Abstract
Fusarium species are among the most important fungal pathogens of maize, where they cause severe reduction of yield and accumulation of a wide range of harmful mycotoxins in the kernels. In order to identify the Fusarium species and their mycotoxin profiles associated to maize ear rot and kernel contamination in Iran, a wide sampling was carried out from field in ten major maize-producing provinces in Iran, during 2015 and 2016. From 182 samples of maize kernels, 551 strains were isolated and identified as belonging to Fusarium genus. Among the 234 representative strains identified at species level by translation elongation factor (EF-1α) sequences, the main Fusarium species were F. verticillioides and F. proliferatum, together representing 90% of the Iranian Fusarium population, and, to a lesser extent, F. incarnatum equiseti species complex (FIESC), F. thapsinum and F. redolens. Fumonisin (FBs) production by F. verticillioides and F. proliferatum representative strains was analysed, showing that all strains produced FB1. None of F. verticillioides strains produced FB2 nor FB3, while both FB2 and FB3 were produced only by F. proliferatum. Total mean of FBs production by F. verticillioides was higher than F. proliferatum. The occurrence of different Fusarium species on Iranian maize is reason of great concern because of the toxigenic risk associated to these species. Moreover, the diversity of the species identified increases the toxigenic risk associated to Fusarium contaminated maize kernels, because of the high possibility that a multi-toxin contamination can occur with harmful consequences on human and animal health.
Keywords: mycotoxin, maize ear rot, fumonisin, trichothecenes, toxigenic risk
1. Introduction
Maize (Zea mays L.) is one of the most important cereals in the world for animal feed and human use, being the third highest produced grain crop, after wheat and rice. About 65% of the total world maize production is used as livestock feed, 15% as human food, and the remaining 20% is mainly addressed to industrial purposes [1]. However, maize is constantly contaminated by toxigenic fungi (TF), which represent a danger for the potential accumulation of the mycotoxins (MTX) that they produce in the grains at the harvest, and could cause a decrease of the storage life of the final products. Moreover, during the post-harvest period, inappropriate environmental conditions of storage could trigger the further development of TF in the grains with consequent production of MTX and further deterioration of maize kernels with low quality and nutritive value [2]. On the other hand, in the field, TF ability to colonise ears of maize is strictly related to climatic parameters, which are also key factors for the MTX production within the kernels. The MTX that cause main concern regarding maize, because of their toxicity and their worldwide distribution on the most common crops, are aflatoxins (AFLA), fumonisins (FBs), ochratoxin A (OTA), trichothecenes and zearalenone (ZEA) [3,4], produced by a wide range of species belonging to three fungal genera, Aspergillus, Fusarium and Penicillium.
In particular, maize ear rot is among the most serious diseases and is caused by a broad range of Fusarium species that significantly reduce the quantity and quality of maize. Commonly, this disease is divided in two types, pink ear rot and red ear rot. Pink ear rot is caused by species belonging to the Fusarium fujikuroi species complex (FFSC) [5] that include species that can colonise temperate and tropical regions and are often regarded as endophytes. On the other hand, red ear rot is caused mostly by F. graminearum and related species such as F. culmorum, and is more developed in the geographical areas where colder temperatures and more humid conditions characterise the environment [6]. In Iran, most of the reports have shown that the species belonging to FFSC were the most common in maize.
Among the FFSC, F. verticillioides and F. proliferatum are those that cause major concern, since they are both the main producing species of the carcinogenic FBs [7] and very common in maize in all regions of the world where this crop is cultivated, included Iran. As a consequence of such colonisation, FBs’ contamination in maize kernels in the fields at maturity and, as carry over, in the silos during storage, is often high [8,9]. Fumonisins have been related to several animal and human diseases such as esophageal cancer (EC), as reported in several countries worldwide [10]. In particular, in Iran, several studies have reported not only high levels of FBs contamination and related Fusarium species occurrence in Iranian maize and maize-based products [11,12,13,14], but also a positive correlation between FBs content in maize kernels and the risk of EC [9]. More detailed studies on the fungal mycoflora associated with maize kernels have also revealed in some maize-producing areas of Iran that F. verticillioides and F. proliferatum were the prevalent species [15,16].
On the other hand, more recently, Amirahmadi et al. [17] reported the occurrence of deoxynivalenol (DON), T-2 toxin and ZEA that are mycotoxins produced by other Fusarium species than F. verticillioides and F. proliferatum. Deoxynivalenol and T-2 are the most important members of trichotecene B and A groups, respectively, and are considered potent inhibitors of proteic synthesis, while ZEA is a metabolite causing estrogenic disorders in human and animals [10]. However, all these reports were limited for the geographical areas investigated in Iran and could not provide a complete overview of the situation in the country. This is extremely important, since a more comprehensive knowledge of the Fusarium species profile occurring on maize kernels, would offer a more reliable assessment of the related mycotoxin risk, since each Fusarium species has its own mycotoxin profile [10].
Therefore, since maize is the most important cereal crop in Iran, and its consumption is related to high risk for the population due to possible Fusarium mycotoxin contamination, there is a need to correctly assess the main Fusarium species occurring on maize in the country, and detect their mycotoxin profile. The aims of this study were: (i) to significantly sample maize kernels at maturity in the all main producing regions in Iran; (ii) to identify the Fusarium species occurring in the samples and (iii) to assess the mycotoxin profiles of the main Fusarium species identified.
2. Results
2.1. Species Identification
In total, 551 Fusarium isolates were obtained from maize samples and, based on morphological characteristics, several different species were identified, namely F. verticillioides, F. proliferatum, F. subglutinans, F. thapsinum, F. redolens, F. sacchari and species belonging to F. solani species complex (FSSC) and F. incarnatum equiseti species complex (FIESC). The number of maize samples and the number of the isolates obtained from the ten major maize-producing Iranian provinces are reported in Table 1.
Table 1.
