Abstract
Background.
Selectively flexible rings, Colvin-Galloway (CG) Future and Carpentier-Edwards (CE) Physio II, are used for annuloplasty during mitral valve repair to facilitate dynamic annular motion while preventing annular dilation. In this study, we assessed the extent and nature of the flexibility of these rings in vivo, which has not been objectively demonstrated.
Methods.
Three-dimensional transesophageal echocardiography was used intraoperatively to acquire data regarding dynamic motion of mitral annuli and annuloplasty rings in 33 patients undergoing mitral repair (15 CG Future and 18 CE Physio II) and in 15 control patients. Data were analyzed to assess the dynamic changes in annular geometry after implantation of selectively flexible rings.
Results.
After annuloplasty, there was an immediate and significant decrease in annular displacement (p < 0.001) and annular displacement velocity (p < 0.01). Dynamic change in multiple variables including anteroposterior diameter (p < 0.001) and annular area (p < 0.001) was also significantly depressed. In comparison with normal mitral valves, partially flexible rings allowed limited dynamic motion: percentage changes in anteroposterior diameter (p < 0.001), anterolateral posteromedial diameter (p < 0.001), and total circumference (p < 0.001) were significantly lower. Compared with each other, the two rings resulted in similar changes in anterior annulus length (p = 0.93), posterior annular length (p = 0.82), and annular area (p = 0.31).
Conclusions.
Mitral annular dynamics were uniformly depressed after implantation of these rings. Selective flexibility could not be demonstrated in vivo using echocardiographic data.
Placement of an annuloplasty device during mitral valve (MV) repair reduces mitral annular (MA) area, provides greater coaptation surface, and prevents recurrent mitral regurgitation [1]. This facilitates reversal of left ventricular (LV) remodeling by reducing volume overload [2–4]. However, there is variation in the surgical approach toward selection of an appropriate annuloplasty device. Devices range from partial to full, completely flexible to rigid, and flat to saddle-shaped [5]. Although rigid rings reliably prevent dilation of the annulus, they can negatively impact LV function, annular stress, and long-term LV remodeling [6–8]. Conversely, nonrigid rings, although they preserve LV function, run a higher risk of continued annular dilation, poor durability, and recurrent mitral regurgitation [9]. Presumably, these rings allow normal dynamic annular function after implantation.
With 3-dimensional transesophageal echocardiography (3D TEE), it is now possible to appreciate the dynamic MA structure during the cardiac cycle with precision [10]. Notably, the MA conformation in 3D space can be measured and tracked in a clinically feasible fashion [11]. The ready availability of specialized software and enhanced computation for analysis allows the demonstration of annular motion in vivo. This provides us with the opportunity to objectively analyze annular behavior after annuloplasty, and to clinically verify MA dynamism and ring flexibility.
In this study, 3D TEE was used intraoperatively to observe the precise geometric changes of the mitral annulus after implantation with selectively flexible annuloplasty rings. Whereas the Colvin-Galloway (CG) Future Ring (Medtronic Inc, Minneapolis, MN) is anteriorly flexible, the Carpentier-Edwards (CE) Physio II Ring (Edwards Lifesciences, Irvine, CA) is posteriorly flexible. Specifically, we wanted to assess the extent and selectivity of the flexibility of these rings in the range of forces generated by the mitral annulus. Such an investigation of the extent and nature of flexibility of these devices has not been previously performed.
Material and Methods
Colvin-Galloway Future
The CG Future ring (Fig 1) is designed to afford anterior flexibility to the annulus while providing posterior and longitudinal rigidity [12]. This is accomplished by the presence of a flexible sewing strip at the anterior portion, whereas the posterior element is stiffened trigone-to-trigone by a cobalt-nickel alloy. This allows the anterior segment to displace above the plane of the device by 7% of its transverse diameter. By allowing anterior mobility, the dynamic interaction and conformational change through the cardiac cycle between the MV annulus and the LV outflow tract is theoretically better preserved. The tilting of the anterior annulus away from the LV outflow tract during systole may also allow the avoidance of LV outflow tract obstruction and decrease the incidence of systolic anterior motion of the anterior mitral leaflet [13, 14]. This corresponds with a number of studies, which indicate the aortomitral continuity may change in shape and have implications for mitral valve behavior throughout the cardiac cycle [15].
