Abstract
Background:
Hypomethylating agents such as decitabine are standard of care for older patients with newly diagnosed acute myeloid leukemia. Single-arm studies have suggested that a 10-day schedule of decitabine may result in better outcomes than the standard 5-day schedule. The aim of this study was to assess the relative efficacy and safety of these two schedules of decitabine in older adults with acute myeloid leukemia unfit for intensive chemotherapy.
Methods:
Between February 28, 2013 and April 12, 2018, older adults with acute myeloid leukemia unfit for intensive chemotherapy were randomized using a Bayesian adaptive design to receive decitabine 20 mg/m2 intravenously as induction for either 5 consecutive days or 10 consecutive days. Initially 40 patients were allocated equally to the two treatment arms using block randomization. After the completion of the equal randomization, the response-adaptive randomization algorithm was employed to unbalance the randomization probabilities in favor of the arm with a superior response rate. Responding patients received decitabine on a 5-day schedule as consolidation for up to 24 cycles. The primary endpoint was composite response rate (defined as complete remission [CR], CR with incomplete platelet recovery [CRp], and CR with incomplete hematologic recovery [CRi]) of the two schedules achieved at any time during therapy in the intention-to-treat population. This trial is closed to new patient entry and was registered at ClinicalTrials.gov (NCT01786343).
Findings:
Seventy one patients received either decitabine for 5 days (n=28) or for 10 days (n=43) and were evaluable for efficacy and safety. The overall response rate at any time during therapy was similar in the 5-day and 10-day arms (43% [95% credible interval (0.26, 0.60)] versus 40% [95% credible interval (0.26, 0.60)], respectively; P=0.78). The difference in overall response rate between groups was 3% (95% credible interval [−0.21, 0.27]). With a total duration of follow-up of 38.2 months, the median overall survival (OS) in the 5-day and 10-day decitabine arms was 5.5 months (interquartile range [IQR], 2.1–11.7) and 6.0 months (IQR, 1.9–11.7), respectively, and 1-year OS rates were 25% in both arms (P=0.47). No significant differences in response rates or OS were observed when stratified by cytogenetics, de novo versus therapy-related/secondary acute myeloid leukemia, or TP53 status. The most common grade 3–4 adverse events were neutropenic fever (7 patients [25%] in the 5-arm and 14 patients [33%] in the 10-day arm) and infection (5 [18%] versus 16 [37%]). In the 5-day arm, 1 patient (4%) each died from sepsis in the context of neutropenic fever, infection, and hemorrhage; in the 10-day arm, 6 patients (14%) died from infection. Early mortality was similar between the two schedules.
Interpretation:
In older patients with newly diagnosed acute myeloid leukemia, decitabine given on either a 5-day or 10-day schedule did not result in significantly different response rates or survival. These findings suggest that these two schedules of decitabine have similar efficacy and that either may be appropriate for use in older patients with acute myeloid leukemia.
Introduction
The outcomes of older patients with acute myeloid leukemia are significantly worse than their younger counterparts. This is due both to their lower tolerance to intensive treatment and also higher rates of adverse-risk cytogenetic and molecular abnormalities in this population.1–3 The hypomethylating agent (HMA) decitabine is commonly used to treat older patients with acute myeloid leukemia who are not candidates for intensive chemotherapy. The combined rate of complete remission (CR) and CR with incomplete hematologic recovery (CRi) with standard doses of HMAs such as decitabine is approximately 20–30%.4 A randomized trial comparing decitabine (20 mg/m2 intravenously [IV] daily for 5 days administered every 4 weeks) versus low-dose cytarabine (20 mg/m2 daily for 10 days administered every 4 weeks) or supportive care in older acute myeloid leukemia patients showed a modest survival benefit for patients treated with decitabine in an unplanned analysis (hazard ratio, 0.82 [95% confidence interval, 0.69 to 0.99], P = 0.037).5
Single-arm studies have suggested that 10-day dosing of decitabine may result in higher response rates than historical reports with standard 5-day dosing.6–9 In a study by Blum et al., a CR rate of 47% was reported with decitabine 20 mg/m2 given for 10 consecutive days, with 64% of patients achieving a morphologic leukemia-free state (with or without count recovery).6 Prolonged exposure to decitabine may be particularly beneficial in patients whose acute myeloid leukemia harbors a TP53 mutation.9 TP53 mutations in acute myeloid leukemia are highly associated with complex karyotypes, resistance to cytotoxic chemotherapy, and poor prognosis.10,11 In one study of a 10-day schedule of decitabine in older patients with acute myeloid leukemia, a subgroup analysis found that all 21 patients with mutated TP53 achieved bone marrow blast clearance (i.e. <5% blasts), compared to only 32 of 78 patients (41%) with wild type TP53.9 Despite this differential response rate between TP53-mutated and wild type cases, the duration of remission for TP53-mutated cases was generally short, and the survivals of these two groups were similar.
Despite evidence suggesting that 10-day schedules of decitabine may be superior to 5-day schedules, no randomized comparison between these regimens has been reported. We therefore designed a randomized phase II trial to evaluate the relative safety and efficacy of decitabine given at a dose of 20 mg/m2 IV for either 5 or 10 consecutive days in older patients with ACUTE MYELOID LEUKEMIA unfit for intensive chemotherapy.
Methods
Study design and participants
This was a single-center, open-label, randomized phase II trial to assess the efficacy of decitabine given in either 5-day or 10-day schedules. This study was conducted at a single academic center (The University of Texas MD Anderson Cancer Center [UTMDACC]).
