Abstract
Objectives: We sought to determine the relative contributions of stroke, dementia, and their combination to disability and racial differences in disability among community-dwelling older adults. Methods: We performed a cross-sectional study of 6848 community-dwelling older adults. We evaluated the associations of stroke, dementia, and their combination with activities of daily living (ADL) limitations (range 0-7). We then explored the impact of stroke and dementia on race differences in ADL limitations using Poisson regression after accounting for sociodemographics and comorbidities. Results: After full adjustment, ADL limitations differed among older adults with stroke and dementia. Older adults without stroke or dementia had 0.32 (95% CI 0.29-0.35) ADL limitations compared to 0.64 (95% CI 0.54-0.73) with stroke, 1.36 (95% CI 1.20-1.53) with dementia and 1.84 (95% CI 1.54-2.15) with stroke and dementia. Overall, blacks had 0.27 (95%CI 0.19-0.36) more ADL limitations than whites. Models accounting for stroke led to a 3.7% (95%CI 2.98%-4.43%) reduction in race differences, while those for dementia led to a 29.26% (95%CI 28.53%-29.99%) reduction and the stroke-dementia combination −1.48% (95%CI −2.21% to −0.76) had little impact. Discussion: Older adults with stroke and dementia have greater disability than older adults with either of these conditions alone. However, the amount of disability experienced by older adults with stroke and dementia is less than the sum of the contributions from stroke and dementia. Dementia is likely a key contributor to race differences in disability.
Keywords: stroke, dementia, disability, race, elderly
Introduction
In 2030, 20% of the US population is projected to be older than 65 years of whom over 20% may have difficulty with activities of daily living (ADLs).1-3 The aging of the population of the United States will give rise to increasing numbers of older adults with disability.4,5 Chronic diseases and more recently multimorbidity, frequently contribute to disability through unknown pathways.6-8 Dementia and stroke, however, have a measurable impact on cognitive and physical function and directly contribute to disability.9-11 Furthermore, there may be a reciprocal relationship between stroke and dementia whereby stroke survivors are more likely to have dementia and vice versa.12,13
While both stroke and dementia are important contributors to disability, little is known about the impact of their co-occurrence on disability.6,14 We hypothesize that stroke and dementia interact to result in disability that is greater than the sum of the independent contributions from stroke and dementia. One mechanism may be via impaired neural reorganization. In the poststroke state, functional neuroimaging studies suggest that neural reorganization through diffusely connected networks has an important role in recovery.15,16 However, older adults with dementia have decreased functional connectivity or synchronization between key neural networks.17,18 Thus, in older adults suffering from both stroke and dementia, there may be decreased neural reorganization necessary for recovery. Similarly, impaired participation or responsiveness to poststroke rehabilitation may contribute to increased physical disability among individuals with stroke and dementia.19-21 Finally, the efficacy of post-stroke rehabilitation for cognitive deficits is not well established.22
Older blacks experience more late-life disability than whites, with black women experiencing markedly less improvement in years of disability-free life than whites and black men.13 Contributors to racial differences in disability among older adults may include differential accumulation of chronic disease. Blacks have a higher incidence of stroke and dementia than whites and may lead to the observed differential in disability.13,23-25 While researchers have suggested that blacks have shown poorer cognitive function following stroke, the combined contribution of stroke and dementia to race differences in disability is unknown.26
In this context, we explore the relative impact of stroke and dementia with disability. We hypothesize that a person with both stroke and dementia will have greater disability than the sum of its parts. We then examine the extent to which stroke, dementia, and the combination contribute to racial differences in disability and other important patient-centered outcomes.
Methods
Overview
We performed a cross-sectional study using data from the National Health and Aging Trends Study (NHATS) to examine 2 research questions. First, we explored the association of stroke, dementia, and the combination of these two entities on disability, as measured by ADL limitations. We then explored the association between race and the following outcomes: ADL limitations, instrumental activities of daily living (IADL) limitations, participation in valued activities restrictions, well-being, and hours of caregiving, as racial disparities in these outcome measures have been previously described in the stroke survivor population.24,27 We examined the extent to which stroke, dementia, or a stroke-dementia interaction contribute to race differences in these outcomes (Figure 1).
Figure 1.
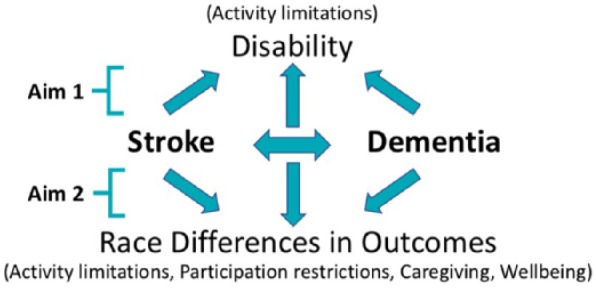
Overview of analyses.