Number of Fusarium isolates from maize kernels collected in 10 different provinces of Iran. The occurrence and the incidence of the most occurring species F. verticillioides (Fv) and F. proliferatum (Fp) are also reported.
| Iranian Provinces | Number of Maize Sample | Number of Fusarium Isolates | Fusarium Species Occurrence (%) | Incidence * (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Fv | Fp | Other Species | Fusarium spp. | Fv | Fp | |||
| Alborz | 30 | 126 | 47 | 48 | 5 | 21 | 10 | 10 |
| Golestan | 15 | 100 | 61 | 28 | 11 | 33 | 20 | 9 |
| Qazvin | 25 | 84 | 56 | 40 | 4 | 17 | 9 | 7 |
| Fars | 30 | 82 | 50 | 34 | 16 | 14 | 7 | 5 |
| Khuzestan | 24 | 62 | 74 | 0 | 26 | 13 | 10 | 0 |
| Ardabil | 20 | 43 | 81 | 12 | 7 | 11 | 9 | 1 |
| Zanjan | 12 | 27 | 78 | 18 | 4 | 11 | 9 | 2 |
| Lorestan | 10 | 11 | 18 | 55 | 27 | 6 | 1 | 3 |
| Esfahan | 10 | 9 | 78 | 0 | 22 | 5 | 3 | 0 |
| Kermanshah | 6 | 7 | 71 | 28 | 0 | 6 | 4 | 2 |
| TOTAL | 182 | 551 | 59 | 31 | 10 | 15 | 9 | 5 |
* Incidence was calculated as percentage of seeds infected on total analysed seeds.
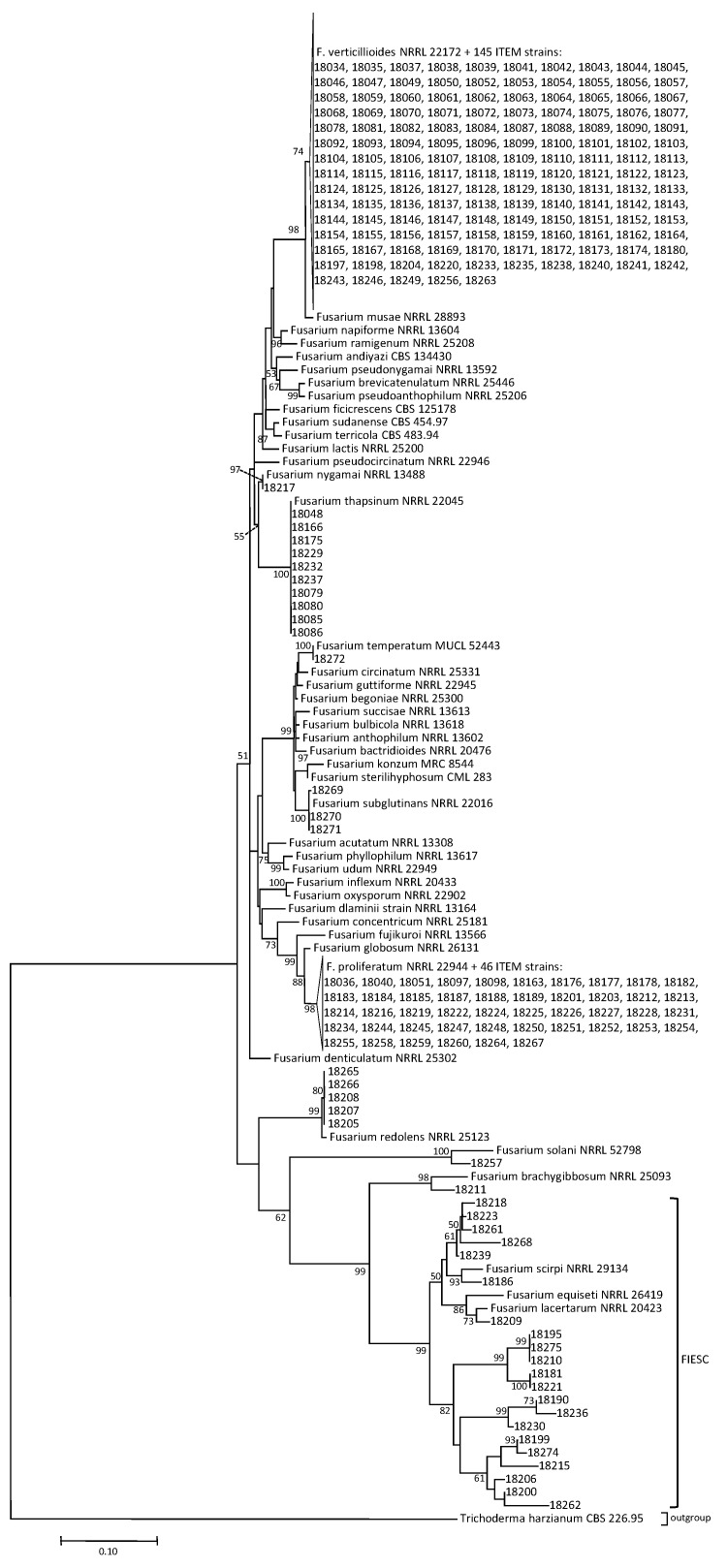
To correctly assign the Fusarium species identification, 234 isolates, selected to be representative of the Fusarium population isolated from Iranian maize, were molecularly identified by DNA sequence analysis of EF-1α gene. The Figure 1 shows the phylogenetic tree, based on EF-1α gene, inferred for 234 sequences compared to Fusarium species reference sequences available on GeneBank. This analysis allowed to identify the isolates as belonging to F. verticillioides, F. proliferatum, F. subglutinans, F. thapsinum, F. temperatum, F.nygamai, FIESC, F. brachygibbosum, F.redolens and FSSC. In particular, 145 isolates were identified as F. verticillioides, 46 as F. proliferatum, 10 F. thapsinum, 3 F. subglutinans, 1 F. temperatum, 1 F. nygamai, 5 F. redolens, 1 isolate belonging to FSSC, 1 F. brachygibbosum and 21 isolates as belonging to FIESC. All isolates described as F. sacchari, based on morphological characteristics, were identified as F. subglutinans through molecular analyses.
