol. Disord. Drug Targets 11(3), 222–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-de-las-Penas C, Fernandez-Lao C, Cantarero-Villanueva I. et al. Catechol-O-methyltransferase genotype (val158met) modulates cancer-related fatigue and pain sensitivity in breast cancer survivors. Breast Cancer Res. Treat. 133(2), 405–412 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Muller T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs 75(2), 157–174 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Foltynie T, Goldberg TE, Lewis SG. et al. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov. Disord. 19(8), 885–891 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Klebe S, Golmard JL, Nalls MA. et al. The val158met COMT polymorphism is a modifier of the age at onset in Parkinson’s disease with a sexual dimorphism. J. Neurol. Neurosurg. Psychiatry 84(6), 666–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan W, Zhao C, Sun L, Tang B. Association between polymorphism of COMT gene (val158met) with Alzheimer’s disease: an updated analysis. J. Neurol. Sci. 361, 250–255 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Finan PH, Zautra AJ. Rheumatoid arthritis: stress affects rheumatoid arthritis, but via what mechanisms? Nat. Rev. Rheumatol. 9(10), 569–570 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naushad SM, Vijayalakshmi SV, Rupasree Y. et al. Multifactor dimensionality reduction analysis to elucidate the cross-talk between one-carbon and xenobiotic metabolic pathways in multi-disease models. Mol. Biol. Rep. 42(7), 1211–1224 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism 57(5), 708–711 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Kanasaki M, Srivastava SP, Yang F. et al. Deficiency in catechol-o-methyltransferase is linked to a disruption of glucose homeostasis in mice. Sci. Rep. 7(1), 7927 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall KT, Jablonski KA, Chen L. et al. Catechol-O-methyltransferase association with hemoglobin A1c. Metabolism 65(7), 961–967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kring SI, Werge T, Holst C. et al. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and Type 2 diabetes. PLoS ONE 4(8), e6696 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall KT, Nelson CP, Davis RB. et al. Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 34(9), 2160–2167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voutilainen S, Tuomainen TP, Korhonen M. et al. Functional COMT val158met polymorphism, risk of acute coronary events and serum homocysteine: the Kuopio ischaemic heart disease risk factor study. PLoS ONE 2(1), e181 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall KT, Buring J, Mukamal K. et al. COMT and alpha-tocopherol effects in cancer prevention: gene-supplement interactions in two randomized clinical trials. JNCI (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukiw WJ, Rogaev EI. Genetics of aggression in Alzheimer’s disease (AD). Front. Aging Neurosci. 9, 87 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 68, 651–668 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armbruster D, Mueller A, Strobel A, Lesch KP, Brocke B, Kirschbaum C. Children under stress – COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. Int. J. Neuropsychopharmacol. 15(9), 1229–1239 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Lovallo WR, Enoch MA, Sorocco KH. et al. Joint impact of early life adversity and COMT val158met (rs4680) genotypes on the adult cortisol response to psychological stress. Psychosom. Med. 79(6), 631–637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montag C, Jurkiewicz M, Reuter M. The role of the catechol-O-methyltransferase (COMT) gene in personality and related psychopathological disorders. CNS Neurol. Disord. Drug Targets 11(3), 236–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papaleo F, Crawley JN, Song J. et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J. Neurosci. 28(35), 8709–8723 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell JM, O’Neil JP, Jagust WJ, Fields HL. Catechol-O-methyltransferase genotype modulates opioid release in decision circuitry. Clin. Transl. Sci. 6(5), 400–403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zubieta JK, Heitzeg MM, Smith YR. et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299(5610), 1240–1243 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Ciszek BP, O’Buckley SC, Nackley AG. Persistent catechol-O-methyltransferase-dependent pain is initiated by peripheral beta-adrenergic receptors. Anesthesiology 124(5), 1122–1135 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JA. COMT gene and risk for Parkinson’s disease: a systematic review and meta-analysis. Pharmacogenet. Genomics 24(7), 331–339 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Williams-Gray CH, Evans JR, Goris A. et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 132(Pt 11), 2958–2969 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Nombela C, Rowe JB, Winder-Rhodes SE. et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 137(Pt 10), 2743–2758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magina S, Moura E, Serrao MP, Moura D, Vieira-Coelho MA. Catechol-O-methyltransferase activity is higher in psoriasis patients and is down-regulated by narrowband ultraviolet B treatment. Eur. J. Dermatol. 