Fig 1.
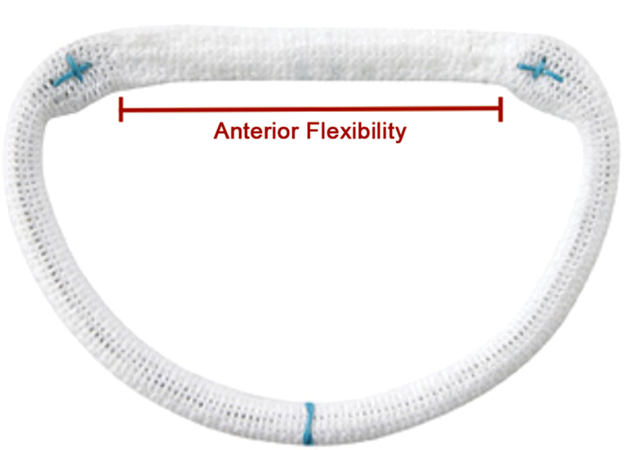
Colvin-Galloway Future ring with anterior flexibility through the flexible sewing strip between the trigones.
Carpentier-Edwards Physio II
The CE Physio II annuloplasty ring (Fig 2) not only incorporates a “double-saddle” shape to reduce leaflet stress [5] but also has a partially flexible element to it. Its design takes into account more current causes for mitral regurgitation, such as ischemic and degenerative. The commissural and posterior sections allow it to change shape during ventricular contraction by means of transverse, but not longitudinal, flexibility [16]. This was designed to reduce stress on the sutures while promoting the possibility of ventricular remodeling. The theory was that the posterior flexibility would allow excursion during diastole to promote filling, and the commissural flexibility would allow annular remodeling. This more circular shape may also have the added benefit of reducing the incidence of systolic anterior motion after repair. Unlike the CG Future rings, this ring demonstrates rigidity at the anterior portion to contrast with the flexibility of its posterior aspect [17]. This differential flexibility was theorized to not only reduce stress on the sutures but also maintain the annulus remodeling effect.
Fig 2.
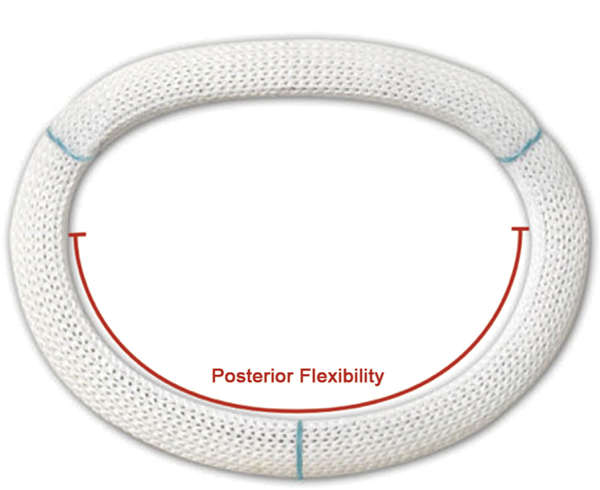
Carpentier-Edwards Physio II ring with posterior flexibility.
Data Collection and Analysis
Data were collected at the Beth Israel Deaconess Medical Center from July 2011 to December 2012 as part of a prospective institutional review board–approved protocol with a waiver of informed consent from patients undergoing MV repair with either CG Future or CE Physio II annuloplasty rings. Only patients with normal LV function with an ejection fraction of more than 0.55 were included. The pre-repair data were acquired after induction of general anesthesia and before institution of cardiopulmonary bypass, and the postrepair data were acquired after the completion of repair and successful separation from cardiopulmonary bypass. The 3D TEE images were acquired by an iE-33 ultrasound system equipped with an X7–2t matrix TEE probe (Philips Medical Systems, Andover, MA). Images were acquired with R-wave gating over 4 beats during a brief period of apnea. For patients in which R-wave gated images could not be acquired because of arrhythmia, 3D live-zoom mode was used to acquire an en face view of the MV such that maximal frame rate could be preserved.
