Abstract
Most cyanobacterial organisms included in the genus Microcystis can produce a wide repertoire of secondary metabolites. In the mid-2010s, summer cyanobacterial blooms of Microcystis sp. occurred regularly in Lake Balaton. During this period, we investigated how the alimentary tract of filter-feeding bigheaded carps could deliver different chemotypes of viable cyanobacteria with specific peptide patterns. Twenty-five Microcystis strains were isolated from pelagic plankton samples (14 samples) and the hindguts of bigheaded carp (11 samples), and three bloom samples were collected from the scums of cyanobacterial blooms. An LC-MS/MS-based untargeted approach was used to analyze peptide patterns, which identified 36 anabaenopeptin, 17 microginin, and 13 microcystin variants. Heat map clustering visualization was used to compare the identified chemotypes. A lack of separation was observed in peptide patterns of Microcystis that originated from hindguts, water samples, and bloom-samples. Except for 13 peptides, all other congeners were detected from the viable and cultivated chemotypes of bigheaded carp. This finding suggests that the alimentary tract of bigheaded carps is not simply an extreme habitat, but may also supply the cyanobacterial strains that represent the pelagic chemotypes.
Keywords: Microcystis, chemotype, bigheaded carp, anabaenopeptin, microginin, microcystin
1. Introduction
Most cyanobacterial species can produce a wide range of secondary metabolites with diversified biological activities. The unique cyanobacterium-specific secondary metabolites originating from variable biosynthetic pathways show great chemical diversity and are common across cyanobacterial taxa. Many of these compounds that are of interest in several scientific fields (pharmacology, toxicology, ecology, etc.) have been isolated from strains cultured under controlled conditions and field samples, and partly or fully characterized [1,2].
Oligopeptides are a major family of cyanobacterial secondary metabolites. They are a highly diverse category of low molecular weight peptides built from proteinogenic and non-proteinogenic amino acids. The most widely accepted classification by Welker and von Döhren [3] is as follows: aeruginosins [4], cyanopeptolins [5], anabaenopeptins [6], microginins [7], microviridins [8], cyclamides [9], and the well-studied and notorious microcystins [10]. These oligopeptides are mainly synthesized by non-ribosomal pathways, assembled by large multifunctional enzyme complexes, regularly non-ribosomal peptide synthases (NRPS), or hybrid NRPS/PKS (polyketide) synthases. These complexes are encoded in large gene clusters with a modular unit, and produce mainly small peptide chains as end-products [11,12].
Oligopeptides are appropriate biomarkers of cyanobacterial subpopulations. LC-MS-based untargeted peptide metabolomic studies can be useful for the separation and identification of unknown metabolites from complex natural matrices, and for metabolite typing at both individual and population levels [13,14].
Microcystis is one of the most widely studied cyanobacterial genera due to its ability to form toxic blooms in freshwater environments across almost all continents. Microcystis blooms have increased in general during recent decades, and are expected to further expand in the near future. Furthermore, several cases of huge biomass production have occurred, which were linked to this genus in strategically important freshwater habitats [10]. High genetic diversity and genotype numbers have been identified in the genus Microcystis [15,16,17,18,19,20,21,22,23]. It is important to note that the reported existence of a number of described morphospecies based on colony/cell morphology is not supported by molecular evidence, forming a clade of nearly identical 16S rDNA sequences [24,25]. Based on the low 16S rRNA sequence variability and DNA–DNA hybridization data, Otsuka et al. [26] suggested merging all morphospecies into a single species following the Bacteriological Code rules.
Climate change and nutrient over-enrichment in waters has led to worldwide proliferation of various geno- and chemo-types of several cyanobacterial species [27]. Although the invasion of microorganisms to new aquatic environments is difficult to observe, several cyanobacteria have shown characteristic microscopic morphological features, or have generated conspicuous negative impacts on the local ecosystem [28,29]. Invasions may also threaten global biodiversity by changing the structure and function of ecosystems and interrupting key biological interactions [30]. Indeed, when invading new areas, cyanobacterial species (including Microcystis spp.) are able to cause fatal environmental changes by defeating native species, disturbing food-web structures [31], or reducing diversity [28,32,33].
Bighead carp (Hypophthalmichthys nobilis R.) and silver carp (H. molitrix V.) are filter feeder cyprinid fish, native to the large freshwater habitats of Asia [34,35]. These species and their hybrids (collectively referred to as filter-feeding Asian or bigheaded carps) are detritivorous, planktivorous, and opportunistic feeders [35,36]. They have been introduced into lakes, rivers, and reservoirs throughout the world since the early 1950s. The usual purpose of introducing and stocking bigheaded carp outside their native range is to increase fishery yields and improve water quality, because it is assumed that that these fish species (especially silver carp) are effective biological control agents for algal blooms [37,38,39,40]. Several promising biomanipulation experiments have been conducted and found that population size and quantity of cyanobacteria were unchanged or even increased by stocking filter-feeding fish [41]. In addition, filter-feeding fish might increase nutrient availability and could thus stimulate the proliferation of cyanobacteria [42,43]. Miura and Wang [44] noted that several cyanobacteria survived after passing through the gut of filter-feeding species, and attained increased photosynthetic activity. Many studies conducted on bigheaded carps reported no negative effects on the viability of some cyanobacterial species after gut passage [45]. Lewin et al. [46] and Görgényi et al. [47] proposed that Microcystis cells were not harmed or damaged after transit through the gut due to their mucous protection. Moreover, direct use of phosphorus by this cyanobacterial species has been detected in fish guts during passage [47].
In the mid-2010s, summer cyanobacterial blooms of Microcystis spp. occurred on a regular basis in Lake Balaton, the largest lake in Central Europe (Figure 1). The main objective of the present work was to investigate Microcystis chemotypes within these waterbodies in the period between 2013–2016. More specifically, we aimed to: (i) Identify new and well known congeners of the cyanobacteria peptide family using a LC-MS-based untargeted approach; and (ii) determine the pattern, abundance, and distribution of Microcystis chemotypes among the pelagial, bloom area, and in the alimentary tracts of filter-feeding bigheaded carps (Figure 2)—as this fish species represent a massive stock in the lake recently.
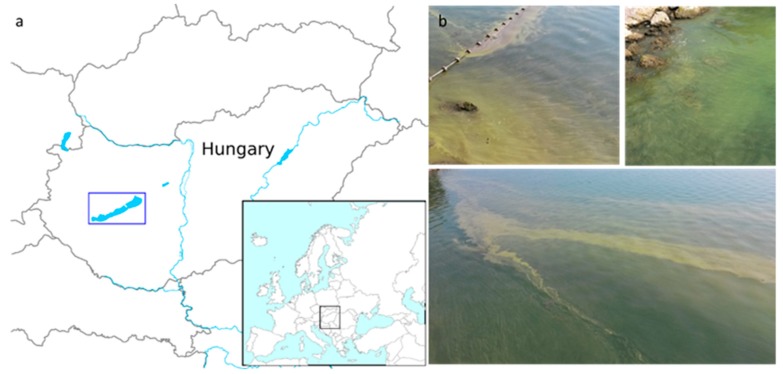
Figure 1.
(a) Location of Lake Balaton in Hungary and Central Europe, indicated by a blue rectangle. (b) The Microcystis bloom in the lake (August 2015).
Figure 2.
(a) A hybrid bigheaded carp (total body length 1.08 m; weight 23 kg) caught in Lake Balaton, Hungary. (b) A dissected male bigheaded carp, showing the testicles and the guts in the abdominal cavity. Foregut (to the right side of the image) and hindgut (to the left) are denoted by white ellipses.
2. Results
2.1. Morphology-Based Identification of Cyanobacterial Bloom Causing Organisms, and the Isolated Strains
Microcystis cells were spherical with a 5–8 µm diameter. Aggregated cells were organized into colonies with very narrow and diffluent colorless mucilage. Light blue-green protoplasts appeared to be light-brown due to the optical effects of gas vesicles which were observed in the cells. Colony sizes ranged from microscopic to macroscopic. The macroscopic colony layer showed a pale green color in the natural blooms and cultures. Morphological features were identical to the characteristics of Microcystis flos-aquae [48]. Based on morphological criteria of the colinies, the dominant morphotype in the bloom samples was identified as Microcystis flos-aquae.
2.2. Molecular Phylogenetic Analyses
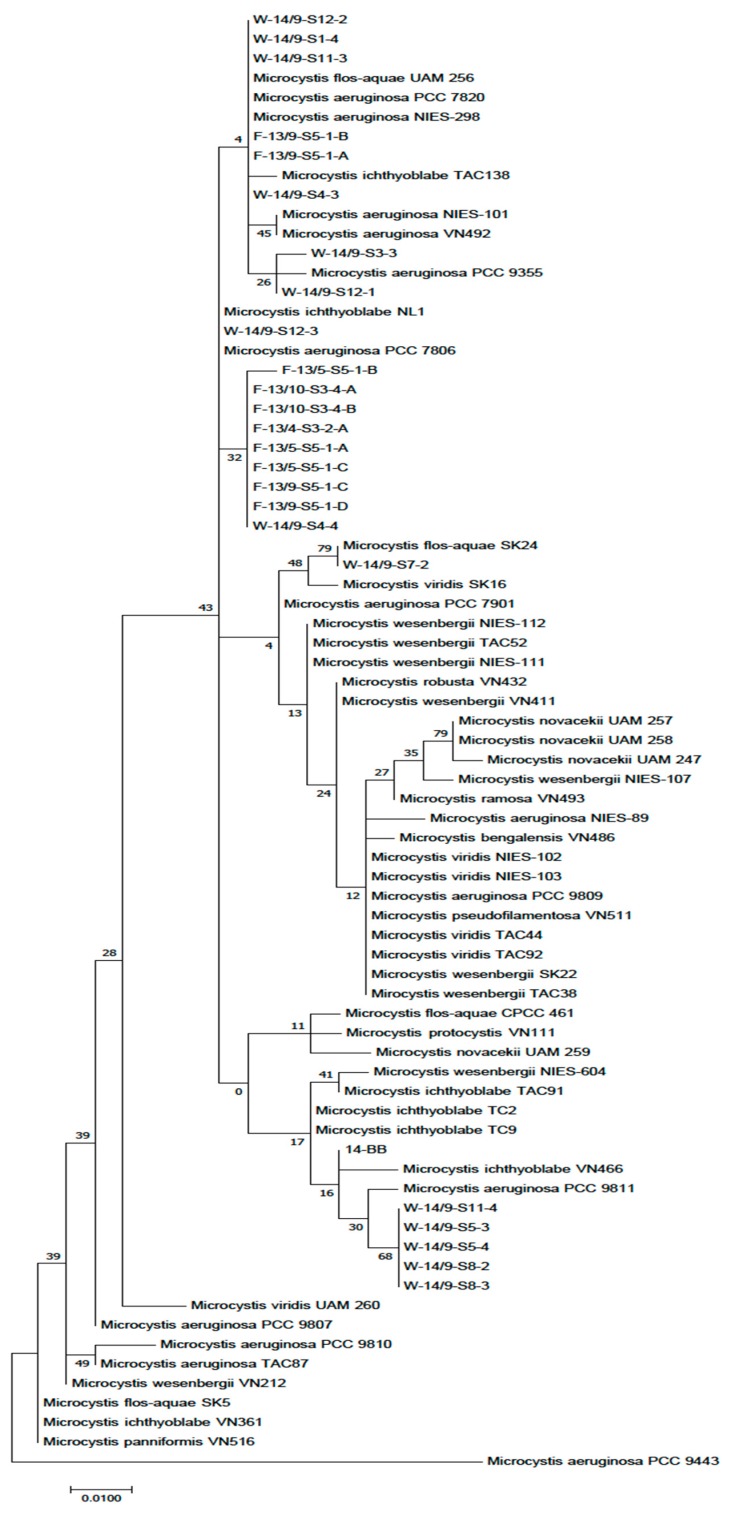
In the phylogenetic analysis, altogether 25 ITS strain sequences were isolated from Lake Balaton and hindgut content of bigheaded carps (Hypophthalmichthys spp.). These were involved with Microcystis ITS sequences originating from six different morphospecies. All ITS sequences in this study formed a distantly related cluster with all but one Microcystis morphospecies (Figure A1). In the phylogenetic tree, the ITS sequences of strains from the lake’s water and hindgut samples were arranged into eight separated lineages, however, no clear distinction according to sample type or sampling time could be observed. Our strains showed the closest phylogenetic relation to morphospecies M. flos-aquae and M. viridis.
2.3. Identification of Peptides and Comparative Analysis of Bloom Samples and M. flos-aquae Strains
The cyanobacterial peptides identified from M. flos-aquae strains and blooms that originated from the pelagic and bigheaded carp matrix are presented in Table 1, Table 2 and Table 3.
Table 1.