23(1), 49–52 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Wieczorek S, Jagiello P, Arning L, Dahmen N, Epplen JT. Screening for candidate gene regions in narcolepsy using a microsatellite based approach and pooled DNA. J. Mol. Med. (Berl). 82(10), 696–705 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Rupasree Y, Naushad SM, Varshaa R. et al. Application of various statistical models to explore gene–gene interactions in folate, xenobiotic, toll-like receptor and STAT4 pathways that modulate susceptibility to systemic lupus erythematosus. Mol. Diagn. Ther. 20(1), 83–95 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Liu C, Kraja AT, Smith JA. et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet. 48(10), 1162–1170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-O-methyl transferase gene val158met polymorphism with blood pressure and prevalence of hypertension: interaction with dietary energy intake. Am. J. Hypertens 24(9), 1022–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Xiu L, Lin M, Liu W. et al. Association of DRD3, COMT, and SLC6A4 gene polymorphisms with Type 2 diabetes in Southern Chinese: a hospital-based case–control study. Diabetes Technol. Ther. 17(8), 580–586 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Mir R, Bhat M, Javid J, Jha C, Saxena A, Banu S. Potential impact of COMT-rs4680 G > a gene polymorphism in coronary artery disease. J.Cardiovas. Develop. Dis. 5(3), pii:E38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almas A, Forsell Y, Millischer V, Moller J, Lavebratt C. Association of catechol-O-methyltransferase (COMT val158met) with future risk of cardiovascular disease in depressed individuals: a Swedish population-based cohort study. BMC Medical Genet. 19(1), 126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 69(9), 3727–3730 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basu S, Nagy JA, Pal S. et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 7(5), 569–574 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Yager JD. Mechanisms of estrogen carcinogenesis: the role of E2/E1-quinone metabolites suggests new approaches to preventive intervention: a review. Steroids 99(Pt A), 56–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu W, Wu Q, Hong X. et al. Catechol-O-methyltransferase, a new target for pancreatic cancer therapy. Cancer Sci. 106(5), 576–583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu W, Wu Q, Hong X. et al. Catechol-O-methyltransferase inhibits colorectal cancer cell proliferation and invasion. Arch. Med. Res. 46(1), 17–23 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Yang EV. Role for catecholamines in tumor progression: possible use for beta-blockers in the treatment of cancer. Cancer Biol. Ther. 10(1), 30–32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J. 16(5), 430–438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafo MR. Association of COMT val108/158met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol. Biomark. Prev. 16(6), 1065–1069 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berrettini WH, Wileyto EP, Epstein L. et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol. Psychiatry 61(1), 111–118 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Yoshida K, Higuchi H, Takahashi H. et al. Influence of the tyrosine hydroxylase val81met polymorphism and catechol-O-methyltransferase val158met polymorphism on the antidepressant effect of milnacipran. Hum. Psychopharmacol. 23(2), 121–128 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Domschke K, Zavorotnyy M, Diemer J. et al. COMT val158met influence on electroconvulsive therapy response in major depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B(1), 286–290 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Narasimhan S, Aquino TD, Multani PK, Rickels K, Lohoff FW. Variation in the catechol-O-methyltransferase (COMT) gene and treatment response to venlafaxine XR in generalized anxiety disorder. Psychiatry Res. 198(1), 112–115 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Tunbridge EM, Attenburrow MJ, Gardiner A. et al. Biochemical and genetic predictors and correlates of response to lamotrigine and folic acid in bipolar depression: analysis of the CEQUEL clinical trial. Bipolar Disord 19(6), 477–486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang E, Zai CC, Lisoway A. et al. Catechol-O-methyltransferase val158met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizo-affective disorder patients: a meta-analysis. Int. J. Neuropsychopharmacol. 