Abstract
More than 2 million people worldwide are being treated for end-stage kidney disease (ESKD). This Series paper provides an overview of incidence, modality use (in-centre haemodialysis, home dialysis, or transplantation), and mortality for patients with ESKD based on national registry data. We also present data from an international cohort study to highlight differences in haemodialysis practices that affect survival and the experience of patients who rely on this therapy, which is both life-sustaining and profoundly disruptive to their quality of life. Data illustrate disparities in access to renal replacement therapy of any kind and in the use of transplantation or home dialysis, both of which are widely considered preferable to in-centre haemodialysis for many patients with ESKD in settings where infrastructure permits. For most patients with ESKD worldwide who are treated with in-centre haemodialysis, overall survival is poor, but longer in some Asian countries than elsewhere in the world, and longer in Europe than in the USA, although this gap has reduced. Commendable haemodialysis practice includes exceptionally high use of surgical vascular access in Japan and in some European countries, and the use of longer or more frequent dialysis sessions in some countries, allowing for more effective volume management. Mortality is especially high soon after ESKD onset, and improved preparation for ESKD is needed including alignment of decision making with the wishes of patients and families.
Introduction
An estimated 2·6 million people worldwide were treated for end-stage kidney disease (ESKD) in 2010, and one to three times that number might have died because they reached ESKD but renal replacement therapy (RRT) was declined or could not be accessed.1 Our Series paper first provides an overview of incidence, modality use (in-centre dialysis, home dialysis, or transplantation), and mortality for patients with ESKD based on data from many national registries. In addition to population differences in the incidence of kidney failure, these data illustrate disparities in access to RRT of any kind, and disparities in the use of transplantation or home dialysis, widely considered preferable to in-centre haemodialysis for many patients with ESKD where infrastructure permits. Worldwide, most patients with ESKD are treated with in-centre haemodialysis, and, though survival outcomes are poor overall, survival in some Asian countries substantially exceeds survival elsewhere. In this Series paper we then present international cohort study data that demonstrate differences in key in-centre haemodialysis practices, highlighting opportunities to improve survival and the experience of the millions of patients who rely on this therapy, which is both life-sustaining and profoundly disruptive to their lives.
Comparisons of national ESKD registries
Data sources
We collected data from the US Renal Data System (USRDS) Annual Data Report,2 which compiles and publishes incidence and prevalence data annually from more than 50 national registries. National ESKD registry data are not available for countries that include at least half of the world’s population, such as in many low-income and middle-income countries in Africa and in the world’s two most populous nations China and India. In China, regional registries are in place and efforts towards a national registry are underway.
ESKD incidence
Registry data are provided for patients with ESKD treated by RRT (defined here as chronic dialysis or kidney transplantation). Table 1 summarises some factors that might affect incidence of treated ESKD. Gross domestic product (GDP) per person and percentage of GDP spent on health care are positively associated with incidence of treated ESKD,3 reflecting the reality that many countries have unrecognised diagnoses of ESKD or reduced access to RRT. As a result, reported ESKD incidence almost certainly underestimates the incidence of kidney failure in these countries. Even in countries where RRT is widely available, ESKD incidence probably underestimates irreversible kidney failure to some extent because some patients choose to decline dialysis or transplantation. The term “conservative kidney management” has been applied to the choice to forego or postpone RRT while continuing active medical care by nephrologists and other providers. Conservative kidney management has been promoted for some time in Australia, the UK, and other countries, and has become an increasingly visible option worldwide.4–7
Table 1:
Examples of factors affecting incidence and prevalence of treated ESKD
| Comments | |
|---|---|
| ESKD incidence | Net trend in ESKD incidence varies (from strongly positive to weakly negative) across countries |
| Factors favouring higher incidence | |
| Increase in population size | Affects incident counts, not rate |
| Increase in population and age, and in prevalence of diabetes, hypertension, and obesity | Greater burden of risk factors for ESKD |
| Longer survival with chronic kidney disease | Yielding more people who can progress to ESKD |
| Increase in access to renal replacement therapy | In many low-income and middle-income countries |
| Larger percentage of gross domestic product spent on health care | Via effect on several other factors listed |
| Earlier start of dialysis therapy | Earlier as defined by higher eGFR at dialysis start |
| Factors favouring lower incidence | |
| Better treatment of diabetes and hypertension | Decreasing incidence of CKD or rates of CKD progression |
| Later start of dialysis therapy | Later as defined by lower eGFR at dialysis start |
| Greater use of conservative kidney management | Conservative kidney management is management without dialysis, for patients reaching ESKD |
| ESKD prevalence | Net trend in ESKD prevalence is increasing in nearly all countries providing data |
| Factors favouring higher prevalence | |
| Rising incidence of treated ESKD | Occurring in most countries |
| Increasing proportion of ESKD patients receiving a kidney transplant | On average patients with kidney transplant survive longer than dialysis patients |
| Longer survival for dialysis and transplant recipients | Documented in many countries |
| Fewer voluntary withdrawals from dialysis | Trends in dialysis withdrawals are uncertain in most countries |
| Factors favouring lower prevalence | |
| Stable or lower incidence of treated ESKD | Occurs in a few countries |
| Time-limited payment for dialysis | Occurs in some countries, where government support for payment is limited to, for example, 1 year of dialysis |
ESKD=end-stage kidney disease. eGFR=estimated glomerular filtration rate. CKD=chronic kidney disease.
Both absolute rates of treated ESKD and trends in these rates are informative. Of higher-income countries, ESKD incidence is lowest in Nordic countries, other European countries, Australia, and New Zealand (figure 1). These countries have nearly universal access to RRT, so these rates are presumably due to relatively low incidence or progression of chronic kidney disease. Selection of conservative kidney management in lieu of RRT is probably not a major determinant of low ESKD incidence, although the extent of its use is not well known. By contrast, ESKD incidence is much higher in the USA and high-income eastern or southeastern Asian countries, presumably reflecting high burden of chronic kidney disease and antecedent risk factors such as diabetes, hypertension, obesity, and glomerular diseases (eg, IgA nephropathy in Asian populations).
Figure 1. Treated end-stage kidney disease incidence and prevalence by country in 2013.
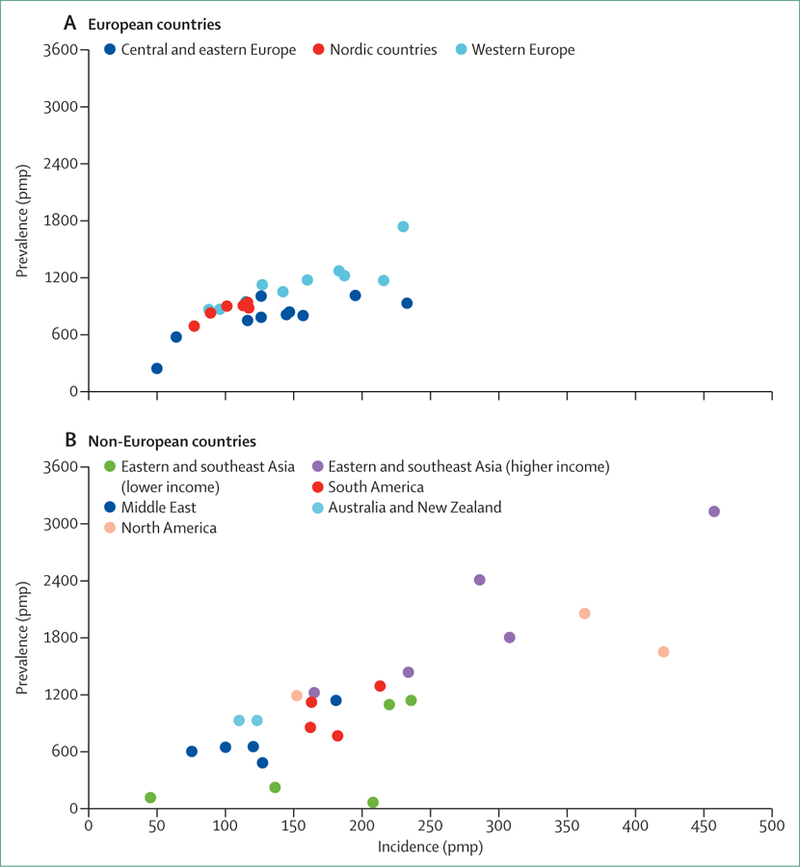
End-stage kidney disease (ESKD) incidence and prevalence calculated for patients using either maintenance dialysis or a kidney transplant for ESKD. Countries listed in order of lowest to highest incidence within each region. (A) Central and eastern Europe: Russia, Estonia, Bosnia and Herzegovina, Poland, Slovenia, Romania, Serbia, Croatia, Czech Republic, and Hungary; Nordic countries: Iceland, Finland, Norway, Sweden, and Denmark; and western Europe: Ireland, Scotland, UK (excluding Scotland), Netherlands, Spain, Austria, France, Belgium (French speaking), Belgium (Dutch speaking), Greece, and Portugal. (B) Eastern and southeastern Asia (gross national income <US$25 000): Bangladesh, Philippines, Indonesia, Thailand, and Malaysia; eastern and southeastern Asia (gross national income ≥$25 000): Hong Kong, South Korea, Japan, Singapore, and Taiwan; the Middle East: Iran, Qatar, Oman, Saudi Arabia, and Israel; South America: Argentina, Uruguay, Brazil, and Chile; North America: Canada, USA, and Mexico (Jalisco). pmp=per million population. Data are from the US Renal Data System.2
Also in higher-income countries, ESKD incidence rates have plateaued during the past decade, not only in Nordic and other European countries but also in the USA, which has a much higher ESKD incidence.2 Stabilisation of ESKD incidence might suggest increased success in prevention of chronic kidney disease or slowing its progression to avoid kidney failure. In support of either possibility, incidence of ESKD due to diabetes has plateaued or declined in nearly all of these countries in 2008–13.2 Both reasons would be important public health achievements. Other factors that might have contributed to reductions in the observed incidence rates of ESKD are increases in the delay to start RRT or in patients who choose to avoid RRT entirely in favour of conservative kidney management. By contrast, incidence of ESKD continues to rise in higher-income eastern and southeast Asian countries.