Identified microginin type peptides from isolated Microcystis strains. Leucine and isoleucine cannot be distinguished from LC-MS/MS data, these amino acids have been deduced from the nearest literary results.
| Compound | [M + H]+ m/z | RT min | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| MG 565 | 565.5 | 6.1 | Ahda | Thr | Pro | Tyr | |
| MG 549 | 549.5 | 6.2 | Ahda | Ala | MePro | Tyr | |
| MG 579 | 579.6 | 6.4 | MeAhda | Thr | Pro | Tyr | |
| MG 579 | 579.6 | 6.3 | Ahda | Thr | MePro | Tyr | |
| MG 581 | 581.5 | 5.5 | Ahda | Thr | Pro | OHTyr | |
| MG 595 | 595.5 | 5.7 | Ahda | Thr | MePro | OHTyr | |
| MG FR3 | 728.6 | 6.3 | Ahda | Thr | Pro | Tyr | Tyr |
| MG 712 | 712.6 | 6.4 | Ahda | Ala | MePro | Tyr | Tyr |
| MG FR4 | 742.6 | 6.6 | MeAhda | Thr | Pro | Tyr | Tyr |
| MG 744 | 744.6 | 6 | Ahda | Thr | Pro | OHTyr | Tyr |
| MG 754 | 754.6 | 6.7 | Ahda | Leu | Pro | Hty | Tyr |
| MG 607 | 607.5 | 6.4 | Ahda | Leu | MeVal | Hty | |
| MG 621 | 621.6 | 6.4 | MeAhda | Leu | MeVal | Hty | |
| MG 591 | 591.5 | 7.4 | MeAhda | Leu | MeVal | Phe | |
| MG 770 | 770.6 | 6.7 | Ahda | Leu | MeVal | Hty | Tyr |
| MG 784 | 784.6 | 6.7 | MeAhda | Leu | MeVal | Hty | Tyr |
| MG 798 | 798.6 | 7 | MeAhda | Leu | MeLeu | Hty | Tyr |
Table 2.
Identified anabaenopeptin type peptides from isolated Microcystis strains. Leucine and isoleucine cannot be distinguished from LC-MS/MS data, these amino acids have been deduced from the nearest literary results. Unidentified amino acids were marked with X.
| Compound | [M + H]+ m/z | RT min | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|
| ANA B | 837.7 | 5 | Arg | CO | Lys | Val | Hty | MeAla | Phe |
| ANA F | 851.7 | 5.3 | Arg | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA A | 844.7 | 6.6 | Tyr | CO | Lys | Val | Hty | MeAla | Phe |
| OSC Y | 858.6 | 7 | Tyr | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA 828 | 828.6 | 7.7 | Tyr | CO | Lys | Val | Hph | MeAla | Phe |
| ANA 842 | 842.7 | 8 | Tyr | CO | Lys | Ile | Hph | MeAla | Phe |
| ANA 916 | 916.9 | 6.9 | Tyr | CO | Lys | Val | Hty | MeHty | Ile |
| ANA 930 | 930.6 | 7.1 | Tyr | CO | Lys | Ile | Hty | MeHty | Ile |
| ANA 852 | 852.8 | 7.3 | MeHty | CO | Lys | Val | Hty | MeAla | MeLeu |
| ANA 866 | 866.7 | 7.6 | MeHty | CO | Lys | Ile | Hty | MeAla | MeLeu |
| ANA 856 | 856.7 | 8.2 | MeHty | CO | Lys | Val | Hph | MeAla | Phe |
| ANA 870 | 870.7 | 8.5 | MeHty | CO | Lys | Ile | Hph | MeAla | Phe |
| ANA 872 | 872.6 | 7.2 | MeHty | CO | Lys | Val | Hty | MeAla | Phe |
| ANA 886 | 886.7 | 7.4 | MeHty | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA 900 | 900.7 | 7.8 | MeHty | CO | Lys | Ile | Hty | MeAla | MePhe |
| ANA 914 | 914.8 | 8 | Tyr | CO | Lys | X | X | X | X |
| ANA 928 | 928.7 | 7.9 | Tyr | CO | Lys | X | X | X | X |
| ANA 892 | 892.7 | 6 | Tyr | CO | Lys | X | X | X | X |
| ANA 938 | 938.5 | 7.7 | Tyr | CO | Lys | X | X | X | X |
| ANA 860 | 860.7 | 6 | Tyr | CO | Lys | X | X | X | X |
| ANA 888 | 888.7 | 6.6 | MeHty | CO | Lys | X | X | X | X |
| ANA 902 | 902.6 | 6.8 | MeHty | CO | Lys | X | X | X | X |
| ANA 904 | 904.6 | 6.6 | MeHty | CO | Lys | X | X | X | X |
| ANA 904 | 904.7 | 7.4 | MeHty | CO | Lys | X | X | X | X |
| ANA 934 | 934.6 | 6.9 | MeHty | CO | Lys | X | X | X | X |
| ANA 854 | 854.6 | 8.1 | X | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA 814 | 814.7 | 7.6 | X | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA 888 | 888.7 | 6.2 | X | CO | Lys | Val | Hty | MeAla | Phe |
| ANA 920 | 920.7 | 8 | X | CO | Lys | Ile | Hty | MeAla | Phe |
| ANA 892 | 892.6 | 7.4 | X | CO | Lys | X | X | X | X |
| ANA 984 | 984.6 | 7.7 | X | CO | Lys | X | X | X | X |
| ANA 902 | 902.7 | 6.5 | X | CO | Lys | X | X | X | X |
| ANA 918 | 918.6 | 6.7 | X | CO | Lys | X | X | X | X |
| ANA 905 | 905.2 | 6.5 | X | CO | Lys | X | X | X | X |
| ANA 922 | 922.1 | 6.6 | X | CO | Lys | X | X | X | X |
| ANA 904 | 904.7 | 5.8 | X | CO | Lys | X | X | X | X |
Table 3.
Identified microcystin-type peptides from isolated Microcystis strains. Leucine and isoleucine cannot be distinguished from LC-MS/MS data, these amino acids have been deduced from the nearest literary results.
| Compound | [M + H]+ m/z | RT min | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|
| MCY-LW | 513.3 1 | 5.4 | Ala | Leu | MeAsp | Trp | Adda | Glu | MeDha |
| MCY-RR | 520.3 1 | 5.6 | Ala | Arg | MeAsp | Arg | Adda | Glu | MeDha |
| [D-Asp3]MCY-LR | 981.9 | 6.6 | Ala | Leu | Asp | Arg | Adda | Glu | MeDha |
| MCY-LR | 995.8 | 6.8 | Ala | Leu | MeAsp | Arg | Adda | Glu | MeDha |
| MCY-HilR | 1009.9 | 7 | Ala | Hil | MeAsp | Arg | Adda | Glu | MeDha |
| [MeSer7]MCY-LR | 1014 | 6.6 | Ala | Leu | MeAsp | Arg | Adda | Glu | MeSer |
| [Dha7]MCY-FR | 1015.8 | 7 | Ala | Phe | MeAsp | Arg | Adda | Glu | Dha |
| MCY-FR | 1029.7 | 7.1 | Ala | Phe | MeAsp | Arg | Adda | Glu | MeDha |
| [Dha7]MCY-YR | 1031.9 | 6.5 | Ala | Tyr | MeAsp | Arg | Adda | Glu | Dha |
| MCY-YR | 1045.5 | 6.6 | Ala | Tyr | MeAsp | Arg | Adda | Glu | MeDha |
| MCY-(H4)YR | 1049.7 | 6.3 | Ala | H4Tyr | MeAsp | Arg | Adda | Glu | MeDha |
| MCY-WR | 1068.8 | 7.2 | Ala | Trp | MeAsp | Arg | Adda | Glu | MeDha |
| [MeSer7]MCY-WR | 1086.9 | 6.9 | Ala | Trp | MeAsp | Arg | Adda | Glu | MeSer |
1 Data are given in [M + 2H]2+.
Seventeen variants from the microginin (MG) class were present in our samples (Table 1). MG FR3 (m/z 728 [M + H]+) and FR4 (m/z 742 [M + H]+) were identified from the MS2 data [49]. MGs with m/z 565 [M + H]+ and m/z 579 [M + H]+ showed identical fragmentation patterns but in both cases, the last tyrosine unit was absent. The 16 Da mass difference in the MS dataset of m/z 581 [M + H]+ and m/z 744 [M + H]+ indicated the presence of an additional hydroxyl group on tyrosine at position 4 in these congeners. MG m/z 754 [M + H]+ contained amino acid modification in two positions compared to FR3. The 26 Da mass increase can be explained by an amino acid exchange from threonine to leucine at position 2, and a substitution of tyrosine with homotyrosine at position 4, which is also supported by MS2 data (Table A1).
The structure of MG m/z 770 [M + H]+ was deduced from the amino acid sequence of MG 478 [50] with the same molecular weight. However, Ahda and leucine amino acids were at positions 1 and 2, respectively, as concluded from the 127 Da mass difference between the product peaks and presence of a fragment with 299 m/z. MGs with m/z 784 [M + H]+ and 798 [M + H]+ were identified as methylated analogs of MG 770 with N-methyl-Ahda at position 1 in both cases and an amino acid exchanged from methyl-valine to methyl-leucine at position 2 for the latter. MG m/z 607 [M + H]+ and MG m/z 621[M + H]+ were characterized as shortened forms of MG 770 and 784, composed of four amino acid units. MG m/z 591 [M + H]+ was found to be an analog of MG 621, and based on its fragmentation pattern (the 30 Da neutral loss affected only position 4), this variant contains phenylalanine at position 4 (Table A1).
MG m/z 712 [M + H]+ was identified as a methylated form of MG T2, whose modification affects proline at position 4 according to the MS2 data. MG m/z 549 [M + H]+ and m/z 595 [M + H]+ were found to be shortened variants of methylated-T2 with tyrosine and hydroxy-tyrosine at position 4. The structure of MG m/z 579 [M + H]+ was derived from MG m/z 549 [M + H]+ with an amino acid substitution from alanine to threonine at position 2 (Table A1).
Fifteen completely characterized—and an additional 21 partially characterized—anabaenopeptin (ANA) variants were identified in our samples. ANA A (m/z 844 [M + H]+) and B (m/z 837 [M + H]+) showed the same MS2 and MS3 fragmentation pattern as previously reported by Mayumi [51]. Other previously known variants include oscillamide (OSC) Y (m/z 858 [M + H]+) and ANA F (m/z 851 [M + H]+). Although ANA F and E variants had the same molecular mass, the two ring structures differed only in their MS3 spectra. In our case, MS3 product ions supported only the structure of ANA F (Table A2). ANAs with m/z 828 [M + H]+ and m/z 842 [M + H]+ as well as m/z 916 [M + H]+ and m/z 930 [M + H]+ showed the same MS3 fragmentation pattern as ANA A and OSC Y. For the first pair, the 16 Da neutral loss indicated the presence of homophenylalanine in position 4 instead of homotyrosine, which was also supported by MS3 data. In the second pair, the difference in molecular mass of 72 Da affected two positions. According to the MS3 information, a 106 Da mass increase at position 5 indicated a substitution from N-methyl-alanine to N-methyl-homotyrosine, and a 34 Da mass reduction at position 6 suggested a change from phenylalanine to isoleucine. Seven completely characterized variants showed unusual product ions in the MS2 experiments. The 26 Da mass difference between the two product ions was linked to the presence of the CO-group between the ring and the side chain, but these products indicated an amino acid with a 209 Da mass in position 1, which could be explained by the presence of a tyrosine derivative like N-methyl-homotyrosine. Among these congeners, ANA m/z 872 [M + H]+ and m/z 886 [M + H]+ showed identical structures to ANA A and OSC Y. In the case of ANA m/z 852 [M + H]+ and m/z 866 [M + H]+, the 20 Da mass loss indicated methyl-leucine at position 6, while the 14 Da mass increase of ANA m/z 900 [M + H]+ suggested methyl-phenylalanine at position 6. ANA m/z 856 [M + H]+ and m/z 870 [M + H]+ have homophenylalanine at position 5 deduced from the loss of 16 Da (Table A2).
Each of the partially characterized ANAs showed characteristic product ions with the 26 Da difference in the MS2 experiences. In four cases, no amino acid or a simple derivative thereof could be assigned to amino acid position 1 based on MS/MS, but their ring structure suggested that these were compounds related to previously known ANAs. For the remaining cases, the MS analysis data were insufficient to determine all the structural elements due to the low peak intensity (Table A2).
In our samples, 13 microcystin (MCY) variants were identified (Table 3). MCY-RR and -WR were found in a double protonated form with m/z 520 [M + 2H]2+ and m/z 513 [M + 2H]2+ [52]. The MCY-LR, -FR, -YR, and -WR forms were singly-charged and gave protonated ions at m/z 995 [M + H]+ and m/z 1029 [M + H]+, respectively. Modification at position 7 was observed in four cases. Peaks with m/z 1015 [M + H]+ and m/z 1031 [M + H]+ were identified as [Dha7]MCY-FR and [Dha7]MCY-YR, respectively. Furthermore, m/z 1014 [M + H]+ and m/z 1086 [M + H]+ were evaluated as [MeSer7]MCY-LR and [MeSer7]MCY-WR, respectively. A non-methylated asparagine was present at position 3 in [D-Asp3]MCY-LR, which gave m/z 981 [M + H]+. Non-proteinogenic amino acids were found at position 2 in two cases. A peak with m/z 1009 [M + H]+ was found to be a homoisoleucine-containing analogue (MCY-HilR), and m/z 1049 [M + H]+ was identified as MCY-(H4)YR [52,53] (Table A3).
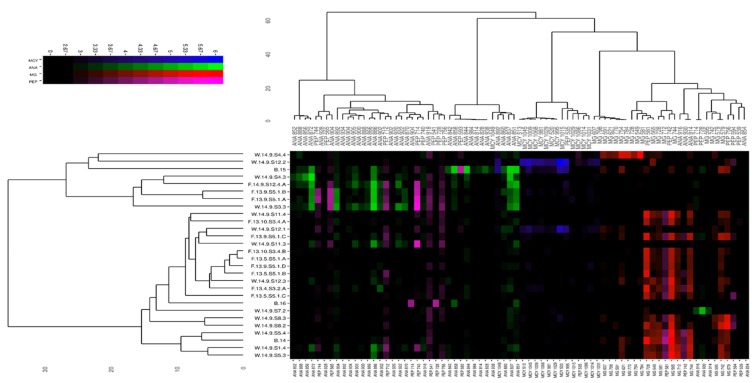
The heatmap shows the relative abundance of given natural products in the Microcystis samples—the color is proportional to the log10 of the area under the curve values.