19(5), pii:pyv132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall KT, Kossowsky J, Oberlander TF. et al. Genetic variation in catechol-O-methyltransferase modifies effects of clonidine treatment in chronic fatigue syndrome. Pharmacogenomics J. 16(5), 454–460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henquet C, Rosa A, Krabbendam L. et al. An experimental study of catechol-o-methyltransferase val158met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31(12), 2748–2757 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Tunbridge EM, Dunn G, Murray RM. et al. Genetic moderation of the effects of cannabis: catechol-O-methyltransferase (COMT) affects the impact of Delta9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. J. Psychopharmacol. 29(11), 1146–1151 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Reilly DK, Hershey L, Rivera-Calimlim L, Shoulson I. On-off effects in Parkinson’s disease: a controlled investigation of ascorbic acid therapy. Adv. Neurol. 37, 51–60 (1983). [PubMed] [Google Scholar]
- 93.Shin SY, Fauman EB, Petersen AK. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46(6), 543–550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nissinen E, Mannisto PT. Biochemistry and pharmacology of catechol-O-methyltransferase inhibitors. Int. Rev. Neurobiol. 95, 73–118 (2010). [DOI] [PubMed] [Google Scholar]
- 95.ClinicalTrials.gov Sleep Disorders and Gastroesophageal Reflux Disease (GERD) (2007). http://clinicaltrials.gov/ct/show/NCT00287391?order=00287391
- 96.Zhang F, Tong J, Hu J. et al. COMT gene haplotypes are closely associated with postoperative fentanyl dose in patients. Anesth. Analg. 120(4), 933–940 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Rakvag TT, Klepstad P, Baar C. et al. The val158met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain 116(1–2), 73–78 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A. The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine. Clin. Pharmacol. Ther. 58(2), 221–227 (1995). [DOI] [PubMed] [Google Scholar]
- 99.Bozek T, Blazekovic A, Perkovic MN. et al. The influence of dopamine-beta-hydroxylase and catechol O-methyltransferase gene polymorphism on the efficacy of insulin detemir therapy in patients with Type 2 diabetes mellitus. Diabetol. Metab. Syndr. 9, 97 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barnea ER, Fakih H, Oelsner G, Walner S, DeCherney AH, Naftolin F. Effect of antihypertensive drugs on catechol-O-methyltransferase and monoamine oxidase activity in human term placental explants. Gynecol. Obstet. Invest. 21(3), 124–130 (1986). [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Bostrom AE, Saeed M, Dubey RK, Waeber G, Vollenweider P. et al. A genetic variant in the catechol-O-methyl transferase (COMT) gene is related to age-dependent differences in the therapeutic effect of calcium-channel blockers. Medicine 96(30), e7029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang L, Platek ME, Yao S, Till C, Goodman PJ, Tangen CM. et al. Associations between polymorphisms in genes related to estrogen metabolism and function and prostate cancer risk: results from the prostate cancer prevention trial. Carcinogenesis 39(2), 125–133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hall KT. . COMT effects on vitamin E in cancer prevention: gene-supplement interactions in two randomized clinical trials. JNCI (2018) (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 32(5), 497–504 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian–American women. Cancer Res. 63(21), 7526–7529 (2003). [PubMed] [Google Scholar]
- 106.Hursel R, Janssens PL, Bouwman FG, Mariman EC, Westerterp-Plantenga MS. The role of catechol-O-methyl transferase val(108/158)met polymorphism (rs4680) in the effect of green tea on resting energy expenditure and fat oxidation: a pilot study. PLoS ONE 9(9), e106220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsai SJ, Gau YT, Hong CJ, Liou YJ, Yu YW, Chen TJ. Sexually dimorphic effect of catechol-O-methyltransferase val158met polymorphism on clinical response to fluoxetine in major depressive patients. J. Affect. Disord. 