Figure 1.
Phylogenetic tree based on EF-1α gene sequences, inferred by using the Maximum Likelihood method, on 234 Fusarium isolated from Iranian maize, compared to reference sequences for Fusarium species. The bootstrap values are shown next to the branches.
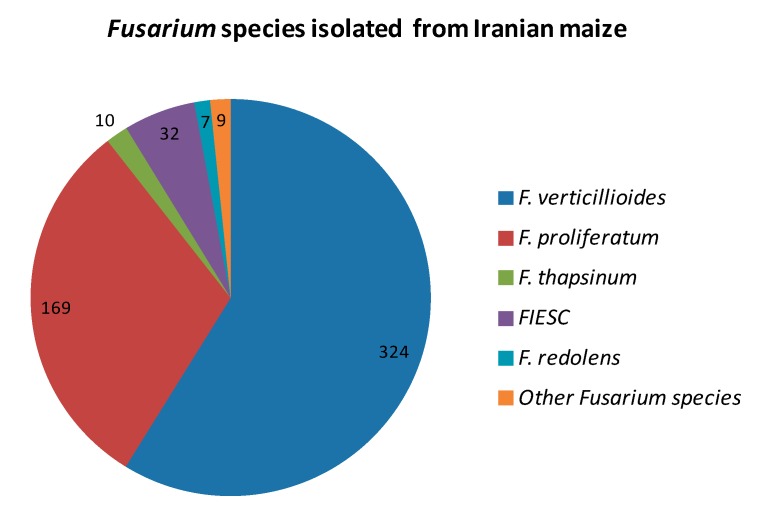
In Figure 2, the distribution of the mainly occurring Fusarium species from Iranian maize has been reported, with the number of strains identified for each species. An overview of the occurrence of the most present species, i.e., F. verticillioides and F. proliferatum, expressed as percentage of these species on total Fusarium species, is shown in Table 1, referred to the considered Iranian provinces. Moreover, based on the samples collected from each province, the incidence of Fusarium species and the incidence of both F. verticillioides and F. proliferatum were calculated as percentage of seeds infected on total analysed seeds, and reported in Table 1.
Figure 2.
Distribution of Fusarium species isolated from 182 maize samples collected in 10 maize producing provinces of Iran. The number of isolates for each species is reported.
Incidence of 15% of seeds infected by Fusarium species was observed in Iranian maize, ranging between 33% in Golestan province and 5% in Esfahan. In Golestan was also observed the highest incidence of F. verticillioides (20%), while the lowest incidence of this species was reported in Lorestan. About F. proliferatum incidence (5%), the highest values were observed in Alborz (10%) and Golestan (9%), while this species was absent in Khuzestan and in Esfahan. In all the Iranian provinces, maize kernels were more contaminated by F. verticillioides than F. proliferatum, except in Alborz, in which the incidence of both the species was the same (10%).
Among the 551 Fusarium strains isolated from maize kernels, F. verticillioides species occurred at 59% and F. proliferatum species at 31%, representing together 90% of the Iranian Fusarium population. In most of the Iranian provinces the percentage of F. verticillioides was higher than F. proliferatum, and above 50%, except in Lorestan, where F. proliferatum represents the most of the Fusarium population (55%) and in Alborz, where both the species occurred at 47 and 48%, respectively (Table 1). Other Fusarium species were isolated from Iranian maize kernels (10%), mostly FIESC (6%), with higher rate in Golestan, Fars, Khuzestan and Lorestan (11, 16, 26, 27%, respectively).
2.2. Mycotoxin Production
Fumonisin production was assessed for all the analysed strains. In particular, as reported in Table 2, all F. verticillioides (67) and F. proliferatum (26) strains produced FB1, with mean values of 505 and 216 µg/g, respectively. All F. verticillioides strains produced FB1 levels above 150 and up to 2232 µg/g, except two strains (about 80 µg/g); but none of them produced detectable levels of FB2 or FB3. Conversely, FB1 production of F. proliferatum strains was more variable, with values ranging from 1 to 1860 µg/g and a mean of 216 µg/g; in addition, 17 F. proliferatum strains on 26 (65%) also produced FB2 and 11 strains (42%) produced FB3, with mean values of 30 and 3 µg/g, respectively. In particular, FB2 production was never higher than 25 µg/g, except for one strain (ITEM 18177) that produced 466 µg/g. Total FBs produced by F. proliferatum ranged between 1 to 2335 µg/g, with mean value of 238 µg/g. In addition to F. verticillioides and F. proliferatum, the only F. nygamai strain (ITEM 18217) was also analysed for FBs production, showing to be able to produce FB1 (38 µg/g).
Table 2.
Fumonisin B1 (FB1), B2 (FB2) and B3 (FB3) production, expressed in µg/g, of F. verticillioides and F. proliferatum strains from Iranian maize kernels. For each mycotoxin, the number of positive strains on total analysed strains, and range and mean of production with standard error (SE) are reported.
| Fumonisin Producing Strains | F. verticillioides ** | F. proliferatum ** | |||
|---|---|---|---|---|---|
| FB1 * | FB1 | FB2 | FB3 | Total FBs | |
| N. positive strains/total | 67/67 | 26/26 | 17/26 | 11/26 | 26/26 |
| Range (µg/g) | 79–2232 | 1–1860 | 0–466 | 0–11 | 1–2335 |
| Mean ± SE (µg/g) | 505 ± 38 | 216 ± 69 | 30 ± 18 | 3 ± 1 | 238 ± 442 |
* FB2 and FB3 were not detected in any F. verticillioides culture. ** For each mycotoxin, the number of positive strains on total analysed strains, and range and mean of production are reported.
