Abstract
Tethered cord syndrome (TCS) is a type of occult spinal dysraphism that may lead to permanent neurologic and orthopedic deficits. Infants with TCS may have lumbosacral cutaneous malformations (LsCMs). We studied 67 infants referred to a single pediatric urology practice for a urological concern unrelated to occult spinal dysraphism with no prior diagnosis of LsCM between March 1, 2015 and September 30, 2018. Each infant underwent a spinal ultrasound. If an abnormality was detected, they were referred to a pediatric neurosurgeon. The most common cutaneous manifestations were duplicated or bifurcated (46%) gluteal folds and gluteal asymmetry (16%). Fourteen (21%) of the 67 patients had an abnormal spinal ultrasound; 5 of the 14 infants underwent a lumbar magnetic resonance imaging. One infant had urodynamics studies and a tethered cord release. Pediatricians should be familiar with TCS and perform lumbar physical examinations for LsCMs suggestive of TCS to ensure prompt diagnosis and management and avoid potentially devastating complications.
Keywords: pediatrics, pediatric urology, pediatric neurosurgery, occult spinal dysraphism, tethered cord syndrome, ultrasound
Introduction
Spinal dysraphisms are a group of congenital spinal anomalies characterized by incomplete fusion of the midline mesenchymal, bony, or neural elements of the spine, which develop during the early stages of fetal development.1-3 During the third to fifth weeks of gestation, both the separation of the neural ectoderm from the epithelial ectoderm and the formation and closure of the neural tube occurs.4,5 Errors during this process may result in anomalies of the central nervous system and skin.5 Patients with spinal dysraphism may also have anorectal or urogenital malformations as these structures arise from a common cloaca that develops adjacent to the caudal portion of the neural tube.6,7 The most common congenital urological anomalies in spinal dysraphism include vesicoureteral reflux and cryptorchidism.8
Spinal dysraphism has an incidence of 0.5 to 0.8 cases per 1000 live births and may present as open (neural tissue is exposed to external environment such as myelomeningocele or myelocele) or occult (skin-covered lesions without exposed neural tissue).1,2,9 Occult spinal dysraphism (OSD) may be harmless—for example, spina bifida occulta, which is more an anatomic variation than a “disease.” One type of OSD, tethered cord syndrome (TCS), may result in permanent neurologic and orthopedic problems if not diagnosed and treated in a timely manner. While open spinal defects receive prompt neurosurgical intervention at birth, TCS (aka neuro-orthopedic syndrome) may present with few clinical findings and a more insidious and delayed appearance of symptoms.
One clinical finding that may allow for timely diagnosis of TCS is a lumbosacral cutaneous malformation (LsCM). LsCMs are located in the midline over the lumbosacral spine and vary, including hypertrichosis, lipomas, hemangiomas, and dermal sinuses.1,2,4,10,11 The presence of a LsCM raises the risk of there being an OSD. Infants and children with a midline lumbosacral infantile hemangioma in addition to other cutaneous findings have an even higher risk for spinal abnormalities.4,7 Presence of a LsCM warrants further investigation for the presence of TCS, including spinal ultrasound (US), lumbar magnetic resonance imaging (MRI), and urodynamic studies (UDS).12
In this article, we discuss the identification of LsCMs that predict OSD and the importance of a lumbosacral cutaneous physical examination to identify these cutaneous lesions. Once identified, we present a management pathway for obtaining imaging and, if necessary, a pediatric neurosurgical consultation.
Materials and Methods
Under the institutional review board–approved protocol, we retrospectively reviewed the presence of LsCMs suggestive of OSD in infants evaluated in our pediatric urology clinic at our institution over a 3½-year period (March 1, 2015 to September 30, 2018). All patients were evaluated on at least one occasion after birth and by their pediatrician. They were subsequently referred to our office for a urological concern unrelated to OSD. None of the infants were diagnosed with OSD prior to pediatric urological evaluation, and the LsCM was first noted in the medical record on routine physical examination by the pediatric urologist.