Immediately after acquisition, the data were exported to a Windows-based computer equipped with the Image Arena software (TomTec GmbH, Munich, Germany). In the Image Arena software, the 3D data were accessed by the Mitral Valve Assessment package versions 1.0 and 2.1. The frames representing end-diastole (the point of MV closure) and end-systole (the point before MV opening) were labeled as the temporal borders for analysis, and the geometric indices for these two points in time were used for comparative analysis. Mitral Valve Assessment version 2.1 is a semiautomated dynamic geometric analysis program, which tracks multiple MV geometric variables during the systolic phase [10]. After image orientation, anterior, posterior, anterolateral and posteromedial landmarks were manually identified, along with the positions of the aortic valve and MV coaptations. Based on these anatomic landmarks and temporal boundaries, we tracked pre-repair and postrepair mitral annuli and leaflets from end-diastole to end-systole (Fig 3).
Fig 3.
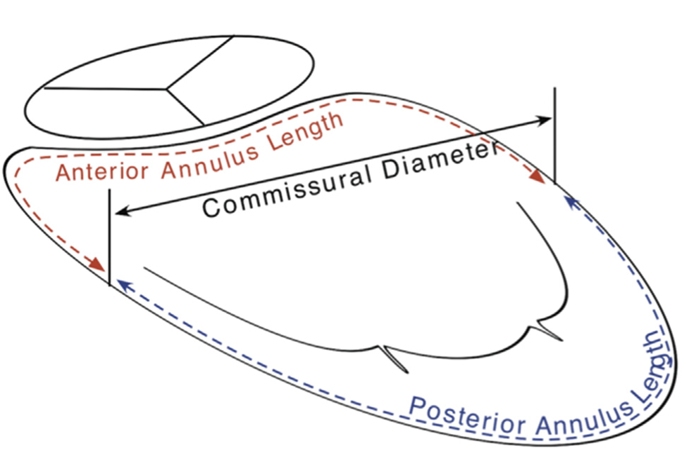
A rendition of the variables measured in Mitral Valve Assessment version 1.0. The anterior annulus length and the posterior annulus length refer to the circumferential length of each of these segments to determine dynamic, in vivo selective flexibility.
To calculate the anterior and posterior annular circumferential dimensions at end-diastole and at endsystole, the postrepair 3D data were accessed with the Mitral Valve Assessment 1.0 software. Data generated from these MV analysis software environments were exported to a comma-separated values file and analyzed with Microsoft Excel for Mac 2011 (Microsoft Corp, Redmond, WA).
Statistical Analysis
For pre-repair versus postrepair analysis, the delta change in dimensions of each measured variable over the cardiac cycle before and after annuloplasty was compared using paired Student’s t test. The variables compared between end-diastole and end-systole were also analyzed with paired Student’s t tests, whereas values analyzed between the two ring types, as well as between each ring type and control patients (patients with normal mitral annuli), were analyzed with unpaired Student’s t tests. Statistical significance was determined at a probability value less than or equal to 0.05.
Thirty-three patients undergoing mitral valve repair (15 CG Future, 18 CE Physio II) with routine 3D TEE images were analyzed during the systolic phase of the cardiac cycle for changes in geometric measurements. For comparison, we also analyzed the MA dynamic geometry in 15 control patients undergoing cardiac surgery with normal systolic function and no valvular abnormalities.
Results
No significant differences were noted in baseline characteristics between the two groups with regard to age, body surface area, or mitral annuloplasty ring size (Table 1).
Table 1.
Baseline Characteristics of Mitral Annuloplasty Patients
| Characteristic | CG Future | CE Physio II | p Value |
|---|---|---|---|
| Sex | 12 M, 3 F | 11 M, 7 F | |
| Cause | IMR, 8; Myxo, 7 | IMR, 9; Myxo, 9 | |
| Age (y) | 60.7 ± 12.1 | 65.0 ± 8.6 | 0.28 |
| BSA (kg/m2) | 1.95 ± 0.14 | 1.93 ± 0.23 | 0.78 |
| MV ring size (mm) | 30.5 ± 3.2 | 30.0 ± 3.0 | 0.63 |
BSA = body surface area; CE = Carpentier-Edwards; CG = Colvin-Galloway; IMR = ischemic mitral regurgitation; MV = mitral valve; Myxo = myxomatous degeneration.
