Abstract
Objective
Antibodies against posttranslationally modified proteins are a hallmark of rheumatoid arthritis (RA), but the emergence and pathogenicity of these autoantibodies are still incompletely understood. The aim of this study was to analyze the antigen specificities and mutation patterns of monoclonal antibodies (mAb) derived from RA synovial plasma cells and address the question of antigen cross‐reactivity.
Methods
IgG‐secreting cells were isolated from RA synovial fluid, and the variable regions of the immunoglobulins were sequenced (n = 182) and expressed in full‐length mAb (n = 93) and also as germline‐reverted versions. The patterns of reactivity with 53,019 citrullinated peptides and 49,211 carbamylated peptides and the potential of the mAb to promote osteoclastogenesis were investigated.
Results
Four unrelated anti–citrullinated protein autoantibodies (ACPAs), of which one was clonally expanded, were identified and found to be highly somatically mutated in the synovial fluid of a patient with RA. The ACPAs recognized >3,000 unique peptides modified by either citrullination or carbamylation. This highly multireactive autoantibody feature was replicated for Ig sequences derived from B cells from the peripheral blood of other RA patients. The plasma cell–derived mAb were found to target distinct amino acid motifs and partially overlapping protein targets. They also conveyed different effector functions as revealed in an osteoclast activation assay.
Conclusion
These findings suggest that the high level of cross‐reactivity among RA autoreactive B cells is the result of different antigen encounters, possibly at different sites and at different time points. This is consistent with the notion that RA is initiated in one context, such as in the mucosal organs, and thereafter targets other sites, such as the joints.
Introduction
Antibodies against citrullinated antigens (anti–citrullinated protein/peptide antibodies [ACPAs]), which were first described in 1998 1, 2, constitute a hallmark of the subset of patients with rheumatoid arthritis (RA) displaying associations with distinct major histocompatibility complex class II genes 3, 4 and with predominantly erosive disease 5, 6. The presence of such antibodies is also part of the American College of Rheumatology/European League Against Rheumatism 2010 classification criteria for RA 7. These antibodies generally develop before the onset of joint inflammation 8 in RA patients. Importantly, in vitro and in vivo models have shown that ACPAs induce phenotypes consistent with symptoms associated with RA, such as bone loss and joint pain 9, 10, 11.
ACPAs also represent a class of autoantibodies to posttranslationally modified (PTM) antigens, for which the actual targets of the antibodies remain incompletely understood. Thus, a number of citrullinated (Cit) peptide antigens recognized by ACPAs have been identified, including citrullinated fibrinogen 12, vimentin 13, α‐enolase peptide (CEP‐1) 14, type II collagen 15, tenascin‐C 16, and histones 17. In addition, over recent years, antibodies recognizing other protein modifications, such as carbamylation (referred to as CarP) and acetylation of amino acids, have been found to be associated with RA 18, 19. These observations have raised a number of new questions concerning the generation, specificity, and function of this group of autoantibodies in their interactions with PTM antigens; this group is sometimes referred to as anti–modified protein antibodies, or AMPAs.
In the present study, we generated monoclonal ACPAs from single plasma cells obtained from an inflamed joint of an anti–cyclic citrullinated peptide (anti‐CCP)–positive RA patient. These autoantibodies were characterized in detail to assess their genetic features, reactivity with large numbers of citrullinated and carbamylated peptides, and critical functional properties.
Materials and Methods
Cell isolation, assays, and cultures
Plasma cells were obtained from the synovial fluid of a patient with anti‐CCP–positive RA, and antibody‐secreting cells were isolated from synovial fluid mononuclear cells using the fluorescent foci method. In addition, single citrulline‐specific B cells were sorted by flow cytometry from the peripheral blood of other RA patients, using an antigen‐tetramer system. Further details on the synovial fluid and serum samples obtained from RA patients and the methods used for plasma cell isolation, blood‐derived memory B cell tetramer isolation, Ig gene sequence analysis, cloning of Ig genes, generation of germline‐reverted antibodies, expression and purification of monoclonal antibodies (mAb), surface plasmon resonance (SPR) assay, ACPA peptide array, PTM peptide enzyme‐linked immunosorbent assay (ELISA), in solution citrullination ELISA, immunoprecipitation and osteoclast cultures, and in vitro bone erosion assay are provided in Supplementary Materials and Methods (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract).
Peptide ELISA
Peptide ELISAs were performed as previously described 20, with some minor modifications. The ELISAs assessed binding to both citrulline‐containing and arginine (Arg)–containing peptides, including filaggrin, α‐enolase, vimentin, fibrinogen, and histones H414–34, H431–50, and H31–30. All peptides assessed by ELISA are described in further detail in Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract. The mAb were added to the wells at a concentration of 5 μg/ml, which was diluted by a factor of 2 down to 0.15 ng/ml. All reactive samples were analyzed in at least 3 independent experiments.
Full‐length protein ELISA
For the full‐length protein ELISA, 10 μg/ml of fibrinogen (isolated from human plasma [Sigma]), recombinant vimentin (InVent), and histone H4 (in‐house produced) were diluted in phosphate buffered saline (PBS) and coated on Nunc Maxisorp 96‐well plates. The plates were blocked with 5% milk powder in PBS and thereafter incubated for 3 hours at 37°C with citrullination buffer (50 mM Tris, 10 mM CaCl2, 1 mM dithiothreitol, 150 mU/ml human peptidylarginine deiminase 2 [hPAD2; Modiquest]) or buffer control, followed by incubation with ACPAs at a concentration of 5 μg/ml, which was diluted by a factor of 2 down to 0.15 ng/ml. Sequential incubation was carried out with secondary Fc‐specific, horseradish peroxidase (HRP)–conjugated anti‐human IgG (Sigma) or biotin‐conjugated human IgG1‐Fc (Invitrogen) plus HRP‐conjugated streptavidin (Dako). This was followed by development with SeramunBlau fast TMB (Seramun Diagnostica), and the results were read with Spectra Fluor (Tecan).
Extracellular matrix peptide microarray
Peptides (16 amino acids in length) that covered all arginine and lysine sites of 1,610 extracellular matrix proteins and RA‐related proteins 21, 22, 23 were synthesized in situ (Roche NimbleGen) as previously described 24. Citrulline (arginine) and homocitrulline (lysine) variants were synthesized, resulting in 53,019 citrullinated peptides and 49,211 carbamylated peptides, along with cognate unmodified peptides (n = 70,535), yielding a total of 172,765 unique peptides. The mAb were diluted to a concentration of 1 μg/ml (except mAb 1325:07E07, which was diluted to 0.55 μg/ml), while synovial fluid and serum samples were diluted 1/100.
Scanning for citrulline and homocitrulline reactivity with each mAb was performed at a resolution of 2 μm, using a NimbleGen MS200 Scanner (Roche NimbleGen). The median fluorescence intensities were calculated based on a peptide signal intensity variation (spot size) of 25 pixels. The cutoff value for positive signals was determined as 5 times the fluorescence intensity of the 98th percentile of values for a set of non‐ACPA mAb.
Western blotting
For Western blot analyses, full‐length proteins (same as those assessed by the full‐length protein ELISA) were solubilized in 8M urea at 1 mg/ml, and 10 μg was electrophoresed on 12.5% sodium dodecyl sulfate–polyacrylamide electrophoresis gels, followed by blotting to a nitrocellulose membrane (GE Healthcare). Thereafter, the blots were incubated overnight at 37°C in citrullination buffer with 150 mU/ml hPAD2 (Modiquest) or with buffer control, followed by incubation with ACPAs at 5 μg/ml and sequential incubation with the same secondary antibody as that used for the full‐length protein ELISA. Visualization was performed using Amersham Hyperfilm.