All infants with a LsCM were referred for a spinal US. The patients younger than the age of 3 months were usually evaluated by the pediatric urologist, and the parents were instructed to perform the spinal US as soon as possible. The determination of an abnormal US was based on either a conus medullaris that terminated at or below the L2-3 level or an echogenic filum terminale. All patients with an abnormal spinal US were referred to a pediatric neurosurgeon, who determined whether a lumbar MRI was required. UDS were performed in certain cases where the lumbar MRI revealed abnormal findings suspicious for TCS. A pediatric radiologist reviewed all of the spinal US and lumbar MRIs.
Results
Patient Demographics and Reason for Pediatric Urological Referral
Of the 67 infants who had LsCMs suggestive of OSD as noted by the pediatric urologist, 57 were male and 10 were female with a mean age of 5.6 weeks (range = 1 day to 13 weeks; Table 1). The most common reasons for a pediatric urology consultation were circumcision and hypospadias (both 24%) followed by hydronephrosis (22%).
Table 1.
Characteristics of Patients With Documented Cutaneous Manifestations Suggestive of Occult Spinal Dysraphism in Our Pediatric Urology Clinic at Our Institution.
| Patient Characteristics | Number of Patients (N = 67), n (%) |
|---|---|
| Sex | |
| Male | 57 (85%) |
| Female | 10 (15%) |
| Mean age at office evaluation | 5.6 weeks (1 day to 13 weeks) |
| Reason for urology evaluation | |
| Circumcision | 16 (24%) |
| Hypospadias | 16 (24%) |
| Hydronephrosis | 15 (22%) |
| Undescended testis | 4 (6%) |
| Hydrocele | 2 (3%) |
| Bilateral vesicoureteral reflux | 2 (3%) |
| Pelviectasis | 2 (3%) |
| Penile torsion | 2 (3%) |
| Micropenis | 2 (3%) |
| Periurethral cyst | 1 (1.5%) |
| Penile swelling | 1 (1.5%) |
| Scrotal hematoma | 1 (1.5%) |
| Ectopic kidney | 1 (1.5%) |
| Horseshoe kidney | 1 (1.5%) |
| Single kidney | 1 (1.5%) |
| Cutaneous manifestation | |
| Duplicated or bifurcated gluteal fold | 31 (46%) |
| Gluteal asymmetry | 11 (16%) |
| Coccygeal pit | 7 (10%) |
| Lumbar hair | 5 (7.5%) |
| Mongolian spot on back | 1 (2%) |
| Sacral dimple | 3 (4.5%) |
| Two of the above-mentioned findings | 9 (13%) |
| Mean age at spinal ultrasound | 7 weeks (1 day to 16 weeks) |
| Spinal ultrasound findings | |
| Normal | 50 (74.5%) |
| Abnormal | 14 (21%) |
| Examination limited by spinal ossification | 2 (3%) |
| Examination limited by patient size and motion | 1 (1.5%) |
| Lumbar MRI following abnormal spinal ultrasound | 5/14 (36%) |
| Evaluated by neurosurgery | 12/67 (18%) |
Abbreviation: MRI, magnetic resonance imaging.
Cutaneous Manifestation of Occult Spinal Dysraphism
A duplicated or bifurcated gluteal fold was the most frequent cutaneous manifestation as observed in 31 (46%) infants and gluteal asymmetry in 11 (16%; Table 1 and Figure 1). Nine patients had 2 LsCMs suggestive of OSD; specifically, gluteal asymmetry and a coccygeal pit (2 infants); lumbar hair and coccygeal pit (2 infants); bifurcated gluteal fold and coccygeal pit (1 infant); gluteal asymmetry and duplicated gluteal fold (1 infant); duplicated gluteal fold and coccygeal pit (1 infants); Mongolian spot and coccygeal pit (1 infant); and sacral dimple and lumbar hair (1 infant).
Figure 1.