Adults ≥60 years of age with newly diagnosed ACUTE MYELOID LEUKEMIA (by World Health Organization criteria, i.e. ≥20 % blasts, excluding acute promyelocytic leukemia) who were deemed unsuitable for intensive chemotherapy12 were eligible for this randomized phase II study. Patients <60 years of age could also be enrolled if deemed not to be a candidate for intensive anthracycline plus cytarabine-based chemotherapy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–3 and adequate renal and hepatic function, including a creatinine <2.5 mg/dL and total bilirubin <2.0 mg/dL. Patients with antecedent hematologic disorder (e.g. myelodysplastic syndrome or myeloproliferative neoplasm) were eligible, but they may not have received any prior HMAs. This study was approved by the Institutional Review Board of UTMDACC. All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki.
Randomization and masking
Patients were randomized to either decitabine given for 5 consecutive days or for 10 consecutive days. Initially 40 patients were randomized equally to the two treatment arms using block randomization with a block size of 40. After the completion of the equal randomization, the adaptive randomization algorithm was employed after every patient in order to unbalance the randomization probabilities in favor of the better arm with the better composite response rate. Response after 3 courses of therapy was used for purposes of the adaptive randomization algorithm. All group assignment was open label, and neither investigators nor patients were masked to allocation. The study was monitored by the Data Safety Monitoring Board (DSMB) of UTMDACC for safety and efficacy with the data blinded to the principal investigator.
Procedures
Patients were randomized to receive decitabine 20 mg/m2 IV daily infused over 1 hour given for either 5 or 10 consecutive days during induction. Patients could receive up to 3 courses of decitabine at the randomly assigned dose. Once CR, CR with incomplete platelet recovery (CRp), or CRi was achieved, or earlier at the discretion of the treating physician, patients could receive decitabine 20 mg/m2 IV daily for 5 consecutive days for subsequent consolidation courses (up to 24 total cycles). Courses were repeated every 4–8 weeks, depending on toxicity and the recovery of neutrophil and platelet counts.
Outcomes
The primary endpoint of this phase II randomized study was the composite response rate of CR, CRp, and CRi of the 5-day and 10-day decitabine schedules assessed at any time during treatment. Secondary endpoints included the remission duration, relapse-free survival (RFS), overall survival (OS), and safety profile of the two schedules.
CR, CRp, CRi, and partial remission (PR) were defined according to the revised International Working Group guidelines for response assessment in ACUTE MYELOID LEUKEMIA.13 Minimal residual disease (MRD) was assessed by multiparameter flow cytometry performed on the BM at the time of remission as previously described.14 MRD positivity was defined as a cluster of at least 20 cells showing altered expression of ≥2 antigens. The sensitivity of this MRD assay was at least 0.01%. Remission duration was calculated from the time of CR/CRp/CRi until relapse (censored for death in remission). RFS was calculated from the time of remission until relapse or death. OS was calculated from the time of treatment initiation until death. Safety was assessed with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Mutation analysis was performed using a 28-gene panel as previously described.15–17 Genomic DNA was extracted from bone marrow aspirates or peripheral blood. Amplicon-based next-generation sequencing (NGS) targeting the entire coding regions of a panel of 28 genes associated with myeloid neoplasms was performed using a MiSeq platform (Illumina, San Diego, CA). The genes analyzed included ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, IKZF2, JAK2, KIT, KRAS, MDM2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53, and WT1. For clinical reporting, a minimum sequencing coverage of 250X (bi-directional true paired-end sequencing) was required. The analytical sensitivity was established at 5% mutant reads in a background of wild-type reads. In addition, single gene sequencing of TP53 covering the entire coding region was performed in available post-treatment samples from patients harboring a baseline TP53 mutation as previously described.18
Statistical analysis
The planned maximum sample size was 100. Based on previous studies, we expected a composite response rate (RR) of about 30% in both arms. Therefore, we assumed RR has a prior Beta distribution (0.6, 1.4) with mean 0.3 for each arm. This prior distribution is updated after every patient’s response outcome is observed. For either arm, after observing x responders and y non-responders, the posterior distribution for its composite response rate is updated as Beta (0.6+x, 1.4+y). Beginning with the 21st patient in each arm and for each subsequent patient, we compared RRa (composite response in arm A) with RRb (composite response in arm B), incorporating data from all patients with evaluable response. In order to avoid favoring one arm earlier in a large trial, we used the following randomization formula to assign patients:
, Ab=1-Aa, where Aa is the probability of assigning patients to arm A, Ab is the probability of assigning patients to arm B, Pa is the posterior probability that arm A is superior to arm B, and Pb is the posterior probability that arm B is superior to arm A.
If at any time the probability that one of the schedules is better is higher than 0.95, then it will be declared as superior. If the maximum of 100 patients is enrolled and the probability that one of the schedules is better is higher than 0.90, then it will be declared as superior. Otherwise, the trial will be inconclusive. Detailed operating characteristics and DSMB report can be found in the Supplemental Statistical Information.
Analyses for response rate comparison between the two treatment arms were done by Chi-square tests. Remission duration, RFS and OS were calculated with Kaplan-Meier estimates, and survival estimates were compared with the log-rank test. Paired t-test was used for compare TP53 variant allelic frequencies (VAFs) in pre-treatment and post-cycle 1 bone marrow samples. The data cutoff for this analysis was May 15, 2018. The data analyses were done using GraphPad Prism 6. This study was registered at ClinicalTrials.gov (NCT01786343).