Data Source and Study Population
NHATS is an ongoing nationally representative cohort study of over 8000 Medicare beneficiaries ages 65 years and older. Participants are interviewed annually in-person by trained staff. Proxy respondents, people familiar with the older adults’ daily routines, were interviewed when participants were unable to participate due to cognitive or physical limitations. The first round of NHATS, fielded in 2011 with oversampling of both the oldest old and African American populations, was utilized in this study. Further details of the study design have been previously published.28 Because of our focus on race differences, races other than self-reported blacks and whites (N = 796) were excluded (eFigure 1, see Supplementary Material available online). Our study was limited to self-reported black and white participants. We also limited our sample to participants residing in the community or a residential care facility who completed a participant interview (eFigure 1, see Supplementary Material available online).
Identifying Stroke, Dementia, and Race
Stroke diagnosis was either self- or proxy-reported affirmative to the question: “Has a doctor ever told you that you had a stroke?”29 Probable dementia, termed dementia throughout the manuscript, was classified based on one or more of the following criteria: self-reported dementia diagnosis, met established AD8 criteria, proxy-reported, or was ≤1.5 standard deviations below the mean in at least two cognitive domains (orientation, memory, executive functioning).30,31
Primary and Secondary Outcome Measures
The primary outcome was disability as defined by receipt of help or forwent activity if help was not available for ADLs which included: eating, showering/taking baths, using the toilet, getting dressed, getting out of bed, moving around within the home, and leaving the home.32 The results for each participant were transformed into a count outcome, ranging from 0 to 7. A secondary outcome of IADL limitations was constructed based on receipt of help performing 5 household activities due to health or functioning or forewent household activities if no one was there to help, including doing the laundry, shopping for groceries/personal items, preparing hot meals, handling bills/banking, and keeping track of/taking medications. This count outcome ranged from 0 to 5.
Additional secondary outcomes in the study included participation restrictions in valued activities due to health or functioning, hours per week of caregiving received, and well-being, which were defined using NHATS measures.32,33 Participation restrictions were a count ranging from 0 to 4 of restrictions in valued activities including visiting with family/friends, attending religious services, participating in clubs/classes, and going out for enjoyment. Well-being included metrics of both emotion and self-realization resulting in a count outcome ranging from 0 to 22, with higher scores representing greater well-being.34 Caregiving quantity was recorded as the number of hours of help per week and whether this help was paid or unpaid.35 There were 7 (0.094 weighted%) participants who were missing data for ADL, 11 (0.16 weighted%) missing participation restriction, 491 (5.26 weighted%) missing well-being, and 27 (0.37 weighted%) missing caregiving quantity.
Covariates: Sociodemographic Factors and Comorbidities
Demographics (age, sex, marital status), and educational attainment were included. Self-reported comorbidities (myocardial infarction, coronary artery disease, hypertension, diabetes, cancer, lung disease, osteoporosis, and arthritis) and symptoms of mood disorders, including depressive and anxiety symptoms were transformed into a comorbidity count, ranging from 0 to 10.36,37 Nonresponse for each covariate was coded as a separate category to include in the model (Appendix Table A1).
Statistical Analysis
After application of survey weights, descriptive statistics were used to compare sociodemographic factors, comorbidities, and stroke and dementia status between blacks and whites. To address our first question of how stroke, dementia, and the combination of both conditions influence disability, we used survey-weighted Poisson regression with a dependent variable of disability, adjusting for sociodemographics and comorbidities. To address our second question, the association of stroke, dementia and their combination on racial disparities, we fit a series of models for each dependent variable (disability, IADL limitations, participation restrictions, well-being, and caregiving hours) with a primary independent variable, race. For each outcome, serial models were constructed to evaluate the effects of covariates. Our first model included sociodemographic factors and comorbidities. We then built models adding a single variable with each subsequent model—stroke, dementia, and the interaction between stroke and dementia. Wald tests were then used to compare the outcomes between the blacks and whites for statistical significance with a P value <.05. We summarized the magnitude of these effects using average marginal effects. To explore the role of individual comorbidities on disability, we performed a sensitivity analysis that included each comorbidity as a separate covariate in the model. All analyses were performed in STATA 14 (StataCorp LLC, College Station, TX).
Ethics and Informed Consent Statement
This study was determined as not regulated by the institutional review board from the University of Michigan. Data are freely available at https://www.nhats.org/.
Results
Baseline Characteristics
A total of 6848 older adults (survey-weighted n = 31 296 131) were included in our study, 9.2% of whom were black. About 10% were stroke survivors, 9% had dementia, and 2.2% had both conditions. Compared with whites, blacks in our study were younger (<74 years: 57.9% vs 52.1%, P < .01); a greater proportion were women (60.2% vs 56.3%, P = .02); fewer were married (38.3% vs 59.1%, P < .01); had less educational attainment (less than high school: 39.2% vs 16.4%, P < .01); had lower income (<$13 000 [lowest quintile]: 29.0% vs 16.5%, P < .01); and had more comorbidities (mean: 2.8 vs 2.6, P < .01). Blacks were more likely to have had a stroke (12.5% vs 9.8%, P < .01), dementia (15.3% vs 8.4%, P < .01) and both stroke and dementia (3.9% vs 2.0%, P < .01) than whites (Table 1).