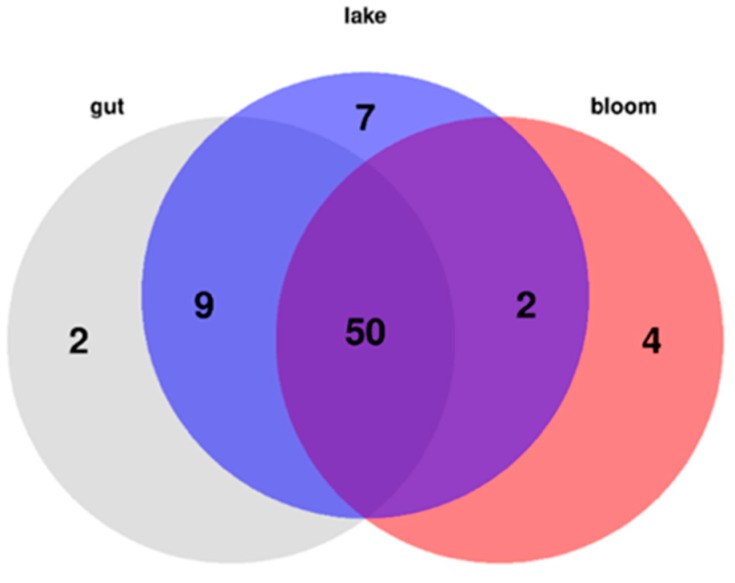
The most important phenomenon is the lack of peptide pattern separation for Microcystis originating from the gut, lake, and bloom-samples. The samples were separated into relatively loose clusters based on their metabolomes, spreading all three functional groups throughout these clusters. No separation by sampling year could be found either (Figure 3 and Figure 4).
Figure 3.
Venn diagram showing the presence/absence of identified peptide natural products in the dataset containing the gut, bloom, and lake samples. A single sample was enough to state that the group contained the metabolite of interest.
Figure 4.
Heatmap of the abundance values for peptide natural products from the bloom, water, and gut samples. Compounds and samples were sorted along the axes after hierarchical clustering in R 3.5.0. Color is proportional to log10(abundance) of the compounds, as obtained by LC-ESI-MS measurements, and indicated in the color gradient legend. The different peptide subclasses were mapped to different colors as follows: microcystins (MCY) are shown in blue; anabenopeptines (ANA) are shown in green; microginins (MG) are shown in red; other peptides (PEP) are shown in magenta. The x-axis contains labels showing metabolite class and m/z of [M + H]+. The y-axis labels show sample IDs. The presence of certain chemotypes (composed of isolates from gut, lake, and bloom samples) can be easily recognized.
2.4. Classification of Oligopeptide Pattern into Chemotypes
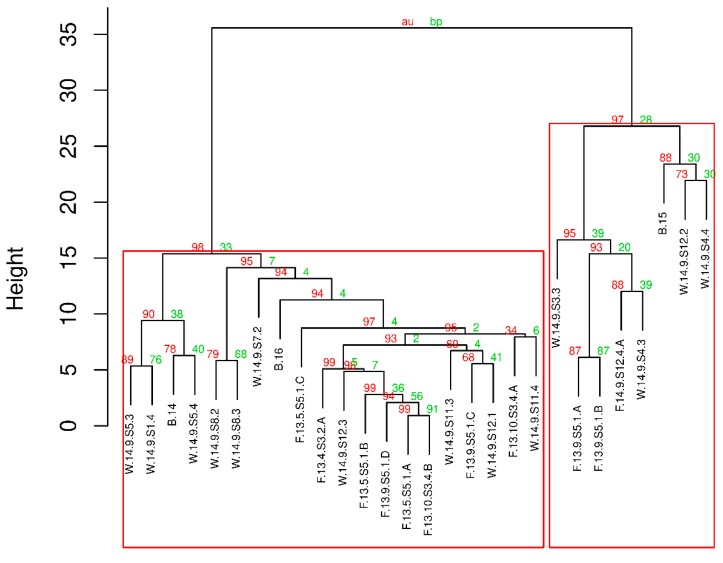
Investigating individual Microcystis strains and samples showed a high number of different, but more or less distinct, chemotypes (with different peptide patterns) that originated from all habitats. On the other hand, more or less distinguishable chemotypes were observed among the samples, which were separated into clusters of varied densities based on the absence or co-occurrence of biosynthetic metabolite clusters. The samples either contained: (1) a variety of ANAs and a few unidentified peptides, (2) MCYs, or (3) MGs as their major compounds, and usually contained significantly lower or no detectable amounts from other peptide groups. For example, in many samples, a group containing ANAs that lack MGs and MCYs were seen to be of this chemotype. The co-occurrence of several MCY types was observed in only a few samples. This was also illustrated by the compact cluster separating MCYs from other natural products. These MCY-rich samples lacked other chief compounds of interest. Another subset of cyanobacteria contained various MGs at high concentrations, but did not contain ANAs or other peptides as major components. These compounds were all present in their producers, resulting in a relatively compact MG cluster. The bootstrapped hierarchical clustering analysis revealed the existence of two main chemotypes: MG-dominant and ANA-dominant (Figure 5). The existence for both clusters was p < 0.05.
Figure 5.
Bootstrapped hierarchical clustering of algal lines according to scaled and centered natural compound abundance values, using the Minkowski distance and Ward’s hierarchical clustering, bootstrapping N = 1 × 106. The clusters highlighted in red rectangles exist at a p-value < 0.05.
2.5. Lack of Phylogenetic Relationship Was Found among the Chemotypes
Although all of the isolates were identified as the M. flos-aquae morphotype by conventional microscopy techniques, several phylogenetic clusters (Figure A1) and more distinct chemotypes (Figure 4 and Figure 5) have also been defined. The lack of a phylogenetic relationship was found among the chemotypes. To test the statistical significance of chemical patterns and other variables (such as origin, phylogenetic position), principal component score values were subjected to the Kruskal–Wallis significance test, where the variable studied was the group-determining factor. This approach for all metabolites allowed higher statistical power compared to direct statistical analyses. No significant association was found between the metabolite pattern, abundance, and genetic background. The 16S–23S ITS phylogenetical group had no significant effect on the metabolite pattern (p > 0.05), represented by one of the 28 PC dimensions in the analysis. No significant association was found between the metabolite pattern, abundance, and source of isolate. Isolate source (water, fish, or bloom) had no significant effect on the metabolite pattern (p > 0.05), represented by one of the 28 PC dimensions in the analysis.
3. Discussion
The detected Microcystis flos-aquae blooms were unexpected but not unique, because at the beginning of the 20th century, Microcystis blooms were observed in Lake Balaton localizing to small areas [54]. In addition, from the middle of the century, mainly nitrogen fixing filamentous cyanobacteria species like Aphanizomenon and Anabaena (Dolichospermum) caused this phenomenon in the lake. In Lake Balaton, the species Cylindrospermopsis raciborskii was identified in the 1970s and initiated whole-lake-area sized water blooms in 1982 and 1994, which adversely affected the tourism and economy of the area [55]. A number of comprehensive water management measures at the end of the 1980s were aimed at curbing eutrophication such as the drainage of communal sewage from the coastal zone. At the same time, the scale of agricultural activity decreased, resulting in lower nutrient loads to the lake. From this period until the present report, there has been no cyanobacterial blooming in Lake Balaton. The reappearance of Microcystis blooms in the lake, which were observed in this study, suggests that external nitrogen loads may have initiated the multiplication of non-diazotrophic cyanobacteria. In our present study, 11 Microcystis strains were isolated from gut samples, 14 from the pelagic plankton from Lake Balaton, and three collected Microcystis bloom samples.
Taxonomic classification of Microcystis is difficult. The combination of microscopic observations with molecular data can be the most adequate method for identifying isolates [56]. Most species of this genera have been described via their morphological characteristics [57,58], however, colony variance can be huge, and the external qualities of many populations overlap the limiting specifications [59,60]. Therefore, it is difficult to find the differences between traditional species [61].
The taxonomic position of the isolated strains was confirmed by phylogenetic analysis. Our strains showed the closest phylogenetic relation to morphospecies M. flos-aquae and M. viridis (Figure A1). Several papers have been published on the correlation between Microcystis morphotypes and genotypes [62,63]. The heterogeneity of this genus between different regions has also been well documented using further genetic markers [16,18,19,20,22,23,29]. Neilan et al. [64] defined genetic similarity and noted that the species had no specific phylogeographic structure, which was in accordance with work described by Bittencourt-Oliveira et al., (2001) [17] where Microcystis strains did not show any distinct phylogenetic pattern.
In our work, we identified a strong mucous envelope characteristic of the studied Microcystis morphotype. This can explain how this cyanobacterial species survive in the alimentary tract of bigheaded carps. Exopolysaccharides (EPS) can be crucial for cellular attachment, adhesion, and survival. This highly hydrated layer provides protection to cyanobacterial cells against desiccation, toxic agents, or the digestive enzymes of other organisms. This role of the EPS has been confirmed in several studies [65].
Thirty-six ANA and 17 MG variants were identified from the isolated strains (Table 1 and Table 2). New MCY congeners are more rarely identified, perhaps because this has been the most investigated cyanobacterial peptide family. However, bioactive peptide families like MGs and ANAs are receiving growing attention. Four known and 32—to the best of our knowledge—previously unknown ANA variants were fully or partially identified in our analysis. Several partly identified peptide fragments were detected (Table 4 and Table A4), which were clustered into this family using heat map analysis.
Table 4.
Unidentified peptides/peptide fragments from isolated Microcystis strains. Leucine and isoleucine cannot be distinguished from LC-MS/MS data. Unidentified amino acids were marked with X.
| Compound | [M + H]+ m/z | RT min | n | n + 1 | n + 2 |
|---|---|---|---|---|---|
| PEP 535 | 535.4 | 6.7 | X | Leu | |
| PEP 539 | 539.4 | 9.4 | X | Leu | |
| PEP 541 | 541.4 | 5 | X | Phe | |
| PEP 565 | 565.5 | 5 | X | MeLeu | |
| PEP 581 | 581.5 | 5.8 | X | Tyr | |
| PEP 593 | 593.5 | 6.1 | X | Met | MeLeu |
| PEP 756 | 756.6 | 6.8 | X | Tyr | Tyr |
| PEP 593 | 593.5 | 6.8 | X | Tyr | |
| PEP 594 | 594.4 | 5.2 | X | Thr | Leu |
| PEP 712 | 712.6 | 5.4 | X | MeLeu | Tyr |
| PEP 714 | 714.6 | 5.2 | X | MeLeu | Tyr |
| PEP 728 | 728.6 | 5.4 | X | MeLeu | Tyr |
| PEP 740 | 740.7 | 8.4 | X | MeLeu | Tyr |
| PEP 742 | 742.6 | 5.5 | X | Met | |
| PEP 744 | 744.5 | 5 | X | MeLeu | |
| PEP 714 | 714.6 | 5.5 | X | MeLeu | Tyr |
| PEP 728 | 728.6 | 5.7 | X | MeLeu | Tyr |
ANA F, OSC B, and C are inhibitors of protein phosphatases (PP). N-MeHty and the positively charged Arg are crucial parts of molecules relating to this activity [66]. ANAs were also found to be active toward proteinase enzymes such as trypsin, chymotrypsin, elastase, and carboxypeptidase A [8,67,68]. The relaxing activity of rat aortic preparations was detected by treatments with ANA B and ANA 906 [69]. ANA B and ANA F, the most frequently found ANAs, were shown to inhibit the growth of many M. aeruginosa strains by inducing the lytic cycle in cyanobacteria [70,71]. Taking into account the published effects of these metabolites, its ecological roles might be important [72].
Two known, and 15—to the best of our knowledge—previously unknown MG variants were identified in our analysis. Several partly identified peptid fragments were also detected as part of this family.
MGs are a 40-member group of linear and nonribosomal peptides, which have been detected and purified from several bloom-forming cyanobacteria isolates. The number of congeners is growing [2,3,7]. These are built by an α-hydroxy-β-amino derivate of decanoic or octanoic acid, which is rarely chlorinated at its terminal methyl group with three to five additional amino acids in the molecules [4,7,73,74]. MGs are zinc metalloprotease inhibitors (e.g., angiotensin-converting enzyme), and aminoproteinases that bind their α-hydroxy-β-amino residue to the zinc at the active site of the enzyme. Our findings indicate that these substances are important candidates for treating hypertension [72]. The patchy distribution of oligopeptide patterns in cyanobacterial populations enables classifying isolates into several oligopeptide-based chemotypes [14,75]. It is important to note that mainly ANA and MG dominant strains were detected from Lake Balaton in this study, but 10 strains from the alimentary tract were MCY producing.
The distinguishable chemotypes we found in our analysis were separated into clusters of varied density based on the absence or co-occurrence of biosynthetic metabolite clusters, similar to several other studies that have investigated Microcystis and other cyanobacterial peptide producers such as Planktothrix sp., Nostoc sp., etc. [14,75,76,77]. These genera often possess variable chemotypes with the ability to produce different peptide families in natural assemblages [3]. In the Microcystis isolates originating from natural populations, four chemotypes were characterized based on the fact that they contained a few or several main peptides, but in many cases, the appearance of several different peptides belonging to different biosynthetic clusters has been observed.