In lower-income countries, reported incidence of ESKD varies greatly (figure 1) and substantial increases in recent years have been reported. From 2000 to 2013, increases of 13 times in Thailand, seven times in Bangladesh, and almost four times in Russia were reported, whereas rises of two to three times were seen in the Philippines, Malaysia, the Jalisco region of Mexico, as well as South Korea. As a whole, expansion of governmental support for dialysis programmes was the largest contributor to the rise in ESKD incidence counts, with smaller contributions from increased population size, rise in ageing populations, and growing prevalence of hypertension and diabetes.8 Although expanded access to RRT in many countries is commendable, reduced or no access to RRT is all too common in other countries. The substantially lower incidence reported for ESKD in Indonesia, the Philippines, and Bangladesh than in higher-income countries in east and southeast Asia presumably reflects, at least in part, reduced access to RRT. Worldwide, and as previously noted, the number of people who reach ESKD without access to RRT is estimated at up to three times higher than the number who receive RRT.1
In many countries, the incidence of ESKD due to diabetes has risen much faster than the overall rise in ESKD incidence, presumably reflecting the rising population burden of diabetes and improving survival for people with diabetes. Diabetes was the primary cause of ESKD in 32% of incident patients in the median country in 2013 from 46 national registries,2 varying from 15–25% in many European countries to about 60% in Malaysia, Singapore, and the Jalisco region of Mexico. The global rise in diabetes and its end-organ complications has also affected the burden of comorbidities and overall frailty of patients with ESKD. The complexity of disease management and risks for adverse outcomes (ie, mortality, admission to hospital, cardiovascular events, stroke, vascular access complications, infections, amputations, and others) are higher in patients with ESKD and diabetes than in those without diabetes.2,9–11
ESKD prevalence
Reported ESKD prevalence varies by almost 50 times between countries (figure 1)2 and is strongly correlated with ESKD incidence (regression coefficient [R2]=0·66 across all countries). Unlike trends in ESKD incidence which vary from strongly positive to negative, the prevalence of ESKD per million population has increased in all 32 registries contributing data,2 with a median increase of 50% (range 29–839) during 2000–13. Several observations are pertinent to this Series paper. First, increases in ESKD prevalence in countries with little change in the incidence are strongly indicative of declines in ESKD mortality, now generally confirmed by data from registries that publish mortality trends. Second, in countries such as Indonesia, the Philippines, and Bangladesh, ESKD prevalence is disproportionately very low in relation to the incidence. In these countries, government or third party payment for dialysis is limited to a finite period of time. Third, overall upward trends in ESKD prevalence, and similar projections for the near future, support the need for expanded access to in-centre dialysis, home dialysis, and kidney transplantation services to meet the growing worldwide burden of ESKD.2,8
ESKD treatment modalities
Kidney transplantation is the treatment of choice for eligible patients with ESKD, which results in a substantially improved quality of life and median survival similar to survival for patients without ESKD. Stark differences exist in access to and use of kidney transplantation. In 2013, transplantation use for patients with ESKD ranged from 57–72% in Nordic countries, Estonia, and the Netherlands, to less than 10% in some Asian and eastern European countries (figure 2). A striking observation is that the countries with the highest proportion of kidney transplants in patients with ESKD—mostly Nordic and several other European countries—also have some of the lowest incidence rates of ESKD. One implication, of public health importance, is that efforts to slow progression of chronic kidney disease might have additional downstream benefits, namely that kidney transplantation can be offered to a higher proportion of patients with ESKD because of a relatively low number of incident cases. Some European countries have a much larger proportion of incident ESKD cases who receive a pre-emptive kidney transplant (18–40% in Denmark, Iceland, Norway, Sweden, the Netherlands, and the UK) than other countries (1–2% in the USA;12 Kramer A and Pippias M, European Renal Association/European Dialysis and Transplant Association (ERA-EDTA) Registry, personal communication). As such, these countries provide a substantial fraction of their incident patients with ESKD the opportunity to avoid dialysis as their first treatment for this disease, and potentially altogether. The optimum timing of pre-emptive transplantation remains controversial, because it balances dialysis avoidance with early exposure to chronic immunosuppressive therapy.
Figure 2. Renal replacement therapy modality used for patients with end-stage kidney disease, by country, in 2013.
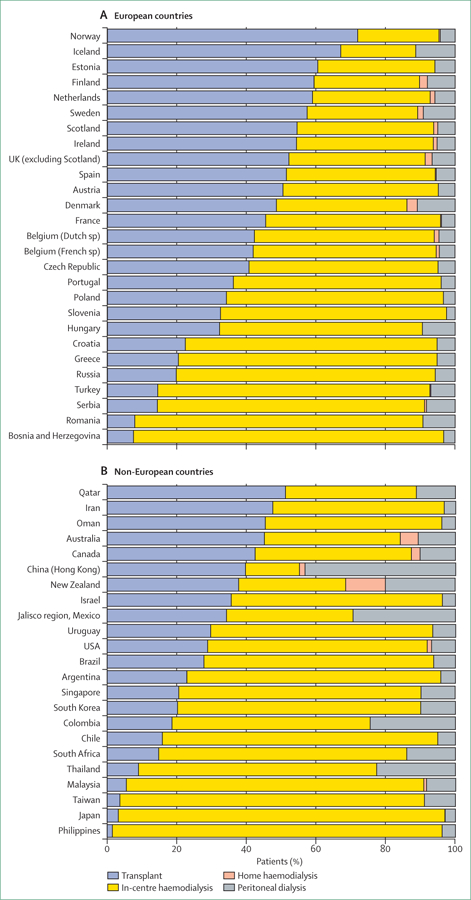
Modality use is shown for all patients reported in each country who received either chronic dialysis or a kidney transplant for treatment of end-stage kidney disease in 2013. Data are from the US Renal Data System.2
Of dialysis modalities, home dialysis options (peritoneal dialysis or home haemodialysis) are considered to have clinical outcomes that are better or at least comparable to in-centre haemodialysis, and are substantially less disruptive to patients’ lives.13 Countries that provide home dialysis to at least 20% of patients with ESKD include Hong Kong (45%), and New Zealand, Colombia, Thailand, and the Jalisco region of Mexico (23–31%; figure 2). These countries’ performances suggest that a large pool of patients receiving in-centre haemodialysis in other countries could use home dialysis if it were made more widely available. For example, peritoneal dialysis use in the USA has risen from 8% in 2009, to 10% in 2015, attributed principally to changes in reimbursement favouring use of peritoneal dialysis. Although this increase can be viewed enthusiastically, this 2% absolute increase could almost certainly be higher still if the culture of in-centre dialysis were less entrenched.
By providing kidney transplantation or home dialysis to a substantial fraction of patients, several nations use in-centre haemodialysis for fewer than a third of their patients with ESKD. These countries include Hong Kong (the lowest use of in-centre haemodialysis at 15%), Estonia, the Netherlands, New Zealand, and some Nordic countries (figure 2). By contrast, in many countries in eastern and southeastern Asia, at least 85% of patients with ESKD receive in-centre haemodialysis. Of these, Japan is notable because it has a large and mature ESKD treatment programme with excellent clinical outcomes, but very low use of transplantation and home dialysis. In-centre dialysis is favoured over home dialysis in Japan partly for historical reasons (many dialysis facilities are available and are easily accessible, with many placed explicitly near public transportation stops), and kidney donation rates are low, which might be because of spiritual or religious beliefs.