Table 1.
Sociodemographic Factors, Comorbidities, and Stroke and Dementia Status by Race.
| Covariate | Total (Survey-Weighted n = 31 296 131), % | White (Survey-Weighted n = 28 433 188), % | Black (Survey-Weighted n = 2 862 942), % | P |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | <.01 | |||
| 65-69 | 27.8 | 27.7 | 29.5 | |
| 70-74 | 24.8 | 24.4 | 28.4 | |
| 75-79 | 19.0 | 19.1 | 17.7 | |
| 80-84 | 14.8 | 15.0 | 13.6 | |
| 85-89 | 9.2 | 9.4 | 7.1 | |
| 90+ | 4.5 | 4.5 | 3.7 | |
| Female | 56.7 | 56.3 | 60.2 | .02 |
| Socioeconomic status | ||||
| Marital STATUS | ||||
| Married | 57.2 | 59.1 | 38.3 | <.01 |
| Not married | 42.8 | 40.9 | 61.4 | |
| Unknown | 0.05 | 0.03 | 0.3 | |
| Education | <.01 | |||
| Less than high school | 18.5 | 16.4 | 39.2 | |
| High school graduate | 28.7 | 29.0 | 25.1 | |
| College graduate | 52.6 | 54.3 | 35.3 | |
| Missing | 0.3 | 0.3 | 0.4 | |
| Income | <.01 | |||
| Less than $13 000 | 18.5 | 16.5 | 29.0 | |
| $13 001-$24 000 | 19.8 | 19.5 | 22.5 | |
| $24 001-$40 000 | 22.0 | 22.3 | 18.9 | |
| $40 000-$68 000 | 18.3 | 17.9 | 11.8 | |
| $68 001+ | 21.4 | 22.8 | 7.7 | |
| Comorbidity count, 0-10, Mean (SD) | 2.6 (1.7) | 2.6 (1.5) | 2.8 (2.6) | <.01 |
| Myocardial infarction | 14.2 | 14.3 | 13.1 | .30 |
| Coronary artery disease | 18.0 | 18.2 | 15.6 | .03 |
| Hypertension | 63.6 | 62.0 | 79.8 | <.01 |
| Arthritis | 54.1 | 53.9 | 55.9 | .16 |
| Osteoporosis | 20.8 | 21.5 | 13.9 | <.01 |
| Diabetes | 22.8 | 21.4 | 37.0 | <.01 |
| Lung disease | 15.5 | 15.6 | 14.9 | .53 |
| Cancer | 27.3 | 28.2 | 18.0 | <.01 |
| Depressiona | 13.7 | 13.1 | 19.4 | <.01 |
| Anxietyb | 11.9 | 11.6 | 14.5 | .01 |
| Stroke | 10.1 | 9.8 | 12.5 | <.01 |
| Dementia | 9.1 | 8.4 | 15.3 | <.01 |
| Stroke and Dementia | <.01 | |||
| No stroke and no dementia | 83.1 | 83.7 | 76.2 | |
| Stroke but no dementia | 7.9 | 7.8 | 8.5 | |
| No stroke but dementia | 6.9 | 6.4 | 11.4 | |
| Stroke and dementia | 2.2 | 2.0 | 3.9 |
0.068% missing (unweighted N = 6).
0.099% missing (unweighted N = 8).
Stroke, Dementia, and Stroke and Dementia With Disability
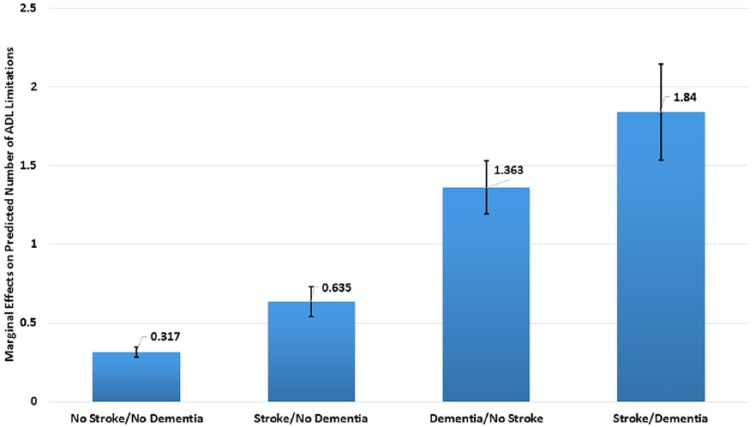
After accounting for age, sex, race, marital status, education, income, and comorbidities, we found that stroke, dementia and the interaction term of stroke and dementia were associated with disability. Older adults without stroke or dementia had 0.32 (95% CI 0.29-0.35) ADL limitations (Figure 2). Older adults with stroke but no dementia had 0.64 (95% CI 0.54-0.73), older adults with dementia but no stroke had 1.36 (95% CI 1.20-1.53), and older adults with stroke and dementia had 1.84 (95% CI: 1.54-2.15) ADL limitations.