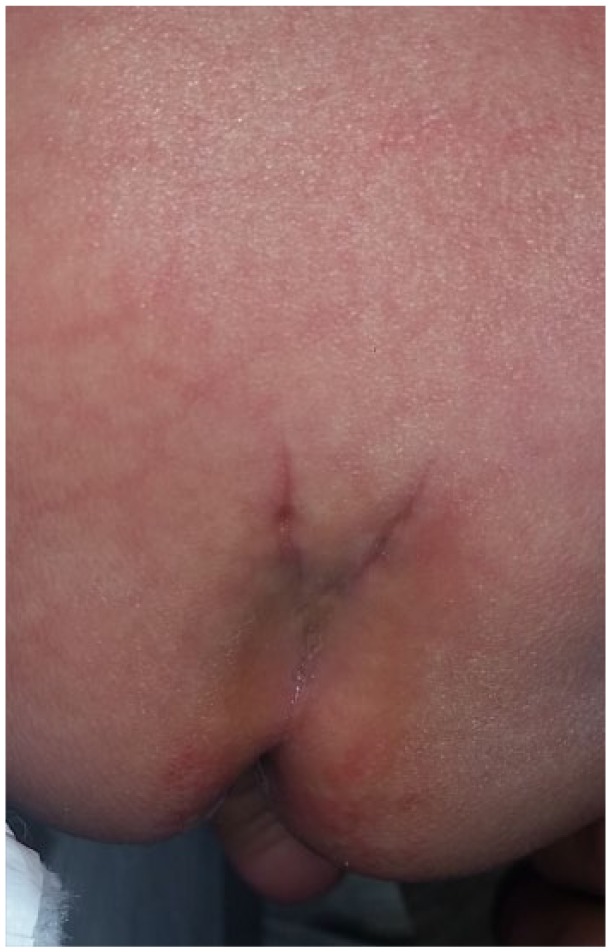
Bifurcated gluteal fold.
Spinal Ultrasound
The mean age of the infants at the time of the spinal US was 7 weeks (range = 1 day to 16 weeks; Table 1). The spinal US demonstrated normal findings in 50 (75%) cases. Of the 14 abnormal spinal US, a low-lying conus medullaris was noted in 13 (93%) patients, and an echogenic filum terminale was observed in 1 (7%) patient. The US examination was too limited to interpret in 3 patients: in 2 due to spinal ossification and in 1 due to patient size and motion.
Lumbar Magnetic Resonance Imaging
Of the 14 patients with abnormal US, 5 (36%) underwent a lumbar MRI. The correlation between the reason for pediatric urology evaluation, cutaneous findings of OSD, abnormal spinal US, lumbar MRI, and neurosurgery consultation are presented in Table 2.
Table 2.
Correlation Between Reason for Pediatric Urology Evaluation, Cutaneous Findings Suggestive of OSD, Abnormal Spinal US, Lumbar MRI, and Neurosurgery Consultation.