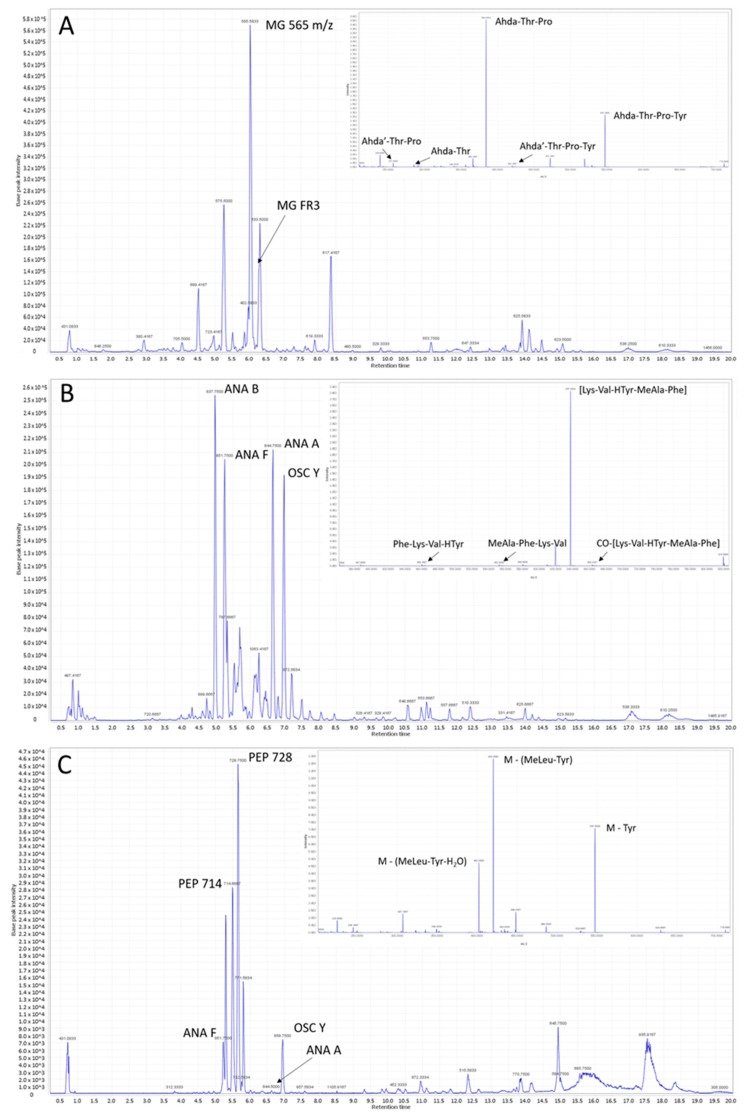
From Lake Balaton, primarily ANA- and MG-dominated strains were detected, with the observation that many lesser-known or new congeners appeared from both groups of peptides. In addition, several partly identified peptide fragments were detected during the analysis whose metabolites seemed to belong to the metabolism (synthesis or degradation) of the two main groups identified as suggested by the heat map visualization. Focusing on the bloom samples from 2014–2016, it is important to note that the naturally collected material of all three samples represent mixed matrices, and each of them contained several chemotypes and/or genotypes of the genus Microcystis. These samples belonged to different chemotype clusters. The bloom samples from the same sampling site showed different bioactive peptide patterns. While the 2014 sample was mainly MG-dominated, the 2015 bloom sample was rather ANA-rich. The 2016 bloom community contained mostly non-identified peptides (Figure A2). Considering all the identified peptides in our samples, we found that the isolated and identified chemotypes originating from the gut and pelagic sample could be involved in the Microcystis community that built the bloom phenomenon.
Altogether, 13 MCY congeners were also identified in this work from phytoplankton and digestive tract strains. There is no doubt that MCYs are the most harmful and notorious family from the described cyanobacterial peptides. All of the detected MCY forms are already known, and no new MCY variant was identified in the present analysis. Although only a few MCY variants have been identified near the large number of MG and ANA, these peptide-producers display a separate cluster in our analysis. It is especially worth paying attention to this group because MCY is the most common toxin produced by cyanobacteria in waters [78,79], and can also cause death, illness, complications, and damage in humans, animals, and plant organs.
In the several cases where Microcystis and Planktothrix oligopeptide patterns have been investigated worldwide [12,13,14,75,80,81,82], and in our local area [83,84,85,86], the numbers of genotypes have been identified with the help of phylogenetic markers [64,87]. The lack of correlation between the Microcystis chemotypes and phylogenetic genotypes found in cases similar to our present study suggest that the synthesis of bioactive peptides is not phylogenetically conserved in this genera. This has also been the conclusion of recent work [88] where Microcystis chemotypes were researched in Spanish freshwater and reservoirs. The findings in our study are consistent with the statement that the distribution of oligopeptide production abilities does not correlate with morphospecies, phylogenies based on commonly used molecular markers, or the geographical origin of the isolated organisms [64,89].
Bigheaded carps were introduced into Lake Balaton (Hungary) in 1972 and were stocked until 1983 [90,91,92,93,94]. These filter-feeder fish species can consume almost all algal and cyanobacterial taxa from ambient water, but the ingested algae are only partially utilized [47]. In fact, a fraction of the consumed phytoplankton cells or colonies (e.g., Microcystis sp., diatoms, volvocalean, and chlorococcalean green algae) may stay alive after passing through the digestive tract of fish as they are protected either by a mucilaginous envelope or by a thick, cellulose-based cell wall [47,95]. In the present study, 11 Microcystis strains were isolated from gut samples and identified as Microcystis flos-aquae with a characteristic mucilage envelope that can be the main protecting layer against the extreme gut environment.
In the scientific literature, there is contradictory information on the abundance and composition of cyanobacteria in the alimentary tract of bigheaded carps. On one hand, a study by Ye et al. [96] found cyanobacteria to be predominant in the gut microbiota of bigheaded carp living in different North American rivers, while Li et al. [97] reported the abundance of cyanobacteria was typically low in the intestines of both silver and bighead carp inhabiting Wuhu Lake (China). Based on the gut content metagenome analysis, Microcystis was identified as the most abundant cyanobacterial genus detected in the gut of bigheaded carp in Lake Balaton [98].
Beyond the outer polysaccharide layer, it is worth noting the detected peptides in this work, mainly the large number of ANAs and MGs (Table 1 and Table 2) and the above discussed bioactivity. Digestive enzymes’ activity in bigheaded carp species have been investigated in several studies [99,100]. Phosphatases and proteases are principal groups of enzymes for the fish species [101]. “The rapid excretion rate of silver carp would require quick digestion and nutrient uptake of foods to support high growth rates” [102]. Several microorganisms that can play a role in digestion and be responsible for higher levels of the above-mentioned digestive enzymes have been identified in the gut of silver carp [96]. Our suggestion is that the detected MGs and ANAs, as potent protease inhibitors, could modify the digestive capacity by binding directly to the enzymes. However, it is known that most oligopeptides stay in the producing cyanobacteria cell and are only released via cell lysis following cell death, and thus, would only provide protection for the surviving cyanobacterial cells in the gut.
While the traditional approaches of toxin and/or bioactive metabolite research of cyanobacteria have mainly focused on individual peptides, exploring their effects or biosynthesis, our chemotyping study with non-targeted analysis investigated the occurrence of various peptides in Microcystis strains that originated from bloom, pelagic plankton samples, and from the gut of a notorious invasive fish species. Except for 13 peptides, all other congeners were detected from viable and cultivated chemotypes originating from bigheaded carps. This finding suggests that the alimentary tract of bigheaded carps is not only a special habitat, but also a supplier for strains that represent the pelagic chemotypes and can initiate blooms in the waterbody. This potentially malicious feature can come from the ability of this fish species to filter plankton efficiently, but a few organisms such as the peptide-producing mucilaginous enveloped cyanobacterial species M. flos-aquae are digested improperly or not at all in the digestive system. In addition, several studies have noted that the toxicity of cyanobacteria remained unaffected or even increased after defecation. Kolmakov et al. [103] demonstrated that the physiology of the investigated cyanobacterial species were not suppressed by passing through fish intestines, but rather enhanced when they returned to the water. Kolar et al. [35] also noted that some Microcystis cells were not eliminated by the digestive processes of fish species. Lewin et al. [46] suspected that Microcystis could survive, and even use the phosphorus in fish guts as nutrients [46].
Wide time interval evacuation rates have been estimated for silver carp at different water temperatures [104]. This is why it is not easy to calculate the retention of viable Microcystis cells in the gut. Although it is worth raising the opportunity that bigheaded carps carrying cyanobacterial chemotypes in their guts from one habitat can invade new areas, and that the viable cyanobacterial cells may be released by defecation from fish [32,33].
In our chemotyping study, Microcystis strains isolated from the invasive non-native bigheaded carps and their peptide patterns were compared to pelagic and bloom material strains. Our results draw attention to the fact that bigheaded carps not only carry and spread viable, mucilaginous envelope-covered Microcystis cells from their alimentary tracts, but harmful cyanobacterial strains can also be found among them according to the chemotypes.
4. Materials and Methods
4.1. Sample Collection and Initial Sample Processing
Bigheaded carp were collected from Lake Balaton (Hungary), which is the largest lake in Central Europe. Its surface area is 596 km2, while the average water depth is about 3 m [105]. Bigheaded carps and water samples were collected from the lake in April, May, June, September, and October in 2013.
The local fishery company (Balaton Fish Management Non-Profit Ltd., Hungary) had a permit to harvest fish by nets (including bigheaded carp) from Lake Balaton in 2013 (permit reg. no.: 2013/N000001, issued by the Fisheries Authority of Somogy County, Hungary). The fishery company provided samples to the researchers from their commercial catches. After receiving the samples, researchers of the Balaton Limnological Institute (Center for Ecological Research, Hungarian Academy of Sciences) transported them to the laboratory within 30 min. The Institute has a permit for the delivery and use of fish for scientific purposes (permit reg. no.: VE-I-001/01890-3/2013, issued in 22 August 2013 by the Food-Security and Animal Health Directorate, Governmental Office of Veszprém County, Hungary). Gut content samples were collected aseptically, as described by Görgényi et al. [47]. Subsamples were taken (cc. 5 g) and stored in sterile Eppendorf tubes at 4 °C until laboratory processing, all done within 24 h.
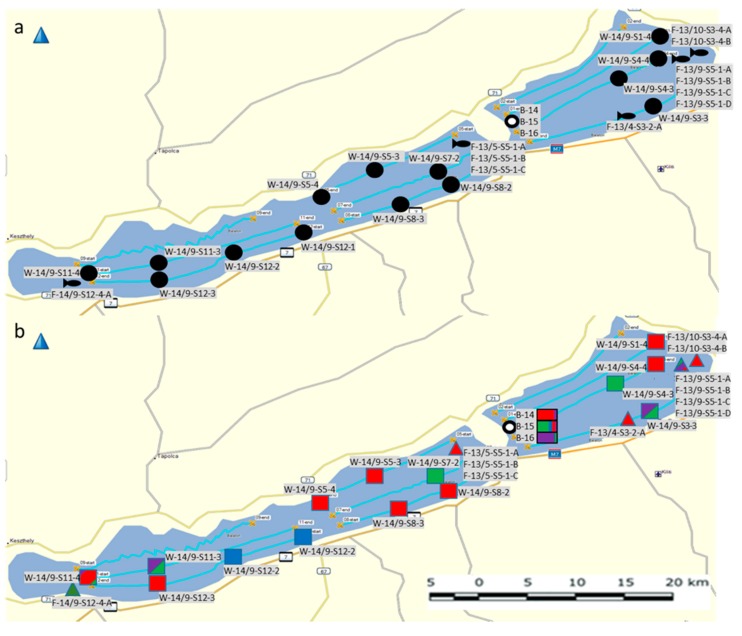
Water samples for chemical and biological analyses were collected by immersion from the upper water layer at the beginning, one-third, two-third, and ending points of each transect (Figure 6a).
Figure 6.
(a) Location of the sampling sites for the isolated Microcystis strains originating from the pelagic plankton samples:  ; hindgut content of bigheaded carp:
; hindgut content of bigheaded carp:  ; and collected bloom samples:
; and collected bloom samples:  . (b): Relative abundance of peptide families (microginins: red; anabaenopeptins: green; microcystins: blue; unidentified peptides: magenta) at the sampling sites: Pelagic plankton samples:
. (b): Relative abundance of peptide families (microginins: red; anabaenopeptins: green; microcystins: blue; unidentified peptides: magenta) at the sampling sites: Pelagic plankton samples:  ; hindgut content of bigheaded carp:
; hindgut content of bigheaded carp:  , and collected bloom samples:
, and collected bloom samples:  .
.
Cyanobacterial bloom samples were collected from blooming waters of Microcystis morphotypes during the summer season (July–August) from 2014 to 2016. Twenty-five isolated strains (11 from the hindgut of bigheaded carps and 14 from free living pelagic plankton) and three collected bloom samples were analyzed in this study. Their origin and localization are shown on the map in Figure 6.
Gut content and water samples were incubated in nitrate containing Allen medium [106] for 5 days and the visible Microcystis colonies were collected and inoculated in nitrate-containing medium at 26 °C under continuous illumination (100 lux m−2s−1) for a week. Prior to the molecular analyses, the collected bloom samples and the cyanobacterial strains were studied using light microscopy.
Cells were collected by centrifugation (J-10 rotor of Beckman Avanti J-25; 4500× g), lyophilized (Christ Alpha 1-2 LD plus), and then 25 mg of each sample were extracted with 80% methanol. After centrifugation (J-18 rotor of Beckman Avanti J-25; 21,000× g), the supernatant was analyzed by HPLC-ESI-MS/MS in positive ion mode.