Comparison of Pre-Repair and Postrepair Dynamics
When preannuloplasty and postannuloplasty dynamics were analyzed, both rings resulted in significant reduction in annular dynamics. This was evidenced by a decrease in both annular displacement (7.79 mm before versus 4.15 mm after; p < 0.001) and annular displacement velocity (36.2 mm/s before versus 21.2 mm/s after; p < 0.01). Dynamic changes in anteroposterior diameter (4.7 mm before versus 1.6 mm after; p < 0.001) and annular area (1.8 cm2 before versus 0.8 cm2 after; p < 0.001) during the cardiac cycle were significantly reduced as well. Both ring types also led to a decrease in delta change in posterior leaflet area after repair (Table 2).
Table 2.
Comparison of Amount of Dynamic Change (Δ) Exhibited by Annuli Before and After Annuloplasty With a Selectively Flexible Device
| Variable | Preannuloplasty | Postannuloplasty | p Value |
|---|---|---|---|
| CG Future (n = 15) | |||
| Annular displacement (cm) | 7.8 | 4.1 | 0.0002 |
| Annular displacement velocity (cm/s) | 36.3 | 21.2 | 0.0022 |
| Δ Anteroposterior diameter (cm) | 0.48 | 0.16 | 0.0001 |
| Δ ALPM diameter (cm) | 0.25 | 0.20 | 0.2274 |
| Δ Nonplanarity angle (degrees) | 8.1 | 6.5 | 0.2793 |
| Δ Annular circumference (cm) | 0.70 | 0.66 | 0.9315 |
| Δ Annular area 2D (cm2) | 1.80 | 0.75 | 0.0009 |
| Δ Annular area 3D (cm2) | 1.80 | 0.83 | 0.0007 |
| Δ Commissural diameter (cm) | 0.18 | 0.19 | 0.7138 |
| Δ Anterior leaflet (cm2) | 0.95 | 0.67 | 0.2568 |
| Δ Posterior leaflet (cm2) | 2.30 | 0.77 | 0.0035 |
| Δ Tenting height (mm) | 3.2 | 2.1 | 0.2357 |
| CE Physio II (n = 18) | |||
| Annular displacement (cm) | 8.9 | 3.9 | 0.0009 |
| Annular displacement velocity (cm/s) | 36.1 | 20.2 | 0.0074 |
| Δ Anteroposterior diameter (cm) | 0.59 | 0.14 | 0.0006 |
| Δ ALPM diameter (cm) | 0.26 | 0.17 | 0.0687 |
| Δ Nonplanarity angle (degrees) | 7.5 | 5.1 | 0.1157 |
| Δ Annular circumference (cm) | 1.08 | 0.52 | 0.0221 |
| Δ Annular area 2D (cm2) | 2.48 | 0.64 | 0.0006 |
| Δ Annular area 3D (cm2) | 2.55 | 0.68 | 0.0006 |
| Δ Commissural diameter (cm) | 0.22 | 0.13 | 0.0302 |
| Δ Anterior leaflet (cm2) | 1.45 | 0.67 | 0.0158 |
| Δ Posterior leaflet (cm2) | 2.32 | 0.59 | 0.0002 |
| Δ Tenting height (mm) | 2.9 | 1.7 | 0.0996 |
ALPM = anterolateral-posteromedial; CE = Carpentier-Edwards; CG = Colvin-Galloway; 3D = three-dimensional; 2D = two-dimensional.
Comparison With Control Patients
When CG Future and CE Physio II rings were compared with normal mitral annuli there was significantly less dynamic expansion in the circumference, anteroposterior, and anterolateral-posteromedial diameters (p < 0.001 for all three). There was also no significant change in nonplanarity angle for either ring compared with control patients (Table 3).
Table 3.