Three isolates of F. subglutinans, one isolate of F. temperatum and 5 of F. redolens were tested for production of enniatins (ENNs) A, A1, B, B1 and beauvericin (BEA). None of them produced ENN A, A1 and B; while only two F. redolens strains out of 5 (ITEM 18207 and ITEM 18208) produced ENN B1, both at values of 124 µg/g.
Production of BEA was detected for one F. subglutinans strain out of 3 (ITEM 18269, 16 µg/g), only for F. temperatum strain ITEM 18272 (302 µg/g), and for 4 F. redolens strains out of 5 (ITEM 18207, ITEM 18208, ITEM 18265 and ITEM 18266). In particular these F. redolens strains were shown to be able to produce high levels of BEA, ranging between 688 and 7936 µg/g, with mean value of 4414 µg/g.
For a detailed description of MTX production of each strain, a Supplementary Table has been provided.
3. Discussion
This study represents the first extended monitoring on the Fusarium species colonising maize in Iran regions where maize is the main cultivation. Several reports have described occurrence and incidence of main Fusarium species in Iran, but they were mostly all limited in regional sampling, that usually provided only a restricted view of the risk related to Fusarium contamination of maize in the country [9,11,12,13,14]. Moreover, most of studies were focused on a single species such as F. verticillioides [13], or a single Fusarium MTX occurrence [9,18,19]. On the other hand, Rahjoo et al. [16] reported, as far as we are aware, the results of the unique large maize kernel sampling in Iran, performed from 11 provinces, in 2004 and 2005. In the mentioned paper, F. verticillioides and, to a lesser extent, F. proliferatum, were the only two species significantly isolated from the maize kernels. Although the number of samples (90) and number of seeds analysed per sample (2) were extremely limited, the data obtained were reason of great concern since both species are well known FB producing species and often co-occur worldwide on maize, making it more difficult the development of resistance program for breeders. Indeed, our study, that expanded the sampling (190 vs. 90) and the number of seeds analysed per sample (20 vs. 2), confirmed F. verticillioides and F. proliferatum as the main species contaminating maize kernels, with a variable distribution of the two species among the regions sampled. Alizadeh et al. [9] related the increased incidence of EC in some areas of the Iranian Province of Golestan, to the high occurrence of fumonisin B1 (FB1) in cereals. In our survey, along with Golestan Province, further provinces Alborz, Qazvin and Fars were highly contaminated by both species, showing that a high risk for human and animal health does exist also in these regions, where possible association between FB1 contaminated maize and EC should be also investigated. Moreover, the frequency of F. proliferatum is high in most of the areas sampled, being very interesting that, in some of the Provinces, F. proliferatum occurrence is higher than F. verticillioides. These data show a significant shift in the ratio between the two most important FBs producing species compared the previous reports from Iran [9,11,13,16].
Climate changes have a significant impact on stages and rates of toxigenic fungi development and can modify host–resistance and host–pathogen interactions, also deeply influencing the conditions for mycotoxin production that vary for each individual pathogen. Moreover, the new combinations mycotoxins/host plants/geographical areas are arousing the attention of the scientific community and require new diagnostic tools and deeper knowledge of both biology and genetics of toxigenic fungi [20]. Therefore, our data trigger further investigations on the possibility that the shift between F. verticillioides and F. proliferatum in the regions sampled in Iran could be related to the eventual environmental changes that occurred in the same areas during the last decade.
On the other hand, since the breeding program, at worldwide level, where and if applied, have been addressed to select more resistant maize genotypes only to the F. verticillioides colonisation of maize [21], the possibility that F. proliferatum could have taken advantage by a reduced occurrence of such competitive fungal species, can also be considered. Finally, the increased incidence of F. proliferatum is of further concern because of the wider ability of this species to produce further mycotoxins than FBs, such as BEA, fusaproliferin and moniliformin [6,22]. At worldwide level, among the MTX produced by F. proliferatum, only FBs are regulated for their occurrence in row maize or maize by-products [23,24,25]. However, the possibility that the other above mentioned MTX could naturally occur in the maize together with FBs, when F. proliferatum colonises the kernels, increases the risk related to the consumption of contaminated maize because of the unpredictable additives, if not synergistic, effects, due to the co-occurrence of multiple MTX produced by this species in the maize [26].
In addition to FBs, the detection of other MTX on maize has been poorly reported in the last decade, in Iran. In this respect, a survey from Iran by Amirahmadi et al. [17] reported the occurrence of the two well-known trichothecenes, DON and T-2 toxin, together with ZEA, in maize kernels collected in Ardabil province and local markets in Teheran, while Karami-Osboo et al. [19] detected DON in maize kernels collected in the Iranian provinces of Golestan and Moquan, and Rashedi et al. [18] detected ZEA in maize collected in Chaharmahal and Bakhtiari Province. In all these studies, no data were provided on the Fusarium species profile associated to the maize kernels analysed. However, at worldwide level, DON and ZEA occurrence in maize have been mostly associated to the contamination of F. graminearum and the related species of the Fusarium sambucinum clade [27], that are all DON and ZEA producing species [6,7,8,28]. In our results, F. graminearum was never isolated from the kernels analysed. This latter species is often associated to maize worldwide mostly in geographical areas where cold and wet environmental conditions characterise the period of maize cultivation. Since the majority of Iranian provinces sampled is characterised by hot and dry climate, the lack of F. graminearum and the predominance of F. verticillioides and F. proliferatum species is not surprising.
Nevertheless, from our samples, we identified, based on the EF-1α gene sequences, with a significant frequency, also 32 strains belonging to FIESC, a complex of 31 phylogenetic species [29], and 3 strains of F. brachygibbosum, a closely related species. This is also reason for further concern, since FIESC isolates were shown to be potentially able to produce B-trichothecenes such as DON, due to the presence of Tri5 gene within trichothecene biosynthetic loci [30]. Moreover, some members of this complex have been reported to be both ZEA and trichothecene producers [31,32], while a recent report by Villani et al. [33] reported the analysis of 13 FIESC members genomes, showing that the complete set of genes involved in the ZEA and trichothecene biosynthesis occurred in all genomes but also that the production of secondary metabolites, included the MTX, could be affected by the different distribution of functional and related gene clusters. The FIESC strains isolated in this study could produce in vitro both trichothecenes and ZEA, however their production was highly variable along the set of strains analysed, confirming the wide bio-diversity of FIESC reported in previous papers [31,32,33].