4.2. Identification of Cyanobacterial Peptides
The optimal ESI ionization parameters were as follows: heater temperature, 250 °C; sheath gas, N2; flow rate, 10 arbitrary units (arb); aux gas flow rate, 5 arb; spray voltage, 5 kV; capillary temperature, 375 °C; capillary voltage, 35.00 V. Sample measurement was run in positive ion mode (MS). The LC-MS measurements were run on a Thermo Accela HPLC (column: Kinetex XB-C18 100 mm × 2.1 mm × 2.6 µm) attached to a Thermo LTQ XL Linear Ion Trap Mass Spectrometer. Gradient components were (A) water with 0.1% HCOOH and (B) MeCN with 0.1% HCOOH. The time program was 10–70% B: 0–10 min, 70–100% B: 10–11 min, 100% B 11–16 min, 100–10% B: 16–18 min, 10% B: 18–20 min. The injected sample amount was 1.0 µL in all cases.
MS data were processed in Thermo Excalibur version 2.2 SP1.48, and MZmine 2.11 freeware. Identification of secondary metabolites from the MS/MS fragmentation patterns was based on literary data [3,49,51,107].
4.3. Statistics
Identified peptides were integrated using targeted peak search in mzMine 2.11 [108]. Thereafter, raw metabolite abundances were scaled and centered separately for each feature in R 3.5.0 [109]. The dataset was hierarchically clustered in both dimensions (samples, metabolites) using the Minkowski distance as the distance measure and Ward’s method. The order of appearance on the presented heatmap’s axes followed that from the clustering. The color strength was proportional to the log10 transformed raw (non-scaled) metabolite abundances, while the color hue was a function of metabolite class: ANA, red; MCY, blue; MG, red; and other peptides, magenta.
The presence of chemotypes was shown by bootstrapped hierarchical clustering analysis of the cyanobacterial lines’ scaled and centered natural compound abundance values using the Minkowski distance and Ward’s hierarchical clustering, bootstrapping N = 1 × 106. The calculation was done with the ‘pvclust’ package in R 3.5.2 [110]. To test the statistical significance of chemical patterns and other variables (such as origin, taxonomic position), principal component score values were subjected to the Kruskal–Wallis significance test, with the variable studied being the group-determining factor. This approach allowed for much more statistical power than that of the direct statistical analyses for all metabolites.
4.4. Phylogenetic Analysis
To explore the phylogenetic relationships between Microcystis strains, amplification and sequence analysis of the 16S–23S internal transcribed spacer region was carried out. DNA amplification was performed by PCR using primers MITS-F (5′-AAGGGAGACCTAATTCVGGT-3′) and MITS-R (5′-TTGCGGTCYTCTTTTTTGGC-3′) [20] in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) with the following temperature protocol: Initial denaturation at 95 °C for 5 min, followed by 30 amplification cycles of 30 s at 94 °C, 30 s at 55 °C, and 30 s at 72 °C, followed by a final extension at 72 °C for 3 min. The PCR reaction mixture contained 200 μM of each deoxynucleoside triphosphate, 1 U of LC Taq DNA Polymerase (recombinant) (Fermentas, Vilnius, Lithuania), 1× Taq buffer with (NH4)2SO4 (Fermentas, Vilnius, Lithuania), 2 mM MgCl2, 0.3 μM of each primer, and about 20 ng of genomic DNA template in a total volume of 50 μL. PCR products were checked on a 1% agarose gel stained with Eco Safe DNA dye (Pacific Image Electronics, New Taipei City, Taiwan), and visualized using UV excitation.
Sequence analysis of the obtained PCR products was accomplished by Sanger sequencing at LGC Genomics (Queens Road, Teddington, Middlesex, UK), using the MITS-F primer.
The phylogenetic dendrogram of Microcystis-related strains was constructed using MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets [111] software. The evolutionary history was inferred by using the maximum likelihood method based on the Jukes–Cantor model [112]. The tree with the highest log likelihood (−716.14) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then by selecting the topology with a superior log likelihood value. A discrete Gamma distribution was used to model the evolutionary rate differences among the sites (five categories (+G, parameter = 0.5472)). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 43.72% sites). The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 75 nucleotide sequences. All positions containing gaps and missing data were eliminated. A total of 239 positions were in the final dataset [111].
Acknowledgments
This work was supported by the Hungarian scientific grants NKFIH K119647 to GV and co-supported by the EFOP-3.6.1-16-2016-00022 and GINOP-2.3.3-15-2016-00021 projects and 20428-3/2018/FEKUTSTRAT.
Appendix A
Table A1.
Product ion data for the microginin type peptides. Masses are given in Dalton rounded to the nearest integer. X1′ indicates core fragment of Ahda after abstraction of the side chain (m = 58 Da). Leucine and isoleucine cannot be distinguished from the LC-MS/MS data, these amino acids have been deduced from the nearest literary results.
| Compound | [M + H]+ m/z | RT min | Amino Acid Sequence | X1-X2-X3-X4 | -H2O | X1’-X2-X3-X4 | -H2O | X1-X2-X3 | -H2O | X1’-X2-X3 | -H2O | X1-X2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG 565 | 565.5 | 6.1 | Ahda-Thr-Pro-Tyr | 384 | 366 | 257 | 239 | 287 | ||||
| MG 549 | 549.5 | 6.2 | Ahda-Ala-MePro-Tyr | 368 | nd | 241 | nd | 257 | ||||
| MG 579 | 579.6 | 6.4 | MeAhda-Thr-Pro-Tyr | 398 | 380 | 257 | 239 | 301 | ||||
| MG 579 | 579.6 | 6.3 | Ahda-Thr-MePro-Tyr | 398 | 380 | 271 | 253 | 287 | ||||
| MG 581 | 581.5 | 5.5 | Ahda-Thr-Pro-OHTyr | 384 | 366 | 257 | 239 | 287 | ||||
| MG 595 | 595.5 | 5.7 | Ahda-Thr-MePro-OHTyr | 398 | 380 | 271 | 253 | 287 | ||||
| MG FR3 | 728.6 | 6.3 | Ahda-Thr-Pro-Tyr-Tyr | 547 | 529 | 420 | 402 | 384 | 366 | 257 | 239 | 287 |
| MG 712 | 712.6 | 6.4 | Ahda-Ala-MePro-Tyr-Tyr | 531 | 513 | 404 | nd | 368 | 350 | 241 | 223 | 257 |
| MG FR4 | 742.6 | 6.6 | MeAhda-Thr-Pro-Tyr-Tyr | 561 | 543 | 420 | nd | 398 | 380 | 257 | 239 | 301 |
| MG 744 | 744.6 | 6 | Ahda-Thr-Pro-OHTyr-Tyr | 547 | 529 | nd | nd | 384 | 366 | 257 | 239 | 287 |
| MG 754 | 754.6 | 6.7 | Ahda-Leu-Pro-Hty-Tyr | 573 | nd | 446 | nd | 396 | nd | 269 | 252 | 299 |
| MG 607 | 607.5 | 6.4 | Ahda-Leu-MeVal-Hty | 412 | 394 | 285 | 267 | 299 | ||||
| MG 621 | 621.6 | 6.4 | MeAhda-Leu-MeVal-Hty | 426 | 408 | 285 | 267 | 313 | ||||
| MG 591 | 591.5 | 7.4 | MeAhda-Leu-MeVal-Phe | 426 | 408 | 285 | 267 | 313 | ||||
| MG 770 | 770.6 | 6.7 | Ahda-Leu-MeVal-Hty-Tyr | 589 | 571 | 462 | 444 | 412 | 394 | 285 | 267 | 299 |
| MG 784 | 784.6 | 6.7 | MeAhda-Leu-MeVal-Hty-Tyr | 603 | 585 | 462 | nd | 426 | 408 | 285 | 267 | 313 |
| MG 798 | 798.6 | 7 | Ahda-Leu-MeLeu-Hty-Tyr | 617 | 599 | 476 | 458 | 440 | 422 | 299 | 281 | 313 |
Table A2.
Product ion data for anabaenopeptin type peptides. Masses are given in Dalton rounded to the nearest integer. Leucine and isoleucine cannot be distinguished from the LC-MS/MS data, these amino acids have been deduced from the nearest literary results.
| Compound | [M + H]+ m/z | RT min | Amino Acid Sequence | Ring-CO | Ring | X6-X2-X3-X4 | X5-X6-X2-X3 | X2-X3-X4 |
|---|---|---|---|---|---|---|---|---|
| ANA B | 837.7 | 5 | Arg-CO-[Lys-Val-Hty-MeAla-Phe] | 663 | 637 | 552 | 460 | 405 |
| ANA F | 851.7 | 5.3 | Arg-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA A | 844.7 | 6.6 | Tyr-CO-[Lys-Val-Hty-MeAla-Phe] | 663 | 637 | 552 | 460 | 405 |
| OSC Y | 858.6 | 7 | Tyr-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA 828 | 828.6 | 7.7 | Tyr-CO-[Lys-Val-Hph-MeAla-Phe] | 647 | 621 | 536 | 460 | 389 |
| ANA 842 | 842.7 | 8 | Tyr-CO-[Lys-Ile-Hph-MeAla-Phe] | 661 | 635 | 550 | 474 | 403 |
| ANA 916 | 916.9 | 6.9 | Tyr-CO-[Lys-Val-Hty-MeHty-Ile] | 735 | 709 | 518 | 532 | 405 |
| ANA 930 | 930.6 | 7.1 | Tyr-CO-[Lys-Ile-Hty-MeHty-Ile] | 749 | 723 | 532 | 546 | 419 |
| ANA 852 | 852.8 | 7.3 | Tyr-CO-[Lys-X3-X4-X5-X6] | 733 | 707 | nd | nd | nd |
| ANA 866 | 866.7 | 7.6 | Tyr-CO-[Lys-X3-X4-X5-X6] | 747 | 721 | nd | nd | nd |
| ANA 856 | 856.7 | 8.2 | Tyr-CO-[Lys-X3-X4-X5-X6] | 711 | 685 | nd | nd | nd |
| ANA 870 | 870.7 | 8.5 | Tyr-CO-[Lys-X3-X4-X5-X6] | 757 | 731 | nd | nd | nd |
| ANA 872 | 872.6 | 7.2 | Tyr-CO-[Lys-X3-X4-X5-X6] | 679 | 653 | nd | nd | nd |
| ANA 886 | 886.7 | 7.4 | MeHty-CO-[Lys-Val-Hty-MeAla-MeLeu] | 643 | 617 | 532 | 440 | 405 |
| ANA 900 | 900.7 | 7.8 | MeHty-CO-[Lys-Ile-Hty-MeAla-MeLeu] | 657 | 631 | 546 | 454 | 419 |
| ANA 914 | 914.8 | 8 | MeHty-CO-[Lys-Val-Hph-MeAla-Phe] | 647 | 621 | 536 | 460 | 389 |
| ANA 928 | 928.7 | 7.9 | MeHty-CO-[Lys-Ile-Hph-MeAla-Phe] | 661 | 635 | 550 | 474 | 403 |
| ANA 892 | 892.7 | 6 | MeHty-CO-[Lys-Val-Hty-MeAla-Phe] | 663 | 637 | 552 | 460 | 405 |
| ANA 938 | 938.5 | 7.7 | MeHty-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA 860 | 860.7 | 6 | MeHty-CO-[Lys-Ile-Hty-MeAla-MePhe] | 691 | 665 | 580 | 488 | 419 |
| ANA 888 | 888.7 | 6.6 | MeHty-CO-[Lys-X3-X4-X5-X6] | 679 | 653 | nd | nd | nd |
| ANA 902 | 902.6 | 6.8 | MeHty-CO-[Lys-X3-X4-X5-X6] | 693 | 667 | nd | nd | nd |
| ANA 904 | 904.6 | 6.6 | MeHty-CO-[Lys-X3-X4-X5-X6] | 695 | 669 | nd | nd | nd |
| ANA 904 | 904.7 | 7.4 | MeHty-CO-[Lys-X3-X4-X5-X6] | 695 | 669 | nd | nd | nd |
| ANA 934 | 934.6 | 6.9 | MeHty-CO-[Lys-X3-X4-X5-X6] | 725 | 699 | nd | nd | nd |
| ANA 854 | 854.6 | 8.1 | X1-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA 814 | 814.7 | 7.6 | X1-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA 888 | 888.7 | 6.2 | X1-CO-[Lys-Val-Hty-MeAla-Phe] | 663 | 637 | 552 | 460 | 405 |
| ANA 920 | 920.7 | 8 | X1-CO-[Lys-Ile-Hty-MeAla-Phe] | 677 | 651 | 566 | 474 | 419 |
| ANA 892 | 892.6 | 7.4 | X1-CO-[Lys-X3-X4-X5-X6] | 677 | 651 | nd | nd | nd |
| ANA 984 | 984.6 | 7.7 | X1-CO-[Lys-X3-X4-X5-X6] | 677 | 651 | nd | nd | nd |
| ANA 902 | 902.7 | 6.5 | X1-CO-[Lys-X3-X4-X5-X6] | 677 | 651 | nd | nd | nd |
| ANA 918 | 918.6 | 6.7 | X1-CO-[Lys-X3-X4-X5-X6] | 677 | 651 | nd | nd | nd |
| ANA 905 | 905.2 | 6.5 | X1-CO-[Lys-X3-X4-X5-X6] | 679 | 653 | nd | nd | nd |
| ANA 922 | 922.1 | 6.6 | X1-CO-[Lys-X3-X4-X5-X6] | 712 | 686 | nd | nd | nd |
| ANA 904 | 904.7 | 5.8 | X1-CO-[Lys-X3-X4-X5-X6] | 721 | 695 | nd | nd | nd |
Table A3.