Average Percentage Increases From End of Diastole to End of Systole Comparing Normal Mitral Annuli (Control Patients) With Colvin-Galloway Future and Carpentier-Edwards Physio II
| CG Future |
CE Physio II |
CG vs P2 |
||||
|---|---|---|---|---|---|---|
| Variable | Control Patients | Average | p Value | Average | p Value | p Value |
| Anteroposterior diameter | 16.8% ± 7.7% | 2.6% ± 3.7% | <0.001 | 3.0% ± 3.0% | <0.001 | 0.75 |
| ALPM diameter | 8.6% ± 5.2% | 1.4% ± 1.5% | <0.001 | 1.9% ± 1.9% | <0.001 | 0.36 |
| 2D area | 5.8% ± 3.6% | 3.2% ± 3.8% | 0.058 | 5.0% ± 3.8% | 0.51 | 0.18 |
| 3D area | 11.8% ± 8.2% | 3.5% ± 3.8% | 0.001 | 4.9% ± 3.7% | 0.003 | 0.31 |
| Total circumference | 14.0% ± 3.6% | 1.5% ± 1.8% | <0.001 | 2.1% ± 1.7% | <0.001 | 0.33 |
| Nonplanarity angle | 0.95% ± 7.4% | 0.0% ± 1.0% | 0.63 | 0.58% ± 1.5% | 0.83 | 0.22 |
| Aorto-mitral angle | 3.1% ± 5.0% | 3.1% ± 3.7% | 0.98 | 3.1% ± 4.0% | 0.99 | 0.96 |
ALPM = anterolateral-posteromedial diameter; CE = Carpentier-Edwards; CG = Colvin-Galloway; P2 = Physio II; 3D = threedimensional; 2D = two-dimensional.
Selective Flexibility
Measurements of anterior annulus length, posterior annulus length, anterior leaflet area, anterior to total circumferential length ratio, and posterior to total circumferential length ratio were unchanged through systole for both CG Future and CE Physio II rings (Table 4). The percentage increases in anterior (3.0% ± 19.9% CG Future versus 3.5% ± 12.7% CE Physio II; p = 0.93) and posterior (2.4% ± 17.7% CG Future versus 1.0% ± 15.5% CE Physio II; p=0.82) annulus lengths also showed no significant difference between the rings. Sphericity and nonplanarity angle were not significantly different for either ring. No other measured change through the cardiac cycle showed any significant difference (Table 4).
Table 4.
Selective Anterior or Posterior Flexibility Analysis
| Variable | End-Diastole | End-Systole | % Change | p Value |
|---|---|---|---|---|
| CG Future (n = 15) | ||||
| Anterior circumferential length (cm) | 4.98 ± 0.94 | 5.06 ± 0.66 | +1.6% | 0.71 |
| Posterior circumferential length (cm) | 4.89 ± 0.34 | 4.93 ± 0.94 | +0.81% | 0.89 |
| Anterior leaflet area (cm2) | 5.18 ± 2.01 | 5.08 ± 1.76 | −2.0% | 0.34 |
| Posterior leaflet area (cm2) | 4.79 ± 1.55 | 4.78 ± 1.42 | −0.21% | 0.93 |
| Anterior to total circumference ratio | 0.54 ± 0.072 | 0.54 ± 0.075 | – | 0.98 |
| Posterior to total circumference ratio | 0.52 ±0.080 | 0.52 ± 0.081 | – | 0.88 |
| CE Physio II (n = 18) | ||||
| Anterior circumferential length (cm) | 5.09 ± 0.75 | 5.21 ± 0.53 | +0.38% | 0.46 |
| Posterior circumferential length (cm) | 4.92 ± 0.89 | 4.88 ± 0.66 | −0.82% | 0.82 |
| Anterior leaflet area (cm2) | 5.47 ± 1.41 | 5.47 ± 1.31 | – | 0.99 |
| Posterior leaflet area (cm2) | 4.36 ± 1.60 | 4.17 ± 1.56 | −4.6% | 0.0079 |
| Anterior to total circumference ratio | 0.54 ± 0.086 | 0.54 ± 0.053 | – | 0.99 |
| Posterior to total circumference ratio | 0.52 ± 0.073 | 0.50 ± 0.055 | −4.0% | 0.47 |
CE = Carpentier-Edwards; CG = Colvin-Galloway.