113(1–2), 183–187 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Benedetti F, Colombo C, Pirovano A, Marino E, Smeraldi E. The catechol-O-methyltransferase val(108/158)met polymorphism affects antidepressant response to paroxetine in a naturalistic setting. Psychopharmacology 203(1), 155–160 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Benedetti F, Dallaspezia S, Colombo C, Lorenzi C, Pirovano A, Smeraldi E. Effect of catechol-O-methyltransferase val(108/158)met polymorphism on antidepressant efficacy of fluvoxamine. Eur. Psychiatry 25(8), 476–478 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Szegedi A, Rujescu D, Tadic A. et al. The catechol-O-methyltransferase Val108/158Met polymorphism affects short-term treatment response to mirtazapine, but not to paroxetine in major depression. Pharmacogenomics J. 5(1), 49–53 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Hopkins SC, Reasner DS, Koblan KS. Catechol-O-methyltransferase genotype as modifier of superior responses to venlafaxine treatment in major depressive disorder. Psychiatry Res. 208(3), 285–287 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Atake K, Yoshimura R, Hori H, Katsuki A, Nakamura J. Catechol-O-methyltransferase val158met genotype and the clinical responses to duloxetine treatment or plasma levels of 3-methoxy-4-hydroxyphenylglycol and homovanillic acid in Japanese patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 11, 967–974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta M, Bhatnagar P, Grover S. et al. Association studies of catechol-O-methyltransferase (COMT) gene with schizophrenia and response to antipsychotic treatment. Pharmacogenomics 10(3), 385–397 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Jornil J, Jensen KG, Larsen F, Linnet K. Identification of cytochrome P450 isoforms involved in the metabolism of paroxetine and estimation of their importance for human paroxetine metabolism using a population-based simulator. Drug Metab. Dispos. 38(3), 376–385 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Kern C, Bernards CM. Ascorbic acid inhibits spinal meningeal catechol-O-methyl transferase in vitro, markedly increasing epinephrine bioavailability. Anesthesiology 86(2), 405–409 (1997). [DOI] [PubMed] [Google Scholar]
- 116.Kaakkola S, Gordin A, Mannisto PT. General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase. Gen. Pharmacol. 25(5), 813–824 (1994). [DOI] [PubMed] [Google Scholar]
- 117.Magalona SC, Rasetti R, Chen J. et al. Effect of tolcapone on brain activity during a variable attentional control task: a double-blind, placebo-controlled, counter-balanced trial in healthy volunteers. CNS Drugs 27(8), 663–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs 21(7), 535–557 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Maser T, Rich M, Hayes D. et al. Tolcapone induces oxidative stress leading to apoptosis and inhibition of tumor growth in neuroblastoma. Cancer Med. 6(6), 1341–1352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Gregori M, Garbin G, De Gregori S. et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur. J. Clin. Pharmacol. 69(9), 1651–1658 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic acid in cancer treatment: let the phoenix fly. Cancer Cell 34(5), 700–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niki E. Interaction of ascorbate and alpha-tocopherol. Ann. NY Acad. Sci. 498, 186–199 (1987). [DOI] [PubMed] [Google Scholar]
- 123.Hall KT, Buring JE, Mukamal KJ. et al. COMT and alpha-tocopherol effects in cancer prevention: gene-supplement interactions in two randomized clinical trials. J. Natl Cancer Inst. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alpha-Tocopherol BCCPSG. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330(15), 1029–1035 (1994). [DOI] [PubMed] [Google Scholar]
- 125.Ridker PM, Cook NR, Lee IM. et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 352(13), 1293–1304 (2005). [DOI] [PubMed] [Google Scholar]
- 126.Lee IM, Cook NR, Gaziano JM. et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA 294(1), 56–65 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Zhu BT, Ezell EL, Liehr JG. Catechol-O-methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids. Metabolic inactivation as a possible reason for their lack of carcinogenicity in vivo. J. Biol. Chem. 269(1), 292–299 (1994). [PubMed] [Google Scholar]
- 128.Wang P, Vadgama JV, Said JW. et al. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 25(1), 73–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilms LC, Boots AW, de Boer VC. et al. Impact of multiple genetic polymorphisms on effects of a 4-week blueberry juice intervention on ex vivo induced lymphocytic DNA damage in human volunteers. Carcinogenesis 28(8), 1800–1806 (2007). [DOI] [PubMed] [Google Scholar]
- 130.Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet 351(9117), 1722–1725 (1998). [DOI] [PubMed] [Google Scholar]