Product ion data for microcystin type peptides. Masses are given in Dalton rounded to the nearest integer.
| Compound | [M + H]+ m/z | RT min | Amino Acid Sequence | X4-X5-X6-X7-X1-X2 | X3-X4-X5-X6 | -H2O | X4-X5-X6-X7 | X4-X5-X6 | X7-X1-X2-X3-X4-NH2 | X7-X1-X2-X3-X4 | X1-X2-X3-X4 | X4-X5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCY-LW | 513.3 1 | 5.4 | [Ala-Leu-MeAsp-Trp-Adda-Glu-MeDha] | 896 | 758 | 740 | 712 | 629 | 600 | 583 | 500 | 500 |
| MCY-RR | 520.3 1 | 5.6 | [Ala-Arg-MeAsp-Arg-Adda-Glu-MeDha] | 910 | 728 | 710 | 682 | 599 | 614 | 597 | 514 | 470 |
| [D-Asp3]MCY-LR | 981.9 | 6.6 | [Ala-Leu-Asp-Arg-Adda-Glu-MeDha] | 866 | 714 | 696 | 682 | 599 | 556 | 539 | 456 | 470 |
| MCY-LR | 995.8 | 6.8 | [Ala-Leu-MeAsp-Arg-Adda-Glu-MeDha] | 866 | 728 | 710 | 682 | 599 | 570 | 553 | 470 | 470 |
| MCY-HilR | 1009.9 | 7 | [Ala-HIle-MeAsp-Arg-Adda-Glu-MeDha] | 880 | 728 | 710 | 682 | 599 | 584 | 567 | 484 | 470 |
| [MeSer7]MCY-LR | 1014 | 6.6 | [Ala-Leu-MeAsp-Arg-Adda-Glu-MeSer] | 884 | 728 | 710 | 700 | 599 | 588 | 571 | 470 | 470 |
| [Dha7]MCY-FR | 1015.8 | 7 | [Ala-Phe-MeAsp-Arg-Adda-Glu-Dha] | 886 | 728 | 710 | 668 | 599 | 590 | 573 | 504 | 470 |
| MCY-FR | 1029.7 | 7.1 | [Ala-Phe-MeAsp-Arg-Adda-Glu-MeDha] | 900 | 728 | 710 | 682 | 599 | 604 | 587 | 504 | 470 |
| [Dha7]MCY-YR | 1031.9 | 6.5 | [Ala-Tyr-MeAsp-Arg-Adda-Glu-Dha] | 902 | 728 | 710 | 668 | 599 | 606 | 589 | 520 | 470 |
| MCY-YR | 1045.5 | 6.6 | [Ala-Tyr-MeAsp-Arg-Adda-Glu-MeDha] | 916 | 728 | 710 | 682 | 599 | 620 | 603 | 520 | 470 |
| MCY-(H4)YR | 1049.7 | 6.3 | [Ala-H4Tyr-MeAsp-Arg-Adda-Glu-MeDha] | 920 | 728 | 710 | 682 | 599 | 624 | 607 | 524 | 470 |
| MCY-WR | 1068.8 | 7.2 | [Ala-Trp-MeAsp-Arg-Adda-Glu-MeDha] | 939 | 728 | 710 | 682 | 599 | 643 | 626 | 543 | 470 |
| [MeSer7]MCY-WR | 1086.9 | 6.9 | [Ala-Trp-MeAsp-Arg-Adda-Glu-MeSer] | 957 | 728 | 710 | 700 | 599 | 661 | 644 | 543 | 470 |
1 Data given in [M + 2H]2+.
Table A4.
Product ion data for unidentified peptide fragments. Masses are given in Dalton rounded to the nearest integer. Leucine and isoleucine cannot be distinguished from the LC-MS/MS data.
| Compound | [M + H]+ m/z | RT min | Amino Acid Sequence | Xn | Xn+1 |
|---|---|---|---|---|---|
| PEP 535 | 535.4 | 6.7 | Xn-Leu | 404 | |
| PEP 539 | 539.4 | 9.4 | Xn-Leu | 408 | |
| PEP 541 | 541.4 | 5 | Xn-Phe | 376 | |
| PEP 565 | 565.5 | 5 | Xn-MeLeu | 420 | |
| PEP 581 | 581.5 | 5.8 | Xn-Tyr | 400 | |
| PEP 593 | 593.5 | 6.1 | Xn-Met-MeLeu | 317 | 448 |
| PEP 756 | 756.6 | 6.8 | Xn-Tyr-Tyr | 412 | 575 |
| PEP 593 | 593.5 | 6.8 | Xn-Tyr | 412 | |
| PEP 594 | 594.4 | 5.2 | Xn -Thr-Leu | 362 | 463 |
| PEP 712 | 712.6 | 5.4 | Xn-MeLeu-Tyr | 404 | 531 |
| PEP 714 | 714.6 | 5.2 | Xn-MeLeu-Tyr | 406 | 533 |
| PEP 728 | 728.6 | 5.4 | Xn-MeLeu-Tyr | 420 | 547 |
| PEP 740 | 740.7 | 8.4 | Xn-MeLeu-Tyr | 432 | 559 |
| PEP 742 | 742.6 | 5.5 | Xn-Met | 593 | |
| PEP 744 | 744.5 | 5 | Xn-MeLeu | 599 | |
| PEP 714 | 714.6 | 5.5 | Xn-MeLeu-Tyr | 406 | 533 |
| PEP 728 | 728.6 | 5.7 | Xn-MeLeu-Tyr | 420 | 547 |
Figure A1.
Phylogenetic dendrogram of the studied Microcystis strains. The dendrogram was based on the 16S–23S internal transcribed spacer region sequences and the evolutionary history was inferred using the maximum likelihood method based on the Jukes–Cantor model.
Figure A2.
LC/MS chromatograms of the bloom samples 2014 (A); 2015 (B); 2016 (C); and the MS/MS fragmentation pattern of the most abundant peak.
Author Contributions
Conceptualization: G.V.; Formal analysis: A.K.-S.; Investigation: M.R., S.G., G.B., and G.V.; Methodology: M.R., A.K.-S., S.G., G.B., Z.V., A.K.B., G.K., G.B., and A.Z.U.; Supervision: G.V.; Writing—original draft: M.R. and G.V.; Writing—review & editing: M.R., G.B., G.B., and G.V.
Funding
This research was funded by the Hungarian scientific grants National Research, Development and Innovation Office (NKFIH K119647), University of Debrecen Debrecen Venture Catapult Program (EFOP-3.6.1-16-2016-00022), Economic Development and Innovation Operational Programme (GINOP-2.3.3-15-2016-00021) and Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary (20428-3/2018/FEKUTSTRAT).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Alimentary tracts of bigheaded carp can supply Microcystis strains that represent pelagic chemotypes. Anabaenopeptin, microginin, and microcystin variants were identified, and heat map clustering visualization was used to compare the identified chemotypes.
References
- 1.Stewart A.K., Ravindra R., Van Wagoner R.M., Wright J.L.C. Metabolomics-Guided Discovery of Microginin Peptides from Cultures of the Cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 2018;81:349–355. doi: 10.1021/acs.jnatprod.7b00829. [DOI] [PubMed] [Google Scholar]
- 2.Lodin-Friedman A., Carmeli S. Microginins from a Microcystis sp. Bloom Material Collected from the Kishon Reservoir, Israel. Mar. Drugs. 2018;16:78. doi: 10.3390/md16030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welker M., von Döhren H. Cyanobacterial Peptides—Nature’s Own Combinatorial Biosynthesis. FEMS Microbiol. Lett. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishida K., Okita Y., Matsuda H., Okino T., Murakami M. Aeruginosins, Protease Inhibitors from the Cyanobacterium Microcystis aeruginosa. Tetrahedron. 1999;55:10971–10988. doi: 10.1016/S0040-4020(99)00621-3. [DOI] [Google Scholar]
- 5.Martin C., Oberer L., Ino T., Konig W.A., Busch M., Weckesser J.C. New Depsipeptides from the Cyanobacterium Microcystis sp. PCC 7806. J. Antibiot. 1993;46:1550–1556. doi: 10.7164/antibiotics.46.1550. [DOI] [PubMed] [Google Scholar]
- 6.Harada K., Fujii K., Shimada T., Suzuki M., Sano H., Adachi K., Carmichael W.W. Two Cyclic Peptides, Anabaenopeptins, a Third Group of Bioactive Compounds from the cyanobacterium Anabaena flos-aquae NRC 525-17. Tetrahedron Lett. 1995;36:1511–1514. doi: 10.1016/0040-4039(95)00073-L. [DOI] [Google Scholar]
- 7.Okino T., Matsuda H., Murakami M., Yamaguchi K. Microginin, an Angiotensin-Converting Enzyme Inhibitor from the Blue-Green Alga Microcystis aeruginosa. Tetrahedron Lett. 1993;34:501–504. doi: 10.1016/0040-4039(93)85112-A. [DOI] [Google Scholar]
- 8.Ishitsuka M.O., Kusumi T., Kakisawa H., Kaya K., Watanabe M.M. Microviridin. A Novel Tricyclic Depsipeptide from the Toxic Cyanobacterium Microcystis viridis. J. Am. Chem. Soc. 1990;112:8180–8182. doi: 10.1021/ja00178a060. [DOI] [Google Scholar]
- 9.Todorova A.K., Jüttner F., Linden A., Pluess T., von Philipsborn W. Nostocyclamide: A New Macrocyclic, Thiazole-Containing Allelochemical from Nostoc sp. 31 (Cyanobacteria) J. Org. Chem. 1995;60:7891–7895. doi: 10.1021/jo00129a032. [DOI] [Google Scholar]
- 10.Carmichael W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992;72:445–459. doi: 10.1111/j.1365-2672.1992.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Dittmann E., Gugger M., Sivonen K., Fewer D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015;23:642–652. doi: 10.1016/j.tim.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Agha R., Quesada A. Oligopeptides as Biomarkers of Cyanobacterial Subpopulations. Toward an Understanding of their Biological Role. Toxins. 2014;6:1929–1950. doi: 10.3390/toxins6061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welker M., Sejnohova L., Nemethova D., von Döhren H., Jarkovsky J., Marsalek B. Seasonal Shifts in Chemotype Composition of Microcystis sp. Communities in the Pelagial and the Sediment of a Shallow Reservoir. Limnol. Oceanogr. 2007;52:609–619. doi: 10.4319/lo.2007.52.2.0609. [DOI] [Google Scholar]
- 14.Agha R., Lezcano M.À., del Mar Labrador M., Cirés S., Quesada A. Seasonal Dynamics and Sedimentation Patterns of Microcystis Oligopeptide-Based Chemotypes Reveal Subpopulations with Different Ecological Traits. Limnol. Oceanogr. 2014;59:861–871. doi: 10.4319/lo.2014.59.3.0861. [DOI] [Google Scholar]
- 15.Neilan B.A., Jacobs D., Goodman A.E. Genetic Diversity and Phylogeny of Toxic Cyanobacteria Determined by DNA Polymorphisms within the Phycocyanin Locus. Appl. Environ. Microbiol. 1995;61:3875–3883. doi: 10.1128/aem.61.11.3875-3883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo R., Kagiya G., Hiroishi S., Watanabe M. Genetic Typing of a Bloom-Forming Cyanobacterial Genus Microcystis in Japan using 16S rRNA Gene Sequence Analysis. Plankton Biol. Ecol. 2000;47:1–6. [Google Scholar]
- 17.Bittencourt-Oliveira M.C., Oliveira M.C., Bolch C.J.S. Genetic Variability of Brazilian Strains of the Microcystis aeruginosa Complex (Cyanobacteria/Cyanophyceae) using the Phycocyanin Intergenic Spacer and Flanking Regions (cpcBA) J. Phycol. 2001;37:810–818. doi: 10.1046/j.1529-8817.2001.00102.x. [DOI] [Google Scholar]
- 18.Wilson A.E., Sarnelle O., Neilan B.A., Salmon T.P., Gehringer M.M., Hay M.E. Genetic Variation of the Bloom-Forming Cyanobacterium Microcystis aeruginosa within and among Lakes: Implications for Harmful Algal Blooms. Appl. Environ. Microbiol. 2005;71:6126–6133. doi: 10.1128/AEM.71.10.6126-6133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Herry S., Nasri H., Bouaïcha N. Morphological and Phylogenetic Analysis of Colonies of Microcystis Morphospecies Isolated from the Lebna Dam, Tunisia. Afr. J. Microb. Res. 2008;2:340–348. [Google Scholar]
- 20.Yoshida M., Yoshida T., Satomi M., Takashima Y., Hosoda N., Hiroishi S. Intra-Specific Phenotypic and Genotypic Variation in Toxic Cyanobacterial Microcystis Strains. J. Appl. Microbiol. 2008;105:407–415. doi: 10.1111/j.1365-2672.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe Y., Kasai F., Watanabe M.M. Fine-Scale Spatial and Temporal Genetic Differentiation of Water Bloom-Forming Cyanobacterium Microcystis aeruginosa: Revealed by Multilocus Sequence Typing. Environ. Microbiol. Rep. 2009;1:575–582. doi: 10.1111/j.1758-2229.2009.00088.x. [DOI] [PubMed] [Google Scholar]
- 22.Fathalli A., Jenhania A.B.R., Moreira C., Welker M., Romdhane M., Antunes A., Vasconcelos V. Molecular and Phylogenetic Characterization of Potentially Toxic Cyanobacteria in Tunisian Freshwaters. Syst. Appl. Microbiol. 2011;34:303–310. doi: 10.1016/j.syapm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Gaevsky N.A., Kolmakov V.I., Belykh O.I., Tikhonova I.V., Joung Y., Ahn T.S., Nabatova V.A., Gladkikh A.S. Ecological Development and Genetic Diversity of Microcystis aeruginosa from Artificial Reservoir in Russia. J. Microbiol. 2011;49:714–720. doi: 10.1007/s12275-011-0523-5. [DOI] [PubMed] [Google Scholar]
- 24.Lepère C., Wilmotte A., Meyer B. Molecular Diversity of Microcystis Strains (Cyanophyceae, Chroococcales) Based on 16S rDNA Sequences. Syst. Geogr. Plants. 2000;70:275–283. doi: 10.2307/3668646. [DOI] [Google Scholar]
- 25.Litvaitis M.K. A Molecular Test of Cyanobacterial Phylogeny: Inferences from Constraint Analyses. Hydrobiologia. 2002;468:135–145. doi: 10.1023/A:1015262621848. [DOI] [Google Scholar]