In our survey, two more species have been detected at a significant incidence: F. thapsinum, a member of FFSC, and the closely related species to FFSC F. redolens, formerly identified as F. oxysporum [34]. All isolates of both species produced enniatins and high level of BEA, which are structurally similar metabolites with demonstrated toxicity on human cell lines [26]. The occurrence of such metabolites cannot be neglected since, although they are not under any regulation by neither National nor International Institutions, they can contribute to increase the risk for human and animal health for the consumption of contaminated maize.
Finally, it is interesting to notice that the profile of FBs produced in vitro by the strains of F. verticillioides and F. proliferatum isolated in our study was highly different between the two species, being both able to produce FB1, while production of FB2 and FB3 was detected only in F. proliferatum cultures. However the mean of FB1 production by F. verticillioides was much higher than F. proliferatum. These results show that, although no production of FB2 and FB3 was detected in vitro cultures of F. verticillioides, this species can be still considered as the main responsible for fumonisin accumulation in maize kernels in Iran. On the other hand, the population of F. verticillioides studied here needs to be further investigated at molecular level, in order to understand the genetic mechanism involved in the inhibition of FB2 and FB3 production. In addition, the extended occurrence on maize in Iran of F. proliferatum strains with full ability to produce in vitro all three more common and toxic FBs, shows that this species can be a very important contributor to the FB1 final contamination of maize kernels, being the in vitro production very useful in forecast contamination in the field.
4. Conclusions
In conclusion, our study showed an increase of F. proliferatum incidence in Iranian maize compared to previous reports and confirmed an extended concern for the potential contamination of maize kernels by FBs in the whole of Iran. Moreover, the occurrence of the FIESC species that are trichothecene and ZEA producers has been recorded for the first time in Iran, showing that a possible association of this complex to the ZEA contamination of maize kernels could occur. Finally, the diversity of Fusarium species isolated, compared the previous reports in Iran, highlights the possible increased risk for human and animal health because of the possible multi-toxin contamination of maize kernels in the whole country.
5. Materials and Methods
5.1. Sampling and Fungal Isolation
Maize samples of two crop seasons, 2015 and 2016, were collected in September–October from fields and maize grain silos of ten provinces of Iran: Khuzestan, Fars, Golestan, Ardabil, Alborz, Qazvin, Zanjan, Kermanshah, Lorestan and Isfahan (Figure 3). A total of 182 maize samples were collected in the main maize producing Iranian regions, as reported in Table 1. The sampling method was based on a hierarchical method [35]. Ears were put in paper boxes, labelled, moved to the laboratory, and air dried for 4–5 days in room temperature.
Figure 3.
Map depicting the location of ten provinces of Iran where maize kernel samples were harvested in two maize growing seasons, 2015 and 2016.
Kernels were surface sterilized for 1 min in 3% sodium hypochlorite solution, rinsed twice in sterile distilled water, dried on filter paper and placed on Nash and Snyder medium containing 15 g peptone, 1 g K2HPO4, 0.5 g MgSO4·7H2O, 15 g agar, 1 g pentachloronitrobenzene (PCNB; Terraclor 75% WP), in 1 L distilled water. Petri dishes containing kernels were incubated at 25 °C in the dark for 5–7 days. All the fungal cultures developed from the kernels were transferred on potato dextrose agar (PDA) using a single-spore technique [36] and incubated at 25 °C for 7 days.
5.2. Morphological Identification
For morphological characterisation, pure cultures were transferred on both carnation leaf-piece agar (CLA; 20.0 g/L agar prepared in distilled water and piece of disinfected carnation leaf) and Synthetic Nutrient Agar (SNA; 1.0 g/L of KH2PO4, 1.0 g/L of KNO3, 0.5 g/L of MgSO4·7H2O, 0.5 g/L of KCl, 0.2 g/L of glucose, 0.2 g/L of sucrose and 20.0 g/L agar). Both CLA and SNA inoculated plates were incubated under the conditions described by Leslie and Summerell [36] for 2 weeks, to allow the fungal colonies growing. Macroscopic traits such as the colony appearance, colour, pigmentation and growth rate were observed on potato dextrose agar (PDA) according to Leslie and Summerell [36]. Morphological identification was performed based on the morphological characteristics observed at optical microscope as described by Booth [37], Gerlach and Nirenberg [38], and Leslie and Summerell [36].
5.3. Molecular Identification
For molecular identification, 234 representative isolates from different morphologically identified Fusarium species, were selected and grown on PDA medium. Mycelium was lyophilized for total DNA extraction, and then 10 mg of lyophilized mycelium, grinded with 5 mm iron beads, was processed with “Wizard® Magnetic DNA Purification System for Food” kit (Promega, Fitchburg, WI, USA). The quality of genomic DNA was determined by agarose gel electrophoresis and the quantification by using the Spectrophotometer ND-1000 (NanoDrop, Thermo-Scientific, Waltham, MA, USA). Molecular identification was carried out based on elongation factor (EF-1α) gene sequencing. The region between primers EF1 and EF2 from O’Donnell et al. [39] was amplified. The reaction mixture for polymerase chain reaction (PCR) was prepared in a final volume of 15 μL, consisting of DNA (1.5 μL), buffer 1X, primers 300 nM, dNTPs 200 nM, Taq DNA polymerase 0.5 U. The PCR conditions were as follows: 95 °C for 2 min; 35 cycles of 95 °C for 30 s, 60 °C for 40 s and 72 °C for 50 s. Final extension was performed at 72 °C for 7 min. The PCR amplification products were verified on 1.5% agarose gel.