Comment
Our study demonstrates that dynamic in vivo analysis of annuloplasty rings in the mitral position can be performed with 3D TEE in a clinically feasible fashion with commercially available software. There is experimental evidence of the flexibility of CG Future and CE Physio II rings, but neither of these rings has been previously studied in regard to their dynamic behavior in real time in humans [13, 18]. Therefore, in our data analysis, we set out to vet two key issues insofar as the use and marketing of these rings are concerned. First, we investigated the extent of flexibility. When compared with pre-repair values, several annular dimensions were significantly restricted. The amount of change in the anteroposterior diameter, for example, was reduced to approximately a third compared with its pre-repair extent of change over the cardiac cycle (Table 2). Compared with control patients, dynamic changes in the annular geometry of our study patients during systole were also significantly reduced for both ring types (Table 3). The nonplanarity angle, which has previously been shown to be characteristic of flexible annuloplasty devices, did not change significantly after repair in either group as compared with control patients [19]. These findings imply that despite manufacturers’ claims, flexibility of these rings is relatively limited, especially in the physiologic force range. The small magnitudes of dynamic changes in MA shape and size in our postrepair study point to the same phenomenon (Table 4). In their in vivo study of different ring types, Rausch and colleagues [18] show that partially and completely rigid rings both considerably limit dynamic annular motion. In view of these findings and our own, it is plausible that in vivo behavior of selectively flexible rings is perhaps similar to that of completely rigid rings.
The second question we dealt with was that of selective flexibility. In our ring-to-ring comparison, the magnitude of changes in anterior and posterior annulus lengths was similar in both groups despite one ring being marketed as anteriorly and the other as posteriorly flexible (Table 4). In fact, the rings behaved similarly in almost all dynamic geometric variables. This is interesting considering not only their claimed selective flexibility but also the fact that they are made of entirely different materials. Both rings had a similar impact on the aorto-mitral angle as well. This finding is of note in that the CG Future ring’s anterior flexibility is reported to be an important consideration in preservation of the dynamic nature of the aortic-mitral continuity. Thus, a “selective” preservation of any specific dimension could not be demonstrated in our in vivo analysis of CG Future and CE Physio II rings using 3D TEE.
It is important to note that diseased valves often lack the normal geometry and dynamism that is characteristic of a normal valve [20, 21]. In this context, valve repair may not be a question of preserving geometry but one of reestablishing lost shape and size. This argument forms the basis for the use of rigid rings, whereby the annulus is fixed in the end-systolic conformation to ensure optimal coaptation during this portion of the cardiac cycle [22]. Our study brings into question the use and marketing of costly and innovative ring designs, which may not always lead to claimed features or outcomes after implantation.
Certain limitations can be appreciated in this study. The size of the sample was relatively small, but a well-established methodology was used for these analyses, and therefore we are confident of the validity of our results. Also, the geometric analyses were not conducted in real time. However, the export and offline analysis can be completed with meaningful results relatively quickly. Also, our results draw from immediate postrepair measurements, which may be liable to change over time. There remains a need to assess the long-term impact of selectively flexible rings on MA dynamics.
In conclusion, it is feasible to dynamically track the mitral annulus after MV repair and annuloplasty. After annuloplasty with selectively flexible rings, dynamic MA behavior was depressed. Both CE Physio II and CG Future devices resulted in identical qualitative and quantitative changes in MA geometry when compared with each other. The geometric changes in mitral annuli were global and not limited to either the anterior or the posterior annulus. Therefore, selective flexibility was not clinically demonstrable with our 3D data, indicating that this may not be an in vivo feature of these annuloplasty rings.
Acknowledgments
The authors thank the Ronald M. Weintraub, MD family research fund for their support.
