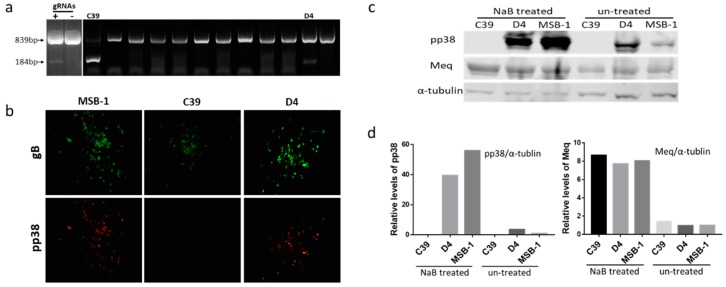
Figure 1.
Deletion of pp38 gene by CRISPR/Cas9 editing in MSB-1 cell. (a) PCR amplification of the edited region, using primers NF and CR on the cell lysates of transfected cells at 24 h post electroporation and single cell clones after sorting. The top band with un-edited/partially-edited sequence with small indels was expected to be around 839 bp, while deletion of the sequence between the Cas9 cleavage sites would result in a 184 bp PCR product. The two clones (C39 and D4) with 184 bp band were indicated. (b) Confirmation of the pp38 gene deletion in MSB-1 by IFA on plaques formed by co-cultivation of edited MSB-1 clones on chick embryo fibroblasts (CEF) monolayer with anti-pp38 monoclonal antibody BD1 (red), anti-gB monoclonal antibody HB3 (green) staining was used as an infection control. Pictures were taken with 100× magnification. The data shown are representative of three independent experiments. (c) Detection of pp38 expression by western blotting with anti-pp38 monoclonal antibody BD1 and anti-Meq monoclonal antibody FD7 before and after NaB treatment on MSB-1 and edited clones C39 and D4. For the loading control, the same blot was stripped and re-probed with anti-α-tubulin antibody. The data shown are representative of three independent experiments. (d) Relative signal intensities of the pp38 and Meq western blot band were quantified using ImageQuant and normalized against the corresponding signal from the α-tubulin band. The signal from the untreated control MSB-1 cells was set as 1.