Abstract
Intrahepatic congenital portosystemic venous shunts are rare vascular anomalies. We report a unique case of a neonate with an intrahepatic congenital portosystemic venous shunt with concurrent congenital duodenal web. Such association has not been previously reported to our knowledge. Interestingly, the shunt became apparent on the seventh day, after a delayed start of oral feeding due to the neonate’s recovery from the duodenal web surgery. The shunt was small and the clinical symptomatology mild. No direct treatment was required. The laboratory and the ultrasound follow-up of the child noted a spontaneous resolution of the shunt by the age of six months.
Keywords: Intrahepatic congenital portosystemic venous shunt, spontaneous portosystemic shunt resolution, duodenal web, association of congenital anomalies, Doppler ultrasound
Introduction
Congenital portosystemic venous shunts (CPSVS) are rare vascular anomalies that occur secondary to abnormal development or involution of fetal vasculature (1). The overall incidence of CPSVS is estimated to be in the range of 1:30,000 and 1:50,000 (2). CPSVS are associated with multiple congenital abnormalities, most commonly involving the cardiovascular system; however, their association with biliary, urogenital, and gastrointestinal anomalies as well as some syndromes has also been described (2–6).
CPSVS are divided into intra- and extrahepatic types. In both types, the portosystemic shunt allows for the blood from the intestine to reach the systemic circulation, bypassing the liver and resulting in a variety of symptoms and complications: clinical encephalopathy that leads to neurodevelopmental delay; hepatopulmonary syndrome; portopulmonary hypertension; and regenerative liver nodules (6, 7). The clinical symptomatology of the two types is similar; however, the differentiation between the types is important because their management differs. All extrahepatic type shunts require treatment, which is occlusion of the shunt or liver transplantation, depending on the subtype. Treatment for the intrahepatic type shunts is required depending on the symptomatology (8). Additionally, it is now known that intrahepatic CPSVS that are diagnosed prenatally or during early infancy do not necessarily require treatment as many will spontaneously close by the age of one year (9).
Upon encountering a child who has suspected CPSVS, a comprehensive work-up comprising biochemical and radiological tests is invariably required to establish the diagnosis and delineate the exact shunt anatomy (8).
We present a case of a newborn with an intrahepatic CPSVS as well as a coexistent congenital duodenal web with an interesting clinical course. The portosystemic shunt became clinically and radiologically apparent on the seventh day, after oral feeding was introduced with delay due to the neonate’s recovery from the surgical correction of the duodenal web.
Case report
In the 32nd week of gestation, prenatal ultrasound (US) of the fetus showed signs of intrauterine growth restriction with suspected duodenal obstruction. In the 39th week of gestation, the labor was induced due to oligohydramnion. The birth weight of the male patient was 1980 g; the Apgar score after 1 and 5 min was 9/9.