Results
Patient Characteristics
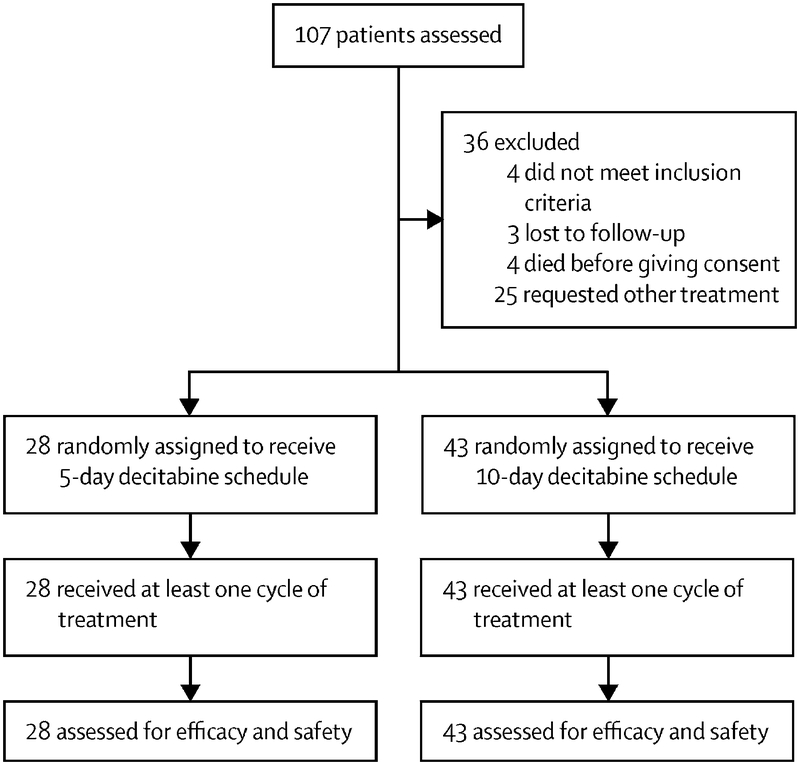
Between February 28, 2013 and April 12, 2018, 71 patients were enrolled and randomized (5-day, n=28; 10-day, n=43; Figure 1). The imbalance of the arms was due to the better performance of the 10-day decitabine schedule during the initial period of the trial. Baseline patient characteristics are shown in Table 1. Treatment arms were well-balanced after randomization. The median age for the 5-day and 10-day arms were 77 (interquartile range [IQR], 70–80) and 78 (IQR, 69–82), respectively; only 1 patient (5-day arm) was <60 years of age. There were high rates of poor-risk features in both treatment arms. Ten patients (36%) and 13 patients (30%) in the 5-day and 10-day arms had an ECOG performance status of 2–3. Thirteen patients (46%) and 24 patients (56%), respectively, had poor-risk cytogenetics (i.e. −5, −7 or complex karyotype). TP53 mutations were detected in 7/24 patients (29%) in the 5-day arm and in 17/41 patients (41%) in the 10-day arm.
Figure 1.
Trial profile
Table 1.
Baseline characteristics
| Characteristica | Decitabine x 5 days (N=28) |
Decitabine x 10 days (N=43) |
|---|---|---|
| Age (years) | 77 [70–80] | 78 [69–82] |
| WBC (109/L) | 2.0 [1.5–3.9] | 3.2 [1.9–10.6] |
| Hemoglobin (g/dL) | 9.4 [8.7–9.8] | 9.2 [9.0–9.7] |
| Platelets (109/L) | 25 [14–60] | 37 [24–83] |
| BM blasts (%) | 40 [29–68] | 46 [25–64] |
| ECOG performance status | ||
| 0–1 | 18 (64) | 30 (70) |
| 2–3 | 10 (36) | 13 (30) |
| s-AML/t-AML | 13 (46) | 18 (42) |
| Cytogenetics | ||
| Diploid | 8 (29) | 10 (23) |
| −5, −7 and/or complex | 13 (46) | 24 (56) |
| Others | 5 (18) | 6 (14) |
| IM/ND | 2 (7) | 3 (7) |
| Mutations | ||
| ASXL1 | 3/19 (16) | 2/34 (6) |
| DNMT3A | 3/24 (13) | 6/42 (14) |
| EZH2 | 1/22 (5) | 1/39 (3) |
| FLT3-ITD | 2/25 (8) | 2/42 (5) |
| NPM1 | 1/24 (4) | 8/42 (19) |
| IDH1/2 | 6/25 (24) | 14/42 (33) |
| JAK2 | 3/24 (13) | 1/42 (2) |
| KRAS/NRAS | 2/24 (8) | 4/42 (10) |
| PTPN11 | 0/21 (0) | 4/38 (6) |
| RUNX1 | 5/19 (26) | 2/35 (6) |
| TET2 | 1/19 (5) | 8/36 (22) |
| TP53 | 7/24 (29) | 17/41 (41) |
Continuous variables are listed as median [interquartile range] and categorical variables as n (%)
WBC, white blood cell; BM, bone marrow; ECOG, Eastern Cooperative Oncology Group; s-AML/t-AML, secondary- or therapy-related AML; IM/ND, insufficient metaphases/not done
Response Rates
Response rates are shown in Table 2. The composite CR + CRp + CRi rate was similar with both decitabine dosing schedules (12/28 [43%, 95% credible interval (0.26, 0.60)] in 5-day arm versus 17/43 [40%, 95% credible interval (0.26, 0.54)] in the 10-day arm; P=0.78). The difference in overall response rate between groups was 3% (95% credible interval [−0.21, 0.27]). Given these overall response rates, if this trial were to continue to the planned maximum sample size (n=100), the probability that the 5-day arm and 10-day arm would be declared superior, were 11% and 0.1%, respectively. Due to both the similar response rates of the two arms and the changing treatment landscape of ACUTE MYELOID LEUKEMIA over the course of this study, it was not felt that this study should continue, as more effective treatments for this population had become available. This study therefore closed based on the above analysis; the closing of this study was endorsed by the institutional DSMB that oversaw it.
Table 2.