| Gender/Age at US (Weeks) | Reason for Urology Evaluation | Cutaneous Finding of OSD | Spinal US | Lumbar MRI | Neurosurgery Evaluation |
|---|---|---|---|---|---|
| Male/8.29 | Hydrocele | Bifurcated gluteal fold | CM ends at L2-3 | CM ends at L2-3 | No clinical TCS; PT |
| Male/10.71 | Penile edema | Lumbar hair, coccygeal pit | FT appears echogenic | N/A | No clinical TCS; PT; constipation |
| Male/2.86 | Penile torsion | Gluteal asymmetry | CM ends at L2-3 | CM ends at L2-3 | No clinical TCS; PT; constipation |
| Male/10.86 | Hydronephrosis | Lumbar hair | CM ends at L2-3 | CM ends at L2-3, filar fibrolipoma | 2 UDS; TCR |
| Male/7.57 | Hydronephrosis | Gluteal asymmetry | CM ends at L2-3 | N/A | No clinical TCS |
| Male/8.57 | Penile torsion | Gluteal asymmetry | CM ends at L2-3 | CM ends at L2-3 | No clinical TCS; PT |
| Male/0.29 | Undescended testes | Lumbar hair | CM ends at L2-3 | CM ends at L2-3 | No clinical TCS; PT |
| Male/13.29 | Hypospadias | Coccygeal pit | CM ends at L2-3 | N/A | No clinical TCS; PT |
| Male/4.71 | Hypospadias | Duplicated gluteal fold | CM ends at L2-3 | N/A | No clinical TCS |
| Male/0.29 | Hypospadias | Bifurcated gluteal fold, coccygeal pit | CM ends at L2-3 | N/A | No clinical TCS |
| Male/3.71 | Circumcision | Duplicated gluteal fold | CM ends at L2-3 | N/A | No clinical TCS |
| Male/3.29 | Hypospadias | Gluteal asymmetry | CM ends at L2-3 | N/A | No clinical TCS |
| Female/10.86 | Hydronephrosis | Coccygeal pit, Mongolian spot | CM ends at L2-3 | N/A | N/A |
| Male/0.71 | Circumcision | Duplicated gluteal fold, coccygeal pit | CM ends at L2-3 | N/A | N/A |
Abbreviations: OSD, occult spinal dysraphism; US, ultrasound; MRI, magnetic resonance imaging; CM, conus medullaris; TCS, tethered cord syndrome; PT, physical therapy; FT, filum terminale; UDS, urodynamics study; TCR, tethered cord release.
Neurosurgical Consultation and Urodynamic Studies
Of the 14 infants who were referred to a pediatric neurosurgeon due to an abnormal spinal US, 11 were evaluated by the pediatric neurosurgeon and had evidence of a low-lying conus medullaris (Tables 1 and 2). One additional infant with a LsCM and negative spinal US was also seen by the pediatric neurosurgeon. Of these 12 infants, 8 were treated with physical therapy (PT), with an emphasis on core strength and exercises of the lower extremities. All 12 patients had met their developmental milestones, and PT could find no clinical evidence of TCS in 11 infants. Caregivers were educated about the signs and symptoms of TCS. Of the 12 patients with a LsCM, only 1 infant underwent a tethered cord release performed by the pediatric neurosurgeon based on the combination of an abnormal spinal US, lumbar MRI findings, and clinical manifestations.
The 6-week-old infant who eventually had a tethered cord release was referred to our pediatric urology clinic for evaluation of hydronephrosis, at which time an abnormal amount of lumbar hair was noted in the midline lumbosacral region during physical examination. A renal and bladder US when he was 1 day old showed mild-to-moderate left pelvocaliectasis, a moderate left hydroureter, and bilateral kidneys below the second standard deviation for his age. He underwent a spinal US at 10 weeks, which demonstrated a low-lying conus medullaris at the L3 level. A lumbar MRI confirmed this finding and also revealed evidence of a filar fibrolipoma. A voiding cystourethrogram and renogram (MAG3) were normal. By 5 months of age, the infant had decreased trunk control in support sitting and trunk righting per PT. UDS performed at 9 months demonstrated detrusor instability and increased bladder pressures. By 1 year of age, he constantly walked on his tiptoes. The infant underwent a tethered cord release at 15 months. Second UDS conducted 4 months after surgery revealed poor bladder compliance but no vesicoureteral reflux.