Sequence logo visualization of the consensus peptide sequences
The 4 flanking amino acids from each posttranslational residue were analyzed using the WebLogo application ( http://weblogo.threeplusone.com/), which allowed us to generate the consensus sequences of the surrounding amino acids.
Statistical analysis
Differences in the number of mutations and third complementarity‐determining region (CDR3) characteristics between ACPAs and non‐ACPAs were determined by Mann‐Whitney nonparametric test. For in vitro experiments, statistically significant differences were calculated using one‐way Kruskal‐Wallis test followed by Dunn's test for multiple comparisons. P values less than or equal to 0.05 were considered significant. GraphPad Prism (version 7.0c) was utilized to calculate and visualize the statistical data.
Results
Production by RA synovial fluid plasma cells of antibodies that recognize multiple PTM peptides and proteins
Plasma cells isolated from cryopreserved synovial fluid cells from a patient with RA exhibited spontaneous secretion of IgG, as identified by the fluorescent foci method 25. Sequences from paired IgG heavy‐ and light‐chain variable regions from 182 plasma cells were generated, and of these, 93 were expressed as recombinant mAb at concentrations of >1 μg/ml; these 93 recombinant mAb were selected for further investigation.
Screening by peptide ELISA revealed that 4 of these mAb (1325:01B09, 1325:04C03, 1325:05C06, and 1325:07E07) showed citrulline reactivity (to peptides Cit‐Vim60–75, Cit‐Fibβ36–52, and CEP‐15–21), whereas none of these antibodies reacted with the respective arginine‐containing peptides. When these 4 mAb were further investigated utilizing an ACPA peptide microarray 26, the ACPAs all reacted against several citrulline‐containing peptides, but each showed a different reactivity pattern (Figure 1A).
Figure 1.
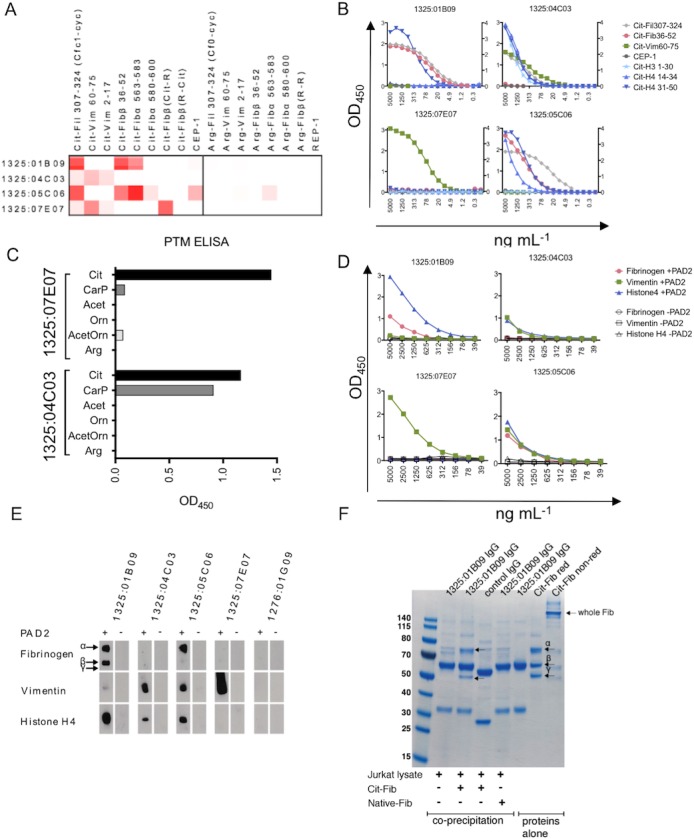
Reactivity pattern of monoclonal antibodies (mAb) against citrullinated proteins/peptides (ACPAs). A, Heatmaps depict ACPA mAb reactivity (at a concentration of 5 μg/ml) with rheumatoid arthritis–associated citrulline (Cit)– and arginine (Arg)–containing peptides. Shades of red indicate increased signal. B, Titration enzyme‐linked immunosorbent assay (ELISA) curves show the dilution (1:2) in 4–14 steps of each mAb (starting at 5 μg/ml) to citrullinated peptides. C, Binding of mAb (at 5 μg/ml) to peptides with various posttranslational modifications (PTMs) was analyzed by ELISA, utilizing a vimentin (Vim)–derived peptide with modifications introduced in position 7 (sequence GRVYATXSSAVR‐OH, with modification denoted by X). D and E, ELISA (D) and Western blotting (E) were used to assess mAb reactivity with full‐length proteins. Antigens (fibrinogen [Fib], vimentin, and histone H4) were treated with or without human peptidylarginine deiminase 2 (PAD2) enzyme to generate citrullination. A concentration of 10 μg of protein was used for Western blots, and 10 μg/ml of antigen for ELISAs. Antibodies were added at 1 μg/ml to detect binding. Arrows in E indicate the size of the α‐, β‐, and γ‐chains of fibrinogen. F, Immunoprecipitation of PAD4‐citrullinated full‐length fibrinogen (5 μg) from spiked Jurkat T cell line lysates with mAb 1325:01B09 (3 μg) was assessed. Stained sodium dodecyl sulfate–polyacrylamide electrophoresis gels of coprecipitated captured proteins or purified proteins alone are shown. Purified citrullinated fibrinogen is also shown as reduced (red) or nonreduced (non‐red) to verify intact protein. Arrows indicate detected fibrinogen chains. Cit‐Fil = citrullinated filaggrin; CEP‐1 = citrulline‐containing α‐enolase peptide; REP = arginine‐containing α‐enolase peptide; CarP = homocitrulline/carbamylated; Acet = acetylated lysine; Orn = unmodified ornithine; AcetOrn = acetylated ornithine; Arg = unmodified arginine.
The results of the peptide microarray were verified and extended by ELISA for the RA candidate autoantigens Cit‐Vim60–75, Cit‐Fibβ36–52, Cit‐Fil307–324, Cit‐H414–34, Cit‐H431–50, Cit‐H31–30, and CEP‐15–21, demonstrating that some of the citrulline peptides were detected at an mAb concentration as low as 5–10 ng/ml (Figure 1B and Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract). The equilibrium constants (KDs) for binding to selected citrulline peptides were determined by SPR assay (of note, mAb 1325:07E07 was not analyzed by SPR assay because of the limited amounts obtained in our expression system). The steady‐state affinities between the ACPAs and the peptide antigens were consistently low, with all KD values in the μM range (Table 1 and Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract), even for antibody–peptide interactions that were positive by ELISA at antibody concentrations in the ng/ml range (Figure 1B).
Table 1.