Response rates
| Best response | Decitabine x 5 days n (%) |
Decitabine x 10 days n (%) |
P |
|---|---|---|---|
| CR | 8 (29) | 13 (30) | 0.88 |
| CRp | 3 (11) | 2 (5) | |
| Cri | 1 (4) | 2 (5) | |
| CR + CRp + CRi | 12 (43) | 17 (40) | 0.78 |
| PR | 0 | 1 (2) | |
| No response | 15 (54) | 22 (51) | |
| Early death | 1 (4) | 3 (7) |
CR, complete remission; CRp, complete remission without platelet recovery; CRi, complete remission with inadequate count recovery; PR, partial remission
The 95% credible interval for the composite response rate of CR + CRp + CRi (primary outcome) is (0.26, 0.60) for the 5-day schedule and (0.26, 0.54) for the 10-day schedule. The difference in overall response rate between groups was 3% (95% credible interval [−0.21, 0.27]).
CR rates were 29% (8/28) and 30% (1¾3), respectively. One patient in the 10-day arm achieved PR as best response. Four patients in the 10-day arm and 3 patients in the 5-day arm did not meet formal criteria for response but received more than 3 courses of decitabine. Among patients who achieved response, there was no difference in MRD negativity rates between the two arms (5/12 [42%] in the 5-day arm versus 6/15 [40%] in the 10-day arm). There was a trend towards earlier responses with the 10-day decitabine schedule. The median number of courses to best response was 2 (IQR, 1–2) for the 5-day schedule and 2 (IQR, 1–3) for the 10-day schedule (P=0.09). Two patients in the 5-day arm and one patient in the 10-day arm achieve best response after more than 3 courses. Response after 3 courses was stable disease in 2 patients (1 in 5-day arm and 1 in 10-day arm) and CRi in 1 patient (5-day arm); all patients eventually achieved CR. The patient in the 10-arm achieved CR after 4 courses of decitabine (2 courses of the 10-day schedule and 2 courses of the 5-day schedule). Only one patient (4%) in the 5-day arm versus 6 patients (14%) in the 10-day arm achieved CR after 1 cycle of induction (P=0.15). No significant differences in response rates were observed when evaluated by disease subgroups. The response rates for patients in the 5-day and 10-day decitabine arms by subgroup were: diploid cytogenetics (50% [4/8] versus 30% [3/10]; P=0.39), adverse-risk cytogenetics (31% [4/13] versus 46% [20/43]; P=0.37), de novo ACUTE MYELOID LEUKEMIA (47% [7/15] versus 36% [9/25]; P=0.50), secondary- or therapy-related ACUTE MYELOID LEUKEMIA (38% [5/13] versus 44% [8/18]; P=0.74), and TP53-mutated ACUTE MYELOID LEUKEMIA (29% [2/7] versus 47% [8/17]; P=0.40).
Survival Outcomes
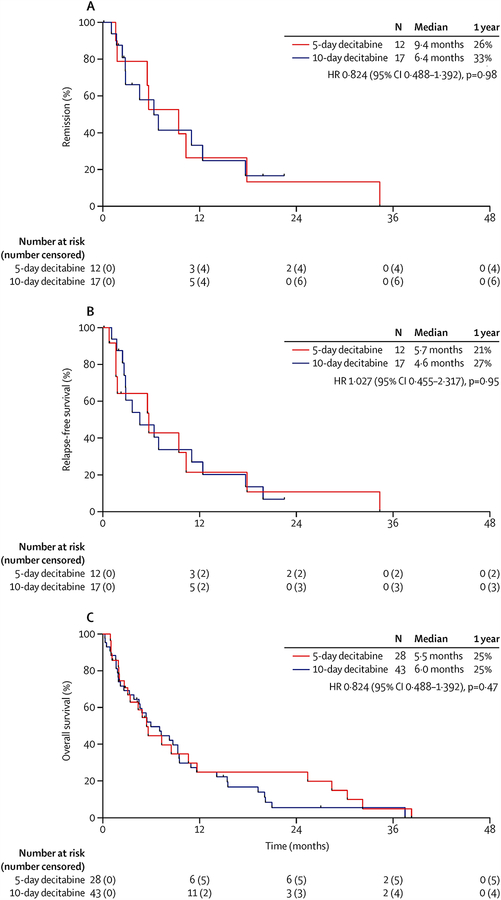
The total duration of follow-up was 38.2 months (median duration of follow-up: 5.3 months [interquartile range: 9.0 months]). The median remission duration for the 5-day and 10-day schedules was 9.4 months (IQR, 5.6–17.9) and 6.4 months (IQR, 2.8–12.4), and 1-year continuous remission rates were 26% and 33%, respectively (P=0.98; Figure 2A). Among 29 responding patients, 19 subsequently relapsed (5-day arm, n=8; 10-day arm, n=11). No patients underwent hematopoietic stem cell transplantation in first remission. The median RFS was 5.7 months (IQR, 1.6–10.4) and 4.6 months (IQR, 2.7–12.4), and 1-year RFS rates were 21% and 27%, respectively (P=0.95; Figure 2B). At last follow-up, 9 patients were still alive (5-day arm, n=5; 10-day arm, n=4). The median OS was 5.5 months (IQR, 2.1–11.7) and 6.0 months (IQR, 1.9–11.7), respectively, and 1-year OS rates were 25% in both arms (P=0.47; Figure 2C).
Figure 2. Outcomes by treatment arm.
(A) Remission duration, (B) relapse-free survival, and (C) overall survival.