ESKD and dialysis mortality
Although most ESKD registries report incidence and prevalence data, survival data are preponderantly from higher-income countries. For those with ESKD onset from 2004 to 2008, unadjusted 5-year survival of all patients with ESKD (treated with dialysis or transplantation) was 41% in the USA, 48% in Europe, and 60% in Japan, despite patients being 2–3 years older on average in Europe and Japan than in the USA, and Japan having very few transplant patients.2,12–15 The survival difference between the USA and Europe is smaller than in previous years, because mortality has declined in both regions but to a greater extent in the USA. In 1996–2000, 5-year survival in patients with incident ESKD was 36% in the USA and 48% in Europe2,12,15 (in Europe, unadjusted survival was 47·6% in 2004 vs 48·3% in 2008; countries in the ERA-EDTA Registry differed slightly).16
Japan substantially outperforms other countries in survival of patients receiving dialysis. Unadjusted 5-year survival was 60% in Japan, 39% in the USA, and 41% in Europe for patients starting dialysis in 2004–08.2,14,15 Where data are available, survival is good in other countries with populations of predominantly Asian ethnicity: unadjusted 5-year survival was 52% in Malaysia (2004–08)17 and 44% in Taiwan (2000–09).18 National dialysis outcomes data are, to our knowledge, not yet available from China or south Asian countries, although steps are underway to obtain such data.19
The reasons for international differences in the survival of dialysis populations are not completely understood. First, patients receiving dialysis in countries with the highest transplantation rates are relatively older and less healthy than those in countries with lower rates of transplantation, so these countries tend to have a lower dialysis population survival. The opposite is true for Japan, which has very few transplant patients and thus retains its healthiest patients on dialysis. However, differences between patient characteristics explain only part of the international variation in survival.10,20 A substantial part of the variability seems to be attributable to variation in cardiovascular21 and all-cause22 background mortality in the general populations. Investigations of the relative frequencies of types of stroke by ethinic origin showed a higher proportion of haemorrhagic versus thrombotic stroke in eastern and southeast Asian populations than in European and US populations,11,23 implying differences in vascular biology or pathophysiological responses to hypertension in different populations.
Intriguingly, adjusted survival on dialysis in Europe and North America is generally shortest in white individuals.2,24–26 In the UK, survival is longer in those of south Asian origin and those who are black compared with white patients. Similarly, in the USA, patients who are Asian, black, or Native American have longer survival than white patients, as do those of Hispanic origin than non-Hispanics. In the USA, nutritional status is healthier and muscle mass is greater in black than in white dialysis patients.26 In the past 5 years, high ESKD incidence in black patients has been attributed to functional variants of the APOL1 gene, which is most common in people of west African descent, and is believed to have evolved as protection from trypanosomal parasitic infections.27–30 Whether APOL1-associated ESKD has a role in explaining racial differences for survival of patients receiving dialysis in the USA is unknown.
International variation in dialysis practices
While international differences in dialysis outcomes derive in part from variation in patient characteristics, evidence over the years indicates that survival differences are, to some extent, affected by modifiable variation in dialysis practices. With regards to in-centre haemodialysis, many data supporting this assertion are from the Dialysis Outcomes and Practice Patterns Study (DOPPS), a series of consecutive, international, prospective cohort studies. The DOPPS is now in its 20th year and is still motivated by the hypothesis that differences in patient longevity, morbidity, and patient experiences are influenced by measurable differences in dialysis facility practices.31–52 The DOPPS studies a random sample of in-centre haemodialysis patients within a random sample of dialysis units in each participating country, stratified to represent facility types (eg, free-standing, hospital-based, or satellite facilities) and geographical regions in each country.53–55 The DOPPS was launched in the USA in 1996, and was expanded to Japan, seven European countries, Canada, Australia, and New Zealand in 1998–2002, China in 2010, and Russia, Turkey, and six Gulf Cooperation Council countries in 2012.31–52,56–58
Contrasting Japanese practices with those of other countries
Because haemodialysis mortality is much lower in Japan than most other countries in the DOPPS, many differences in practices and clinical measures have been studied (table 2). Anaemia management is less intensive, with lower erythropoietin stimulating agent doses and less intravenous iron use; targets for haemoglobin, serum ferritin, and parathyroid hormone are lower; dialysate composition is produced centrally without the chance to modify it at the bedside; dialysate water standards are stricter; and cardiovascular screening tests in the dialysis unit are routine. C-reactive protein concentrations, measured routinely in dialysis units outside the USA, are five times lower in Japan than in Europe, likely reflecting both genetic influences60 and dialysis practices (eg, high use of surgical vascular access and ultrapure water). Although the previously stated differences are of interest, for many the causal associations with survival are uncertain, illustrating the dearth of definitive clinical trial data in the field.61 We discuss selected high profile practice areas with some support from higher-level evidence.
Table 2:
Haemodialysis practice areas and clinical measures that differ in Japan from Europe and the USA
| Japan | Europe* | USA | |
|---|---|---|---|
| Vascular access | |||
| Higher arteriovenous fistula use | 91% | 69% | 68% |
| Anaemia | |||
| Lower epoetin dose† (median units per week) | 5000 | 7176 | 9000 |
| Lower intravenous iron use‡ | 27% | 69% | 66% |
| Lower haemoglobin (g/dL) | 10.5 | 11.3 | 10.9 |
| Lower ferritin (ng/mL) | 144 | 523 | 758 |
| Mineral bone disorder | |||
| Lower intravenous vitamin D use (vs USA) | 36% | 18% | 60% |
| Lower parathyroid hormone (ng/L) | 118 | 240 | 303 |
| Dialysis prescription | |||
| Lower blood flow rate (mL/min) | 208 | 333 | 419 |
| Lower Kt/Vurea | 1.42 | 1.57 | 1.57 |
| Longer dialysis session length (vs USA; min) | 239 | 244 | 218 |
| Dialysis routinely in supine position§ | 93% | 53% | 3% |
| Dialysate composition | |||
| Higher dialysate sodium¶ (mmol/L) | 140 | 139 | 138 |
| Lower dialysate bicarbonate‖ (mmol/L) | 29 | 34 | 36 |
| Dialysate water | |||
| JSDT standard for dialysis fluid is <0.050 EU/mL of endotoxin and a bacterial count <100 colony forming units per mL, the strictest in the world15,59 | .. | .. | .. |
| Cardiovascular factors | |||
| Higher blood pressure (mm Hg) | 146 | 138 | 145 |
| Lower median CRP concentrations** (mg/L) | 1.0 | 5.2 | .. |
| Use of tests | |||
| Routine measurement of CRP** (% of facilities) | 73% | 70% | 0 |
| Routine chest radiographs†† (% of facilities) | 98% | 59% | 40% |
| Yearly screening for vascular calcification†† (% of facilities) | 38% | 35% | 7% |
| Patient preparation for dialysis‡‡ | |||
| Higher proportion with pre-dialysis care§§ | 70% | 76% | 69% |
| Higher proportion with arteriovenous fistula use at haemodialysis initiation§§ | 84% | 50% | 28% |
| Lower estimated glomerular filtration rate at dialysis start¶¶ (mL/min per 1.73 m2) | 6.8 | 9.5 | 10.1 |
Data are from the initial cross-section of patients enrolled in the Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 5 (2012–15). Unless otherwise noted, values in the table represent the mean or prevalence weighted by the fraction of patients sampled in each participating facility. Kt/Vurea=a unitless measure representing clearance of urea (K) over the duration of a haemodialysis treatment (t), divided by the urea volume of distribution (Vurea). JSDT=Japanese Society for Dialysis Therapy. CRP=C-reactive protein.
DOPPS phase 5 European countries were Belgium, Germany, Italy, Spain, Sweden, and the UK. France was excluded because of low DOPPS phase 5 enrolment at time of publication.
Converted to intravenous epoetin equivalent dose; darbepoetin doses converted in ratio of 250:1; pegylated epoetin β (MIRCERA) doses converted in ratio of 208:1; subcutaneous doses converted in ratio of 1·15:1.
Prescription in the month before DOPPS enrolment.
On the basis of DOPPS 3 (2006) enrolment data.
Excluding patients in whom dialysate sodium concentrations varied during treatment (sodium modelling or profiling).
Does not account for dialysate acetate concentration; median of 8·0 mmol/L for Japan, 3·0 mmol/L for Europe, and 4·0 mmol/L for the USA.
Restricted to facilities routinely measuring CRP at least once every 4 months for more than 75% of facility patients during DOPPS phase 5 follow-up.
Used data from DOPPS phase 5 year 3 (2014) medical director survey.
≥4 months of prenephrology care.
Among patients on dialysis ≤60 days at DOPPS enrolment.