- 131.Kaptchuk TJ, Miller FG. Placebo effects in medicine. N. Engl. J. Med. 373(1), 8–9 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Kaptchuk TJ, Miller FG. Open label placebo: can honestly prescribed placebos evoke meaningful therapeutic benefits? BMJ 363, k3889 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ongaro G, Kaptchuk TJ. Symptom perception, placebo effects, and the Bayesian brain. Pain (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci. 16(7), 403–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hrobjartsson A, Kaptchuk TJ, Miller FG. Placebo effect studies are susceptible to response bias and to other types of biases. J. Clin. Epidemiol. 64(11), 1223–1229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaptchuk TJ, Kelley JM, Conboy LA. et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336(7651), 999–1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kam-Hansen S, Jakubowski M, Kelley JM. et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci. Transl. Med. 6(218), 218ra5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain 157(12), 2766–2772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beard DJ, Rees JL, Cook JA. et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 391(10118), 329–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boehm K, Berger B, Weger U, Heusser P. Does the model of additive effect in placebo research still hold true? A narrative review. JRSM Open 8(3), 2054270416681434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kleijnen J, de Craen AJ, van Everdingen J, Krol L. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet 344(8933), 1347–1349 (1994). [DOI] [PubMed] [Google Scholar]
- 142.Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature 312(5996), 755–756 (1984). [DOI] [PubMed] [Google Scholar]
- 143.Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS ONE 9(1), e84104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maciukiewicz M, Marshe VS, Tiwari AK. et al. Genome-wide association studies of placebo and duloxetine response in major depressive disorder. Pharmacogenomics J. 18(3), 406–412 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, Colloca L. The placebo effect: from concepts to genes. Neuroscience 307, 171–190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang RS, Hall KT, Giulianini F, Passow D, Kaptchuk TJ, Loscalzo J. Network analysis of the genomic basis of the placebo effect. JCI Insight 2(11), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maciukiewicz M, Marshe VS, Tiwari AK. et al. Genetic variation in IL-1beta, IL-2, IL-6, TSPO and BDNF and response to duloxetine or placebo treatment in major depressive disorder. Pharmacogenomics 16(17), 1919–1929 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Forsberg JT, Gjerstad J, Flaten MA, Aslaksen PM. Influence of catechol-O-methyltransferase val158met on fear of pain and placebo analgesia. Pain 159(1), 168–174 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Hall KT, Lembo AJ, Kirsch I. et al. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS ONE 7(10), e48135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT val(158)met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol. Psychiatry 71(6), 538–544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leuchter AF, McCracken JT, Hunter AM, Cook IA, Alpert JE. Monoamine oxidase a and catechol-o-methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J. Clin. Psychopharmacol. 29(4), 372–377 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Aslaksen PM, Forsberg JT, Gjerstad J. The opioid receptor mu 1 (OPRM1) rs1799971 and catechol-O-methyltransferase (COMT) rs4680 as genetic markers for placebo analgesia. Pain 159( 12), 2585– 2592 ( 2018). [DOI] [PubMed] [Google Scholar]
- 153.Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Hum. Brain Mapp. 35(9), 4583–4593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haug-Baltzell A, Bhangale TR, Chang D. et al. Previously reported placebo-response-associated variants do not predict patient outcomes in inflammatory disease Phase III trial placebo arms. Genes Immun. 20( 2), 172 ( 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wendt L, Albring A, Benson S. et al. Catechol-O-methyltransferase Val158Met polymorphism is associated with somatosensory amplification and nocebo responses. PLoS ONE 9(9), e107665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 2(8091), 654–657 (1978). [DOI] [PubMed] [Google Scholar]
- 157.Grevert P, Albert LH, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain 16(2), 129–143 (1983). [DOI] [PubMed] [Google Scholar]
- 158.Benedetti F, Amanzio M, Casadio C, Oliaro A, Maggi G. Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain 71(2), 135–140 (1997). [DOI] [PubMed] [Google Scholar]
- 159.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64(3), 535–543 (1996). [DOI] [PubMed] [Google Scholar]
- 160.Dinnerstein AJ, Halm J. Modification of placebo effects by means of drugs: effects of aspirin and placebos on self-rated moods. J. Abnormal Psychol. 75(3), 308–314 (1970). [DOI] [PubMed] [Google Scholar]
- 161.Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends Mol. Med. 21(5), 285–294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garmaroudi FS, Handy DE, Liu YY, Loscalzo J. Systems pharmacology and rational polypharmacy: nitric oxide-cyclic GMP signaling pathway as an illustrative example and derivation of the general case. PLoS Comput. Biol. 12(3), e1004822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]