Characteristics of the human anti–citrullinated protein mAb generated from synovial fluid plasma cells from a rheumatoid arthritis patient*
| mAb 1325:01B09 | mAb 1325:04C03 | mAb 1325:05C06 | mAb 1325:07E07 | |
|---|---|---|---|---|
| Distribution, no. isolated cells/total | 1/182 | 3/182 | 1/182 | 1/182 |
| Genetic profile | ||||
| VH | IGHV4–38 | IGHV1–2 | IGHV4–39 | IGHV4–39 |
| VL/κ | IGLV1–44 | IGKV1–5 | IGLV1–51 | IGLV3–21 |
| Isotype | IgG1 | IgG2 | IgG1 | NA |
| γ‐chain CDR3 | CATDGEGVLFDEW | CARTNFSPFRHW | CAKLGCSGGGCYFDFDYW | CARLDPFDYW |
| Light‐chain CDR3 | CAVWDDDLSGVVI | CQQYNGPSETF | CGTWDSSLSAGLF | CQVYDRKTDHQVF |
| Reactivity profile, μM affinity | ||||
| Cit‐Fil307–324 | 42 | 23 | 1.3 | NA |
| Cit‐Fibβ36–52 | 20 | NB | NB | NA |
| Cit‐Vim60–75 | NA | 25 | NB | NA |
| CEP‐15–21 | NB | NB | 50 | NA |
| Arg‐Fibβ36–52 | NB | NB | NB | NA |
| Arg‐Vim60–75 | NA | NB | NB | NA |
| REP‐15–21 | NB | NB | NB | NA |
Genetic information on the variable (V) regions was generated from Immunogenetics V‐Quest. Affinities were determined from surface plasmon resonance steady‐state affinity measurements with biotinylated peptides immobilized and monoclonal antibodies (mAb) in solution at a maximum concentration of 100 μM (mAb 1325:01B09) or 50 μM (mAb 1325:04C03 and 1325:05C06). Lack of binding was defined as a sensogram showing such low affinity that no equilibrium constant could be determined. NA = not analyzed; CDR3 = third complementarity‐determining region; Cit‐Fil307–324 = citrullinated filaggrin307–324; Cit‐Fibβ36–52 = citrullinated fibrinogen β36–52; NB = no binding; Cit‐Vim60–75 = citrullinated vimentin60–75; CEP‐15–21 = citrullinated α‐enolase peptide5–21; Arg‐Fibβ36–52 = arginine‐containing fibrinogen β36–52; Arg‐Vim60–75 = arginine‐containing vimentin60–75; REP‐15–21 = arginine‐containing α‐enolase peptide5–21.
Ability of monoclonal ACPAs to cross‐react with other PTM antigens
As other PTM peptides, in addition to those modified by citrullination, have been proposed to generate epitopes recognized by autoantibodies in RA 18, 19, the 4 plasma cell–derived ACPAs were also investigated for binding to synthetic peptides, which had additional PTMs based on a common backbone amino acid sequence derived from vimentin. In this analysis, only 1 of the Cit‐Vim–reactive mAb, 1325:04C03, but not mAb 1325:07E07, cross‐reacted with the CarP‐Vim peptide (Figure 1C), whereas the 2 other mAb (1325:01B09 and 1325:05C06) were negative for both the Cit‐Vim and CarP‐Vim peptides (Supplementary Figure 3A, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract). No reactivity toward acetylated peptides or peptides with other modifications was seen.
Cross‐reactivity of monoclonal ACPAs with various citrullinated and carbamylated peptides
To investigate the cross‐reactivity of the monoclonal ACPAs in greater detail, and also to detect reactivity with additional target peptides, a multipeptide array was designed. This custom‐made array consisted of peptides from 1,610 different extracellular matrix and RA‐related proteins 21, 22, 23, i.e., potential targets for autoantibodies. Arginine‐ and lysine‐containing peptides were duplicated with a citrulline respective homocitrulline (the PTM amino acid generated by carbamylation) version, which thus generated an array of 53,019 citrullinated, 49,211 carbamylated, and 70,535 unmodified peptides.
The reactivity patterns of the 4 RA synovial fluid plasma cell–derived monoclonal ACPAs were investigated, and all showed extensive reactivity to citrullinated peptides (Figures 2A–D and Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract). Two of these mAb (1325:01B09 and 1325:05C06) also showed robust signals for carbamylated peptides. The reactivity toward native, unmodified target peptides was consistently low, at <0.06% (Figures 2A–D and Supplementary Table 2). Thus, it may be concluded that the cross‐reactivity of these mAb was exclusively confined to PTM peptides. Overall, only 0.14% of all detected citrulline peptides were shared by all 4 mAb (Figure 2E, left panel). The mAb 1325:04C03 had the most distinct repertoire, with 32% of its reactivity being with uniquely detected citrullinated peptides. The homocitrulline‐reactive mAb 1325:01B09 and 1325:05C06 demonstrated 2.7% shared carbamylated target peptides (Figure 2E, right panel).
Figure 2.
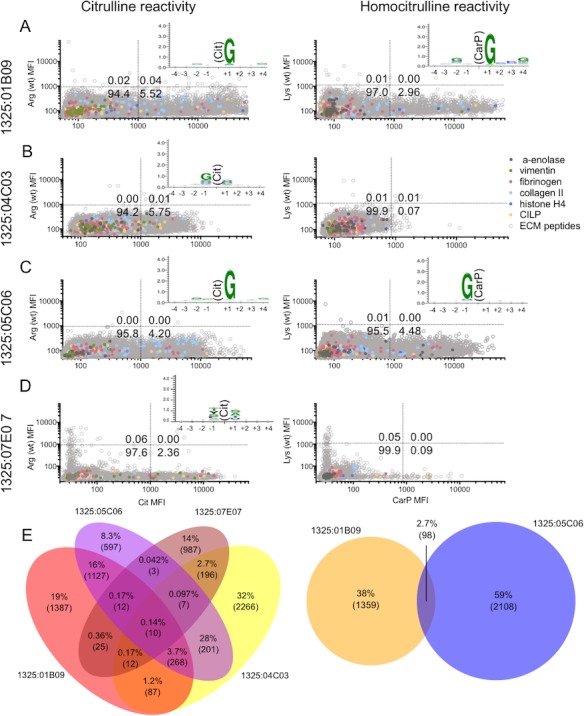
A broad repertoire of posttranslationally modified peptides are detected by mAb against ACPAs, and the mAb have distinct consensus sequence recognition. The mAb 1325:01B09, 1325:04C03, 1325:05C06, and 1325:07E07 were analyzed on an array of 53,019 citrullinated, 49,211 carbamylated, and 70,535 cognate unmodified (wild‐type [wt]) peptides. Peptides (16 amino acids in length) were synthesized on the array, and reactivity with 1 μg/ml antibody (0.55 μg/ml for mAb 1325:07E07) was analyzed. A–D, The mean fluorescence intensity (MFI) of the modified (citrullinated or carbamylated) peptide is shown relative to the MFI of the cognate unmodified peptide. Various colors indicate peptides derived from some previously described rheumatoid arthritis–related antigens. Dotted lines show the cutoff for reactivity based on values for non‐ACPA controls. Numbers in the quadrants are the percentage of detected peptides. Insets, Citrulline (Cit) and homocitrulline (CarP) amino acid positions in the consensus sequence motif are shown, as determined using WebLogo versions 3.5.0. and 3.6.0. E, Overlapping peptides with citrulline recognition by mAb 1325:01B09, 1325:04C03, 1325:05C06, and 1325:07E07 are shown (left), and the Venn diagram shows overlapping carbamylated peptides detected by mAb 1325:01B09 and 1325:05C06 (right). Numbers in parentheses indicate the number of overlapping peptides, and percentages are the fraction of all detected peptides in that section. Arg = arginine; Lys = lysine; CILP = cartilage intermediate layer protein; ECM = extracellular matrix (see Figure 1 for other definitions).
The monoclonal ACPAs together covered 28% of the citrullinated targets and 30% of the carbamylated targets that were recognized by the RA synovial fluid (polyclonal IgG [pAb]) from the same patient (Supplementary Figure 4C, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract).