- 26.Otsuka S., Suda S., Shibata S., Oyaizu H., Matsumoto S., Watanabe M.M. A Proposal for the Unification of Five Species of the Cyanobacterial Genus Microcystis Kützing Ex Lemmermann 1907 Under the Rules of the Bacteriological Code. Int. J. Syst. Evol. Microbiol. 2001;51:873–879. doi: 10.1099/00207713-51-3-873. [DOI] [PubMed] [Google Scholar]
- 27.O’Neil J.M., Davis T.W., Burford M.A., Gobler C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae. 2012;14:313–334. doi: 10.1016/j.hal.2011.10.027. [DOI] [Google Scholar]
- 28.Ribeiro K.F., Duarte L., Crossetti L.O. Everything is Not Everywhere: A Tale on the Biogeography of Cyanobacteria. Hydrobiologia. 2018;820:23–48. doi: 10.1007/s10750-018-3669-x. [DOI] [Google Scholar]
- 29.Padisák J., Vasas G., Borics G. Phycogeography of Freshwater Phytoplankton: Traditional Knowledge and New Molecular Tools. Hydrobiologia. 2016;764:3–27. doi: 10.1007/s10750-015-2259-4. [DOI] [Google Scholar]
- 30.Traveset A., Richardson D.M. Biological Invasions as Disruptors of Plant Reproductive Mutualisms. Trends Ecol. Evol. 2006;21:208–216. doi: 10.1016/j.tree.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Dufour P., Sarazin G., Quiblier C., Sane S., Leboulanger C. Cascading Nutrient Limitation of the Cyanobacterium Cylindrospermopsis raciborskii in a Sahelian Lake (North Senegal) Aquat. Microb. Ecol. 2006;44:219–230. doi: 10.3354/ame044219. [DOI] [Google Scholar]
- 32.Borics G., Grigorszky I., Szabó S., Padisák J. Phytoplankton Associations in a Small Hypertrophic Fishpond in East Hungary during a Change from Bottom-Up to Top-Down Control. Hydrobiologia. 2000;424:79–90. doi: 10.1023/A:1003948827254. [DOI] [Google Scholar]
- 33.Borics G., Tóthmérész B., Lukács B.A., Várbíró G. Functional Groups of Phytoplankton Shaping Diversity of Shallow Lake Ecosystems. Hydrobiologia. 2012;698:251–262. doi: 10.1007/s10750-012-1129-6. [DOI] [Google Scholar]
- 34.Jennings D.P. Bighead Carp (Hypophthalmichthys nobilis): A Biological Synopsis. Fish. Wildl. Serv. Biol. Rep. 1988;88:1–47. [Google Scholar]
- 35.Kolar C.S., Chapman D.C., Courtenay J.W.R., Housel C.M., Williams J.D., Jennings D.P. Bigheaded Carps: A Biological Synopsis and Environmental Risk Assessment. Volume 33. American Fisheries Society Special Publication; Bethesda, MD, USA: 2007. p. 204. [Google Scholar]
- 36.Lieberman D.M. Use of Silver Carp (Hypophthalmichthys molotrix) and Bighead Carp (Aristichthys nobilis) for Algae Control in a Small Pond: Changes in Water Quality. J. Freshw. Ecol. 1996;11:391–397. doi: 10.1080/02705060.1996.9664466. [DOI] [Google Scholar]
- 37.Cremer M.C., Smitherman R.O. Food Habits and Growth of Silver and Bighead Carp in Cages and Ponds. Aquaculture. 1980;20:57–64. doi: 10.1016/0044-8486(80)90061-7. [DOI] [Google Scholar]
- 38.Xie P., Liu J. Practical Success of Biomanipulation using Filter-Feeding Fish to Control Cyanobacteria Blooms: A Synthesis of Decades of Research and Application in a Subtropical Hypereutrophic Lake. Sci. World J. 2001;1:337–356. doi: 10.1100/tsw.2001.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampson S.J., Chick J.H., Pegg M.A. Diet Overlap among Two Asian Carp and Three Native Fishes in Backwater Lakes on the Illinois and Mississippi Rivers. Biol. Invasions. 2009;11:483–496. doi: 10.1007/s10530-008-9265-7. [DOI] [Google Scholar]
- 40.Radke R.J., Kahl U. Effects of a Filter-Feeding Fish Silver Carp, Hypophthalmichthys molitrix (Val.)] on Phyto- and Zooplankton in a Mesotrophic Reservoir: Results from an Enclosure Experiment. Freshw. Biol. 2002;47:2337–2344. doi: 10.1046/j.1365-2427.2002.00993.x. [DOI] [Google Scholar]
- 41.Triest L., Stiers I., Van Onsem S. Biomanipulation as a Nature-Based Solution to Reduce Cyanobacterial Blooms. Aquat. Ecol. 2016;50:461–483. doi: 10.1007/s10452-015-9548-x. [DOI] [Google Scholar]
- 42.Mátyás K., Oldal I., Korponai J., Tátrai I., Paulovits G. Indirect Effect of Different Fish Communities on Nutrient Chlorophyll Relationship in Shallow Hypertrophic Water Quality Reservoirs. Hydrobiologia. 2003;504:231–239. doi: 10.1023/B:HYDR.0000008523.83752.14. [DOI] [Google Scholar]
- 43.Ma H., Cui F., Liu Z., Fan Z., He W., Yin P. Effect of Filter-Feeding Fish Silver Carp on Phytoplankton Species and Size Distribution in Surface Water: A Field Study in Water Works. J. Environ. Sci. 2010;22:161–167. doi: 10.1016/S1001-0742(09)60088-7. [DOI] [PubMed] [Google Scholar]
- 44.Miura T., Wang J. Chlorophyll a found in Feces of Phytoplanktivorous Cyprinids and its Photosynthetic Activity. Verh. Int. Ver. Limnol. 1985;22:2636–2642. doi: 10.1080/03680770.1983.11897742. [DOI] [Google Scholar]
- 45.Zeng Q., Gu X., Mao Z., Chen X. In Situ Growth and Photosynthetic Activity of Cyanobacteria and Phytoplankton Dynamics After Passage through the Gut of Silver Carp (Hypophthalmichthys molitrix), Bighead Carp (Aristichthys nobilis), and Nile Tilapia (Oreochromis niloticus) Hydrobiologia. 2014;736:51–60. doi: 10.1007/s10750-014-1886-5. [DOI] [Google Scholar]
- 46.Lewin W.C., Kamjunke N., Mehner T. Phosphorus Uptake by Microcystis during Passage through Fish Guts. Limnol. Oceanogr. 2003;48:2392–2396. doi: 10.4319/lo.2003.48.6.2392. [DOI] [Google Scholar]
- 47.Görgényi J., Boros G., Vitál Z., Mozsár A., Várbíró G., Vasas G., Borics G. The Role of Filter-Feeding Asian Carps in Algal Dispersion. Hydrobiologia. 2016;764:115–126. doi: 10.1007/s10750-015-2285-2. [DOI] [Google Scholar]
- 48.Komárek J., Anagnostidis K. Cyanoprokaryota: Teil 1/Part. 1: Chroococcales (Suesswasserflora Von Mitteleuropa) Springer Spektrum; Heidelberg, Germany: 2008. p. 548. [Google Scholar]
- 49.Welker M., Maršálek B., Šejnohová L., von Döhren H. Detection and Identification of Oligopeptides in Microcystis (Cyanobacteria) Colonies: Toward an Understanding of Metabolic Diversity. Peptides. 2006;27:2090–2103. doi: 10.1016/j.peptides.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Ishida K., Kato T., Murakami M., Watanabe M., Watanabe M.F. Microginins, Zinc Metalloproteases Inhibitors from the Cyanobacterium Microcystis aeruginosa. Tetrahedron. 2000;56:8643–8656. doi: 10.1016/S0040-4020(00)00770-5. [DOI] [Google Scholar]
- 51.Mayumi T., Kato H., Kawasaki Y., Harada K. Formation of Diagnostic Product Ions from Cyanobacterial Cyclic Peptides by the Two-Bond Fission Mechanism using Ion Trap Liquid Chromatography/Multi-Stage Mass Spectrometry. Rapid. Commun. Mass. Spectrom. 2007;21:1025–1033. doi: 10.1002/rcm.2920. [DOI] [PubMed] [Google Scholar]
- 52.Poon G.K., Griggs L.J., Edwards C., Beattie K.A., Codd G.A. Liquid Chromatography-Electrospray Ionization-Mass Spectrometry of Cyanobacterial Toxins. J. Chromatogr. A. 1993;628:215–233. doi: 10.1016/0021-9673(93)80005-S. [DOI] [Google Scholar]
- 53.Miles C.O., Sandvik M., Nonga H.E., Rundberget T., Wilkins A.L., Rise F., Ballot A. Identification of Microcystins in a Lake Victoria Cyanobacterial Bloom using LC–MS with Thiol Derivatization. Toxicon. 2013;70:21–31. doi: 10.1016/j.toxicon.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Hajnal É., Padisák J. Analysis of Long-Term Ecological Status of Lake Balaton Based on the ALMOBAL Phytoplankton Database. Hydrobiologia. 2008;599:227–237. doi: 10.1007/s10750-007-9207-x. [DOI] [Google Scholar]
- 55.Padisák J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya Et Subba Raju, an Expanding, Highly Adaptive Cyanobacterium: Worldwide Distribution and Review of its Ecology. Arch. Hydrobiol. Suppl. Monogr. Stud. 1997;107:563–593. [Google Scholar]
- 56.Meriluoto J., Spoof L., Codd G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. John Wiley & Sons, Ltd.; London, UK: 2017. p. 576. [Google Scholar]
- 57.Komárek J. Die taxonomische Revision der planktischen Blaualgen der Tschechoslowakei. In: Komárek J., Ettl H., editors. Algologische Studien. Academia; Praha, Czech Republic: 1958. pp. 10–206. [Google Scholar]
- 58.Komárek J. A Review of Water-Bloom Forming Microcystis Species, with Regard to Populations from Japan. Arch. Hydrobiol./Algolog. Stud. 1991;64:115–127. [Google Scholar]
- 59.Cronberg G., Komárek J. Planktic Cyanoprokaryotes found in South Swedish Lakes during the XIIth International Symposium on Cyanophyte Research, 1992. Arch. Hydrobiol./Algolog. Stud. 1994;105:323–352. [Google Scholar]
- 60.Otsuka S., Suda S., Li R., Matsumoto S., Watanabe M.M. Morphological Variability of Colonies of Microcystis Morphospecies in Culture. J. Gen. Appl. Microbiol. 2000;46:39–50. doi: 10.2323/jgam.46.39. [DOI] [PubMed] [Google Scholar]
- 61.Komárek J., Komárková J. Review of the European Microcystis Morphospecies (Cyanoprokaryotes) from Nature. Czech. Phycol. 2002;2:1–24. [Google Scholar]
- 62.Otsuka S., Suda S., Li R., Watanabe M., Oyaizu H., Matsumoto S., Watanabe M.M. Phylogenetic Relationships between Toxic and Non-Toxic Strains of the Genus Microcystis Based on 16S to 23S Internal Transcribed Spacer Sequence. FEMS Microbiol. Lett. 1999;172:15–21. doi: 10.1111/j.1574-6968.1999.tb13443.x. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y., Yang F., Liu Y., Wang Z., Wang J., Wang G., Li R. Genetic Diversity of Microcystis Populations in a Bloom and its Relationship to the Environmental Factors in Qinhuai River, China. Microbiol. Res. 2011;167:20–26. doi: 10.1016/j.micres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Neilan B.A., Jacobs D., DelDot T., Blackall L.L., Hawkins P.R., Cox P.T., Goodman A.E. rRNA Sequences and Evolutionary Relationships among Toxic and Nontoxic Cyanobacteria of the Genus Microcystis. Int. J. Syst. Evol. Microbiol. 1997;47:693–697. doi: 10.1099/00207713-47-3-693. [DOI] [PubMed] [Google Scholar]
- 65.Kehr J.C., Dittmann E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life. 2015;5:164–180. doi: 10.3390/life5010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sano T., Usui T., Ueda K., Osada H., Kaya K. Isolation of New Protein Phosphatase Inhibitors from Two Cyanobacteria Species, Planktothrix spp. J. Nat. Prod. 2001;64:1052–1055. doi: 10.1021/np0005356. [DOI] [PubMed] [Google Scholar]
- 67.Murakami M., Suzuki S., Itou Y., Kodani S., Ishida K. New Anabaenopeptins, Potent Carboxypeptidase-A Inhibitors from the Cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000;63:1280–1282. doi: 10.1021/np000120k. [DOI] [PubMed] [Google Scholar]
- 68.Sano T., Kaya K. Oscillamide Y, a Chymotrypsin Inhibitor from Toxic Oscillatoria agardhii. Tetrahedron Lett. 1995;36:5933–5936. doi: 10.1016/0040-4039(95)01198-Q. [DOI] [Google Scholar]
- 69.Harada K.I., Mayumi T., Shimada T., Suzuki M., Kondo F., Watanabe M.F. Occurrence of Four Depsipeptides, Aeruginopeptins, Together with Microcystins from Toxic Cyanobacteria. Tetrahedron Lett. 1993;34:6091–6094. doi: 10.1016/S0040-4039(00)61736-7. [DOI] [Google Scholar]
- 70.Sedmak B., Carmeli S., Elersek T. “Non-Toxic” Cyclic Peptides Induce Lysis of Cyanobacteria-an Effective Cell Population Density Control Mechanism in Cyanobacterial Blooms. Microb. Ecol. 2008;56:201–209. doi: 10.1007/s00248-007-9336-9. [DOI] [PubMed] [Google Scholar]