Figure 2.
Marginal effects of stroke and dementia on disability.
Description: Predicted number of activities of daily living (ADL) limitations in the overall National Health and Aging Trends Study (NHATS) cohort.
Racial Differences
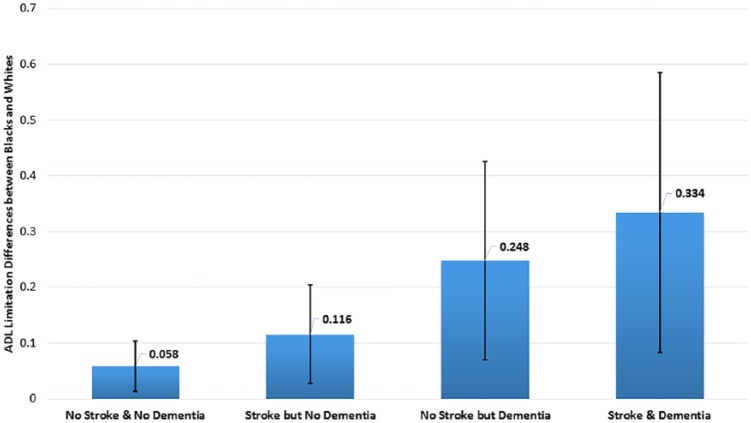
Overall, blacks had 0.27 (95% CI 0.19-0.36) more unadjusted ADL limitations than whites (P < .01). After accounting for sociodemographics and comorbidities, stroke had little impact on race differences in disability (racial difference changes in ADLs = 3.70%; 95%CI 2.98%-4.43%). Dementia had a larger impact on race differences 29.26% (95% CI 28.53%-29.99%) that was overall unchanged with the inclusion of the stroke and dementia interaction −1.48% (95%CI −2.21% to −0.76%) (Table 2). Compared with whites with stroke and dementia, blacks with stroke and dementia had 0.33 (95% CI 0.08-0.59, P = .01) more ADL limitations (Figure 3).
Table 2.
Predicted Primary and Secondary Outcomes by Race (Black vs White) (Unweighted N = 6848, Sample-Weighted N = 31 296 131).
| Outcome |
Unadjusted
a
|
Sociodemographic/Comorbidities
b
|
Stroke
c
|
Dementia
d
|
Stroke/Dementia
e
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMEf | P | AME | P | AME | P | AME | P | AME | P | |
| Disability (ADL limitations) g | 0.27 (0.19-0.36) | <.01 | 0.18 (0.1-0.26) | <.01 | 0.17 (0.09-0.25) | <.01 | 0.091 (0.02-0.17) | .02 | 0.095 (0.02-0.17) | .01 |
| Total variation explained adjustment, % | 33.33 (30.79-35.87) | 37.04 (34.53-35.75) | 66.30 (63.81-65.03) | 64.81 (62.37-63.56) | ||||||
| Each additional variation explained, % | 3.70 (2.98-4.43) | 29.26 (28.53-29.99) | −1.48 (−2.21 to −0.76) | |||||||
| IADL limitations | 0.41 (0.31-0.51) | <.01 | 0.26 (0.17-0.35) | <.01 | 0.25 (0.16-0.34) | <.01 | 0.17 (0.08-0.25) | <.01 | 0.17 (0.09-0.26) | <.01 |
| Total variation explained adjustment, % | 36.59 (34.82-38.35) | 39.02 (37.26-40.79) | 58.54 (56.78-60.30) | 58.54 (56.78-60.29) | ||||||
| Each additional variation explained, % | 2.44 (1.94-2.94) | 19.51 (19.02-20.01) | 0 (−0.5 to 0.5) | |||||||
| Participation restrictions h | 0.082 (0.032-0.13) | <.01 | 0.03 (−0.01 to 0.07) | .18 | 0.03 (−0.01 to 0.07) | .18 | 0.02 (−0.02 to 0.06) | .35 | 0.02 (−0.02 to 0.06) | .37 |
| Well-being i | −0.077 (−0.35 to 0.19) | .57 | 0.45 (0.18-0.72) | <.01 | 0.45 (0.18-0.72) | <.01 | 0.48 (0.22-0.75) | <.01 | 0.48 (0.22-0.75) | <.01 |
| Coef.j | P | Coef. | P | Coef. | P | Coef. | P | Coef. | P | |
| Caregiving, h/wk k | 4.37 (1.12-7.62) | <.01 | 5.65 (2.54-8.75) | <.01 | 5.55 (2.45-8.65) | <.01 | 4.30 (1.08-7.52) | .01 | 4.28 (1.05-7.5) | .01 |
Abbreviations: AME, average marginal effects; ADL, activities of daily living; IADL, instrumental activities of daily living; Coef., coefficient; h/wk, hours per week.