Discussion
TCS is one of the most common types of OSD and is a condition in which loss of elasticity of the filum terminale puts abnormal traction on the spinal cord.12 This causes decreased blood flow triggering metabolic derangements that culminate in motor, sensory, and urinary neurological deficits, which run a higher risk of becoming permanent the longer they remain untreated.12,13 TCS is often associated with gait abnormalities (toe-walking and clumsiness), back and leg pain, neurogenic bladder with repeated urinary tract infections, any urinary incontinence, back or leg pain, easy leg fatiguability, and scoliosis.4,14,15 Surgical untethering may either ameliorate these issues or halt their worsening if they have been long-standing for many years.14
Unlike open spinal dysraphism that is apparent at birth and requires immediate neurosurgical intervention, many infants with TCS lack obvious cutaneous, neurological, or urological signs or symptoms at birth. Sometimes infants are sign- and symptom-free until later in life, when the faster relative growth of the bony spine induces spinal cord traction. Signs and symptoms may also be occult because infants can neither walk, talk (“my back hurts”), nor remain continent of their urine. The initial presentation of TCS may be neurological dysfunction.16 It is essential to monitor urinary function to evaluate for new-onset urinary incontinence, changes in voiding patterns, or recurrent urinary tract infections.9 Early detection of worsening urological function permits timely tethered cord release prior to irreversible urological damage.4,9,12,17
A wide spectrum of cutaneous findings may be observed in the lumbosacral region of an infant, with varying degrees of suspicion for TCS. Many studies in the literature report that approximately 80% of patients diagnosed with TCS have evidence of midline cutaneous lesions over the lumbosacral spine.1,2,4,10,11 Lesions with a high index for suspicion include hypertrichosis (a silky “hairy patch”), lipomas, dermoid cysts or dermal sinuses, and 2 or more cutaneous lesions.2,4,7,16,18 Hypertrichosis is commonly observed with other cutaneous manifestations of OSD, which may be indicative of an underlying spinal defect, and intraspinal lipomas are often associated with a tethered cord.4
The categorization of a “dimple” and its pathological significance is determined by its size and location. Any dimple lying superior to the gluteal cleft, outside the midline, and with a diameter greater than 5 mm commonly accompanies a spinal anomaly and warrants radiological investigation such as an MRI.4,16,18,19 A simple sacral dimple is located in the midline, within the gluteal cleft, has a diameter less than 5 mm, and is less than 2.5 cm from the anus.10,18,20 Simple isolated sacral dimples, more properly called coccygeal pits, are usually not associated with OSD; therefore, spinal imaging is often not recommended.2,7,10,20,21 Other cutaneous observations such as hyperpigmentation, melanocytic nevi, and telangiectasia have a low index for suspicion of OSD.2,4,7
A spinal US is a simple, inexpensive, and noninvasive screening tool for detecting OSD in infants less than 3 months of age when the vertebrae have not completely ossified.2,4,7,10-12,16,22 A neonatal US has a 50% to 70% sensitivity in detecting any OSD.4,22 However, the use of LsCMs as a screening tool for OSD results in many more false positives than true positives.23 While there is a risk of overdiagnosing TCS and the associated cost of the diagnostic workup in terms of financial implications, office visits, referrals, and need for sedation for an MRI, the negative repercussions of not recognizing TCS and failing to surgically treat it outweigh the risks. If the US is abnormal, equivocal, or technically limited due to ossification of the spine, an MRI is recommended. While the MRI is the imaging modality of choice and most sensitive for detecting OSD in infants, the primary drawbacks are the need for general anesthesia with its inherent risk factors, higher cost, decreased availability, and heart-generated motion of the infants’ small bodies.7 To compensate for this, unless there is urgent worry, delaying the lumbar MRI until 6 months of age or later can increase the chances that a high-quality MRI is obtained. It has been suggested that further investigation for the presence of a tethered cord is recommended when the spinal US reveals that the conus medullaris is below L2 in infants.10,12,14,24
UDS represent the gold standard urological evaluation of infants with suspected TCS.12,25 This test provides details about the lower urinary tract function that assists neurosurgeons in their decision to perform a tethered cord release. A controversy exists regarding UDS indications for surgery in asymptomatic or stable infants who have evidence of OSD.12 Once deficits develop such as lower extremity motor weakness, foot deformity, and/or urinary and bowel dysfunction, they are often irreversible following surgery.10 Furthermore, deficits due to TCS run larger risks of becoming refractory to surgical management the longer they are untreated over months to years. While an infant may have TCS with an abnormal spinal US and lumbar MRI, it is the neurosurgeon’s clinical judgment that plays the most important role in determining surgical intervention.