In patients enrolled in DOPPS within 120 days of starting dialysis; estimated glomerular filtration rate calculated with the Modification of Diet in Renal Disease (version 4) variable formulae, with variables of creatinine concentration, age, black ethnicity, and sex; qualitatively similar results obtained when also adjusting for Japanese ethnic origin.
The crucial role of vascular access
Of haemodialysis practices, variation in vascular access is undoubtedly one of the most important determinants of patient outcomes.42 The native arteriovenous fistula (AVF) is widely recognised as the access of first choice for most patients, providing better outcomes than arteriovenous grafts or central venous catheters (CVCs). CVC use has been associated with substantially higher mortality, medical complications, and costs.42,62–69 Since joining the DOPPS more than 15 years ago, AVF use has been highest in Japan—at more than 90%—than any other country except for Russia, which had more than 90% AVF use at study entry in 2012–13 (table 2; figure 3).70 (Of note, mortality in patients receiving haemodialysis in the Russian registry is very low at 7·2 deaths per 100 patient-years during 2009–13; although this mortality might reflect excellent practice, patient selection for dialysis could contribute, and greater understanding is needed.71) In the late 1990s to early 2000s, AVF use was lower in the USA (24% in 1997) than all other DOPPS countries.52,70 Shorter survival in the USA compared with Europe was explained largely by differences in vascular access use.42
Figure 3. Selected practices or measures in prevalent haemodialysis patients, by country (2012–15).
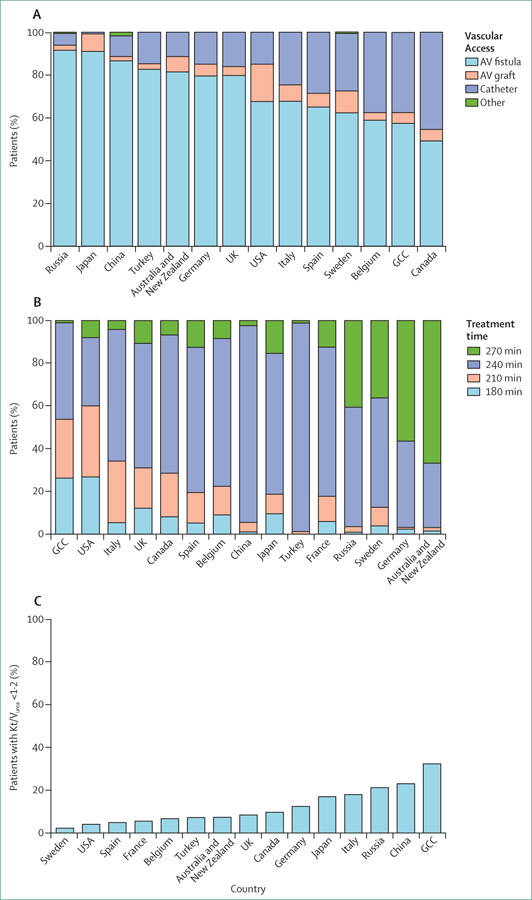
(A) Type of vascular access used. Catheter is a central venous catheter. Data from Gulf Cooperation Council (GCC; Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates), Russia, Turkey, Belgium, Sweden, and China are based on vascular access at the initial cross-section of the Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 5; data from remaining countries based on cross-section of haemodialysis patients in August, 2013. (B) Haemodialysis session duration (treatment time) in patients receiving dialysis three times a week; treatment time was defined as a categorical variable (<200, 200–225, 226–250, and >250 min). Because treatment time for most patients was at exactly 30 min intervals, these categories are labelled as 180, 210, 240, and 270 min, respectively. (C) Single pool Kt/Vurea in patients receiving haemodialysis three times a week, and receiving dialysis for at least 1 year. AV=arteriovenous. Kt/Vurea=a unitless measure representing clearance of urea (K) over the duration of an haemodialysis treatment (t), divided by the urea volume of distribution (Vurea). Some countries are omitted from some figures because of missing data. Figure adapted from Pisoni and colleagues,70 by permisson of Elsevier.
Because high AVF use is of crucial clinical importance and has been achieved in some countries for many years, practice guidelines, policy changes, and quality initiatives have been directed toward this goal in countries with lower rates of AVF use in the past 10–15 years.72–76 However, in 2013,70 AVF use in prevalent haemodialysis patients varied from 49% to 92% across 20 countries, and catheter use ranged from 2% to 45% (figure 3A). In countries such as the UK and USA, changes spurred by policy interventions have had commendable effects. In the UK, CVC use declined from 28%70,77,78 to 16% after application of a tariff to CVC use in 2011–12·79 In the USA, greater AVF use is largely attributed to the Centers for Medicare & Medicaid Services’ (CMS) Fistula First Breakthrough Initiative (FFBI) launched in 2003. From 2003 to 2013, AVF use increased from 32% to 68%, arteriovenous graft use declined from 38% to 18%, and CVC use declined from 27% to 15%.2,70 The FFBI spurred quality initiatives by dialysis companies, regional ESKD quality networks, and by the CMS.73,80 By contrast, fistula use has fallen and catheter use has risen in other countries.77 CVC use is now 38% in Belgium and 45% in Canada, roughly three times higher than in the USA and some European countries.
Drivers of vascular access success, or underperformance, are complex. Social determinants and the dialysis unit culture might be a factor. Although some patients state a preference for CVCs—perhaps because surgical vascular access (AVF or arteriovenous grafts) requires large-needle venipuncture three times a week, often bleeds after dialysis, and can be physically disfiguring81—these preferences are more common in CVC users at dialysis centres with high CVC use, suggesting that these centres foster a culture of catheter use. Many additional observations and questions about current vascular access practice remain regarding: why differences in AVF failure rates between centres and countries are so large;34 whether use of upper arm AVFs (now more common than forearm AVFs in the USA82) accelerates loss of sites for future surgical vascular access; why vascular access procedure rates have risen dramatically, and the implications for AVF durability;83 whether vascular access outcomes can be improved by better coordination of care between the dialysis unit, surgeon, and interventional suite; what are the best approaches to vascular access for dialysis patients with a very short life expectancy; and how patients’ preferences can be honoured through the complex and frequently invasive processes associated with establishment and use of vascular access.
Dialysis adequacy and dialysis session duration
Dialysis session length for in-centre haemodialysis has received considerable attention because in the past decade sessions have become longer in most DOPPS countries, but have shortened in the USA (figure 3B). Reasons for these changes reflect interplay between clinical practice recommendations, reimbursement incentives, unit policies, and clinician and patient preferences, all in absence of definitive evidence from clinical trials. For many years, dialysis adequacy in everyday clinical practice has been measured primarily by dialytic clearance of blood urea, expressed typically as Kt/Vurea (a unitless measure representing clearance of urea [K] over the duration of a haemodialysis treatment [t], divided by the urea volume of distribution [Vurea]). Clinical trial data support achievement of single pool (unequilibrated) Kt/Vurea values of more than 1·2, but not necessarily a cutpoint greater than 1·2 for most patients.50,84,85 Kt/Vurea can be raised by increasing the dialyser size or blood flow rate through the dialyser (to increase Kurea), or by lengthening the dialysis session (ie, increase in treatment time).
In the absence of definitive clinical trial data, widespread opinion holds that longer treatment time confers clinical benefits beyond Kt/Vurea, including clearance of toxins substantially larger than urea (so-called middle molecules) and removal of target fluid volume while reducing haemodynamic instability (ie, intradialytic hypotension).86,87 Observational data indicate that longer treatment time is associated with longer survival, better volume management, better blood pressure control, better phosphorus control, and fewer cardiovascular events than shorter dialysis sessions.23,88–90 In this context, median dialysis treatment time in those patients receiving in-centre dialysis three times weekly is now 4 h (total of 12 h per week) or longer in many high-income countries, despite the logistical challenges to management of dialysis shifts for health-care staff in busy dialysis units.35,48 Dialysis session duration of 4 h or more was tied to reimbursement measures in Japan in 2008, and Germany in 2009,91 and sessions in both these countries are now some of the longest in DOPPS countries (figure 3B).
By contrast, performance measures are not pegged to session duration in the USA, but instead to Kt/Vurea72 achieved in dialysis units in the USA via short dialysis sessions, but high blood flow rate and large dialyser size (figure 3C). Shorter dialysis sessions yield many operational advantages for patient flow over three shifts a day in busy dialysis units. Although treatment time is nearly the shortest in the USA of DOPPS countries, average blood flow rate is roughly 50% higher than in Europe and double that used in Japan (table 2).34 Japanese practice guidelines stress the importance of dialysis that is longer and gentler (ie, uses a lower blood flow rate), on the premise that this approach best ensures haemodynamic stability, despite greater likelihood of having Kt/Vurea values of less than 1·2.33,49,92 Uncertainty about optimum dialysis session length, and metrics for dialysis adequacy and fluid volume management in general, continue to merit research and policy attention. Results from an ongoing pragmatic trial in the USA of dialysis session duration in incident haemodialysis patients (NCT02019225), with randomisation at the centre level to standard session length or 4·25 h, will be of interest.