For sequencing, PCR products were purified with the enzymatic mixture EXO/FastAP (Exonuclease I, FastAP thermosensitive alkaline phosphatase, Thermo Scientific, Waltham, MA, USA). BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Thermo Scientific, Waltham, MA, USA) was used for sequence reactions of both strands, and then the PCR products were purified by gel filtration through Sephadex G-50 (5%) (Sigma Aldrich, Milan, Italy) and run on the 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA). DNA sequences were analysed with the Sequencing Analysis 5.2 software (Applied Biosystems, Waltham, MA, USA). Each consensus sequence was generated from forward and reverse strand with Bionumerics software (Applied Maths, Kortrijk, Belgium). To obtain a previous species identification in order to select reference sequences for Fusarium species to be used in the phylogenetic analysis, the EF-1α sequences were searched on GeneBank database by using the Basic Local Alignment Tool (BLAST). In addition to the EF-1α sequences of 234 isolates from Iranian maize, 39 reference sequences for Fusarium species and one Trichoderma sequence used as outgroup, were downloaded from GeneBank and used for phylogenetic analysis. All the sequences were aligned by using the MUSCLE algorithm [40] with MEGA7 software ver. 7.0.14 [41]. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura–Nei model [42] in MEGA 7 software. To evaluate the support for inferred topologies, the percentage of trees in which the associated taxa clustered together was evaluated by using bootstrapping [43] with 1000 replicates.
5.4. Mycotoxins Analysis
Selected 104 Fusarium isolates, representative of the Fusarium species belonging to F. fujikuroi species complex (FFSC) and related species (F. redolens), were examined for mycotoxin production. In particular, 68 isolates of F. verticillioides, 26 F. proliferatum, 3 F. subglutinans,1 F. temperatum, 1 F. nygamai and 5 F. redolens were analysed.
For mycotoxin production assays, we used 30 g rice in PYREX Glass Erlenmeyer Flasks, added with 13.5 mL of distilled water, standing overnight, and then autoclaved at 121 °C for 30 min. The flasks containing autoclaved rice were inoculated with piece of fungal cultures grown on PDA and incubated at 25 °C for 21 days in order to allow fungal development and mycotoxin production. High-performance liquid chromatography (HPLC) analytical methods were used to detect fumonisins B1, B2 and B3 (FB1, FB2, FB3), beauvericin (BEA), enniatins (ENNs) A, A1, B and B1.
5.4.1. Fumonisin Production
Fumonisin production was analysed for F. verticillioides (68 isolates), F. proliferatum (26 isolates) and F. nygamai (1 isolate). One gram of inoculated rice culture was used for toxin extraction with 5 mL of methanol/water (75/25, v/v). Samples were placed for 60 min in an orbital shaker, and then filtered using Whatman no. 4 filter papers (Maidstone, UK). 500 µL was diluted with 500 µL ultrapure water (Millipore, Bedford, MA, USA). An aliquot of 50 µL of the extract was derivatized with 50 µL of o-phtaldialdehyde (OPA) using the HPLC autosampler Agilent 1100 (Agilent, Waldbronn, Germany). The column Symmetry shield RP18 15 cm × 4.6 mm, 5 µm (Waters, Milford, MA, USA) with a guard column inlet filter (0.5 µm × 3 mm diameter, Rheodyne Inc. Rohnert Park, CA, USA) was thermostat set at 30 °C, and FLD detector was set at ex = 335 nm, em = 440 nm. A volume of 100 µL was injected in the HPLC at 3 min after adding the OPA reagent. The mobile phase consisting of a binary gradient was applied as follows: the initial composition of the mobile phase 57% of A water-acetic acid (99/1, v/v)/43% of B acetonitrile-acetic acid (99/1, v/v) was kept constant for 5 min, then B solvent was linearly increased to 54% in 21 min, then up to 58% at 25 min and kept constant for 5 min. The flow rate of the mobile phase was 0.8 mL/min. The retention time of the FB1 was about 16.6 min, 24.6 min for FB2 and 26.0 min for FB3. The mycotoxins were quantified by comparing peak areas with a calibration curves obtained with standard solutions (Romer Labs Diagnostic GmbH, Tulln, Austria). The detection limits (LOD) of the method based on signal-to-noise ratio of 3:1 was 12.5 µg/kg for FB1, FB2 and FB3.
5.4.2. Beauvericin and Enniatins Production
Production of BEA and ENNs was assayed for F. redolens (5 isolates), F. subglutinans (3 isolates) and F. temperatum (1 isolate). Fungal culture on rice (1 g) was used for toxin extraction with 5 mL of methanol/water (70/30, v/v) on an orbital shaker for 60 min, and then was filtered using Whatman no. 4 filter papers (Waters, Milford, MA, USA). The sample (100 µL) was diluted with 900 µL ultrapure water (Millipore, Bedford, MA, USA) and filtered using RC through 0.20 µm regenerated cellulose filter (Phenomenex, Torrance, CA, USA). A volume of 100 µL was injected into HPLC apparatus (Agilent 1260 Series, Agilent Technology, Santa Clara, CA, USA). The analytical column was a Gemini (150 × 4.6 mm, 5 μm, Phenomenex) preceded by a pre-column Gemini (4 × 3 mm, Phenomenex). The mobile phase consisting of a binary gradient was applied as follows: the initial composition of the mobile phase 30% of A water/70% of B acetonitrile was kept constant for 5 min, then B solvent was lineary increased to 90% in 10 min. The flow rate of the mobile phase was 1 mL/min. Retention time was 11.4 min for BEA, 9 min for ENN B, 10.3 min for ENN B1, 12 min for ENN A1 and 13 min for ENN A. The mycotoxins were quantified by comparing peak areas with a calibration curves obtained with standard solutions (Sigma-Aldrich, Milan, Italy). The detection limits (LOD) of the method based on signal-to-noise ratio of 3:1, were the following: BEA = 10 µg/kg, ENN B = 60 µg/kg, ENN B1 = 70 µg/kg, ENN A = 200 µg/kg, ENN A1 = 500 µg/kg.