Abbreviations and Acronyms
- CE
Carpentier-Edwards
- CG
Colvin-Galloway
- LV
left ventricle
- MA
mitral annular
- MV
mitral valve
- TEE
transesophageal echocardiography
- 3D
three-dimensional
References
- 1.Schwartz CF, Gulkarov I, Bohmann K, Colvin SB, Galloway AC. The role of annuloplasty in mitral valve repair. J Cardiovasc Surg (Torino) 2004;45:419–25. [PubMed] [Google Scholar]
- 2.DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010;139:76–84. [DOI] [PubMed] [Google Scholar]
- 3.Fedak PWM, McCarthy PM, Bonow RO. Evolving concepts and technologies in mitral valve repair. Circulation 2008;117: 963–74. [DOI] [PubMed] [Google Scholar]
- 4.Unger P, Magne J, Dedobbeleer C, Lancellotti P. Ischemic mitral regurgitation: not only a bystander. Curr Cardiol Rep 2012;14:180–9. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MO, Jensen H, Smerup M, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation 2008;118(14 Suppl 1):S250–5. [DOI] [PubMed] [Google Scholar]
- 6.Bothe W, Kuhl E, Kvitting J-PE, et al. Rigid, complete annuloplasty rings increase anterior mitral leaflet strains in the normal beating ovine heart. Circulation 2011;124(11 Suppl):S81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David TE, Komeda M, Pollick C, Burns RJ. Mitral valve annuloplasty: the effect of the type on left ventricular function. Ann Thorac Surg 1989;47:524–8. [DOI] [PubMed] [Google Scholar]
- 8.Okada Y, Shomura T, Yamaura Y, Yoshikawa J. Comparison of the Carpentier and Duran prosthetic rings used in mitral reconstruction. Ann Thorac Surg 1995;59:658–63. [DOI] [PubMed] [Google Scholar]
- 9.Spoor MT, Geltz A, Bolling SF. Flexible versus nonflexible mitral valve rings for congestive heart failure: differential durability of repair. Circulation 2006;114(1 Suppl):I67–71. [DOI] [PubMed] [Google Scholar]
- 10.Warraich HJ, Shahul S, Matyal R, Mahmood F. Bench to bedside: dynamic mitral valve assessment. J Cardiothorac Vasc Anesth 2011;25:863–6. [DOI] [PubMed] [Google Scholar]
- 11.Khabbaz KR, Mahmood F, Shakil O, et al. Dynamic 3-dimensional echocardiographic assessment of mitral annular geometry in patients with functional mitral regurgitation. Ann Thorac Surg 2013;95:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasol R, Meinhart J, Deutsch M, Binder T. Mitral valve repair with the Colvin-Galloway Future Band. Ann Thorac Surg 2004;77:1985–8. [DOI] [PubMed] [Google Scholar]
- 13.Redmond J, Christiansen D, Bergin C, et al. In-vivo motion of mitral valve annuloplasty devices. J. Heart Valve Dis 2008;17: 110–8. [PubMed] [Google Scholar]
- 14.Lange R, Guenther T, Kiefer B, et al. Mitral valve repair with the new semirigid partial Colvin-Galloway Future annuloplasty band. J Thorac Cardiovasc Surg 2008;135: 1087–93.e4. [DOI] [PubMed] [Google Scholar]
- 15.Veronesi F, Corsi C, Sugeng L, et al. A study of functional anatomy of aortic-mitral valve coupling using 3D matrix transesophageal echocardiography. Circ Cardiovasc Imaging 2009;2:24–31. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier AF, Lessana A, Relland JYM, et al. The “PhysioRing”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995;60:1177–86. [DOI] [PubMed] [Google Scholar]
- 17.Vohra HA, Whistance RN, Bezuska L, Livesey SA. Initial experience of mitral valve repair using the Carpentier-Edwards Physio II annuloplasty ring. Eur J Cardiothorac Surg 2011;39:881–5. [DOI] [PubMed] [Google Scholar]
- 18.Rausch MK, Bothe W, Kvitting J-PE, Swanson JC, Miller DC, Kuhl E. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann Biomed Eng 2012;40:750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmood F, Subramaniam B, Gorman JH 3rd, et al. Threedimensional echocardiographic assessment of changes in mitral valve geometry after valve repair. Ann Thorac Surg 2009;88:1838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levack MM, Jassar AS, Shang EK, et al. Three-dimensional echocardiographic analysis of mitral annular dynamics: implication for annuloplasty selection. Circulation 2012;126(11 Suppl 1):S183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jassar AS, Vergnat M, Jackson BM, et al. Regional annular geometry in patients with mitral regurgitation: implications for annuloplasty ring selection. Ann Thorac Surg 2014;97: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med 2009;361:2261–9. [DOI] [PubMed] [Google Scholar]