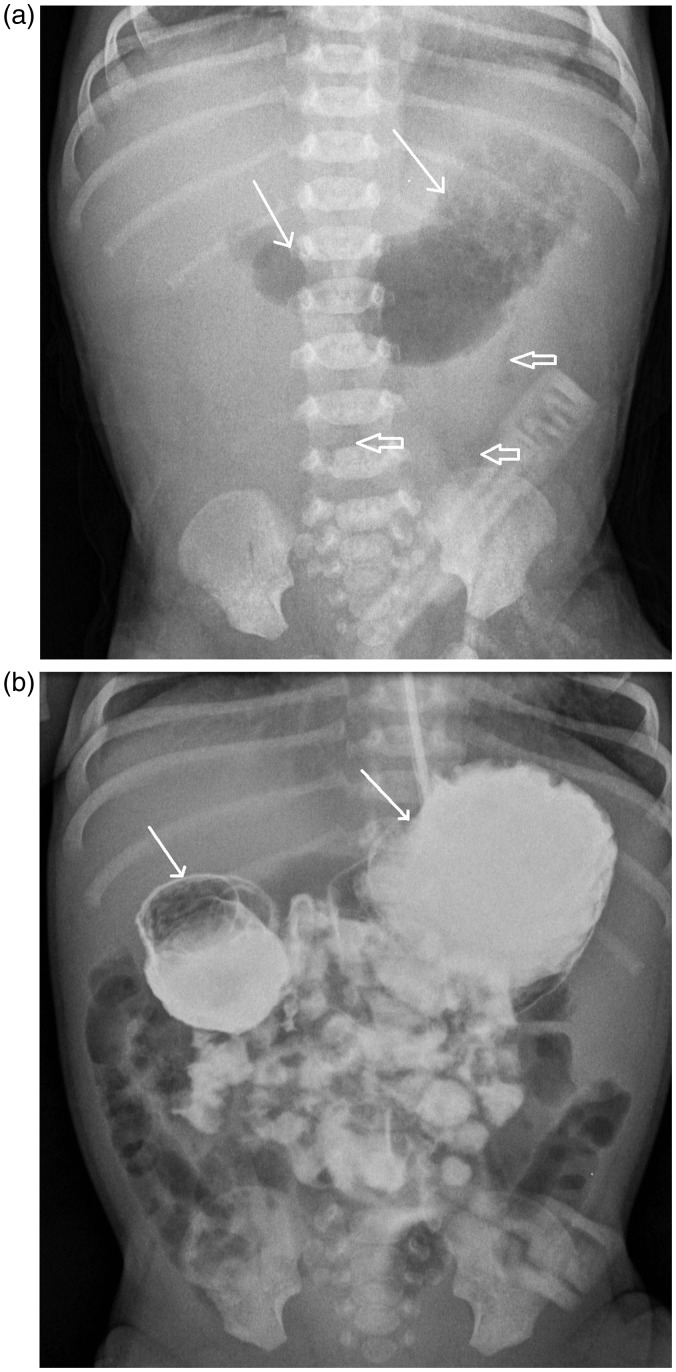
The first chest radiograph, performed 1 h after birth, showed a dilated stomach and the proximal part of the duodenum (“double bubble”) and only minimal gas distally within the bowels (Fig. 1a). Abdominal US also showed a dilated stomach and a proximal duodenum; no other pathologic findings in the abdomen were found. The patient did not tolerate oral feeding; vomiting happened after <10 mL of the milk intake. Duodenal web was suspected. A barium contrast study was performed and showed signs of relative obstruction at the level of the duodenum (Fig. 1b).
Fig. 1.
(a) Chest radiograph, performed 1 h after the birth, shows a dilated stomach and the proximal part of the duodenum (white arrows) and only minimal gas distally within the bowels (void arrows). (b) Barium contrast study shows a dilated stomach and the proximal part of the duodenum with slow emptying and some contrast present in the distal parts of the bowel. Both studies are suggestive of a relative obstruction at the level of the duodenum – a duodenal web.
The child had surgery, which confirmed the duodenal web and corrected the anomaly. Following the surgery, the boy developed sepsis and required antibiotic and inotropic support. Abdominal US, performed at that time, showed normal results. The condition of the patient improved and six days after the surgery, oral feeding was reinitiated. After the start of oral feeding, signs of cholestasis appeared: the skin and the whites of the eyes looked yellow; the urine was dark; and the stools were light-colored. Laboratory exams showed direct hyperbilirubinemia (226 µmol/L; reference value: 0–5 µmol/L); mild ammoniemia (47 µmol/L; reference value: 9–33 µmol/L); and secondary galactosuria (1.08 mmol/L; reference value: 0–0.83 mmol/L). The patient received phototherapy, which was only partially successful in lowering bilirubin levels (173 µmol/L) and direct hyperbilirubinemia further persisted. Numerous laboratory studies were performed to test for the presence of various hepatic, hematologic, metabolic, enzymic, or infectious diseases. Gestational alloimmune liver disease was suspected and an exchange transfusion was performed.