Our 3½-year multidisciplinary study of cutaneous manifestations suggestive of OSD detected on routine physical examination by a pediatric urologist demonstrates the importance of both conducting an adequate lumbosacral cutaneous examination and of obtaining a spinal US and/or MRI when a LsCM is observed. In Guggisberg and colleagues’ review of 54 cases of congenital midline lumbosacral cutaneous lesions observed by a pediatric dermatologist, OSD was detected in 11 of 18 patients with 2 or more different skin lesons.2 These authors suggest that a combination of 2 or more congenital midline skin lesions is the strongest marker of OSD.2 As solitary coccygeal pits often have no prognostic value for OSD, their appearance with another lesion is a source of concern. In our study, a coccygeal pit was found in conjunction with another cutaneous marker of OSD in 4 patients who had abnormal US. Of the 14 abnormal spinal US, the conus medullaris terminated at L2-3 in 6 of these cases, suggestive of TCS. Of the 12 patients evaluated by the pediatric neurosurgeon, only one infant had an abnormal spinal US, an abnormal lumbar MRI, and clinical manifestations of TCS to undergo UDS and a tethered cord release. One should not be discouraged by the low rate of pediatric neurosurgical concurrence with the presence of a LsCM. As a pediatrician, the expectation is that any candidate’s LsCMs should be noticed; leaving accurate assessment to the pediatric neurosurgeons is entirely appropriate. A pediatrician should not be discouraged by several neurosurgical referrals, which yield an assessment of “no cutaneous evidence of dysraphism.”
An additional strength of our study is the heightened awareness of neurogenic bladders and OSD among pediatric urologists, which accounts for the high detection rate. We encourage pediatricians to incorporate physical examination of the lumbar region for cutaneous manifestations suggestive of OSD into their routine and have a higher index of suspicion for these markers. Consequently, there will be a higher detection rate, and infants will be diagnosed and treated for OSD and TCS at a younger age.
Arising from our experience, we recommend the management pathway for patients with a LsCM synopsized in Table 3. An infant with a possible LsCM should get either of the following: (1) US if younger than 3 months; or (2) MRI of the lumbar spine without contrast if older than 6 months; or (3) a referral to pediatric neurosurgery. Unless there is clinical concern of a symptomatic tethered cord, it is acceptable to wait 3 months to get an MRI between age 3 and 6 months. It is always acceptable to refer to pediatric neurosurgery before obtaining imaging, although having imaging can facilitate the patient’s and family’s experience. The same 3 options are appropriate if the patient does not have a LsCM but has constipation, urinary retention, or abnormal muscle tone. Since it is difficult in an office encounter to assess the developmental appropriateness of an infant, a referral to PT is also appropriate. Hospital-based PTs with their deep experience with neurological disorders are usually best for this. If the LsCM is not concerning and the patient has no clinical evidence of TCS but there is some uncertainty over the significance of the LsCM, referral to pediatric neurosurgery is appropriate.
Table 3.
Indications for Imaging and Pediatric Neurosurgical Consultation When a Lumbosacral Cutaneous Malformation Is Noted on Physical Examination in an Infant.
| 1. If an infant has a possible LsCM, obtain an ultrasound if the patient is younger than 3 months and an MRI if older than 6 months or refer to a pediatric neurosurgeon. |
| 2. If the LsCM does not look concerning, but the patient has some clinical evidence of TCS (constipation, urinary retention, high or low tone), obtain an ultrasound if the infant is younger than 3 months and an MRI if older than 6 months or refer to a pediatric neurosurgeon. |
| 3. If the cutaneous abnormality does not look concerning and the patient has no clinical evidence of TCS, do not image although a referral to pediatric neurosurgery is an option |
Abbreviations: LsCM, lumbosacral cutaneous malformation; MRI, magnetic resonance imaging; TCS, tethered cord syndrome.