No significant difference in OS was observed between dosing schedules when stratified by disease subgroups. The median OS for patients in the 5-day and 10-day decitabine arms by subgroup were: diploid cytogenetics (4.7 months [IQR, 1.6–18.1] versus 7.2 months [IQR, 2.6–9.5]; P=0.98), adverse-risk cytogenetics (5.5 months [IQR, 2.7–11.7] versus 5.4 months [IQR, 1.9–15.5]; P=0.76), de novo ACUTE MYELOID LEUKEMIA (7.3 months [IQR, 3.4–28.5] versus 7.1 months [IQR, 1.9–14.2]; P=0.19), secondary- or therapy-related ACUTE MYELOID LEUKEMIA (4.4 months [IQR, 1.9–8.5] versus 5.4 months [IQR, 2.2–9.3]; P=0.19), and TP53-mutated ACUTE MYELOID LEUKEMIA (5.5 months [IQR, 1.9–8.5] versus 4.9 months [IQR, 1.9–9.5]; P=0.55). Among patients in the 5-day arm, there was no difference in OS between patients with and without TP53 mutations (median OS: 5.5 months [IQR, 1.9–8.5] versus 4.9 months [IQR, 3.1–10.6]); however, in patients who received the 10-day schedule of decitabine, there was a trend towards worse OS in patients with TP53-mutated ACUTE MYELOID LEUKEMIA compared to wild type TP53 ACUTE MYELOID LEUKEMIA (median OS: 4.7 months [IQR, 1.9–9.5] versus 8.3 months [IQR, 3.0–15.5]; P=0.16).
Treatment Intensity and Early Mortality
The median number of cycles received were 2 (IQR, 2–5.75) in 5-day decitabine arm and 3 (IQR, 2–6) in 10-day arm. Among responding patients the median number of cycles of decitabine received were 5 (IQR, 2.5–5) and 6 (IQR, 4–9.5), respectively. Of 33 patients in 10-day arm who received ≥2 cycles, 23 (70%) received 10-day regimen only with first course. Among these 23 patients, the decitabine schedule of the second course was decreased to 5 days because of achievement of CR/CRp in 7 patients and because of treating physician’s decision that dose reduction was in the best interest of the patient due to concern for prolonged myelosuppression in the other 16 patients.
Most adverse events were grade 1–2 (Table 3). The most common grade 3–4 adverse events were neutropenic fever (7 patients [25%] in the 5-arm and 14 patients [33%] in the 10-day arm) and infection (5 [18%] versus 16 [37%]). In the 5-day arm, 1 patient (4%) each died from sepsis in the context of neutropenic fever, infection, and hemorrhage; in the 10-day arm, 6 patients (14%) died from infection. No deaths in either arm were considered to be treatment-related. Early mortality was similar between the two decitabine schedules. The 30-day mortality rates for the 5-day and 10-day arms were 4% (1/28) and 9% (4/43), and the 60-day mortality rates were 21% (6/28) and 25% (1¼3), respectively.
Table 3.
Non-hematologic adverse events by grade
| Decitabine 5-day schedule (n=28) | Decitabine 10-day schedule (n=43) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
| Acute kidney injury | 5 (18) | 0 | 0 | 0 | 1 (2) | 2 (5) | 1 (2) | 0 |
| Altered mental status | 1 (4) | 0 | 0 | 0 | 1 (2) | 2 (5) | 0 | 0 |
| Anorexia | 0 | 1 (4) | 0 | 0 | 2 (5) | 0 | 0 | 0 |
| Cholecystitis | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Constipation | 2 (7) | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Diarrhea | 2 (7) | 1 (4) | 0 | 0 | 1 (2) | 0 | 0 | 0 |
| Dysphagia | 0 | 1 (4) | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Fatigue | 3 (11) | 2 (7) | 0 | 0 | 4 (9) | 2 (5) | 0 | 0 |
| Febrile neutropenia | 0 | 7 (25) | 0 | 1 (4) | 0 | 12 (28) | 2 (5) | 0 |
| Hearing loss | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 |
| Hemorrhage | 1 (4) | 0 | 1 (4) | 1 (4) | 0 | 1 (2) | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Hypoglycemia | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 2 (5) | 0 | 0 |
| Hypotension | 0 | 1 (4) | 0 | 0 | 1 (2) | 2 (5) | 0 | 0 |
| Increased bilirubin | 1 (4) | 1 (4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 1 (4) | 4 (14) | 1 (4) | 1 (4) | 3 (7) | 16 (37) | 0 | 6 (14) |
| Myocardial infarction | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Nausea | 1 (4) | 1 (4) | 0 | 0 | 4 (9) | 0 | 0 | 0 |
| Pain | 1 (4) | 1 (4) | 0 | 0 | 8 (19) | 2 (5) | 0 | 0 |
| Pleural effusion | 0 | 2 (7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Syncope | 0 | 1 (4) | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Transient ischemic attack | 0 | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| Venous thromboembolism | 0 | 1 (4) | 0 | 0 | 1 (2) | 0 | 0 | 0 |
Data are n (%). Any grade 1–2 adverse event occuring in ≥10% of patients in either arm, and all grade 3, 4, and 5 adverse events are listed.