- 71.Sedmak B., Eleršek T., Grach-Pogrebinsky O., Carmeli S., Sever N., Lah T. Ecotoxicologically Relevant Cyclic Peptides from Cyanobacterial Bloom (Planktothrix Rubescens)—A Threat to Human and Environmental Health. Radiol. Oncol. 2008;42:102–113. doi: 10.2478/v10019-008-0001-9. [DOI] [Google Scholar]
- 72.Elersek T., Bláha L., Mazur-Marzec H., Schmidt W., Carmeli S. Other cyanobacterial bioactive substances. In: Meriluoto J., Spoof L., Codd G.A., editors. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. John Wiley & Sons, Ltd.; London, UK: 2017. pp. 179–196. [Google Scholar]
- 73.Bannage M.E., Burke A.J., Davies S.G., Goodwin C.J. Asymmetric Synthesis of (2S,3R)-3-Amino-2-Hydroxydecanoic Acid: The Unknown Amino Acid Component of Microginin. Tetrahedron Asymmetry. 1994;5:203–206. doi: 10.1016/S0957-4166(00)86173-X. [DOI] [Google Scholar]
- 74.Lifshits M., Zafrir-Ilan E., Raveh A., Carmeli S. Protease Inhibitors from Three Fishpond Water Blooms of Microcystis spp. Tetrahedron. 2011;67:4017–4024. doi: 10.1016/j.tet.2011.04.042. [DOI] [Google Scholar]
- 75.Rohrlack T., Edvardsen B., Skulberg R., Halstvedt C.B., Utkilen H.C., Ptacnik R., Skulberg O.M. Oligopeptide Chemotypes of the Toxic Freshwater Cyanobacterium Planktothrix can Form Sub-Populations with Dissimilar Ecological Traits. Limnol. Oceanogr. 2008;53:1279–1293. doi: 10.4319/lo.2008.53.4.1279. [DOI] [Google Scholar]
- 76.Sakamoto T., Hashimoto A., Yamaba M., Wada N., Yoshida T., Inoue-Sakamoto K., Nishiuchi T., Matsugo S. Four Chemotypes of the Terrestrial Cyanobacterium Nostoc commune Characterized by Differences in the Mycosporine-Like Amino Acids. Phycol. Res. 2018 doi: 10.1111/pre.12333. [DOI] [Google Scholar]
- 77.Sharp K., Arthur K.E., Gu L., Ross C., Harrison G., Gunasekera S.P., Meickle T., Matthew S., Luesch H., Thacker R.W., et al. Phylogenetic and Chemical Diversity of Three Chemotypes of Bloom-Forming Lyngbya species (Cyanobacteria: Oscillatoriales) from Reefs of Southeastern Florida. Appl. Environ. Microbiol. 2009;75:2879–2888. doi: 10.1128/AEM.02656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rastogi R.P., Madamwar D., Incharoensakdi A. Bloom Dynamics of Cyanobacteria and their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015;6:1254. doi: 10.3389/fmicb.2015.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babica P., Bláha L., Maršálek B. Exploring the Natural Role of Microcystins—A Review of Effects on Photoautotrophic Organisms. J. Phycol. 2006;42:9–20. doi: 10.1111/j.1529-8817.2006.00176.x. [DOI] [Google Scholar]
- 80.Agha R., Cires S., Wormer L., Dominguez J.A., Quesada A. Multi-Scale Strategies for the Monitoring of Freshwater Cyanobacteria: Reducing the Sources of Uncertainty. Water Res. 2012;46:3043–3053. doi: 10.1016/j.watres.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Fastner J., Erhard M., von Dohren H. Determination of Oligopeptide Diversity within a Natural Population of Microcystis spp. (Cyanobacteria) by Typing Single Colonies by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. Appl. Environ. Microbiol. 2001;67:5069–5076. doi: 10.1128/AEM.67.11.5069-5076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harustiakova D., Welker M. Chemotype Diversity in Planktothrix rubescens (Cyanobacteria) Populations is Correlated to Lake Depth. Environ. Microbiol. Rep. 2017;9:158–168. doi: 10.1111/1758-2229.12519. [DOI] [PubMed] [Google Scholar]
- 83.Vasas G., Farkas O., Borics G., Felföldi T., Sramkó G., Batta G., Bácsi I., Gonda S. Appearance of Planktothrix rubescens Bloom with D-Asp3, Mdha7]MC-RR in Gravel Pit Pond of a Shallow Lake-Dominated Area. Toxins. 2013;5:2434–2455. doi: 10.3390/toxins5122434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farkas O., Gyémánt G., Hajdú G., Gonda S., Parizsa P., Horgos T., Mosolygó Á., Vasas G. Variability of Microcystins and its Synthetase Gene Cluster in Microcystis and Planktothrix Waterblooms in Shallow Lakes of Hungary. Acta Biol. Hung. 2014;65:227–239. doi: 10.1556/ABiol.65.2014.2.10. [DOI] [PubMed] [Google Scholar]
- 85.Bell T.A.S., Sen-Kilic E., Felfoldi T., Vasas G., Fields M.W., Peyton B.M. Microbial Community Changes during a Toxic Cyanobacterial Bloom in an Alkaline Hungarian Lake. Antonie Van Leeuwenhoek. 2018;111:2425–2440. doi: 10.1007/s10482-018-1132-7. [DOI] [PubMed] [Google Scholar]
- 86.Ujvarosi A.Z., Riba M., Garda T., Gyemant G., Vereb G., M-Hamvas M., Vasas G., Mathe C. Attack of Microcystis aeruginosa Bloom on a Ceratophyllum submersum Field: Ecotoxicological Measurements in Real Environment with Real Microcystin Exposure. Sci. Total Environ. 2019;662:735–745. doi: 10.1016/j.scitotenv.2019.01.226. [DOI] [PubMed] [Google Scholar]
- 87.Sanchis D., Padilla C., Del Campo F.F., Quesada A., Sanz-Alférez S. Phylogenetic and Morphological Analyses of Microcystis Strains (Cyanophyta/Cyanobacteria) from a Spanish Water Reservoir. Nova Hedwig. 2005;81:431–448. doi: 10.1127/0029-5035/2005/0081-0431. [DOI] [Google Scholar]
- 88.Lezcano M.Á., Agha R., Cirés S., Quesada A. Spatial-Temporal Survey of Microcystis Oligopeptide Chemotypes in Reservoirs with Dissimilar Waterbody Features and their Relation to Genetic Variation. Harmful Algae. 2019;81:77–85. doi: 10.1016/j.hal.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 89.Mazur-Marzec H., Kaczkowska M.J., Blaszczyk A., Akcaalan R., Spoof L., Meriluoto J. Diversity of Peptides Produced by Nodularia spumigena from various Geographical Regions. Mar. Drugs. 2013;11:1–19. doi: 10.3390/md11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Specziár A. Fish Fauna of Lake Balaton: Stock Composition, Living Conditions of Fish and Directives of the Modern Utilization of the Fish Stock. Acta Biol. Debr. Suppl. Oecol. Hung. 2010;23:7–185. [Google Scholar]
- 91.Virág A. A Balaton Múltja És Jelene. (The Past and the Present of Lake Balaton) Egri Nyomda Kft; Eger, Hungary: 1998. p. 904. [Google Scholar]
- 92.Boros G., Mozsár A., Vitál Z., Nagy A.S., Specziár A. Growth and Condition Factor of Hybrid (Bighead Hypophthalmichthys nobilis Richardson, 1845 × silver Carp H. Molitrix Valenciennes, 1844) Asian Carps in the Shallow, Oligo-Mesotrophic Lake Balaton. J. Appl. Ichthyol. 2014;30:546–548. doi: 10.1111/jai.12325. [DOI] [Google Scholar]
- 93.Boros G., Vitál Z., Mozsár A., Borics G., Görgényi J., Józsa V., Tóth L., Lehoczky I., Kovács B., Vasas G., et al. Ecological Impacts of Filter-Feeding Asian Carps (Hypophthalmichthys spp.) in Lake Balaton, Hungary. Front. Mar. Sci. 2015 doi: 10.3389/conf.FMARS.2015.03.00019. [DOI] [Google Scholar]
- 94.Battonyai I., Specziár A., Vitál Z., Mozsár A., Görgényi J., Borics G., Tóth L.G., Boros G. Relationship between Gill Raker Morphology and Feeding Habits of Hybrid Bigheaded Carps (Hypophthalmichthys spp.) Knowl. Manag. Aquat. Ecosyst. 2015;416:36. doi: 10.1051/kmae/2015031. [DOI] [Google Scholar]
- 95.Gavel A., Maršálek B., Adámek Z. Viability of Microcystis Colonies is Not Damaged by Silver Carp (Hypophthalmichthys molitrix) Digestion. Algol. Stud. /Arch. Hydrobiol. 2004;113:189–194. doi: 10.1127/1864-1318/2004/0113-0189. [DOI] [Google Scholar]
- 96.Ye L., Amberg J., Chapman D., Gaikowski M., Liu W.T. Fish Gut Microbiota Analysis Differentiates Physiology and Behavior of Invasive Asian Carp and Indigenous American Fish. ISME J. 2014;8:541–551. doi: 10.1038/ismej.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J., Ni J., Li J., Wang C., Li X., Wu S., Zhang T., Yu Y., Yan Q. Comparative Study on Gastrointestinal Microbiota of Eight Fish Species with Different Feeding Habits. J. Appl. Microbiol. 2014;117:1750–1760. doi: 10.1111/jam.12663. [DOI] [PubMed] [Google Scholar]
- 98.Borsodi A.K., Szabó A., Krett G., Felföldi T., Specziár A., Boros G. Gut Content Microbiota of Introduced Bigheaded Carps (Hypophthalmichthys spp.) Inhabiting the Largest Shallow Lake in Central Europe. Microbiol. Res. 2017;195:40–50. doi: 10.1016/j.micres.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Bitterlich G. Digestive Enzyme Pattern of Two Stomachless Filter Feeders, Silver Carp, Hypophthalmichthys molitrix Val., and Bighead Carp, Aristichthys Nobilis Rich. J. Fish. Biol. 2006;27:103–112. doi: 10.1111/j.1095-8649.1985.tb04013.x. [DOI] [Google Scholar]
- 100.Ni S., Gui Y., Liu H. A Comparative Research on Amylase Activities among Grass Carp, Common Carp, Silver Carp, Bighead Carp, Tilapia Nilotica. J. Dalian Fish. Univ. 1992;7:24–31. [Google Scholar]
- 101.Opuszynski K., Shireman J.V. Food Passage Time and Daily Ration of Bighead Carp, Aristichthys nobilis, Kept in Cages. Environ. Biol. Fish. 1991;30:387–393. doi: 10.1007/BF02027982. [DOI] [Google Scholar]
- 102.Williamson C.J., Garvey J.E. Growth, Fecundity, and Diets of Newly Established Silver Carp in the Middle Mississippi River. Trans. Am. Fish. Soc. 2005;134:1423–1430. doi: 10.1577/T04-106.1. [DOI] [Google Scholar]
- 103.Kolmakov V.I., Gladyshev M.I., Kravchuk E.S., Chuprov S.M., Anishchenkob O.V., Ivanova E.A., Trusova M.Y. Species-Specific Stimulation of Cyanobacteria by Silver Carp Hypophthalmichthys molitrix (Val.) Dokl. Biol. Sci. 2006;408:223–225. doi: 10.1134/S0012496606030069. [DOI] [PubMed] [Google Scholar]
- 104.Kolar C.S., Chapman D., Walter R.C., Jr., Housel C.M., Williams J., Jennings D.P. Asian Carps of the Genus Hypophthalmichthys (Pisces, Cyprinidae)—A Biological Synopsis and Environmental Risk Assessment. Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska-Lincoln; Lincoln, NE, USA: 2005. [Google Scholar]
- 105.Szabó G., Khayer B., Rusznyák A., Tátrai I., Dévai G., Márialigeti K., Borsodi A.K. Seasonal and Spatial Variability of Sediment Bacterial Communities Inhabiting the Large Shallow Lake Balaton. Hydrobiologia. 2011;663:217–232. doi: 10.1007/s10750-010-0574-3. [DOI] [Google Scholar]
- 106.Allen M.M. Simple Conditions for Growth of Unicellular Blue-Green Algae on Plates. J. Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 107.Sanz M., Andreote A.P., Fiore M.F., Dorr F.A., Pinto E. Structural Characterization of New Peptide Variants Produced by Cyanobacteria from the Brazilian Atlantic Coastal Forest using Liquid Chromatography Coupled to Quadrupole Time-of-Flight Tandem Mass Spectrometry. Mar. Drugs. 2015;13:3892–3919. doi: 10.3390/md13063892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pluskal T., Castillo S., Villar-Briones A., Orešič M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 110.Suzuki R., Shimodaira H. Pvclust: An R Package for Assessing the Uncertainty in Hierarchical Clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 111.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jukes T.H., Cantor C.R. Evolution of Protein Molecules. In: Munro H.N., editor. Mammalian Protein Metabolism. Academic Press; Cambridge, MA, USA: 1969. pp. 21–132. [Google Scholar]