We next analyzed synovial fluid and serum samples obtained 10 years later from the same RA patient. The patient had untreated chronic RA at both time points. A stable reactivity pattern was seen for PTM peptide reactivities (especially for citrullinated peptides) at both time points, with only a moderate number of new peptides (range 7.1–9.8%) being recognized at the second time point (Supplementary Figures 4D and E). In contrast, a large fraction of carbamylated peptides that were detected at the first time point were not detected at the 10‐year follow‐up (44% in synovial fluid and 58% in sera), implying that these reactivities were not as stable as those toward citrullinated peptides (for which 22% of the citrulline peptides in synovial fluid and 30% of the citrulline peptides in sera were detected at the first time point only). The 200 highest‐binding PTM peptides for each mAb, as well as for the pAb in synovial fluid and sera of the same patient, all contained never before–described peptide targets for the ACPAs (Supplementary Tables 3–8, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract) as well as reactivity against previously known RA targets such as vimentin and fibrinogen.
To confirm our finding that the high multireactivity of the ACPAs was not restricted to a single patient, we next tested 2 mAb that were generated from citrulline‐selected memory B cells isolated from the peripheral blood of 2 other RA patients 27 (Supplementary Figure 5 and Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract). These independently generated ACPAs displayed extensive citrulline multireactivity. The ACPAs from 1 of the patients also detected multiple carbamylated peptides at levels of reactivity comparable to those observed for the synovial plasma cell–derived ACPAs described above.
Overrepresentation of glycine residues in consensus epitopes of monoclonal ACPAs, but with distinct features for each mAb
The consensus sequences recognized by the mAb (Figures 2A–D and Supplementary Figure 5) and the pAb in the sera and synovial fluid samples from the same patient (Supplementary Figures 4A and B) were assessed using a WebLogo analysis. Among the mAb, a clear and shared motif was apparent for mAb 1325:01B09, mAb 1325:05C06, and the comparator ACPAs generated from the peripheral blood of 2 other RA patients, with a dominant glycine residue in position +1 (Figures 2A and C and Supplementary Figure 5). A glycine residue in position +1 was also the dominant consensus sequence for citrulline recognition of pAb in synovial fluid and sera from the same patient (Supplementary Figures 4A and B), suggesting that this is a dominant specificity. The mAb 1325:01B09 also had a similar consensus epitope for CarP recognition (Figure 2A). In contrast, the mAb 1325:05C06 CarP consensus epitope was different, in that it preferred a glycine in the −1 position (Figure 2C), and mAb 37CEPT2C04 had a lysine in the +4 position (Supplementary Figure 5). The mAb 1325:04C03, which only recognized the citrullinated and not the carbamylated peptides, displayed a consensus epitope with glycine being overrepresented in position −1. Lastly, the mAb 1325:07E07, which also only recognized citrullinated peptides, had a different preference, with small amino acids (serine, alanine, and threonine) flanking at the +1 position (Figure 2D).
Recognition by monoclonal ACPAs of multiple citrullinated full‐length protein antigens
Next, in vitro citrullinated candidate full‐length proteins were studied to validate the capacity of the mAb to bind epitopes of the intact autoantigens. Binding of full‐length proteins correlated well with the peptide binding of the ACPAs, according to the results of both ELISA (Figure 1D and Supplementary Figure 6, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract) and Western blotting (Figure 1E). An interesting feature was that mAb 1325:01B09 bound to both the citrullinated α‐chain and citrullinated β‐chain of fibrinogen, whereas mAb 1325:05C06 displayed reactivity restricted to the α‐chain (Figure 1E).
We could also show that the ACPAs had the potential to detect citrullinated protein targets in complex cell lysates (Figure 1F). Consistently, no reactivity toward unmodified proteins was detected.
Synovial ACPAs carrying more mutations, but shorter CDR3 regions in their B cell receptor heavy chain, compared to non‐ACPAs
The 4 RA synovial fluid–derived monoclonal ACPAs, as well as the non‐ACPAs isolated from the same donor (n = 78; 11 non‐ACPA sequences were excluded from the analysis because either the heavy‐ and/or light‐chain sequences were of poor quality), were investigated for mutation frequencies, gene usage, and CDR3 characteristics. The ACPAs consistently displayed a large number of mutations, with a median frequency of nonconservative replacement mutations in the heavy‐chain variable region of 19.5, as compared to 7.0 in the non‐ACPAs (P = 0.0008). Heavy‐chain silent mutations were also more numerous in the ACPAs compared to the non‐ACPAs (median frequency 11 versus 1.5; P < 0.0001) (Figure 3A). To summarize, the total frequency of mutations in the heavy chain was more than 3 times higher in ACPAs as compared to non‐ACPAs.
Figure 3.
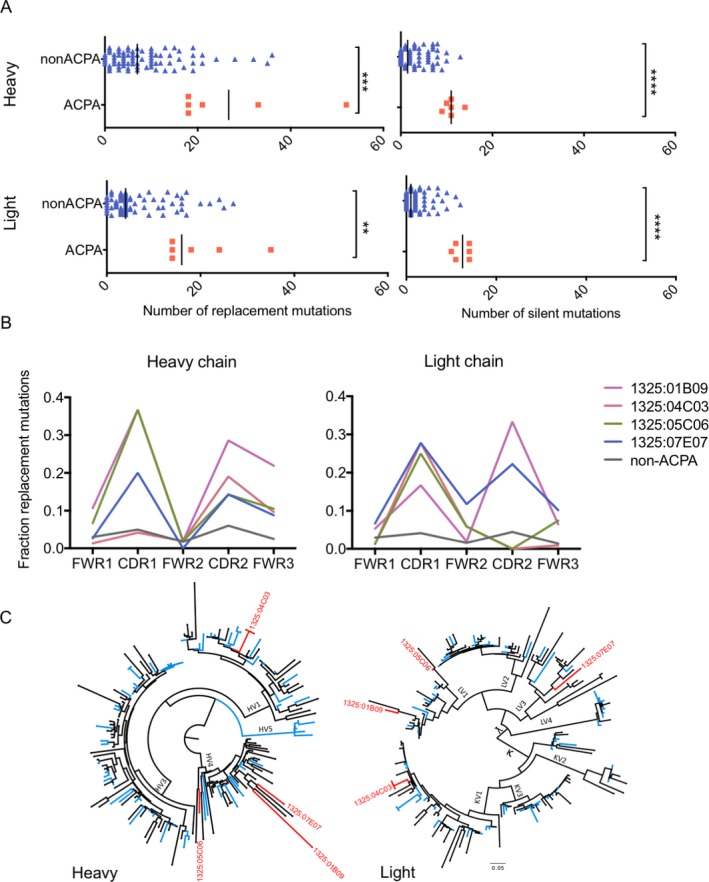
Mutation characteristics and phylogenetic relationship of Ig variable regions coding for ACPAs compared to non‐ACPAs. A, The number of replacement and silent mutations in the heavy‐ and light‐chain variable (V) regions are depicted for ACPAs (n = 6) and non‐ACPAs (n = 78). B, Distributions of mutations within the heavy‐ and light‐chain variable regions are shown for the individual ACPAs compared to non‐ACPAs. All data was extracted from the Immunogenetics V‐Quest database. Differences in the fraction of replacement mutations in the framework regions (FWRs) and complementarity‐determining regions (CDRs) were calculated by Mann‐Whitney nonparametric test. C, Phylogenetic relationships for heavy‐chain and light‐chain sequences are shown. Antibodies binding to citrullinated antigens (ACPAs) are indicated in red, whereas non‐ACPAs are indicated in blue. Antibody sequences with unknown reactivity are labeled in black. The phylogenetic trees were generated utilizing Phylogeny.fr with MUSCLE alignment and visualized by FigTree. ** = P < 0.01; *** = P < 0.001; **** = P < 0.0001. See Figure 1 for other definitions.