Acknowledgments
We thank the University of Tehran, the INSF Iran National Science Foundation and The CNR-ISPA for providing Facilities for us to conduct this research. This work was supported by the Horizon 2020 EU Project 678781 MycoKey.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/11/5/297/s1, Table S1: Fumonisin B1 (FB1), B2 (FB2) and B3 (FB3) production by F. verticillioides and F. proliferatum strains from Iranian maize kernels.
Author Contributions
Conceptualization, M.F., H.S., A.M. and S.S.; methodology, M.F., S.S. and M.H.; validation, M.F., S.S. and M.H.; formal analysis, M.F., S.S. and M.H.; investigation, M.F., S.S. and M.H.; resources, M.F., S.S., A.M., H.S., M.J.-N., M.H. and A.F.L.; data curation, M.F and S.S.; writing – original draft preparation, M.F., S.S., H.S. and M.J.-N.; writing – review and editing, A.M.; visualization, M.F., S.S., A.M., H.S., M.J.-N., M.H. and A.F.L.; supervision, H.S. and A.M.; project administration, H.S. and A.M.; funding acquisition, A.M., H.S. and A.F.L.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
A wide sampling of maize kernels was carried out from ten major maize-producing provinces of Iran. The prevalence of F. verticillioides species in maize samples was generally observed. In all region sampled, a significant co-occurrence of F. proliferatum was detected with, in some cases, a shift in F. verticillioides/F. proliferatum ratio towards F. proliferatum. FIESC species, potentially trichothecene and zearaleone producers, have been recorded for the first time in Iran. The diversity of Fusarium species isolated from Iranian maize highlights the possible increased risk of multi-toxin contamination.
References
- 1.Abbassian A. Background Paper for the Competitive Agriculture in Sub-Saharan African, Economic and Social Department. Trade and Markets Division, Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2006. Maize: International Market Profile. [Google Scholar]
- 2.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. 2nd ed. Blackie Academic and Professional; London, UK: 1997. [Google Scholar]
- 3.Marasas W.F.O., Thiel P.G., Rabie C.J., Nelson P.E., Toussoun T.A. Moniliformin production in Fusarium section Liseola. [(accessed on 20 May 2019)];Mycologia. 1986 78:242–247. doi: 10.1080/00275514.1986.12025235. Available online: https://www.jstor.org/stable/3793169. [DOI] [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) Mycotoxins and human health. In: Pitt J.I., Wild C.P., Baan R.A., Gelderblom W.C.A., Miller J.D., Riley R.T., Wu F., editors. Improving Public Health through Mycotoxin Control. International Agency for Research on Cancer; Lyon, France: 2012. pp. 87–104. IARC Scientific Publication No. 158. [Google Scholar]
- 5.Al-Hatmi A.M., Normand A.C., van Diepeningen A.D., Hendrickx M., de Hoog G.S., Piarroux R. Rapid species-level identification of opportunists in the Fusarium fujikuroi species complex using MALDI-TOF mass spectrometry. Future Microbiol. 2015;10:1939–1952. doi: 10.2217/fmb.15.108. [DOI] [PubMed] [Google Scholar]
- 6.Logrieco A., Mule G., Moretti A., Bottalico A. Mycotoxins in Plant Disease. Springer; Dordrecht, The Netherlands: 2002. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe; pp. 597–609. [Google Scholar]
- 7.Goertz A., Zuehlke S., Spiteller M., Steiner U., Dehne H.W., Waalwijk C., de Vries I., Oerke E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010;128:101–111. doi: 10.1007/s10658-010-9634-9. [DOI] [Google Scholar]
- 8.Aguín O., Cao A., Pintos C., Santiago R., Mansilla P., Butrón A. Occurrence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathol. 2014;63:946–951. doi: 10.1111/ppa.12151. [DOI] [Google Scholar]
- 9.Alizadeh A.M., Roshandel G., Roudbarmohammadi S., Roudbary M., Sohanaki H., Ghiasian S.A., Taherkhani A., Semnani S., Aghasi M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pac. J. Cancer Prev. 2012;13:2625–2628. doi: 10.7314/APJCP.2012.13.6.2625. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology. American Phytopathological Society (APS Press); St. Paul, MN, USA: 2006. [Google Scholar]
- 11.Ghiasian S.A., Kord-Bacheh P., Rezayat S.M., Maghsood A.H., Taherkhani H. Mycoflora of Iranian maize harvested in the main production areas in 2000. Mycopathologia. 2004;158:113–121. doi: 10.1023/B:MYCO.0000038425.95049.03. [DOI] [PubMed] [Google Scholar]
- 12.Ghiasian S.A., Rezayat S.M., Kord-Bacheh P., Maghsood A.H., Yazdanpanah H., Shephard G.S., Van der Westhuizen L., Vismer H.F., Marasas W.F.O. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia. 2005;159:31–40. doi: 10.1007/s11046-004-3899-5. [DOI] [PubMed] [Google Scholar]
- 13.Ghiasian S.A., Maghsood A.H., Yazdanpanah H., Shephard G.S., Van Der Westhuizen L., Vismer H.F., Rheeder J.P., Marasas W.F. Incidence of Fusarium verticillioides and levels of fumonisins in corn from main production areas in Iran. J. Agric. Food Chem. 2006;54:6118–6122. doi: 10.1021/jf061248w. [DOI] [PubMed] [Google Scholar]
- 14.Shephard S., Marasas W.F.O., Leggott N.L., Yazdanpanah H., Rahimian H., Safavi N. Natural occurrence of fumonisins in corn from Iran. J. Agric. Food Chem. 2000;48:1860–1864. doi: 10.1021/jf991196t. [DOI] [PubMed] [Google Scholar]
- 15.Aliakbari F., Mirabolfathy M., Emami M., Mazhar S.F., Osboo R.K. Natural occurrence of Fusarium species in maize kernels at Gholestan province in Northern Iran. Asian J. Plant Sci. 2007;6:1276–1281. doi: 10.3923/ajps.2007.1276.1281. [DOI] [Google Scholar]