Impact of Decitabine on TP53-Mutated ACUTE MYELOID LEUKEMIA
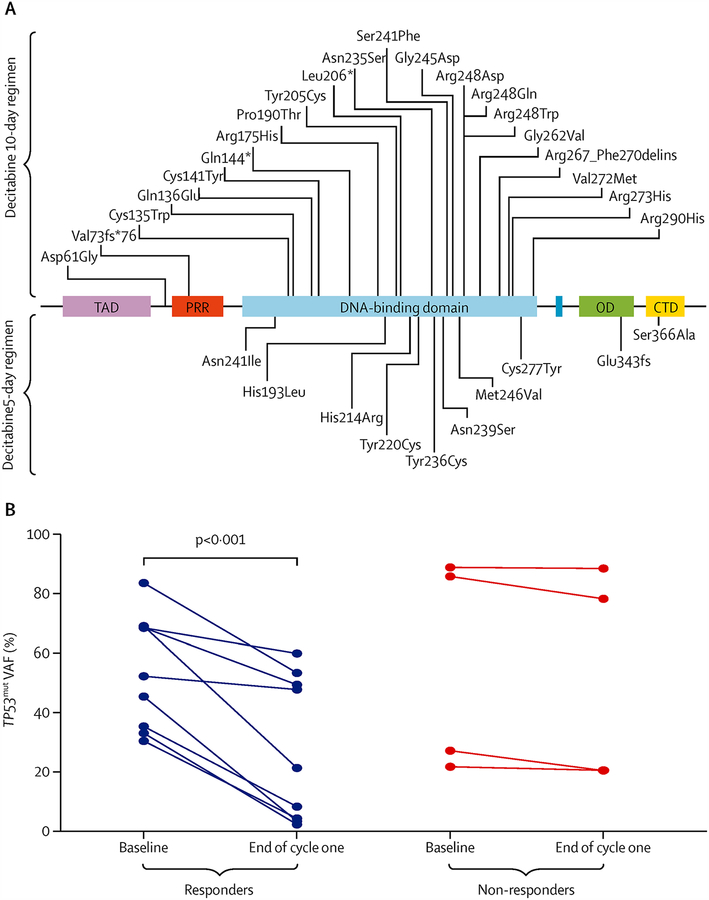
Given reports that a 10-day schedule of decitabine may have particular efficacy in TP53-mutated ACUTE MYELOID LEUKEMIA9, we performed an exploratory post-hoc analysis of the effect of decitabine on TP53 mutation VAFs in post-treatment samples. A total of 31 TP53 mutations (including 29 unique mutations) were detected in 24 of 65 patients (37%) who had baseline sequencing performed (Figure 3A). Six of the tested patients (9%) had more than one TP53 mutation detected. The pre-treatment median VAF of mutated TP53 was higher in the 10-day arm (50.3%, IQR, 33.3%−78.8%) than in the 5-day arm (23.1%, IQR, 11.7%−50.9%).
Figure 3. TP53 mutations in 24 patients.
(A) Schematic of TP53 protein showing mutational spectrum in 24 patients. Two patients had H193L and V272M TP53 mutations. TAD - transactivation domain (1–42); PRD - proline-rich domain (40–92); DBD - DNA-binding domain (101–306); OD - oligomerization domain (307–355); CTD - C-terminal regulatory domain (356–393). (Adapted with permission from http://p53.free.fr). (B) Change in variant allele frequencies of TP53 by response versus no response to decitabine in 13 patients with paired samples.
Seventeen patients had sequencing for TP53 performed at one or more post-treatment time points (Supplemental Table 1). Among the 9 responders who had TP53 sequencing at the time of remission, the median VAF at the time of remission was 8.4% (IQR, 4.1–47.9) and 4 patients had a VAF ≥20% at the time of remission. There were no differences in baseline TP53 VAF in patients who responded to decitabine compared to those who did not respond (P=0.70). Responding patients had a significant decline in TP53 VAF at the end of cycle 1 (mean ± standard deviation [SD] baseline VAF 53.2% ± 19.7% versus end-of-cycle 1 VAF 27.5% ± 23.9%, P<0.001; Figure 3B). In contrast, non-responding patients did not have a significant change in the TP53 VAF (mean ± SD baseline VAF 52.0% ± 33.8% versus end-of-cycle 1 VAF 51.2% ± 35.8%. Patients who received the 10-day schedule of decitabine had significant decline in VAF after 1 cycle (mean ± SD baseline VAF 43.0% ± 27.1% versus end-of-cycle 1 31.3% ± 28.9%, P<0.001). There were too few patients in the 5-day arm with post-cycle 1 TP53 sequencing to assess the effect of this decitabine schedule on TP53 VAF or to compare with the 10-day schedule.
In all three patients who had TP53 sequencing performed at the time of relapse, the previously detected TP53 clone (i.e. same variant) was detected in the relapse sample. Two patients had no TP53 mutation detected in a post-treatment sample (both 10-day arm and both first undetectable at the end of cycle 4). These two patients were the longest survivors in the mutant TP53 cohort. One patient died in CR after 19.9 months of continuous remission; the other eventually relapsed after a CR duration of 12.4 months.
Discussion
Here we report the first reported randomized study of two schedules of decitabine as frontline therapy for older patients with ACUTE MYELOID LEUKEMIA. Previous studies have suggested that 10-day schedules of decitabine may be associated with superior response rates compared with standard 5-day schedules, with response rates as high as 64%.6–9 There is also a theoretical rationale for a 10-day versus 5-day schedule of decitabine, as decitabine has a short half-life in vivo and would be expected to capture a larger proportion of leukemic cells as they asynchronously enter S-phase when delivered over a more prolonged period.19 However, in this randomized, prospective study, we observed no difference between 5-day and 10-day schedules of decitabine with respect to response rates, remission duration or survival. Although there was a trend towards earlier response with the 10-day schedules, there was no difference in response rates between the two decitabine schedules overall or when patients were stratified by cytogenetic risk, de novo versus therapy- or secondary-ACUTE MYELOID LEUKEMIA, or TP53 mutation status.
In previous phase III randomized studies of HMAs in elderly patients with ACUTE MYELOID LEUKEMIA, a median OS of 7.7 months was observed with a 5-day schedule of decitabine and a median OS of 10.4 months with the standard 7-day schedule of azacitidine.5,20 In contrast, the median OS was approximately 6 months in our study, which is likely explained by the high incidence of poor-risk features, including a median age of 78 years, approximately one-third of patients with performance status ≥2, and over half of patients with adverse cytogenetic abnormalities. Furthermore, while the incidence of TP53 mutations with newly diagnosed ACUTE MYELOID LEUKEMIA is generally reported as approximately 5–20%11,21, our cohort was particularly enriched for TP53 mutations (present in 37% of tested patients), which are associated with poor prognosis in ACUTE MYELOID LEUKEMIA. In light of these poor-risk features and the relatively poor OS in both arms, it is notable that the response rate with the 5-day decitabine regimen was 43% (compared to 17.8% with the same schedule in a large randomized phase III trial in a similar patient population5).