The same pattern was observed for the light‐chain variable region, with ACPAs being almost 4 times more mutated as compared to non‐ACPAs. In the light‐chain variable region, the median frequency of replacement mutations was 16.0 in the ACPAs as compared to 4.0 in the non‐ACPAs (P = 0.0010), and the median frequency of light‐chain silent mutations was 12.5 in ACPAs and 1.0 in non‐ACPAs (P < 0.0001) (Figure 3A).
Compared to non‐ACPAs, the mutations in the ACPAs were distributed within the variable region, with enrichment of replacement mutations in the CDRs. However, ACPAs also carried enriched mutations in the framework regions (FWRs), especially in heavy‐chain FWR3 (Figure 3B).
We next assessed the features of the CDR3 in ACPAs compared to non‐ACPAs (Supplementary Table 9, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract). The synovial fluid–derived monoclonal ACPAs consistently had a short heavy‐chain CDR3, consisting of a median of 10 amino acids, while the heavy‐chain CDR3 of non‐ACPAs had a median of 15 amino acids (P = 0.0011). In contrast, the length of the light‐chain CDR3 displayed no differences, with a median of 9.7 amino acids in the non‐ACPAs as compared to 10 amino acids in the ACPAs (P = 0.5615). Negatively charged amino acids were overrepresented in ACPAs as compared to non‐ACPAs in the light‐chain CDR3 (P = 0.0002). No difference between ACPAs and non‐ACPAs could be detected with regard to the number of positive or hydrophobic amino acids.
Presence of both citrulline‐reactive and citrulline‐nonreactive clonal expansions in RA synovial fluid plasma cells
Next, the phylogenetic relationships of the heavy‐chain and light‐chain variable regions were investigated. Three of the ACPAs were found to belong to the VH4 gene family, whereas 1 of the ACPAs was of VH1 origin (mAb 1325:04C03) (Table 1). The variable‐region gene usage was never ancestral for any of the 4 ACPAs (Figure 3C).
The clonality of all 182 isolated Ig clones was investigated using a definition of clonality in which 2 or more single cells generated a clonotype characterized by identical V–(D)–J gene usages and identical CDR3 nucleotide sequences. One of the 4 ACPAs was clonally expanded, i.e., the sequence coding for mAb 1325:04C03 was detected in 3 isolated antibody‐secreting cells, which corresponded to 1.6% (3 of 182) of the isolated plasma cells. The variable regions of the other 3 ACPAs could only be detected in single cells (0.5%) (Table 1). Five additional clonalities were identified within the plasma cells in the non‐ACPA group, and 1 additional clone was found within the unexpressed sequences, i.e., with unknown reactivity. Intriguingly, 1 of the non‐ACPA expanded clones was found in 8 individual cells (4.4% of all isolated cells). In total, 11.4% of the generated IgG sequences were expanded.
Reduced or lost citrulline reactivity in germline‐reverted ACPAs
New sets of recombinant antibodies were constructed from the RA synovial fluid–derived plasma cells, in which the heavy and light chains of the monoclonal ACPAs were reverted to their predicted germline sequences. Two of the antibodies (mAb 1325:01B09 and 1325:05C06) completely lost their reactivity when converted back to germline (G + G in Figures 4A and B). However, the germline mAb 1325:07E07 maintained citrulline reactivity, although at a reduced level, as compared to the fully mutated antibody (Figure 4C). Germline mAb 1325:04C03 did not express functional antibodies in our system, and thus could not be investigated.
Figure 4.
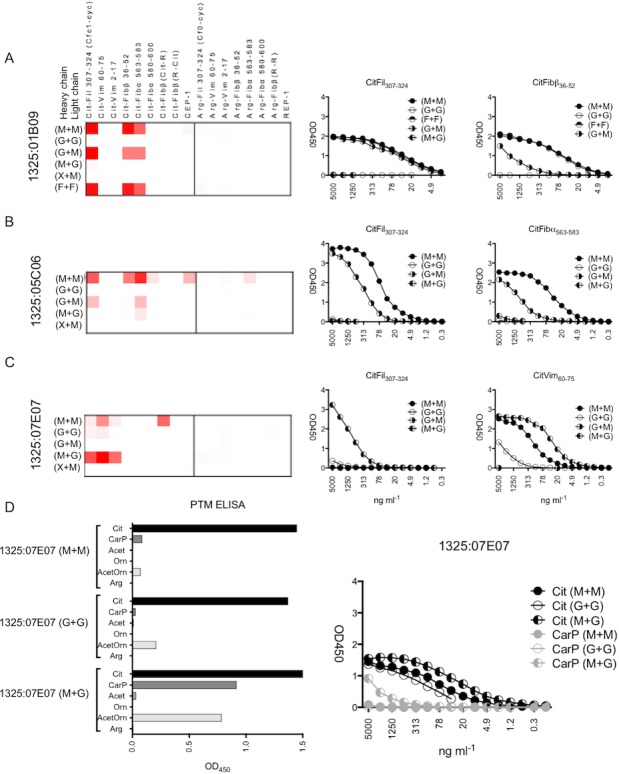
Binding pattern of the mAb with variable regions reverted to germline. A–D, Left, Heatmap summaries show ACPA peptide array antibody binding to the different citrullinated peptides (A–C) or PTMs (D). Right, Titration ELISA dilution curves show mAb (at different dilutions) binding to selected peptides. M + M = fully mutated antibody; G + G = fully germline antibody; M + G = heavy‐chain mutated and light‐chain germline; G + M = heavy‐chain germline and light‐chain mutated; X + M = heavy‐chain unrelated (different VH gene, third complementarity‐determining region length, and other ACPAs) and light‐chain mutated; F + F = framework regions converted to germline in heavy and light chains. In the ACPA peptide array, the mAb were analyzed at 5 μg/ml, and the ELISA titration curves start at an mAb concentration of 5 μg/ml and were diluted one‐half in 12 steps. See Figure 1 for other definitions.
Variations between ACPAs in the contributions of heavy‐ versus light‐chain mutations to antigen recognition
As a further investigation of the molecular basis for citrulline recognition, we produced converted and chain‐exchanged recombinant antibodies with germline‐reverted (G) sequences in either only the heavy chains or only the light chains.
For mAb 1325:01B09, there was a strong dependency on the light‐chain mutations for citrulline recognition. The citrulline reactivity was thus sustained in the variant, where only the heavy chain was converted back to germline, while the mutations were retained in the light chain (G + M in Figure 4A). In contrast, all reactivity diminished in the opposite chain–exchanged version (heavy chain mutated and light chain germline) (M + G in Figure 4A).
Since this mAb displayed many mutations in the FWRs (Figure 3B), an additional construct was produced in which only the FWRs were reverted to germline, with mutations in the CDRs remaining. This construct displayed citrulline reactivity without any loss in sensitivity (F + F in Figure 4A).