The urological symptoms of TCS, specifically, voiding irregularities, neurogenic bladder, and frequent urinary tract infections are often not apparent in the first months of life. Due to the numerous neurologic or urologic deficits potentially associated with TCS, multidisciplinary and long-term management of patients with TCS is crucial. Infants with cutaneous findings suggestive of OSD and an abnormal spinal US necessitate clinical surveillance as they are unable to communicate and are not potty trained. Additionally, children who have been diagnosed with TCS should be monitored closely, especially during puberty when there are significant and sudden alterations in height and weight.12 Observation of urological abnormalities is imperative in infants and children as any variations may reflect OSD and TCS. We intend to follow the infants in our study who had evidence of abnormal cutaneous stigmata longitudinally through adolescence by a team approach with other specialists. These infants may not have displayed indications of TCS early in life yet warrant continued observation as they age.
This study is inherently limited by its retrospective nature. Additionally, since TCS may only present later in life as the growth of the bony spine outpaces the lengthening of the spinal cord itself, the lack of long-term patient follow-up limits our ability to correlate LsCM with the presence of a TCS. Our correlation of LsCM with TCS is further limited by the dearth of patients who underwent UDS. Without a wider application of UDS, it is difficult to know whether other infants in our study were in fact potentially symptomatic.
It is important for pediatricians to perform a complete cutaneous examination when an infant is evaluated for a urological complaint. Particular attention should be focused on cutaneous abnormalities in the lumbosacral area with regard to size, location, shape, texture, and color. A cursory examination of the skin may not reveal pathologic findings; however, a more comprehensive analysis may uncover cutaneous markers of OSD (Figure 2).
Figure 2.
(A) No discernable pathology with cursory cutaneous examination. (B) Cutaneous marker of occult spinal dysraphism uncovered with more comprehensive analysis.
Conclusion
Pediatricians should be aware of the wide array of cutaneous stigmata with their varying risks of suspicion for OSD. A spinal US or lumbar MRI should be performed in cases with a high level of suspicion for TCS, such as suspicious cutaneous manifestations or urinary, bowel, or gait abnormalities in infants and children. A close collaboration between pediatricians, pediatric urologists, and pediatric neurosurgeons is integral to the early diagnosis, treatment, and management of TCS in order to avoid the devastating sequelae of TCS.
Footnotes
Author Contributions: LBES contributed to the conception, design, acquisition, analysis, and interpretation as well as drafted and critically revised the manuscript. ISM, DSP, and ER contributed to the conception, design, acquisition, analysis, and interpretation as well as critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: As the infants presented in this study were less than 1 year of age, we have obtained written permission from the infants’ mothers.
ORCID iD: Lisa B. E. Shields  https://orcid.org/0000-0002-1526-4063
https://orcid.org/0000-0002-1526-4063
References
- 1. Drolet BA, Chamlin SL, Garzon MC, et al. Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr. 2010;157:789-794. [DOI] [PubMed] [Google Scholar]
- 2. Guggisberg D, Hadj-Rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol. 2004;140:1109-1115. [DOI] [PubMed] [Google Scholar]
- 3. Sardana K, Gupta R, Garg VK, et al. A prospective study of cutaneous manifestations of spinal dysraphism from India. Pediatr Dermatol. 2009;26:688-695. [DOI] [PubMed] [Google Scholar]