Use of haemodiafiltration for chronic dialysis has gained much attention with its rapidly increased use in many countries, spurred by the availability of on-line replacement fluid, dialysis machines that can be readily adapted to haemodiafiltration, and accompanying industry interests.87 Haemodiafiltration use in the DOPPS countries is now 26% in Europe and 7% in Japan, but less than 1% in North America. Haemodiafiltration relies on convective dialysis, which might more closely mimic glomerular filtration than conventional diffusive-based dialysis. Despite widespread uptake of haemodiafiltration, com parative effectiveness studies have, so far, reported mixed results.
Extended duration dialysis
Haemodialysis is an intermittently delivered therapy, and thus an inherently unnatural approach to replacement of kidney function. Approaches to substantially extend haemodialysis duration—eg, from 4 h three times a week to a total of 15 h or more per week—include frequent dialysis (more than three sessions a week), long dialysis (≥5 h per session), or combinations thereof, provided during the day or overnight, and in the dialysis unit or at home. Despite the theoretical benefits of extended duration dialysis, supporting evidence is derived from observational data and from the relatively small, and logistically challenging, Frequent Hemodialysis Network (FHN) trials in the USA.93–96 The two trials6,94 in this network showed better composite outcomes for short daily (in-centre) dialysis versus conventional three times a week haemodialysis, and did not show a survival benefit for long-hours dialysis (predominantly home nocturnal dialysis). Higher mortality rates were reported for the long-hours dialysis group during the year after study completion.97,98 Although conclusive data are absent, use of extended duration haemodialysis is very common in countries experienced with this technique (eg, Canada, Australia, and New Zealand; figure 2), where practitioners believe in its beneficial effects on dialysis outcomes and patients’ everyday experiences.
For most patients with ESKD who are still undergoing standard dialysis three times a week, the once weekly 2-day interval between dialysis sessions (long gap) is now recognised to have increased risk of complications and mortality (often volume overload related).40,99 Beyond counselling patients to restrict salt and water intake at weekends, the practice of dialysing every other day, rather than three times a week, is physiologically appealing but rare due to scheduling challenges. A notable exception is Australia where 6–15% of haemodialysis patients receive dialysis on alternate days and predominantly in the home setting.100
By contrast, some authorities now advocate incremental dialysis, typically twice a week, for people starting dialysis principally as a strategy to reduce dialytic complications and help preserve residual kidney function. Although residual kidney function is associated with increased survival and a favourable patient experience, the value of incremental dialysis is uncertain and controversial.101 Twice weekly dialysis is also common in countries with reduced access to dialysis or where patients have to pay for dialysis treatment. Use of dialysis twice weekly is less than 3% in Europe, Japan, and North America, but is 20% in China, and is more common in China among patients who report lower incomes.102
Outcomes in the early dialysis period
Patients starting chronic haemodialysis are faced with very high mortality rates in the first few months (figure 4).33,103,104 As with overall dialysis mortality, mortality in the early dialysis period is lowest in Japan, and is higher in the USA than in many European DOPPS countries.33 However, early mortality, if adjusted for age, is also especially high in Belgium and Canada. Catheter use by incident patients receiving dialysis is particularly high, and use of AVF is low in Canada, Belgium, and the USA (figure 5A).2,70
Figure 4. Mortality in time periods after the start of haemodialysis, by country in the Dialysis Outcomes and Practice Patterns Study (2002–15).
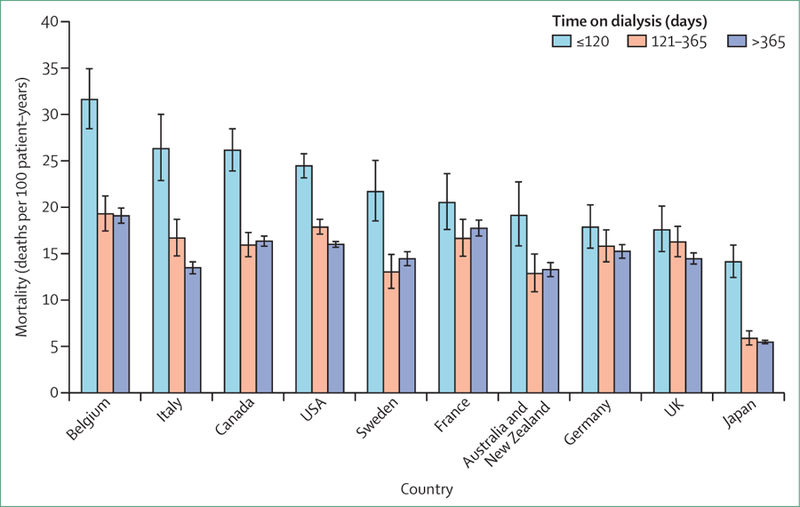
Countries ordered by mortality at 120 days. Error bars show 95% CIs, calculated with the Byar approximation. Data are of 86 886 patients at facilities participating in the Dialysis Outcomes and Practice Patterns Study, phases 2–5 (2002–15). Figure adapted from Robinson and colleagues,33 by permission of Elsevier.
Figure 5. Selected practices or measures in incident haemodialysis patients, by country (2012–15).
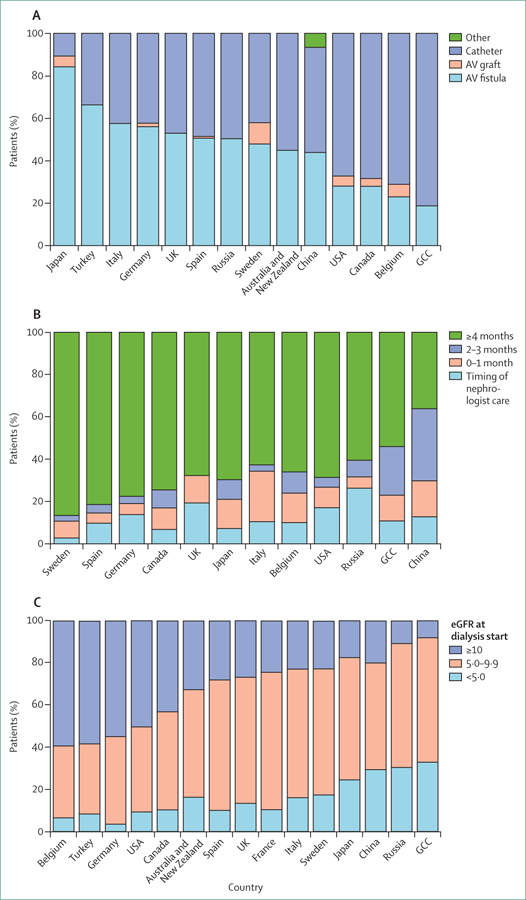
(A) Type of vascular access used in patients on dialysis ≤60 days at Dialysis Outcomes and Practice Patterns Study (DOPPS) enrolment. (B) Timing of first nephrology care before dialysis initiation in patients on dialysis ≤60 days at DOPPS enrolment; on the basis of responses to the question, “How many months before start of chronic dialysis did the patient first see a nephrologist?”, collected at enrolment. (C) Estimated glomerular filtration rate just before patients started haemodialysis. Some countries are omitted from some figures because of missing data.Figure reproduced from Pisoni and colleagues,70 by permission of Elsevier. AV=arteriovenous. GCC=Gulf Cooperation Council (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and United Arab Emirates).