Leukemic clones harboring TP53 mutations are relatively resistant to chemotherapy and therefore have a competitive advantage over healthy hematopoietic stem cells when exposed to cytotoxic agents.10 As such, non-intensive chemotherapeutic agents, including HMAs, are commonly used for patients with TP53-mutated ACUTE MYELOID LEUKEMIA. Thus, the question of the most effective schedule of decitabine in this subgroup is particularly relevant. Although this study was not designed specifically to evaluate the impact of these two schedules of decitabine on TP53-mutated ACUTE MYELOID LEUKEMIA, the high rate of TP53 mutations allowed for sufficient numbers to provide some comparisons. In a previous single-arm phase II study of a 10-day schedule of decitabine, Welch et al. reported a 100% response rate (21 of 21 responders) in patients with TP53-mutated ACUTE MYELOID LEUKEMIA.9 Notably, in their cohort, no difference in OS was observed between TP53-mutated and TP53 wild type cases. In contrast, in our study, the response rate for TP53-mutated ACUTE MYELOID LEUKEMIA with the 10-day schedule of decitabine was 47% (8 responders out of 17). While out findings do not confirm the findings of Welch et al., they are generally consistent with several other studies reporting that response rates to HMAs are similar in TP53-mutated and TP53 wild type ACUTE MYELOID LEUKEMIA and myelodysplastic syndromes.22–25 Acknowledging that our study was not powered to definitively answer the question of the optimal decitabine schedule for TP53-mutated ACUTE MYELOID LEUKEMIA, the similar response rates and survival observed in the 5-day and 10-day arms are suggestive that neither of these schedules is superior in this disease subgroup.
We performed targeted TP53 sequencing of bone marrow specimens to correlate clinical response parameters with TP53 VAF at baseline and in response to decitabine exposure. Although numbers were limited for comparison between the schedules of decitabine, we observed several preliminary findings that should be further evaluated in larger cohorts. First, we did not observe a correlation between baseline TP53 VAF and response. This is notable because it has been suggested that TP53-containing clones may be particularly sensitive to decitabine and therefore it might be expected that higher TP53 allelic burden may lead to increased likelihood of response.9 We also observed that the median TP53 VAF at the time of remission was 8.4%, with 4 patients with a VAF of ≥20% at the time of remission, suggesting that some of these TP53 mutations may be present in pre-leukemic clones and not exclusively in myeloblasts.10 Finally, it is notable that the 2 patients in whom a TP53 mutation could no longer be detected in the remission bone marrow had the longest durations of remission. This finding is consistent with previous reports of the importance of “mutation clearance” as a predictor of prolonged remission in ACUTE MYELOID LEUKEMIA.26–28
As almost half of patients with ACUTE MYELOID LEUKEMIA are >70 years of age at the time of diagnosis and frequently have significant comorbidities and poor performance status, many are considered unfit for intensive chemotherapy.29,30 Additionally, although the response rates with HMAs are generally lower than observed with intensive chemotherapy, survival is similar or possibly superior compared to what can be expected with intensive chemotherapy in these older patients with ACUTE MYELOID LEUKEMIA, likely driven by lower treatment-related mortality with HMAs.4,31,32 As such, the use of HMAs such as decitabine as induction therapy has become a commonly employed strategy for older adults with ACUTE MYELOID LEUKEMIA. To improve upon the outcomes with single-agent HMAs, several combinatorial approaches have been investigated. To date, perhaps the most notable is the combination of the oral Bcl-2 inhibitor venetoclax with either azacitidine or decitabine, which is emerging as a potential new standard of care in this older population. The response rate with single-agent venetoclax in older patients with relapsed ACUTE MYELOID LEUKEMIA is only 19% with a median OS of 4.7 months.33 In contrast, the combination of venetoclax with either azacitidine or decitabine resulted in very promising CR/CRi rates of 66% in older patients with ACUTE MYELOID LEUKEMIA (57% in those with poor-risk cytogenetics) with a median OS of 17.5 months.34 Therefore, despite their modest single agent activity, decitabine and azacitidine are becoming a backbone for new and more effective strategies used to treat elderly patients with ACUTE MYELOID LEUKEMIA. Our study is of significant relevance as to what would be the best duration of decitabine therapy in such future combination regimens, suggesting that 5 or 10 day regimens are equivalent in efficacy. These results also have implications for delivery of cost-effective care, as they suggest that the additional doses of decitabine may add additional treatment cost without significantly impacting clinical outcomes.
There are several limitations to this study. In the frontline setting, azacitidine is also commonly used for older patients with ACUTE MYELOID LEUKEMIA who are unfit for chemotherapy and has been shown to improve outcomes compared to induction chemotherapy, low-dose cytarabine or supportive care.20 No large randomized trials have been performed to compare decitabine and azacitidine in this setting, and therefore it remains unclear whether either hypomethylating agent is superior to the other. It is also notable that our results may not directly translate to patients with relapsed or refractory ACUTE MYELOID LEUKEMIA, as a recent retrospective analysis reported that a 10-day schedule of decitabine was independently associated with higher response rates (compared to a 5-day schedule of decitabine or 7-day schedule of azacitidine) when used as salvage therapy.35 However, despite the increased responses with the 10-day schedule, no survival benefit was observed. Future prospective studies in patients with relapsed or refractory disease will be needed to corroborate these findings. Finally, it is important to note that despite the adaptive randomization design, there may have been some imbalance of high-risk features in the 10-day arm compared to the 5-day arm (adverse cytogenetics in 56% versus 46%, and TP53 mutations in 41% versus 29%), which should be considered when interpreting these findings of this study.
In conclusion, in this randomized trial of 5-day and 10-day schedules of decitabine in older patients with newly diagnosed ACUTE MYELOID LEUKEMIA deemed unfit for intensive chemotherapy, these two schedules resulted in similar response rates and survival. Notably, there was no subgroup identified who benefited preferentially from either schedule of decitabine, including patients TP53-mutated ACUTE MYELOID LEUKEMIA. These results suggest that both schedules of decitabine are safe and effective in older patients with ACUTE MYELOID LEUKEMIA and may be considered as reasonable non-intensive backbone regimens for future investigational combinations with novel agents.