Unadjusted: no adjustment for covariates.
Sociodemographic/Comorbidities: adjusted for age, sex, marital status, education, income, and comorbidity score.
Stroke: adjusted for age, sex, marital status, education, income, comorbidity score, and stroke.
Dementia: adjusted for age, sex, marital status, education, income, comorbidity score, stroke, and dementia.
Stroke/Dementia: adjusted for age, sex, marital status, education, income, comorbidity score, stroke, dementia, and interaction term of stroke and dementia.
Values are average marginal effects (black vs white).
Unweighted N = 6841, survey-weighted N = 31 266 588.
Unweighted N = 6837, survey-weighted N = 31 246 328.
Unweighted N = 6357, survey-weighted N = 29 650 216.
Values are regression coefficients (black vs white).
Unweighted N = 6821, survey-weighted N = 31 181 149.
Figure 3.
Marginal differences in disability by race (black vs white).
Description: Race differences (blacks vs whites) in the predicted number of activities of daily living (ADL) limitations in those with no stroke or dementia, stroke, dementia, and stroke + dementia.
A similar pattern was observed for IADL limitations, with the addition of dementia to the models that accounted for sociodemographic factors and comorbidities and stroke having had the largest reduction of the race difference (racial difference changes in IADLs = 19.51% [95% CI 19.02%-20.01%]) with little change due to the inclusion of stroke (2.44% [95%CI 1.94%-2.94%]) and the stroke and dementia interaction (0% [95% CI: −0.5% to 0.5%]). Before adjustment for covariates, blacks had significantly greater participation restrictions in valued activities due to health and functioning than whites (P < .01). However, this difference no longer persisted after adjustment for sociodemographic factors and comorbidities. There was no statistically significant difference in the well-being scores between blacks and whites before adjustment for covariates. Small differences emerged after adjustment for sociodemographic factors and comorbidities, blacks experienced greater well-being than whites (P < .01), and this difference persisted with minimal change after further adjustment. Finally, blacks received more hours of caregiving than whites with the inclusion of dementia decreasing the race difference in adjusted models. In the sensitivity analysis that included each comorbidity as a separate covariate in the model, the results were similar to those from primary analyses (Appendix Table A2).
Discussion
In this nationally representative sample of older, community-dwelling adults, we found that older adults with stroke and dementia have greater disability than older adults with either of these conditions alone. However, the amount of disability experienced by older adults with stroke and dementia is less than the sum of the combined contributions from stroke and dementia. Additionally, while dementia was an important contributor to race differences in community-dwelling older adults, the combination of stroke and dementia had little impact on race differences.
We found that older community-dwelling adults who had both stroke and dementia did not have ADL limitations that were greater than the sum of their stroke and dementia limitations, rejecting our hypothesis. Reasons why this may be the case warrant careful consideration. First, this could be a true null hypothesis. Alternately, by excluding older adults residing in nursing homes, we likely excluded the most severely affected older adults with both stroke and dementia.38 Second, the cross-sectional study design is a limitation. It is plausible that on average, older adults with both stroke and dementia were earlier in their course of dementia relative to the dementia-only cohort. If this were the case, it could lead to an underestimation of disability among older adults with both stroke and dementia. Further studies with a longitudinal design that includes older adults residing in a nursing home are warranted to explore the interactions between stroke and dementia and their effect on disability.
Since the stroke-dementia interaction was less prominent than hypothesized, it had correspondingly little impact on racial differences in disability. Taken independently from one another, stroke and dementia each impacted the observed race differences in disability, with dementia reducing the point estimates of racial differences in disability more than stroke. This finding may be related to the differential prevalence of stroke and dementia between the races in our study. Whereas blacks had roughly a 30% greater prevalence of strokes than whites (12.5% vs 9.8%), blacks had almost twice the prevalence of dementia (15.3% vs 8.3%) compared with whites. Our findings do not suggest that for an individual that stroke has a smaller impact on their disability than dementia, but that stroke contributes less to the racial differences in outcome at a population level. As discussed previously, the cross-sectional study design may contribute to our finding, namely racial differences between incident and prevalent stroke and dementia. Whereas incident stroke presents with peak disability and prevalent stroke with lesser disability,39 incident and prevalent dementia have the exact opposite relationship. Because of the cross-sectional nature of our study, it is unclear where in the incident-prevalent time course our participants were when their disability was measured.
Our findings have clinical and policy-relevant implications. First, given the prevalence of stroke and dementia as well as their contribution to disability among older adults, novel treatment strategies that prevent and reduce disability should be encouraged. Second, known risk factors, such as cardiovascular risk factors, that decrease the incidence of incident and recurrent stroke and may decrease or delay the incidence of dementia should be managed.12,22 Our findings may influence the provider-patient conversation. Rather than focus on control of vascular risk factors to promote disease prevention, control of vascular risk factors could be framed as disability prevention, likely a more patient-centered outcome, which may result in more patient engagement in self-management. From a policy perspective, providing care for the growing number of stroke and dementia survivors, many of whom will be disabled, is critical.