- 16.Rahjoo V., Zad J., Javan-Nikkhah M., Gohari A.M., Okhovvat S.M., Bihamta M.R., Razzaghian J., Klemsdal S.S. Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 2008;90:463–468. doi: 10.4454/jpp.v90i3.688. [DOI] [Google Scholar]
- 17.Amirahmadi M., Shoeibi S., Rastegar H., Elmi M., Mousavi Khaneghah A. Simultaneous analysis of mycotoxins in corn flour using LC/MS-MS combined with a modified QuEChERS procedure. Toxin Rev. 2018;37:187–195. doi: 10.1080/15569543.2017.1354306. [DOI] [Google Scholar]
- 18.Rashedi M., Sohrabi H.R., Ashjaazadeh M.A., Azizi H., Rahimi E. Zearalenone contamination in barley, corn, silage and wheat bran. Toxicol. Ind. Health. 2012;28:779–782. doi: 10.1177/0748233711422733. [DOI] [PubMed] [Google Scholar]
- 19.Karami-Osboo R., Mirabolfathy M., Aliakbari F. Natural Deoxynivalenol Contamination of Corn Produced in Golestan and Moqan Areas in Iran. [(accessed on 20 May 2019)];J. Agrscitechnol. 2010 12:233–239. Available online: http://journals.modares.ac.ir/article-23-1426-en.html. [Google Scholar]
- 20.Moretti A., Pascale M., Logrieco A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Tech. 2018;84:38–40. doi: 10.1016/j.tifs.2018.03.008. [DOI] [Google Scholar]
- 21.Lanubile A., Maschietto V., Borrelli V.M., Stagnati L., Logrieco A.F., Marocco A. Molecular Basis of Resistance to Fusarium Ear Rot in Maize. Front. Plant Sci. 2017;8:1–13. doi: 10.3389/fpls.2017.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moretti A., Logrieco A., Bottalico A., Ritieni A., Fogliano V., Randazzo G. Diversity in beauvericin and fusaproliferin production by different populations of Gibberella fujikuroi (Fusarium section Liseola) Sydowia. 1996;48:44–56. [Google Scholar]
- 23.Commission of the European Communities (CEC) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. [(accessed on 20 May 2019)];Off. J. Eur. Union. 2006 L364:5–24. Available online: http://data.europa.eu/eli/reg/2006/1881/oj. [Google Scholar]
- 24.World Health Organization (WHO) Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series 906. World Health Organization; Geneva, Switzerland: 2002. Evaluation of certain mycotoxins in food; p. 70. [PubMed] [Google Scholar]
- 25.Food and Agriculture Organization (FAO) FAO Food and Nutrition Paper 81. Food and Agriculture Organization of the United Nations; Rome, Italy: 2004. Worldwide regulations for mycotoxins in food and feed in 2003. [Google Scholar]
- 26.Bertero A., Moretti A., Spicer L.J., Caloni F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins. 2018;10:244. doi: 10.3390/toxins10060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell K., Rooney A.P., Proctor R.H., Brown D.W., McCormick S.P., Ward T.J., Frandsen R.J.N., Lysøe E., Rehner S.A., Aoki T., et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013;52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J.B., Li H.P., Dang F.J., Qu B., Xu Y.B., Zhao C.S., Liao Y.C. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 2007;111:967–975. doi: 10.1016/j.mycres.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell K., Sutton D.A., Rinaldi M.G., Gueidan C., Crous P.W., Geiser D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009;47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proctor R.H., McCormick S.P., Alexander N.J., Desjardins A.E. Evidence that a secondary metabolic biosynthetic gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol. Microbiol. 2009;74:1128–1142. doi: 10.1111/j.1365-2958.2009.06927.x. [DOI] [PubMed] [Google Scholar]
- 31.Villani A., Moretti A., De Saeger S., Han Z., Di Mavungu J.D., Soares C.M.G., Proctor R.H., Venâncio A., Lima N., Stea G., et al. A polyphasic approach for characterization of a collection of cereal isolates of the Fusarium incarnatum-equiseti species complex. Int. J. Food Microbiol. 2016;234:24–35. doi: 10.1016/j.ijfoodmicro.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell K., McCormick S.P., Busman M., Proctor R.H., Ward T.J., Doehring G., Geiser D.M., Alberts J.F., Rheeder J.P. Marasas et al. 1984 “Toxigenic Fusarium Species: Identity and Mycotoxicology” revisited. Mycologia. 2018;110:1058–1080. doi: 10.1080/00275514.2018.1519773. [DOI] [PubMed] [Google Scholar]
- 33.Villani A., Proctor R.H., Kim H.-S., Brown D.W., Logrieco A.F., Amatulli M.T., Moretti A., Susca A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019;20:314. doi: 10.1186/s12864-019-5567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson P.E., Toussoun T.A., Marasas W.F.O. Fusarium Species: An Illustrated Manual for Identification. Pennsylvania State University Press; University Park, FL, USA: London, UK: 1983. [Google Scholar]
- 35.McDonald B.A., Zhan J., Burdon J.J. Genetic structure of Rhynchosporiumsecalisin Australia. Phytopathology. 1999;89:639–645. doi: 10.1094/PHYTO.1999.89.8.639. [DOI] [PubMed] [Google Scholar]
- 36.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. Wiley-Blackwell; Oxford, UK: 2006. [Google Scholar]
- 37.Booth C. The Genus Fusarium. Commonwealth Mycological Institute; Kew, Surray, UK: 1971. [Google Scholar]
- 38.Gerlach W., Nirenberg H. The genus Fusarium a pictorial atlas. Mitteilungen aus der Biologischen Bundesanstalt fur Land-und Forstwirtschaft Berlin-Dahlem. 1982;209:406. doi: 10.2307/3792677. [DOI] [Google Scholar]
- 39.O’Donnell K., Kistler H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 43.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.