A similar pattern of reactivity was found for the chain‐exchanged variant mAb 1325:05C06, with a strong dependence of the light‐chain mutations for its peptide reactivity. The citrulline binding was more profoundly reduced in this variant construct (Figure 4B), implying that the dependency was less absolute.
To further understand the function of the heavy chain of the 2 mAb in which only the mutations in the light chain contributed to citrulline binding, we next exchanged the original heavy chain to unrelated heavy‐chain sequences, and thus addressed the contributions of various VH genes, CDR3 lengths, or other investigated ACPA heavy chains. Interestingly, none of these combinations generated any citrulline binding, indicating that the original heavy‐chain V–D–J rearrangement (even if germline) is essential for the citrulline binding for mAb 1325:01B09 and mAb 1325:05C06 (X + M in Figures 4A and B).
The mAb 1325:07E07 had the interesting feature of retaining some citrulline binding for the germline‐converted mAb. Notably, the heavy chain of germline‐converted mAb 1325:07E07 was more important than the light chain for binding (Figure 3C). The variant with germline light chain, as compared to the fully mutated 1325:07E07, demonstrated stronger citrulline binding for the chain‐exchanged antibody (mAb 1325:07E07 [M + G] versus mAb 1325:07E07 [M + M] in Figure 4C), and also obtained reactivity with the peptides with PTMs CarP and acetylated ornithine (Figure 4D).
For mAb1325:04C03, only the germline heavy‐chain construct, and not constructs with germline light chain, was productive. Based on the reactivity of the expressed mAb, we conclude that hypermutations of the light chain may not contribute significantly to the citrulline binding for this antibody (Supplementary Figure 7, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract).
Differential functional effects of monoclonal ACPAs on osteoclasts
The investigated monoclonal ACPAs had several features in common, such as high mutation frequencies and overlap in reactivities, and yet they also displayed essentially unique patterns of citrulline and homocitrulline reactivity with peptides and/or full‐length proteins. To further understand whether these differences could result in different functions, we tested the 4 antibodies in an in vitro assay for antibody‐dependent effects on osteoclastogenesis and in vitro bone destruction 10.
Of the 4 tested antibodies, mAb 1325:04C03 promoted osteoclastogenesis (P = 0.0049 at a concentration of 10 μg/ml) and increased erosion of an artificial bone surface (P = 0.0005 at a concentration of 10 μg/ml), whereas no such stimulatory effects were seen for the other 3 monoclonal ACPAs (Figure 5). Instead, mAb 1325:05C06 displayed an inhibitory effect on osteoclast‐mediated bone erosion in vitro (P = 0.0221 at a concentration of 1 μg/ml, P = 0.0067 at a concentration of 10 μg/ml), but without a concomitant effect on osteoclast numbers (P = 0.9 at a concentration of 10 μg/ml) (Figure 5). Notably, polyclonal IgG ACPAs from RA patients have been shown to exert effects on osteoclast activation that were similar to those of mAb 1325:04C03 10.
Figure 5.
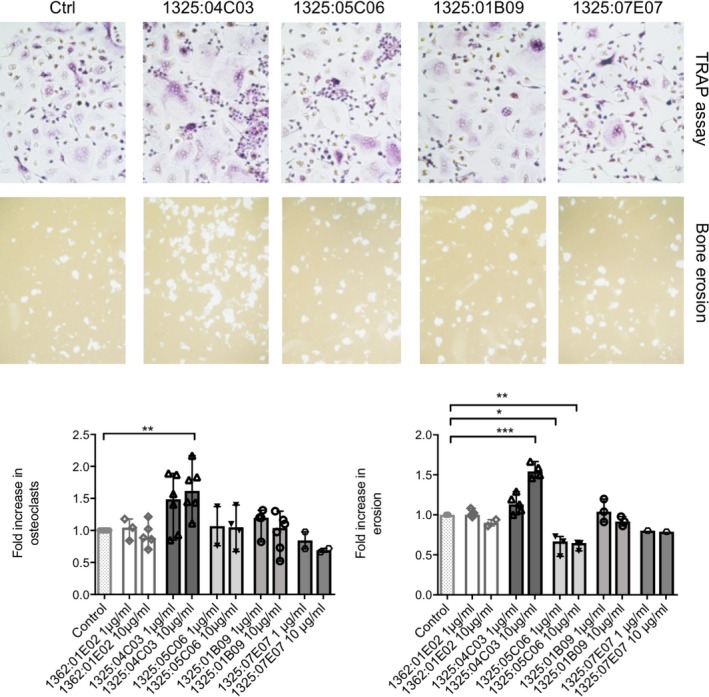
Effects of the 4 ACPA mAb on osteoclast differentiation and bone erosion. Top, Osteoclast differentiation in the presence of the 4 mAb (or control antibody [Ctrl]) was measured using a combination of tartrate‐resistant acid phosphatase (TRAP) staining and >3 nuclei per cell (representative examples shown in upper panels). Effects of the 4 mAb on artificial bone erosion were investigated using a previously described in vitro assay 10 (representative examples shown in lower panels). Bottom, The effects of the 4 ACPA mAb (at 2 different concentrations) on osteoclast numbers and bone erosion in peripheral blood samples from several rheumatoid arthritis donors are summarized, showing the fold increase in osteoclast numbers and bone resorption. Results are the mean ± SEM of 1–6 samples per group. * = P < 0.05; ** = P < 0.01; *** = P < 0.001, by Kruskal‐Wallis test followed by Dunn's test for multiple comparisons. See Figure 1 for other definitions.
Discussion
A major finding from our study of spontaneous production of antibody‐secreting cells from the active RA joint was that the ACPAs are both highly specific and refined for recognition of PTMs, mainly citrullination, while at the same time they display extensive, but still partly unique, cross‐reactivity patterns that are dependent on linear consensus sequences rather than distinct proteins. We also demonstrated that these features are confined neither to cells from inflamed joints nor to disease‐modifying antirheumatic drug–naive disease, as we could replicate the extensive cross‐reactivity also for citrulline‐reactive B cells from the peripheral blood of additional RA patients.
We are intrigued by our finding that the combination of high numbers of somatic mutations and broad cross‐reactivity against thousands of PTM peptides, and at least several PTM proteins, differed from what has been described for most previously investigated autoantibodies associated with organ‐specific and systemic autoimmune diseases 28, 29, 30, 31. For the presently investigated ACPAs, we hypothesize that multiple T cell–dependent selection processes with somatic Ig mutations may happen sequentially, for example, in germinal center–like structures located in mucosal tissues, in lymphoid organs, and in joints. Such germinal center–like structures have previously been described in these sites in RA patients 32, 33, 34, 35, 36. In addition, there are indications that immune responses toward citrullinated antigens may be initiated in mucosal tissues, for example, in the lungs. It will thus be very interesting to further analyze the emergence of somatic mutations at these different sites in order to better understand the mechanisms behind the development of the somatically hypermutated ACPAs described in the inflamed joints of patients with established RA.
Concerning the T cells that may have provided help to the generation of the B cells/plasma cells in this study, we note that some of the ACPA targets (α‐enolase, vimentin, and fibrinogen, as well as cartilage intermediate layer protein) overlap with well‐defined HLA–DRB1*04:01–associated T cell epitopes studied in ACPA+ RA 37, 38. The difference in the underlying germinal center reactions, as compared to classic autoimmune reactions, would be that different PTM proteins in different organs may contribute to a consecutive selection of B cells that ultimately mature into plasma cells in the inflamed joint.