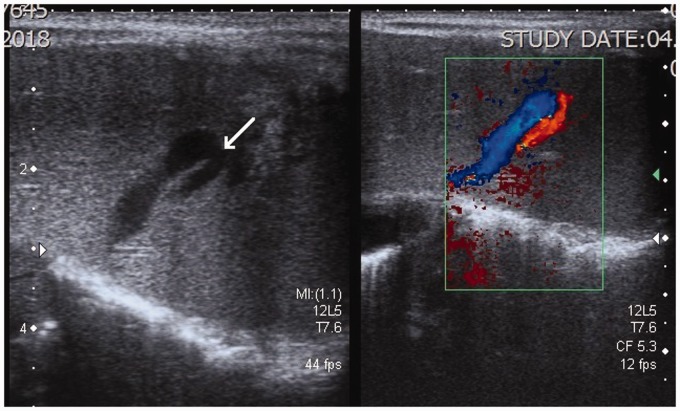
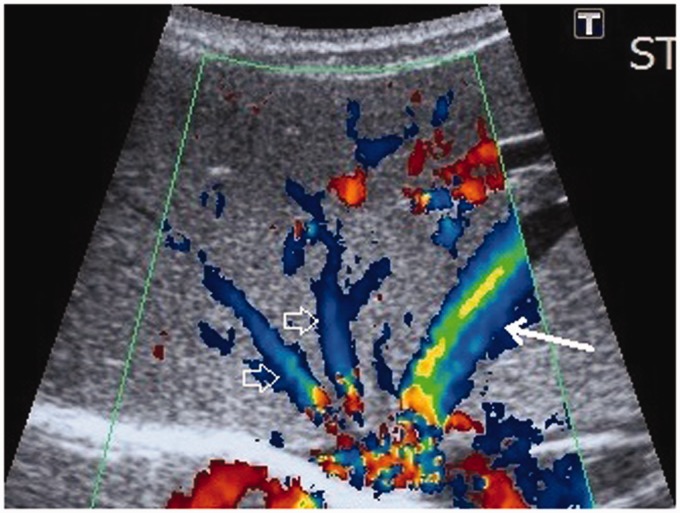
Abdominal US was also repeated. The repeated US showed that there were two strong branches of the left portal vein which communicated directly to the branches of the left hepatic vein at the periphery of the left liver lobe – the intrahepatic portosystemic venous shunts (Fig. 2). The left hepatic vein was dilated and double in diameter in comparison to other two hepatic veins (Fig. 3). Color and pulsed Doppler examinations were performed and the measurements of the blood flow were taken. It was calculated that around 30% of the portal blood flow was bypassing the liver through the shunts. Magnetic resonance imaging (MRI) was also performed, as per the parents’ decision without anesthesia and contrast media application. The intrahepatic portosystemic shunts, which were located peripherally within the left liver lobe, were not visualized because of the strong breathing and pulsation artifacts. The MRI, however, did confirm that the left hepatic vein and the left portal vein were wider. The final diagnosis of a small intrahepatic CVPS was accepted by the clinicians and the patient was discharged home at the age of one month. The US at discharge showed no changes with the shunts.
Fig. 2.
US image of the left liver lobe with a high-frequency linear array transducer. Unusually large veins are shown in the periphery of the liver. The color Doppler examination shows the veins belonging to the portal (red) and hepatic venous (blue) systems. The direct communication between the veins, the portosystemic venous shunt, is marked with a white arrow.
Fig. 3.
US image of the liver with a convex array transducer with color Doppler. The image shows the enlarged left hepatic vein (white arrow) in comparison to the right and middle hepatic veins (void arrows).
At the ages of two and three months, direct hyperbilirubinemia (168 and 133 µmol/L) and mild ammoniemia (66 and 59 µmol/L) were still present in the laboratory tests. Abdominal US was not performed. At the age of six months, the laboratory results showed no more signs of hyperbilirubinemia (5 µmol/L) or ammoniemia (16 µmol/L). Abdominal US was again performed and revealed normal vascular anatomy of the liver; no signs of shunts within the liver were present. The control Doppler examination also yielded normal results.