In addition to the limitations of our study already noted, we used patient-reported identification of race, stroke, and outcome measures. However, stroke diagnosis by self-report has been shown to have relatively high fidelity in the older population.40 Moreover, while there may be inherent variability in self-reports, prior studies have demonstrated the advantage of such measures of disability, as they provide a more patient-centric approach to outcomes.41 The NHATS team did not interview nursing home patients, limiting the generalizability of our results to the community-dwelling elderly population and likely underestimating the disability measures. Finally, assessments of the severity of comorbidities are not available.
In summary, among community-dwelling older adults, we found that stroke and dementia were associated with greater disability than either condition alone, but less than expected. Stroke, and to a greater extent dementia, contribute to race differences in disability among older community-dwelling adults. Future studies should include older adults residing in nursing homes and incorporate a longitudinal study design to understand the full scope of disability.
Supplemental Material
Supplemental material, JPC852507_Supplemental_Material_CLN for Disability in Community-Dwelling Older Adults: Exploring the Role of Stroke and Dementia by Brian J. Stamm, James F. Burke, Chun Chieh Lin, Rory J. Price and Lesli E Skolarus in Journal of Primary Care & Community Health
Author Biographies
Brian J. Stamm is a recent graduate of the University of Michigan Medical School and is currently a resident at Northwestern University in Chicago. He is interested in stroke and health services research.
James F. Burke is an associate professor of Neurology at the University of Michigan. He is a health services researcher with a focus on understanding differences in long-term outcomes in stroke and other neurologic conditions and applying advanced statistical methodology to optimizing treatment decisions for individual patients.
Chun Chieh Lin is a sr. analyst in the University of Michigan Medical School Department of Neurology Health Services Research program. She had her PhD in Health Services Organization and Research from the Virginia Commonwealth University and her research interests include health services research, clinical epidemiology, workforce demand/supply, comparative treatment effectiveness studies, health outcomes research, and examination in the factors associated with treatment disparities among underserved patients.
Rory J. Price is a recent graduate of the University of Michigan School of Public Health with a Master of Science in Health Behavior and Health Education. She is interested in social determinants of health in rural communities, addressing health disparities related to socioeconomic status and the interplay between functional health literacy and health outcome.
Lesli E. Skolarus is an associate professor of Neurology at the University of Michigan. Her research focuses on behavioral trials to promote health equity, community based participatory research and health services research.
Appendix
Table A1.
Nonresponse Percentage of Missing Cases for Each Variable.
| Variables | N (weighted %) |
|---|---|
| ADL | 7 (0.094) |
| Eating | 1 (0.03) |
| Bathing | 0 (0) |
| Toileting | 6 (0.084) |
| Dressing | 0 (0) |
| Getting out of bed | 3 (0.058) |
| Getting around inside house | 2 (0.047) |
| Getting outside one’s house | 0 (0) |
| IADL | 0 (0) |
| Laundry | 0 (0) |
| Shopping | 0 (0) |
| Preparing meals | 0 (0) |
| Bank | 0 (0) |
| Medication | 0 (0) |
| Participation restrictions | 11 (0.16) |
| Visit family/friend | 7 (0.1) |
| Religious services | 5 (0.083) |
| Participate in club/class | 4 (0.061) |
| Fun outside | 6 (0.088) |
| Well-being index | 491 (5.26) |
| Caregiving quantity | 27 (0.37) |
| Marital status | 7 (0.06) |
| Comorbidity | |
| Myocardial infarction | 7 (0.12) |
| Coronary artery disease | 15 (0.22) |
| Hypertension | 10 (0.17) |
| Arthritis | 16 (0.25) |
| Osteoporosis | 24 (0.32) |
| Diabetes | 2 (0.0079) |
| Lung disease | 5 (0.12) |
| Cancer | 3 (0.047) |
| Depression (PHQ-2 ≥3) | 6 (0.068) |
| Anxiety (GAD-2 ≥3) | 8 (0.099) |
| Education | 9 (0.27) |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; PHQ-2, Patient Health Questionnaire–2; GAD-2, Generalized Anxiety Disorder–2.
Table A2.