- 4. Drolet B. Birthmarks to worry about. Cutaneous markers of dysraphism. Dermatol Clin. 1998;16:447-453. [DOI] [PubMed] [Google Scholar]
- 5. Hall DE, Udvarhelyi GB, Altman J. Lumbosacral skin lesions as markers of occult spinal dysraphism. JAMA. 1981;246:2606-2608. [PubMed] [Google Scholar]
- 6. Kaufman BA. Neural tube defects. Pediatr Clin North Am. 2004;51:389-419. [DOI] [PubMed] [Google Scholar]
- 7. Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170. [DOI] [PubMed] [Google Scholar]
- 8. Netto JM, Bastos AN, Figueiredo AA, Pérez LM. Spinal dysraphism: a neurosurgical review for the urologist. Rev Urol. 2009;11:71-81. [PMC free article] [PubMed] [Google Scholar]
- 9. Amarante MA, Shrensel JA, Tomei KL, Carmel PW, Gandhi CD. Management of urological dysfunction in pediatric patients with spinal dysraphism: review of the literature. Neurosurg Focus. 2012;33:E4. [DOI] [PubMed] [Google Scholar]
- 10. Gibson PJ, Britton J, Hall DM, Hill CR. Lumbosacral skin markers and identification of occult spinal dysraphism in neonates. Acta Paediatr. 1995;84:208-209. [DOI] [PubMed] [Google Scholar]
- 11. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmão S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol. 2005;63(suppl 1):S8-S12. [DOI] [PubMed] [Google Scholar]
- 12. Tuite GF, Thompson DNP, Austin PF, Bauer SB. Evaluation and management of tethered cord syndrome in occult spinal dysraphism: recommendations from the International Children’s Continence Society. Neurourol Urodyn. 2018;37:890-903. [DOI] [PubMed] [Google Scholar]
- 13. Filippidis AS, Kalani MY, Theodore N, Rekate HL. Spinal cord traction, vascular compromise, hypoxia, and metabolic derangements in the pathophysiology of tethered cord syndrome. Neurosurg Focus. 2010;29:E9. [DOI] [PubMed] [Google Scholar]
- 14. Sahin F, Selcuki M, Ecin N, et al. Level of conus medullaris in term and preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1997;77:F67-F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarica K, Erbağci A, Yağci F, Yurtseven C, Buyukbebeci O, Karakurum G. Multidisciplinary evaluation of occult spinal dysraphism in 47 children. Scand J Urol Nephrol. 2003;37:329-334. [DOI] [PubMed] [Google Scholar]
- 16. Drolet BA, Boudreau C. When good is not good enough: the predictive value of cutaneous lesions of the lumbosacral region for occult spinal dysraphism. Arch Dermatol. 2004;140:1153-1155. [DOI] [PubMed] [Google Scholar]
- 17. White JT, Samples DC, Prieto JC, Tarasiewicz I. Systematic review of urologic outcomes from tethered cord release in occult spinal dysraphism in children. Curr Urol Rep. 2015;16:78. [DOI] [PubMed] [Google Scholar]
- 18. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol. 1998;171:1687-1692. [DOI] [PubMed] [Google Scholar]
- 19. Gomi A, Oguma H, Furukawa R. Sacrococcygeal dimple: new classification and relationship with spinal lesions. Childs Nerv Syst. 2013;29:1641-1645. [DOI] [PubMed] [Google Scholar]
- 20. Albert GW. Spine ultrasounds should not be routinely performed for patients with simple sacral dimples. Acta Paediatr. 2016;105:890-894. [DOI] [PubMed] [Google Scholar]
- 21. Weprin BE, Oakes WJ. Coccygeal pits. Pediatrics. 2000;105:E69. [DOI] [PubMed] [Google Scholar]
- 22. Hughes JA, De BR, Patel K, Thompson D. Evaluation of spinal ultrasound in spinal dysraphism. Clin Radiol. 2003;58:227-233. [DOI] [PubMed] [Google Scholar]
- 23. Chern JJ, Kirkman JL, Shannon CN, et al. Use of lumbar ultrasonography to detect occult spinal dysraphism. J Neurosurg Pediatr. 2012;9:274-279. [DOI] [PubMed] [Google Scholar]
- 24. Korsvik HE, Keller MS. Sonography of occult dysraphism in neonates and infants with MR imaging correlation. Radiographics. 1992;12:297-308. [DOI] [PubMed] [Google Scholar]
- 25. Lavallee LT, Leonard MP, Dubois C, Guerra LA. Urodynamic testing—is it a useful tool in the management of children with cutaneous stigmata of occult spinal dysraphism? J Urol. 2013;189:678-683. [DOI] [PubMed] [Google Scholar]