Early and frequent pre-ESKD nephrology care is associated with improved patient preparedness, experiences, and survival in the early dialysis period. However, in many countries a high proportion of patients start dialysis within a few months of their first visit to a nephrologist—ie, too soon to establish surgical vascular access for use at dialysis initiation (figure 5B).45,105,106 And even early nephrological care does not guarantee readiness for dialysis. In the USA, AVF use at dialysis incidence is low (38%) even in patients who had seen a nephrologist 4 months or more before ESKD.2,70 This poor performance is partly due to disincentives in payment structure, as patients aged younger than 65 years become eligible for Medicare reimbursement at 90 days after ESKD onset.107
Recognition that many patients are poorly prepared to start dialysis has led to scrutiny of estimated glomerular filtration rate (eGFR) at dialysis start. The IDEAL clinical trial,108 corroborated by observational studies, demonstrated no clinical benefit in starting dialysis at higher or lower eGFR concentrations (ie, earlier vs later start).109–111 Yet the mean eGFR concentration at dialysis start rose in the USA and elsewhere in the first decade of this century, in part in response to practice guidelines (now altered), but perhaps in some places also driven by practitioners’ desire to keep dialysis clinics operating at high capacity.112 Mean eGFR at dialysis start in the USA has been stable since roughly 2010,113 but the difference between DOPPS countries in mean eGFR at dialysis start of 3–4 mL/min per 1·73 m2 in eGFR concentration at dialysis start (figure 5C, table 2) conservatively indicates that some patients might start dialysis 6 months earlier than needed or more. Multidisciplinary programmes to start dialysis when the patient is prepared, rather than on the basis of eGFR concentrations alone, might help reduce urgent or unnecessarily early dialysis starts and improve patient outcomes.114,115
Future perspectives and conclusions
The upward trends in ESKD prevalence, and projections for the near future, support the need for expanded dialysis and kidney transplantation services to meet the disease’s growing burden in much of the world. In some high-income countries progress is being made in slowing the development of kidney failure, an important public health achievement. At the same time, because patients with ESKD are surviving for longer and demand for transplantation is met by supply only in a few countries, the numbers of patients needing dialysis and transplantation will continue to rise even in countries with stable ESKD incidence. In many low-income and middle-income countries incremental increases in governmental support for chronic dialysis are positive developments. However, inequities in access to this life-saving therapy remain a major challenge in the context of competing priorities for resources.
In countries with universal access to ESKD therapies, large differences exist in the proportional use of transplantation or home dialysis (widely considered preferable to in-centre haemodialysis for many patients with ESKD). Overall survival of patients with ESKD is longer in Europe than in the USA, although this gap has been largely closed by incrementally greater improvements in the USA in the past few decades. Survival of patients receiving dialysis in Japan, and several other eastern or southeastern Asian countries with available data, substantially exceeds other regions partly due to lower background mortality and fewer transplant recipients. However, major differences in dialysis practices between countries highlight opportunities to improve outcomes. A commendable achievement is the exceptionally high use of surgical vascular access in Japan, some European countries, and some centres in countries with less favourable overall surgical access use, showing that excellence is a realistic expectation. Another laudable progression in some countries is the very common use of longer dialysis sessions, allowing for more gradual fluid removal and achievement of target weight with greater haemodynamic stability. By contrast, in countries where short dialysis sessions are the norm, complications of chronic volume overload remain a predominant concern. Mortality is especially high soon after onset of ESKD. Improved access to care and coordination of care to assure patients are adequately prepared to start dialysis are both feasible and necessary.
Modern dialysis is an expensive, intrusive, and physiologically inadequate treatment, and most uraemic toxins are only partly removed by the dialysers used. Nanotechnology and miniaturisation might simplify blood purification and bioengineered kidneys might provide restorative kidney functions, but these technologies are all in their infancy.116 Until game-changing innovations are successfully developed and become widely available dialysis will remain life-saving but incredibly disruptive to patients’ lives. A priority should be to improve patients’ experiences by extending access to kidney transplantation, providing true choice between dialysis modalities, rehabilitating frail and poorly nourished patients, and aligning decision making with the wishes of patients and families, including the options to forego or stop dialysis if desired.
Search strategy and selection criteria.
We searched MEDLINE for articles published between Jan 1, 2006, and Jan 1, 2016, with terms related to specific content areas (including worldwide treatment end-stage renal disease [ESRD]; dialysis conservative care; APO L1 and kidney disease; haemodialysis treatment time; Frequent Haemodialysis Network; and HEMO trial). We also searched for relevant information in national ESRD or renal society registry reports, and for particular relevant health initiatives in some countries (UK: end of life planning; US Renal Physicians Association: shared decision making and dialysis withdrawal). We also cited selected publications from the Dialysis Outcomes and Practice Patterns Study, as this was a particular focus for this Series paper. We largely selected publications from the past 5 years, but did not exclude commonly referenced older publications. Reviews are cited to provide readers with more details and references than this Series paper has room for.
Key messages.
Although the majority of patients reaching end-stage kidney disease (ESKD) worldwide die because renal replacement therapy (RRT; eg, dialysis or transplantation) cannot be accessed, the incidence of ESKD treated with RRT is rising rapidly in many countries because of increased availability of these services and increasingly older populations with multiple comorbidities.
ESKD incidence is stable or declining in many countries with long-standing access to RRT, presumably due, in part, to increased success in prevention of chronic kidney disease or slowing of its progression to avoid end-stage kidney failure.
Transplantation or home dialysis are widely considered preferable to in-centre haemodialysis for many patients with ESKD, yet use of these modalities ranges from more than two-thirds of patients in some countries to fewer than 10% in many others.
Worldwide survival is poor in most patients with ESKD who are treated with in-centre haemodialysis, but generally survival is improving, and is longest in some Asian countries.
International variation in haemodialysis practices and outcomes highlights opportunities to improve care; commendable practice patterns include exceptionally high use of surgical vascular access in Japan and some European countries, and high use of longer or more frequent dialysis sessions in countries such as Germany, Australia, and New Zealand, allowing for more effective management of volume status.
Mortality is especially high soon after onset of ESKD therapy, and improved preparation for ESKD is needed, including alignment of decision making (eg, modality selection, patient choice to decline or withdraw from dialysis, and timing of RRT initiation) with wishes of patients and their families.
Acknowledgments
Information regarding registry data in this Series paper are supported in whole or partly by funds from the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Institutes of Health, Department of Health and Human Services (Contract number HHSN276201400001C; US Renal Data System), and from the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA Registry). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. The DOPPS programme is supported by Amgen, Kyowa Hakko Kirin, AbbVie, Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma. Additional support for specific projects and countries is also provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme, Proteon Therapeutics, Relypsa, and F Hoffmann-LaRoche; in Canada by Amgen, BHC Medical, Janssen, Takeda, and Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire, and WiNe Institute; for PDOPPS in Japan by the Japanese Society for Peritoneal Dialysis. All support is provided without restrictions on publications. Grants are made to Arbor Research Collaborative for Health and not to individual investigators. We thank Brian Bieber, Alan Leichtman, Keith McCullough, Friedrich Port, Sarah White, and Lindsay Zepel for their supportive contributions to this Series paper, and Shauna Leighton for editorial assistance.
Footnotes
Declaration of interests
BMR and RLP report grants from Amgen, Kyowa Hakko Kirin, Baxter Healthcare, European Renal Association/European Dialysis and Transplant Association (ERA-EDTA), Vifor Pharma Ltd, Hexal AG, AbbVie, Shire, German Society of Nephrology, Società Italiana di Nefrologia, Japanese Society for Peritoneal Dialysis, Keryx, and Genzyme Corporation. BMR reports personal fees from University of Toronto, Rhode Island Hospital, and Kyowa Hakko Kirin, outside of the submitted work. TA reports personal fees from Kyowa Hakko Kirin, and AbbVie Inc, during the conduct of the study; and reports personal fees from JT Pharmaceuticals Corporate, Kissei Pharmaceutical, Nipro Medical, Ono Pharmaceutical, Astellas, Bayer HealthCare, Chugai Pharmaceutical, Torii Pharmaceutical, Fuso Pharmaceutical, Teijin Pharma, and GlaxoSmithKline, outside of the submitted work. KJJ reports grants from ERA-EDTA, European Society for Paediatric Nephrology, International Pediatric Nephrology Association, and the Dutch Kidney Foundation, outside of the submitted work. PGK reports personal fees from Fresenius Australia and Quanta Fluid Solutions, outside of the submitted work. RLP reports personal fees from Kyowa Hakko Kirin and reports non-financial support from National Kidney Foundation, outside of the submitted work. RS declares no competing interests.
For more information about the Fistula First Breakthrough Initiative see http://www.fistulafirst.org
Contributor Information
Bruce M Robinson, Arbor Research Collaborative for Health, Ann Arbor, MI, USA; Department of Internal Medicine and Nephrology, University of Michigan, Ann Arbor, MI, USA.
Tadao Akizawa, Showa University School of Medicine, Shinagawa, Tokyo, Japan.
Kitty J Jager, ERA-EDTA Registry, Department of Medical Informatics, Academic Medical Center, University of Amsterdam, Amsterdam-Zuidoost, Netherlands.
Peter G Kerr, Monash Medical Centre and Monash University Clayton, Clayton, VIC, Australia.
Rajiv Saran, Department of Internal Medicine and Nephrology, University of Michigan, Ann Arbor, MI, USA.
Ronald L Pisoni, Arbor Research Collaborative for Health, Ann Arbor, MI, USA.