Predicted Primary and Secondary Outcomes by Race (Black Versus White) (Unweighted N = 6848, survey-weighted N = 31 296 131) Adjusting for Individual Comorbid Conditions (Except in Unadjusted Model).
| Outcome |
Unadjusted
a
|
Sociodemographic/Comorbidities
b
|
Stroke
c
|
Dementia
d
|
Stroke/Dementia
e
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMEf | P | AME | P | AME | P | AME | P | AME | P | |
| Disability (ADL limitations) g | 0.27 (0.19-0.36) | <.01 | 0.16 (0.07-0.25) | <.01 | 0.17 (0.08-0.25) | <.01 | 0.087 (0.01-0.17) | .04 | 0.091 (0.01-0.17) | .02 |
| IADL limitations | 0.41 (0.31-0.51) | <.01 | 0.24 (0.15-0.34) | <.01 | 0.25 (0.16-0.34) | <.01 | 0.16 (0.08-0.25) | <.01 | 0.17 (0.08-0.25) | <.01 |
| Participation restrictions h | 0.082 (0.032-0.13) | <.01 | 0.04 (−0.01 to 0.08) | .12 | 0.04 (−0.01 to 0.08) | .11 | 0.03 (−0.02 to 0.08) | .20 | 0.03 (−0.02 to 0.07) | .21 |
| Well-being i | −0.077 (−0.35 to 0.19) | .57 | 0.43 (0.17-0.69) | <.01 | 0.43 (0.17-0.69) | <.01 | 0.46 (0.20-0.72) | <.01 | 0.46 (0.20-0.72) | <.01 |
| Coef.j | P | Coef. | P | Coef. | P | Coef. | P | Coef. | P | |
| Caregiving, h/wk k | 4.37 (1.12-7.62) | <.01 | 5.29 (2.08-8.49) | <.01 | 5.26 (2.05-8.46) | <.01 | 3.98 (0.63-7.34) | .02 | 3.97 (0.61-7.32) | .02 |
Abbreviations: AME, average marginal effects; ADL, activities of daily living; IADL, instrumental activities of daily living; Coef., coefficient; h/wk, hours per week.
Unadjusted: no adjustment for covariates.
Sociodemographic/Comorbidities: adjusted for age, sex, marital status, education, income, myocardial infarction, coronary artery disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, cancer, depression, and anxiety.
Stroke: adjusted for age, sex, marital status, education, income, myocardial infarction, coronary artery disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, cancer, depression, and anxiety, and stroke.
Dementia: adjusted for age, sex, marital status, education, income, myocardial infarction, coronary artery disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, cancer, depression, and anxiety, stroke, and dementia.
Stroke/Dementia: adjusted for age, sex, marital status, education, income, myocardial infarction, coronary artery disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, cancer, depression, and anxiety, stroke, dementia, and interaction term of stroke and dementia.
Values are average marginal effects (black vs white).
Unweighted N = 6841, survey-weighted N = 31 266 588.
Unweighted N = 6837, survey-weighted N = 31 246 328.
Unweighted N = 6357, survey-weighted N = 29 650 216.
Values are regression coefficients (black vs white).
Unweighted N = 6821, survey-weighted N = 31 181 149.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institute on Minority Health and Health Disparities (R01 MD008879).
ORCID iDs: Brian J. Stamm  https://orcid.org/0000-0002-0862-9650
https://orcid.org/0000-0002-0862-9650
Lesli E Skolarus  https://orcid.org/0000-0002-3088-9838
https://orcid.org/0000-0002-3088-9838
Supplemental Material: Supplemental material for this article is available online.
References
- 1. US Census Bureau. 2012. National Population Projections Tables. https://www.census.gov/data/tables/2012/demo/popproj/2012-summary-tables.html. Accessed March 17, 2019.
- 2. US Census Bureau. Table 2. Projections of the population by selected age groups and sex for the United States: 2015 to 2060 (NP2012-T2). https://www.census.gov/data/tables/2012/demo/popproj/2012-summary-tables.html. Accessed March 12, 2019.
- 3. Freedman VA, Carr D, Cornman JC, Lucas RE. Impairment severity and evaluative and experienced well-being among older adults: assessing the role of daily activities. Innov Aging. 2017;1:igx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freedman VA, Spillman BC, Andreski PM, et al. Trends in late-life activity limitations in the United States: an update from five national surveys. Demography. 2013;50:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colby SL, Ortman JM. The baby boom cohort in the United States: 2012 to 2060. https://www.census.gov/library/publications/2014/demo/p25-1141.html. Published May 2014. Accessed May 9, 2019.