Discussion
We presented a unique case of a neonate with an intrahepatic CPSVS with concurrent congenital duodenal web that had an interesting clinical course. Intrahepatic CPSVS are rare; it is even more rare for them to be discovered in a newborn. During the literature review, we found only 26 such cases (9–20). In our case, the intrahepatic CPSVS was associated with a congenital duodenal web. To the best of our knowledge, the association between gastrointestinal congenital anomalies and the intrahepatic type of CPSVS has not been previously reported. All the previously reported associations of congenital gastrointestinal anomalies with CPSVS were of the extrahepatic type, which is more commonly reported in association with other congenital anomalies or syndromes (8). The reason for this could be a minor or complete lack of clinical symptomatology of small intrahepatic CPSVS and their spontaneous resolution within the first year of life. Thus, the affected neonates often do not require detailed imaging diagnostics and consequently, the shunts are very rarely diagnosed and reported.
What made the clinical course in our case interesting was that the shunt became clinically and radiologically apparent only after the normal oral feeding was introduced to the child on day 7. The oral feeding within the first week of life was not possible due to the congenital duodenal web and the neonate’s recovery time after the surgery. During the first week, the child received three abdominal US that did not show the shunt within the liver. One of the US examinations was performed by the same radiologist who later diagnosed the shunt; therefore, it is most likely that the shunt veins indeed enlarged in diameter with time, most probably after the start of oral feeding. The effect of feeding on the hemodynamics of the splanchnic and portal vasculature has been previously researched and reported (21, 22).
The likelihood of clinical symptoms in a child with CPSVS is proportional to the shunt size (19). The shunts with the ratio < 30% of portal blood flow bypassing the liver are often asymptomatic. In cases with mild clinical symptomatology, only a laboratory and a US follow-up are recommended because it is known that intrahepatic shunts, diagnosed during early infancy, often spontaneously close by the age of one year (9). In our case, because of the relatively low shunt ratio, mild symptomatology, and exclusion of other potential diseases with similar symptomatology, the patient was discharged from the hospital at the age of one month, with regular follow-up appointments required. At the age of six months, the laboratory results and the US of the liver were normal – a sign of spontaneous resolution of the intrahepatic CPSVS. The finding of a spontaneous resolution of the shunt within the first six months of life has also been reported in 19 of the other 26 documented cases of intrahepatic CPSVS in neonates. In four children, the shunt remained open after the age of one year; in two children, the shunt was closed by an interventional procedure before the age of six months; and in the case of one child, the outcome at six months was not reported (9–20).
In conclusion, we described a unique case of a newborn with an intrahepatic CPSVS in association with a duodenal web. The manifestation of the portosystemic shunt became clinically and radiologically apparent with a delay, after the start of oral feeding on day 7. This delay shows the influence of feeding on physiology of splanchnic and portal vasculature. No specific treatment for the shunt was required; it spontaneously resolved by the age of six months.