References
- 1.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–82. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System. 2015 USRDS Annual Data Report: epidemiology of kidney disease in the United States, vol 2 Bethesda, MD: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2015. [Google Scholar]
- 3.Caskey FJ, Kramer A, Elliott RF, et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011; 26: 2604–10. [DOI] [PubMed] [Google Scholar]
- 4.van de Luijtgaarden MW, Noordzij M, van Biesen W, et al. Conservative care in Europe--nephrologists’ experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant 2013; 28: 2604–12. [DOI] [PubMed] [Google Scholar]
- 5.UK NHS. End of life care in adavanced kidney disease: a framework for Implementation http://www.ncpc.org.uk/sites/default/files/EndOfLifeCareInAdvancedKidneyDisease.pdf (accessed March 7, 2016).
- 6.Watanabe Y, Hirakata H, Okada K, et al. , for the Japanese Society for Hemodialysis Therapy Guideline Commission of Maintenance Hemodialysis Investigation, Subgroup Commission on withholding and withdrawal from dialysis. Proposal for the shared decision-making process regarding initiation and continuation of maintenance hemodialysis. Ther Apher Dial 2015; 19 (suppl 1): 108–17. [DOI] [PubMed] [Google Scholar]
- 7.The Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis, second edition. 2010. http://www.thaddeuspope.com/images/RPA_-_2010_no_dialysis_PVS_or_dementia.pdf (accessed March 7, 2016). [DOI] [PubMed]
- 8.Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol 2015; 26: 2621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combe C, Albert JM, Bragg-Gresham JL, et al. The burden of amputation among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Am J Kidney Dis 2009; 54: 680–92. [DOI] [PubMed] [Google Scholar]
- 10.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2003; 14: 3270–77. [DOI] [PubMed] [Google Scholar]
- 11.Herrington W, Haynes R, Staplin N, et al. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial 2015; 28: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pippias M, Jager KJ, Kramer A, et al. The changing trends and outcomes in renal replacement therapy: data from the ERA-EDTA registry. Nephrol Dial Transplant 2016; 31: 831–41. [DOI] [PubMed] [Google Scholar]
- 13.Thodis ED, Oreopoulos DG. Home dialysis first: a new paradigm for new ESRD patients. J Nephrol 2011; 24: 398–404. [DOI] [PubMed] [Google Scholar]
- 14.ERA-EDTA Registry. ERA-EDTA Registry annual report 2013 Amsterdam: The European Renal Association–European Dialysis and Transplant Association, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masakane I, Nakai S, Ogata S, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2013). Ther Apher Dial 2015; 19: 540–74. [DOI] [PubMed] [Google Scholar]
- 16.ERA-EDTA Registry. ERA-EDTA Registry 2005 annual report Amsterdam: The European Renal Association–European Dialysis and Transplant Association, 2007. [Google Scholar]
- 17.Seng WH, Meng OL. Chapter 3: death and survival on dialysis. In: Goh BL, Ong LM, Lim YM, eds. 21st report of the Malaysian dialysis and transplant registry 2013 Kuala Lumpur, 2014. [Google Scholar]
- 18.Wu M-S, Wu I-W, Hsu K-H. Survival analysis of Taiwan renal registry data system (TWRDS) 2000–2009. Acta Nephrol 2012; 26: 104–08. [Google Scholar]
- 19.Jha V, John O, Joshi R, et al. Dialysis outcomes in India: a pilot study. Nephrology (Carlton) 2015; 20: 329–34. [DOI] [PubMed] [Google Scholar]
- 20.van Manen JG, van Dijk PC, Stel VS, et al. Confounding effect of comorbidity in survival studies in patients on renal replacement therapy. Nephrol Dial Transplant 2007; 22: 187–95. [DOI] [PubMed] [Google Scholar]
- 21.Yoshino M, Kuhlmann MK, Kotanko P, et al. International differences in dialysis mortality reflect background general population atherosclerotic cardiovascular mortality. J Am Soc Nephrol 2006; 17: 3510–19. [DOI] [PubMed] [Google Scholar]
- 22.van Dijk PC, Zwinderman AH, Dekker FW, et al. Effect of general population mortality on the north-south mortality gradient in patients on replacement therapy in Europe. Kidney Int 2007; 71: 53–59. [DOI] [PubMed] [Google Scholar]
- 23.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118: 2702–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roderick P, Byrne C, Casula A, et al. Survival of patients from South Asian and Black populations starting renal replacement therapy in England and Wales. Nephrol Dial Transplant 2009; 24: 3774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Beukel TO, Dekker FW, Siegert CE. Increased survival of immigrant compared to native dialysis patients in an urban setting in the Netherlands. Nephrol Dial Transplant 2008; 23: 3571–77. [DOI] [PubMed] [Google Scholar]
- 26.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 2006; 17: 2910–18. [DOI] [PubMed] [Google Scholar]
- 27.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosset S, Tzur S, Behar DM, Wasser WG, Skorecki K. The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat Rev Nephrol 2011; 7: 313–26. [DOI] [PubMed] [Google Scholar]
- 30.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant 2016: 31: 349–58. [DOI] [PubMed] [Google Scholar]
- 31.Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS Study. Clin J Am Soc Nephrol 2015; 10: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailie GR, Larkina M, Goodkin DA, et al. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 2015; 87: 162–68. [DOI] [PubMed] [Google Scholar]
- 33.Robinson B, Zhang J, Morgenstern H, et al. Worldwide, mortality is a high risk soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asano M, Thumma J, Oguchi K, et al. Vascular access care and treatment practices associated with outcomes of arteriovenous fistula: International comparisons from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephron Clin Pract 2013; 124: 23–30. [DOI] [PubMed] [Google Scholar]
- 35.Tentori F, Zhang J, Li Y, et al. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the DOPPS. Nephrol Dial Transplant 2012; 27: 4180–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson BM, Bieber B, Pisoni RL, Port FK. Public policy series. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol 2012; 7: 1897–905. [DOI] [PubMed] [Google Scholar]
- 37.Robinson B, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2012; 82: 570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes AA, Tong L, Thumma J, et al. Phosphate binder use and mortality among hemodialysis patients in the DOPPS: evaluation of possible confounding by nutritional status. Am J Kidney Dis 2012; 60: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis (HD) patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2012; 7: 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Schaubel DE, Kalbfleisch JD, et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int 2012; 81: 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol 2012; 7: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 2009; 53: 475–91. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez SP, Albert JM, Blayney MJ, et al. Rosiglitazone is associated with mortality in chronic hemodialysis patients. J Am Soc Nephrol 2009; 20: 1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopes AA, Bragg-Gresham JL, Ramirez SPB, et al. Prescription of antihypertensive agents to hemodialysis patients: time trends and associations with patient characteristics, country, and survival in the DOPPS. Nephrol Dial Transplant 2009; 24: 2809–16. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa T, Bragg-Gresham JL, Yamazaki S, et al. Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol 2009; 4: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelssohn DC, Pisoni RL, Arrington CJ, et al. A practice-related risk score (PRS): a DOPPS-derived aggregate quality index for haemodialysis facilities. Nephrol Dial Transplant 2008; 23: 3227–33. [DOI] [PubMed] [Google Scholar]
- 47.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–30. [DOI] [PubMed] [Google Scholar]
- 48.Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg 2008; 247: 885–91. [DOI] [PubMed] [Google Scholar]
- 49.Port FK. Practice-based versus patient-level outcomes research in hemodialysis: the DOPPS (Dialysis Outcomes and Practice Patterns Study) experience. Am J Kidney Dis 2014; 64: 969–77. [DOI] [PubMed] [Google Scholar]
- 50.Port FK, Wolfe RA, Hulbert-Shearon TE, Ashby VB, McCullough KP, Held PJ. High dialysis dose is associated with lower mortality among women but not among men. Am J Kidney Dis 2004; 43: 1014–23. [DOI] [PubMed] [Google Scholar]
- 51.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2003; 64: 339–49. [DOI] [PubMed] [Google Scholar]
- 52.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 2002; 61: 305–16. [DOI] [PubMed] [Google Scholar]
- 53.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000; 57: S74–81. [Google Scholar]
- 54.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 2004; 44: 7–15. [DOI] [PubMed] [Google Scholar]
- 55.Port FK, Wolfe RA, Held PJ, Young EW. Random sample (DOPPS) versus census-based registry approaches to kidney disease research. Blood Purif 2003; 21: 85–88. [DOI] [PubMed] [Google Scholar]
- 56.Hecking M, Karaboyas A, Saran R, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2012; 59: 238–48. [DOI] [PubMed] [Google Scholar]
- 57.Fuller DS, Bieber BA, Pisoni RL, et al. International comparisons to assess effects of payment and regulatory changes in the United States on anemia practice in patients on hemodialysis: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 2015; 10.1681/ASN.2015060673. [DOI] [PMC free article] [PubMed]
- 58.Hasegawa T, Bragg-Gresham JL, Pisoni RL, et al. Changes in anemia management and hemoglobin levels following revision of a bundling policy to incorporate recombinant human erythropoietin. Kidney Int 2011; 79: 340–46. [DOI] [PubMed] [Google Scholar]
- 59.Sehulster L, Chinn RY, US Centers for Disease Control and Prevention, Healthcare Infection Control Practices Advisory Committee. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52: 1–42. [PubMed] [Google Scholar]
- 60.Kelley-Hedgepeth A, Lloyd-Jones DM, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem 2008; 54: 1027–37. [DOI] [PubMed] [Google Scholar]
- 61.Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis 2014; 63: 771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. CHOICE study. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study. J Am Soc Nephrol 2005; 16: 1449–55. [DOI] [PubMed] [Google Scholar]
- 63.Bradbury BD, Chen F, Furniss A, et al. Conversion of vascular access type among incident hemodialysis patients: description and association with mortality. Am J Kidney Dis 2009; 53: 804–14. [DOI] [PubMed] [Google Scholar]
- 64.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 2003; 42: 1013–19. [DOI] [PubMed] [Google Scholar]
- 65.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004; 15: 477–86. [DOI] [PubMed] [Google Scholar]
- 66.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 2006; 47: 469–77. [DOI] [PubMed] [Google Scholar]
- 67.Lacson E Jr, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 2009; 53: 79–90. [DOI] [PubMed] [Google Scholar]
- 68.Engemann JJ, Friedman JY, Reed SD, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol 2005; 26: 534–39. [DOI] [PubMed] [Google Scholar]
- 69.Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 2002; 40: 611–22. [DOI] [PubMed] [Google Scholar]
- 70.Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis 2015; 65: 905–15. [DOI] [PubMed] [Google Scholar]
- 71.Bikbov BT, Tomilina NA. Renal replacement therapy for ESRD patients in Russian Federation, 1998–2013. Report of Russian RRT registry. Part 2. Nephrol Dial (in press).