Another striking feature of ACPAs, both on a serologic and monoclonal level, is the recently discovered accumulation of mutations that introduces N‐linked Fab glycosylation sites 39, 40. Indeed, ACPAs (including the ones described herein) have been verified to carry Fab glycosylations in both their heavy‐ and light‐chain variable regions 39, and it is possible that this confers an advantage in the B cell selection process that partly compensates for the relatively low affinities observed.
Although the affinities of our ACPAs were found to be in the μM range, the ELISAs were also effective in mAb concentrations down to the ng/ml range, thus ensuring that we detected antigen recognition and not classic polyreactivity. We also acknowledge that the measured affinities may not properly reflect the binding strength for targeted modified intact proteins in vivo. Furthermore, consistent with this notion, not all peptides in the extracellular matrix array should be regarded as physiologic target antigens for ACPAs, until further validated. Importantly, the autoantigenic peptides/proteins we used in our ELISAs and Western blots all originated from in situ identification of citrullination in serum and synovial fluid samples from RA patients.
The presently described monoclonal ACPAs all showed consistent and clear specific reactivity with citrullinated peptides and intact proteins in several assays in which we used low concentrations of the respective antibodies. This is true also for another set of recently published monoclonal ACPAs 27, from which 2 were also used in the validation experiment of our large peptide array (see Supplementary Figure 5 [http://onlinelibrary.wiley.com/doi/10.1002/art.40699/abstract]). Previously, studies utilizing human monoclonal ACPAs 41, 42, 43, *, including those from our own laboratory, have used single and more limited detection systems and often high concentrations of the antibodies, which may yield false‐positive responses. Such false‐positive signals may stem from specific types of polyreactive antibodies, which are common in systemic lupus erythematosus 44. These may very well be overrepresented in RA as well, and such reactivities, although potentially pathogenetically relevant, are very different from those described in the present report. In the current study, we therefore decided to use several different approaches, including detection of binding to intact proteins as complement to the peptide‐based assays. We thus recommend that optimization of assays for reactivity against PTM peptides/proteins will be necessary for all future studies of ACPAs, and that some previously described antibodies with reported citrulline reactivity may not fulfill the more stringent criteria used in the present investigation. We consider such differences to be a main explanation for the differences in frequency of synovial B cells with citrulline reactivity in our present study as compared to that in our previous report 41.
Concerning the potential pathogenic role of ACPAs, it is interesting to note that 1 of them (mAb 1325:04C03), but not the other 3 multireactive ACPAs, was able to induce osteoclast activation and in vitro bone resorption. In addition, 1 ACPA described by Titcombe et al 27 (which was also 1 of the validation ACPAs [37CEPT2C04] used in our extracellular matrix peptide array) has been demonstrated to enhance joint inflammation in mice receiving a lipopolysaccharide challenge 27. At this point, it is not possible to identify the precise features of these 2 ACPAs that result in these effector functions. Instead, these observations further emphasize the importance of analyzing ACPAs at the monoclonal level, both concerning triggering events and concerning disease‐causing mechanisms. The growing list of reported monoclonal ACPAs 27, 41, 42, 43, 45, 46, 47, 48 represents an important resource for such studies.
The observation that 2 of the predicted germline ACPAs did not recognize the investigated citrulline targets is consistent with the findings in other studies in different disease settings 41, 49, 50, 51, and is consistent with the expected low affinity in germline‐encoded, naive, IgM‐positive B cells before antigen selection. Still, one of the constructs using the germline sequence of the investigated antibodies (germline‐reverted mAb 1325:07E07) recognized some of the citrullinated autoantigens.
Interestingly, some of the ACPA mAb displayed a higher light‐chain somatic hypermutation dependency than heavy‐chain somatic hypermutation dependency. While the antigen‐binding surface of immunoglobulins is predominantly built up by the interface of the light‐chain and heavy‐chain CDR loops, the heavy‐chain CDRs, especially HCDR3, are generally thought to be most critical for the binding in a majority of antibodies. Notably, the results of our chain‐swapping experiments showed that the ancestral heavy‐chain V–D–J rearrangement was essential, even though the binding was independent of heavy‐chain somatic hypermutations in these particular ACPA mAb.
In summary, we have demonstrated how plasma cells from an RA inflamed joint, as well as B cells from the circulation of RA patients, can produce antibodies that, on the one hand, have undergone extensive hypermutation and, on the other hand, react with a massive number of different PTM peptides and proteins with similar, but distinct, recognition amino acid patterns for each of the investigated mAb. We envision that such scenarios may also be valid for other autoimmune diseases in which potentially pathogenic antibodies, which develop over several years, may recognize different targets in different organs. Thus, studies on cross‐reactivity patterns, in particular in the context of PTMs or other protein modifications, may provide us with new leads concerning both triggering and targeting of B cells involved in complex inflammatory diseases like RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Malmström had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design.
Steen, Forsström, Rapecki, Bang, Mueller, Catrina, Grönwall, Skriner, Nilsson, Lightwood, Klareskog, Malmström.
Acquisition of data.
Steen, Forsström, Sahlström, Odowd, Israelsson, Krishnamurthy, Badreh, Mathsson Alm, Compson, Ndlovu, Hansson, Titcombe, Grönwall.
Analysis and interpretation of data.
Steen, Forsström, Sahlström, Israelsson, Krishnamurthy, Mathsson Alm, Compson, Ramsköld, Hansson, Titcombe, Bang, Mueller, Catrina, Grönwall, Skriner, Lightwood, Klareskog, Malmström.
Additional Disclosures
Authors Odowd, Compson, Ndlovu, Rapecki, and Lightwood are employees of UCB Pharma. Author Mathsson Alm is an employee of Thermo Fisher Scientific. Author Bang is an employee of Orgentec Diagnostika.
Supporting information
Acknowledgments
The authors are grateful to Ragnhild Stålesen, Dr. Yvonne Sundström, and Dr. Danika Schepis for technical assistance. We thank Dr. Khaled Amara for the Histone H31–30 peptide and for providing scientific advice. We are grateful to Dr. Jeremie Buratto and Dr. Anders Olsson for assistance with the SPR experiments. Finally, we thank Aase Hensvold for summarizing the medical information on the RA patient.
Supported by the Knut and Alice Wallenberg Foundation, the European Research Council (grant 250167), the Innovative Medicines Initiative–supported BTCure program (grant 115142‐2), the Swedish Association Against Rheumatism, King Gustaf V's 80‐year Foundation, and the Swedish Medical Research Council.
Drs. Klareskog and Malmström contributed equally to this work.
Drs. Rapecki and Lightwood own stock or stock options in UCB Pharma. Dr. Mathsson Alm owns stock or stock options in Thermo Fischer Scientific.
Footnotes
*Reference 41 has been retracted since the time of initial publication of this article.