Declaration of conflicting interests
The authors declare no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kim MJ, Ko JS, Seo JK, et al. Clinical features of congenital portosystemic shunt in children. Eur J Pediatr 2012; 171:395–400. [DOI] [PubMed] [Google Scholar]
- 2.Bernard O, Franchi-Abella S, Branchereau S, et al. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis 2012; 32:273–287. [DOI] [PubMed] [Google Scholar]
- 3.Guérin F, Blanc T, Gauthier F, et al. Congenital portosystemic vascular malformations. Semin Pediatr Surg 2012; 21:233–244. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Yoshida E, Sakanishi Y, et al. Congenital multiple intrahepatic portosystemic shunt: an autopsy case. Int J Clin Exp Pathol 2013; 7:425–431. [PMC free article] [PubMed] [Google Scholar]
- 5.Lautz TB, Tantemsapya N, Rowel E, et al. Management and classification of type II congenital portosystemic shunts. J Pediatr Gastroenterol Nutr 2011; 46:308–314. [DOI] [PubMed] [Google Scholar]
- 6.Sokollik Cm Bandsma RH, Gana JC, et al. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr 2013; 56:675–681. [DOI] [PubMed] [Google Scholar]
- 7.Grimaldi C, Monti L, Falappa P, et al. Congenital intrahepatic portohepatic shunt managed by interventional radiologic occlusion: a case report and literature review. J Pediatr Surg 2012; 47:e27–e31. [DOI] [PubMed] [Google Scholar]
- 8.Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr 2018; 177:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paganelli M, Lipsich JE, Sciveres M, et al. Predisposing factors for spontaneous closure of congenital portosystemic shunts. J Pediatr 2015; 167:931–935. [DOI] [PubMed] [Google Scholar]
- 10.Valls E, Ceres L, Urbaneja A, et al. Color Doppler sonography in the diagnosis of neonatal intrahepatic portosystemic shunts. J Clin Ultrasound 2000; 28:42–46. [DOI] [PubMed] [Google Scholar]
- 11.Santamaria G, Pruma X, Serres X, et al. Congenital intrahepatic portosystemic shunt: sonographic and magnetic resonance imaging. Eur Radiol 1996; 6:76–78. [DOI] [PubMed] [Google Scholar]
- 12.Jabra AA, Taylor GA. Ultrasound diagnosis of congenital portosystemic venous shunt. Pediatr Radiol 1991; 21:529–530. [DOI] [PubMed] [Google Scholar]
- 13.Lewis AM, Aquino NM. Congenital portohepatic vein fistula that resolved spontaneously in a neonate. Am J Roentgenol 1992; 159:837–838. [DOI] [PubMed] [Google Scholar]
- 14.Saxena AK, Sodhi KS, Arora J, et al. Congenital intrahepatic portosystemic venous shunt in an infant with Down syndrome. Am J Roentgenol 2004; 183:1783–1784. [DOI] [PubMed] [Google Scholar]
- 15.Han BH, Park SB, Song MJ, et al. Congenital portosystemic shunts: prenatal manifestations with postnatal confirmation and follow-up. J Ultrasound Med 2013; 32:45–52. [DOI] [PubMed] [Google Scholar]
- 16.Delle Chiaie L, Neuberger P, Von Kalle T. Congenital intrahepatic portosystemic shunt: prenatal diagnosis and possible influence on fetal growth. Ultrasound Obstet Gynecol 2008; 32:233–235. [DOI] [PubMed] [Google Scholar]
- 17.Yamagami T, Nakamura T, Tokiwa K, et al. Intrahepatic portosystemic venous shunt associated with biliary atresia: case report. Pediatr Radiol 2000; 30:489–491. [DOI] [PubMed] [Google Scholar]
- 18.Gitzelmann R, Forster I, Willi UV. Hypergalactosaemia in a newborn:self-limiting intrahepatic portosystemic venous shunt. Eur J Pediatr 1997; 156:719–722. [DOI] [PubMed] [Google Scholar]
- 19.Yoon HK, Choo SW, Do YS, et al. Congenital intrahepatic portosystemic shunts in the neonate: coil embolization via the umbilical vein. J Vasc Interv Radiol 1998; 9:509–511. [DOI] [PubMed] [Google Scholar]
- 20.Kim IO, Cheon JE, Kim WS, et al. Congenital intrahepatic portohepatic venous shunt: treatment with coil embolization. Pediatr Radiol 2000; 30:336–338. [DOI] [PubMed] [Google Scholar]
- 21.Kao SCS, Bell EF, Brown BP, et al. Duplex Doppler sonography of changes in portal vein flow in healthy term newborn infants after feeding. J Ultrasound Med 1996; 15:121–125. [DOI] [PubMed] [Google Scholar]
- 22.Lane AJP, Coombs RC, Evans DH, et al. Effect of feed interval and feed type on splanchnic haemodynamics. Arch Dis Child Fetal Neonatal Ed 1998; 79:F49–F53. [DOI] [PMC free article] [PubMed] [Google Scholar]