Supplementary Material
Research in Context.
Evidence before this study
A systematic review was not performed before starting this trial. However, we searched Pubmed for studies on the outcomes of adults with acute myeloid leukemia treated with hypomethylating agents without date limitations. The response rate a standard 5-day schedule of decitabine is approximately 20–30%. Some small, single-arm studies have reported higher response rates with a 10-day schedule of decitabine, with particularly high responses seen in patients with TP53-mutated acute myeloid leukemia. However, no published study had directly compared the 5-day and 10-day schedules of decitabine in patients with acute myeloid leukemia.
Added value of this study
In this randomized, phase II study in older adults with newly diagnosed acute myeloid leukemia, no difference in response rates or survival were observed between a 5-day and 10-day schedule of decitabine. Neither pretreatment cytogenetics, type of acute myeloid leukemia (i.e. de novo versus secondary- or therapy-related), nor TP53 status were associated with differential outcomes with either schedule. These data suggest that both 5-day and 10-day schedules of decitabine result in similar response rates, survival and early mortality in an unselected population of older adults with newly diagnosed acute myeloid leukemia.
Implications of all the available evidence
Despite its modest single agent activity, decitabine is commonly used as a backbone regimen for combination approaches for older patients with acute myeloid leukemia. This study, which suggests that 5- or 10-day schedules of decitabine have similar efficacy, may therefore inform future studies combining decitabine with novel agents. Future studies of these two schedules in TP53-mutated ACUTE MYELOID LEUKEMIA may be warranted, given previous evidence suggesting particular efficacy of 10-day decitabine schedule in this specific population.
Role of the Funding Source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (F.R.) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: Supported by the MD Anderson Cancer Center Support Grant CA016672 and SPORE
Footnotes
Disclosure of Conflicts of Interest
The authors declare no conflicts of interest.
Data sharing statement: Patient-level data will not be shared.
References
- 1.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014; 32(24): 2541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015; 125(9): 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Reinhardt D, et al. Changes in cytogenetics and molecular genetics in acute myeloid leukemia from childhood to adult age groups. Cancer 2016; 122(24): 3821–30. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. The New England journal of medicine 2015; 373(12): 1136–52. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012; 30(21): 2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences of the United States of America 2010; 107(16): 7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leukemia & lymphoma 2013; 54(9): 2003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leukemia & lymphoma 2014; 55(7): 1533–7. [DOI] [PubMed] [Google Scholar]
- 9.Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. The New England journal of medicine 2016; 375(21): 2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015; 518(7540): 552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer 2006; 106(5): 1090–8. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2003; 21(24): 4642–9. [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. British journal of haematology 2016; 172(3): 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel KP, Ravandi F, Ma D, et al. Acute myeloid leukemia with IDH1 or IDH2 mutation: frequency and clinicopathologic features. American journal of clinical pathology 2011; 135(1): 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh RR, Bains A, Patel KP, et al. Detection of high-frequency and novel DNMT3A mutations in acute myeloid leukemia by high-resolution melting curve analysis. The Journal of molecular diagnostics : JMD 2012; 14(4): 336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014; 99(3): 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanagal-Shamanna R, Jain P, Takahashi K, et al. TP53 mutation does not confer a poor outcome in adult patients with acute lymphoblastic leukemia who are treated with frontline hyper-CVAD-based regimens. Cancer 2017; 123(19): 3717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004; 103(5): 1635–40. [DOI] [PubMed] [Google Scholar]
- 20.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015; 126(3): 291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou HA, Chou WC, Kuo YY, et al. TP53 mutations in de novo acute myeloid leukemia patients: longitudinal follow-ups show the mutation is stable during disease evolution. Blood cancer journal 2015; 5: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bally C, Ades L, Renneville A, et al. Prognostic value of TP53 gene mutations in myelodysplastic syndromes and acute myeloid leukemia treated with azacitidine. Leukemia research 2014; 38(7): 751–5. [DOI] [PubMed] [Google Scholar]
- 23.Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014; 124(17): 2705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Patel K, Bueso-Ramos C, et al. Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents. Oncotarget 2016; 7(12): 14172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung SH, Kim YJ, Yim SH, et al. Somatic mutations predict outcomes of hypomethylating therapy in patients with myelodysplastic syndrome. Oncotarget 2016; 7(34): 55264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klco JM, Miller CA, Griffith M, et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. Jama 2015; 314(8): 811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita K, Kantarjian HM, Wang F, et al. Clearance of Somatic Mutations at Remission and the Risk of Relapse in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018: Jco2017776757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. The New England journal of medicine 2018; 378(13): 1189–99. [DOI] [PubMed] [Google Scholar]
- 29.Institute NC. Cancer Stat Facts: Acute Myeloid Leukemia. 2017. https://seer.cancer.gov/statfacts/html/amyl.html (accessed 9/21/2017.
- 30.Podoltsev NA, Stahl M, Zeidan AM, Gore SD. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood reviews 2017; 31(2): 43–62. [DOI] [PubMed] [Google Scholar]
- 31.Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012; 120(24): 4840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhulipala VC, Extermann M, Al Ali N, et al. Survival Comparison Amongst Commonly Used Frontline Regimens in Patients Age 70 Years and Older with Acute Myeloid Leukemia (AML): A Single-Institution Study of over 600 Patients. Blood 2015; 126(23): 2505–. [Google Scholar]
- 33.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer discovery 2016; 6(10): 1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. The Lancet Oncology 2018; 19(2): 216–28. [DOI] [PubMed] [Google Scholar]
- 35.Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv 2018; 2(8): 923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.