- 72.US Centers for Medicare & Medicaid Services. Technical specifications for ESRD QIP measures www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/061_TechnicalSpecifications.html (accessed March 4, 2016).
- 73.Vassalotti JA, Jennings WC, Beathard GA, et al. Fistula first breakthrough initiative community education committee (2012) fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial 2012; 25: 303–10. [DOI] [PubMed] [Google Scholar]
- 74.National Kidney Foundation. The National Kidney Foundation kidney disease outcomes quality initiative, 2006. Vascular access guidelines http://www.kidney.org/professionals/kdoqi/pdf/12–50–0210_JAG_DCP_Guidelines-VA_Oct06_SectionC_ofC.pdf (accessed July 23, 2013).
- 75.Kumwenda M, Mitra S, Reid C. Clinical practice guideline: vascular access for haemodialysis Hampshire: UK Renal Association, 2015. [DOI] [PubMed] [Google Scholar]
- 76.The Japanese Society for Dialysis Therapy. Guidelines pertaining to creating and repairing vascular access for chronic hemodialysis. J Jpn Soc Dial Ther 2011; 44: 855–937. [Google Scholar]
- 77.Rayner HC, Pisoni RL. The increasing use of hemodialysis catheters: evidence from the DOPPS on its significance and ways to reverse it. Semin Dial 2010; 23: 6–10. [DOI] [PubMed] [Google Scholar]
- 78.UK NHS Department of Health. Payment by results guidance for 2011–12 http://webarchive.nationalarchives.gov.uk/20130402234253/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_126157.pdf (accessed March 8, 2016).
- 79.Abma I, Jayanti A, Bayer S, et al. Perceptions and experiences of financial incentives: a qualitative study of dialysis care in England. BMJ Open 2014; 4: e004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fistula First: Fistula first national vascular access improvement initiative http://www.fistulafirst.org (accessed March 4, 2016).
- 81.Fissell RB, Fuller DS, Morgenstern H, et al. Hemodialysis patient preference for type of vascular access: variation and predictors across countries in the DOPPS. J Vasc Access 2013; 14: 264–72. [DOI] [PubMed] [Google Scholar]
- 82.Pisoni R, Zepel L, Tentori F, et al. International variability in arteriovenous fistula maturation and placement: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 2015; 26: 285A–86A. [Google Scholar]
- 83.Lok CE, McCullough K, Fluck RJ, et al. Rising vascular access (VA) procedure rates over time internationally: findings from the DOPPS. J Am Soc Nephrol 2012; 23: 268A. [Google Scholar]
- 84.Owen WF Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993; 329: 1001–06. [DOI] [PubMed] [Google Scholar]
- 85.Depner T, Daugirdis J, Greene T, et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int 2004; 65: 1386–94. [DOI] [PubMed] [Google Scholar]
- 86.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 2008; 3: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ok E, Asci G, Chazot C, Ozkahya M, Dorhout Mees EJ. Dialysis 2. Controversies and problems of volume control and hypertension in haemodialysis. Lancet 2016; 10.1016/S0140-6736(16)30389-0. [DOI] [PubMed]
- 88.Flythe JE, Curhan GC, Brunelli SM. Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int 2013; 83: 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brunelli SM, Chertow GM, Ankers ED, Lowrie EG, Thadhani R. Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int 2010; 77: 630–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daugirdas JT. Dialysis time, survival, and dose-targeting bias. Kidney Int 2013; 83: 9–13. [DOI] [PubMed] [Google Scholar]
- 91.Kleophas W, Karaboyas A, Li Y, et al. Changes in dialysis treatment modalities during institution of flat rate reimbursement and quality assurance programs. Kidney Int 2013; 84: 578–84. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe Y, Kawanishi H, Suzuki K. Japanese Society for Hemodialysis therapy clinical guideline for proposal for “Maintenance Hemodialysis; Hemodialysis Prescriptions”. Ther Apher Dial 2015; 19 (suppl 1): 67–92. [DOI] [PubMed] [Google Scholar]
- 93.FHN Trial Group Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007; 298: 1291–99. [DOI] [PubMed] [Google Scholar]
- 95.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol 2012; 23: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2011; 58: 782–93. [DOI] [PubMed] [Google Scholar]
- 97.Rocco MV, Daugirdas JT, Greene T, et al. , for the FHN Trial Group. Long-term effects of frequent nocturnal hemodialysis on mortality: the Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am J Kidney Dis 2015; 66: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rocco MV, Lockridge RS Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–107. [DOI] [PubMed] [Google Scholar]
- 100.ANZDATA Registry. Chapter 4: haemodialysis. In: The 37th annual ANZDATA report Adelaide: Australia and New Zealand Dialysis and Transplant Registry, 2014. [Google Scholar]
- 101.Kalantar-Zadeh K, Unruh M, Zager PG, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis 2014; 64: 181–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bieber B, Qian J, Anand S, et al. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics, and quality of life in the China Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2014; 29: 1770–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99. [DOI] [PubMed] [Google Scholar]
- 104.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int 2014; 86: 392–98. [DOI] [PubMed] [Google Scholar]
- 105.Gillespie BW, Morgenstern H, Hedgeman E, et al. Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clin Kidney J 2015; 8: 772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watanabe Y, Yamagata K, Nishi S, et al. Japanese Society for Dialysis therapy clinical guideline for “Hemodialysis Initiation for Maintenance Hemodialysis”. Ther Apher Dial 2015; 19: 93–107. [DOI] [PubMed] [Google Scholar]
- 107.Allon M, Dinwiddie L, Lacson E Jr, et al. Medicare reimbursement policies and hemodialysis vascular access outcomes: a need for change. J Am Soc Nephrol 2011; 22: 426–30. [DOI] [PubMed] [Google Scholar]
- 108.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609–19. [DOI] [PubMed] [Google Scholar]
- 109.Crews DC, Scialla JJ, Liu J, et al. Predialysis health, dialysis timing, and outcomes among older United States adults. J Am Soc Nephrol 2014; 25: 370–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crews DC, Scialla JJ, Boulware LE, et al. Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. Am J Kidney Dis 2014; 63: 806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehrotra R, Rivara M, Himmelfarb J. Initiation of dialysis should be timely: neither early nor late. Semin Dial 2013; 26: 644–49. [DOI] [PubMed] [Google Scholar]
- 112.NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. National Kidney Foundation. Am J Kidney Dis 1997; 30 (3 suppl 2): S67–136. [DOI] [PubMed] [Google Scholar]
- 113.US Renal Data System. USRDS 2010 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States Bethesda, MD: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases, 2010. [Google Scholar]
- 114.Lacson E Jr, Wang W, DeVries C, et al. “Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes.” Am J Kidney Dis 2011; 58: 235–42. [DOI] [PubMed] [Google Scholar]
- 115.National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930. [DOI] [PubMed] [Google Scholar]
- 116.Remuzzi G, Benigni A, Finkelstein FO, et al. Kidney failure: aims for the next 10 years and barriers to success. Lancet 2013; 382: 353–62. [DOI] [PubMed] [Google Scholar]