- 6. Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92-100. [DOI] [PubMed] [Google Scholar]
- 7. Guralnik JM. Understanding the relationship between disease and disability. J Am Geriatr Soc. 1994;42:1128-1129. [DOI] [PubMed] [Google Scholar]
- 8. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430-439. [DOI] [PubMed] [Google Scholar]
- 9. Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham study. Am J Public Health. 1994;84:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu L, Fratiglioni L, Guo Z, Agüero-Torres H, Winblad B, Viitanen M. Association of stroke with dementia, cognitive impairment, and functional disability in the very old: a population-based study. Stroke. 1998;29:2094-2099. [DOI] [PubMed] [Google Scholar]
- 11. Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman VA, Spillman BC. Active life expectancy in the older US population, 1982-2011: differences between blacks and whites persisted. Health Aff (Millwood). 2016;35:1351-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hénon H, Pasquier F, Leys D. Poststroke dementia. Cerebrovasc Dis. 2006;22:61-70. [DOI] [PubMed] [Google Scholar]
- 15. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126(pt 6):1430-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861-872. [DOI] [PubMed] [Google Scholar]
- 17. de Haan W, Pijnenburg YA, Strijers RL, et al. Functional neural network analysis in frontotemporal dementia and Alzheimer’s disease using EEG and graph theory. BMC Neurosci. 2009;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wee CY, Yap PT, Zhang D, et al. Identification of MCI individuals using structural and functional connectivity networks. Neuroimage. 2012;59:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zinn S, Dudley TK, Bosworth HB, Hoenig HM, Duncan PW, Horner RD. The effect of poststroke cognitive impairment on rehabilitation process and functional outcome. Arch Phys Med Rehabil. 2004;85:1084-1090. [DOI] [PubMed] [Google Scholar]
- 20. Heruti RJ, Lusky A, Dankner R, et al. Rehabilitation outcome of elderly patients after a first stroke: effect of cognitive status at admission on the functional outcome. Arch Phys Med Rehabil. 2002;83:742-749. [DOI] [PubMed] [Google Scholar]
- 21. Skidmore ER, Whyte EM, Holm MB, et al. Cognitive and affective predictors of rehabilitation participation after stroke. Arch Phys Med Rehabil. 2010;91:203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38-45. [DOI] [PubMed] [Google Scholar]
- 23. Schoeni RF, Martin LG, Andreski PM, Freedman VA. Persistent and growing socioeconomic disparities in disability among the elderly: 1982-2002. Am J Public Health. 2005;95:2065-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burke JF, Freedman VA, Lisabeth LD, Brown DL, Haggins A, Skolarus LE. Racial differences in disability after stroke Results from a nationwide study. Neurology. 2014;83:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49-56. [DOI] [PubMed] [Google Scholar]
- 26. Johnson NX, Marquine MJ, Flores I, et al. Racial differences in neurocognitive outcomes post-stroke: the impact of healthcare variables. J Int Neuropsychol Soc. 2017;23:640-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skolarus LE, Freedman VA, Feng C, Burke JF. African American stroke survivors: more caregiving time, but less caregiving burden. Circ Cardiovasc Qual Outcomes. 2017;10:e003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montaquila J, Freedman VA, Kasper JD. National Health and Aging Trends Study (NHATS): Round 1 Income Imputation. Baltimore, MD: Johns Hopkins University School of Public Health; 2012. [Google Scholar]
- 29. Kasper JD, Freedman VA. National Health and Aging Trends Study Round 1 User Guide: Final Release. Baltimore, MD: Johns Hopkins University School of Public Health; 2012. http://www.NHATS.org. Accessed November 16, 2015. [Google Scholar]
- 30. Kasper JD, Freedman VA, Spillman BC. Classification of Persons by Dementia Status in the National Health and Aging Trends Study, Technical Paper #5. Baltimore, MD: Johns Hopkins University School of Public Health; 2013. [Google Scholar]
- 31. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67:1942-1948. [DOI] [PubMed] [Google Scholar]
- 32. Freedman VA, Kasper JD, Cornman JC, et al. Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci. 2011;66:1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freedman VA, Kasper JD, Spillman BC, et al. Behavioral adaptation and late-life disability: a new spectrum for assessing public health impacts. Am J Public Health. 2014;104:e88-e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zahuranec DB, Skolarus LE, Feng C, Freedman VA, Burke JF. Activity limitations and subjective well-being after stroke. Neurology. 2017;89:944-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skolarus LE, Freedman VA, Feng C, Wing JJ, Burke JF. Care received by elderly US stroke survivors may be underestimated. Stroke. 2016;47:2090-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292. [DOI] [PubMed] [Google Scholar]
- 37. Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317-325. [DOI] [PubMed] [Google Scholar]
- 38. Blackburn J, Albright KC, Haley WE, et al. Men lacking a caregiver have greater risk of long-term nursing home placement after stroke. J Am Geriatr Soc. 2018;66:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835-841. [DOI] [PubMed] [Google Scholar]
- 40. Horner RD, Cohen HJ, Blazer DG. Accuracy of self-reported stroke among elderly veterans. Aging Ment Health. 2001;5:275-281. [DOI] [PubMed] [Google Scholar]
- 41. Ellis C, Boan AD, Turan TN, Ozark S, Bachman D, Lackland DT. Racial differences in poststroke rehabilitation utilization and functional outcomes. Arch Phys Med Rehabil. 2015;96:84-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JPC852507_Supplemental_Material_CLN for Disability in Community-Dwelling Older Adults: Exploring the Role of Stroke and Dementia by Brian J. Stamm, James F. Burke, Chun Chieh Lin, Rory J. Price and Lesli E Skolarus in Journal of Primary Care & Community Health