References
- 1. Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis‐specific autoantibodies. J Clin Invest 1998;101:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Girbal‐Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, et al. The epitopes targeted by the rheumatoid arthritis‐associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol 1999;162:585–94. [PubMed] [Google Scholar]
- 3. Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High‐density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 2012;44:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Padyukov L, Seielstad M, Ong RT, Ding B, Ronnelid J, Seddighzadeh M, et al. A genome‐wide association study suggests contrasting associations in ACPA‐positive versus ACPA‐negative rheumatoid arthritis. Ann Rheum Dis 2011;70:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van der Helm‐van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, et al. Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford) 2007;46:342–9. [DOI] [PubMed] [Google Scholar]
- 7. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 8. Rantapaa‐Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 9. Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 2012;122:1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, et al. Identification of a novel chemokine‐dependent molecular mechanism underlying rheumatoid arthritis‐associated autoantibody‐mediated bone loss. Ann Rheum Dis 2016;75:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wigerblad G, Bas DB, Fernades‐Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine‐dependent mechanism. Ann Rheum Dis 2016;75:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masson‐Bessiere C, Sebbag M, Girbal‐Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis‐specific antifilaggrin autoantibodies are deiminated forms of the α‐ and β‐chains of fibrin. J Immunol 2001;166:4177–84. [DOI] [PubMed] [Google Scholar]
- 13. Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti‐Sa antibodies target citrullinated vimentin. Arthritis Res Ther 2004;6:R142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, et al. Identification of citrullinated α‐enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther 2005;7:R1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burkhardt H, Sehnert B, Bockermann R, Engstrom A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol 2005;35:1643–52. [DOI] [PubMed] [Google Scholar]
- 16. Schwenzer A, Jiang X, Mikuls TR, Payne JB, Sayles HR, Quirke AM, et al. Identification of an immunodominant peptide from citrullinated tenascin‐C as a major target for autoantibodies in rheumatoid arthritis. Ann Rheum Dis 2016;75:1876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khandpur R, Carmona‐Rivera C, Vivekanandan‐Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011;108:17372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juarez M, Bang H, Hammar F, Reimer U, Dyke B, Sahbudin I, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis 2016;75:1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum 2010;62:44–52. [DOI] [PubMed] [Google Scholar]
- 21. Bennike T, Lauridsen K, Olesen M, Andersen V, Birkelund S, Stensballe A. Optimizing the identification of citrullinated peptides by mass spectrometry: utilizing the inability of trypsin to cleave after citrullinated amino acids. J Proteomics Bioinform 2013;6:288–95. [Google Scholar]
- 22. Balakrishnan L, Bhattacharjee M, Ahmad S, Nirujogi RS, Renuse S, Subbannayya Y, et al. Differential proteomic analysis of synovial fluid from rheumatoid arthritis and osteoarthritis patients. Clin Proteomics 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol 2016;49:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forsstrom B, Axnas BB, Stengele KP, Buhler J, Albert TJ, Richmond TA, et al. Proteome‐wide epitope mapping of antibodies using ultra‐dense peptide arrays. Mol Cell Proteomics 2014;13:1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clargo AM, Hudson AR, Ndlovu W, Wootton RJ, Cremin LA, O'Dowd VL, et al. The rapid generation of recombinant functional monoclonal antibodies from individual, antigen‐specific bone marrow‐derived plasma cells isolated using a novel fluorescence‐based method. MAbs 2014;6:143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansson M, Mathsson L, Schlederer T, Israelsson L, Matsson P, Nogueira L, et al. Validation of a multiplex chip‐based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res Ther 2012;14:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Titcombe PJ, Wigerblad G, Sippl N, Zhang N, Shmagel AK, Sahlstrom P, et al. Pathogenic citrulline‐multispecific B cell receptor clades in rheumatoid arthritis. Arthritis Rheumatol 2018;70:1933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Thomson CA, Allan LL, Jackson LM, Olson M, Hercus TR, et al. Characterization of pathogenic human monoclonal autoantibodies against GM–CSF. Proc Natl Acad Sci U S A 2013;110:7832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, et al. Diversity, cellular origin and autoreactivity of antibody‐secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chardes T, Chapal N, Bresson D, Bes C, Giudicelli V, Lefranc MP, et al. The human anti‐thyroid peroxidase autoantibody repertoire in Graves’ and Hashimoto's autoimmune thyroid diseases. Immunogenetics 2002;54:141–57. [DOI] [PubMed] [Google Scholar]
- 31. Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, et al. High abundance of plasma cells secreting transglutaminase 2‐specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 2012;18:441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reynisdottir G, Olsen H, Joshua V, Engstrom M, Forsslund H, Karimi R, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis 2016;75:1722–7. [DOI] [PubMed] [Google Scholar]
- 33. Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti–citrullinated protein antibody–positive rheumatoid arthritis. Arthritis Rheumatol 2014;66:31–9. [DOI] [PubMed] [Google Scholar]
- 34. Rangel‐Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus‐associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006;116:3183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramwadhdoebe TH, Hahnlein J, Maijer KI, van Boven LJ, Gerlag DM, Tak PP, et al. Lymph node biopsy analysis reveals an altered immunoregulatory balance already during the at‐risk phase of autoantibody positive rheumatoid arthritis. Eur J Immunol 2016;46:2812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dennis G Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 2014;16:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, et al. Citrulline‐specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol 2014;66:1712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gerstner C, Dubnovitsky A, Sandin C, Kozhukh G, Uchtenhagen H, James EA, et al. Functional and structural characterization of a novel HLA‐DRB1*04:01‐restricted α‐enolase T cell epitope in rheumatoid arthritis. Front Immunol 2016;7:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lloyd KA, Steen J, Amara K, Titcombe PJ, Israelsson L, Lundstrom SL, et al. Variable domain N‐linked glycosylation and negative surface charge are key features of monoclonal ACPA: implications for B‐cell selection. Eur J Immunol 2018;48:1030–45. [DOI] [PubMed] [Google Scholar]
- 40. Rombouts Y, Willemze A, van Beers JJ, Shi J, Kerkman PF, van Toorn L, et al. Extensive glycosylation of ACPA‐IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis 2016;75:578–85. [DOI] [PubMed] [Google Scholar]
- 41. Amara K, Steen J, Murray F, Morbach H, Fernandez‐Rodriguez BM, Joshua V, et al. Monoclonal IgG antibodies generated from joint‐derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med 2013;210:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Li S, Yu Y, Yue Y, Liao H, Xie W, Thai J, et al. Autoantibodies from single circulating plasmablasts react with citrullinated antigens and porphyromonas gingivalis in rheumatoid arthritis. Arthritis Rheumatol 2016;68:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis 2016;75:1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005;201:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raats JM, Wijnen EM, Pruijn GJ, van den Hoogen FH, van Venrooij WJ. Recombinant human monoclonal autoantibodies specific for citrulline‐containing peptides from phage display libraries derived from patients with rheumatoid arthritis. J Rheumatol 2003;30:1696–711. [PubMed] [Google Scholar]
- 46. Van de Stadt LA, van Schouwenburg PA, Bryde S, Kruithof S, van Schaardenburg D, Hamann D, et al. Monoclonal anti‐citrullinated protein antibodies selected on citrullinated fibrinogen have distinct targets with different cross‐reactivity patterns. Rheumatology (Oxford) 2013;52:631–5. [DOI] [PubMed] [Google Scholar]
- 47. Tan YC, Kongpachith S, Blum LK, Ju CH, Lahey LJ, Lu DR, et al. Barcode‐enabled sequencing of plasmablast antibody repertoires in rheumatoid arthritis. Arthritis Rheumatol 2014;66:2706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsuda R, Ozawa T, Kobayashi E, Hamana H, Taki H, Tobe K, et al. Monoclonal antibody against citrullinated peptides obtained from rheumatoid arthritis patients reacts with numerous citrullinated microbial and food proteins. Arthritis Rheumatol 2015;67:2020–31. [DOI] [PubMed] [Google Scholar]
- 49. Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV‐1 by antibody VRC01. Science 2010;329:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011;333:1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Karner J, et al. AIRE‐deficient patients harbor unique high‐affinity disease‐ameliorating autoantibodies. Cell 2016;166:582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


