Abstract
Background
Fetal growth restriction is associated with higher risks of childhood respiratory morbidity. Fetal blood flow adaptations might contribute to these associations. We examined the associations of fetal umbilical, cerebral, and pulmonary blood flow with wheezing patterns, lung function, and asthma in childhood.
Methods
In a population‐based prospective cohort study among 903 children, we measured fetal umbilical, cerebral, and pulmonary blood flow by pulsed‐wave Doppler at a median gestational age of 30.3 (95% range 28.8‐32.3) weeks. We obtained information about wheezing patterns until the age of 6 years by questionnaires. Lung function was measured by spirometry and information about current asthma was obtained by questionnaire at the age of 10 years.
Results
Results showed a non‐significant relationship between a higher umbilical artery pulsatility index (PI) and umbilical artery PI/cerebral artery PI ratio, indicating fetal blood flow redistribution at the expense of the trunk, with higher risks of early wheezing (OR [95% CI]: 2.07 (0.70‐6.10) and 2.74 (0.60, 12.62) per unit increase, respectively). A higher pulmonary artery time velocity integral, indicating higher pulmonary vascular resistance, was associated with a higher risk of late/persistent wheezing (Z‐score 1.14 [1.01‐1.29]). A higher middle cerebral artery PI was associated with a higher FEV1/FVC (Z‐score [95% CI]: 0.21 [0.01‐0.42]). Results did not materially change after additional adjustment for birth and growth characteristics.
Conclusion
Third‐trimester fetal blood flow patterns might be related to childhood respiratory health. These findings should be considered as hypothesis generating and need further replication.
Keywords: asthma, epidemiology, fetal blood flow, lung function, wheezing
Short abstract

Key Message.
An adverse intrauterine environment may have consequences on respiratory health and disease across the life course. We assessed whether fetal blood flow adaptations are related to lung function development and asthma risk in childhood. Non‐significant tendencies towards associations for fetal blood flow redistribution with higher risk of early wheezing were found, a higher pulmonary vascular resistance was associated with higher risk of late/persistent wheezing. This study is the first that examines these associations. Our findings are important from an etiological and developmental perspective, providing evidence that changes in fetal blood patterns may lead to altered respiratory health.
1. INTRODUCTION
Pregnancy is a critical period for fetal lung development. In late fetal life, the small airways and alveoli are formed.1 An adverse intrauterine environment in this period seems to have persistent effects on respiratory health and disease across the life course.2 Fetal blood flow adaptations are important mechanisms by which the fetus protects the most important organs such as the brain and heart from an adverse fetal environment.3 A preferential fetal blood flow to the brain at the expense of the trunk is characterized by changes in fetal blood flow including a higher umbilical and lower cerebral arterial resistance.4, 5 This redistribution of fetal blood flow may be beneficial for short‐term survival but may lead to a lower delivery of oxygen and nutrients to the trunk, including the lungs and airways.6, 7 A potential consequence of fetal blood flow redistribution is a reduction in number and metabolism of alveolar type II cells, fewer but larger alveoli, and impaired growth and maturation of the airways and lungs.8, 9 Impaired fetal development of the airways and lungs could predispose individuals to a higher risk of lung disease in later life.8 Previous studies reported associations of fetal growth restriction with impaired lung function and respiratory diseases in later life.10, 11 We previously showed that fetal growth restriction and being born small for gestational age were associated with higher airway resistance and lower lung function in childhood.12 Fetal blood flow adaptations related to fetal growth restriction may underlie these associations. Although the effects of fetal umbilical and cerebral blood flow adaptations on fetal and childhood growth are well known, it is unknown whether fetal blood flow adaptations affect childhood respiratory morbidity. Also, the role of a suboptimal fetal pulmonary blood flow on the development of respiratory morbidity is unclear.
Therefore, we examined in a population‐based prospective cohort study among 903 children the associations of fetal umbilical, cerebral, and pulmonary blood flow with wheezing at age 6 years, and lung function and asthma in children aged 10 years. We also explored whether birthweight, gestational age at birth, or childhood growth mediated these associations.
2. METHODS
2.1. Design and study population
This study was embedded in the Generation R Study, a population‐based prospective cohort study from fetal life onward in Rotterdam, the Netherlands.13 The Medical Ethics Committee of the Erasmus MC, University Medical Center, Rotterdam, has approved the study. Written informed consent was obtained from all participants. Detailed assessments of fetal growth and development were conducted in a random subgroup of 1232 Dutch mothers and children born between April 2002 and January 2006.4 The present analyses were performed on 903 children (Figure 1).
Figure 1.
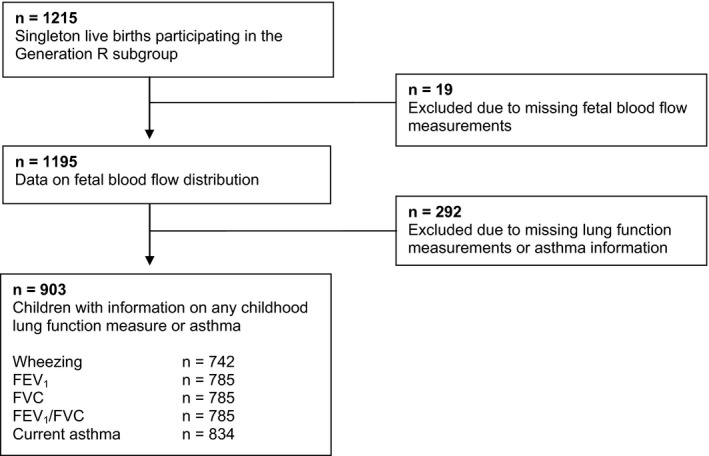
Flowchart of participants included in the analysis
2.2. Third‐trimester fetal blood flow
Fetal blood flow measures were assessed by pulsed‐wave Doppler at a median gestational age of 30.3 (95% range 28.8‐32.3) weeks.
Feto‐placental vascular resistance was evaluated with recorded flow‐velocity waveforms from the umbilical artery. Umbilical artery pulsatility index (PI) was determined in a free‐floating loop of the umbilical cord. A higher umbilical artery PI indicates a higher peripheral vascular resistance.14 Middle cerebral artery Doppler measurements were performed with visualization of the circle of Willis in the fetal brain, and flow‐velocity waveforms were obtained in the proximal part of the cerebral arteries. The middle cerebral artery PI quantifies the redistribution of blood flow, and when lower, in favor of the fetal brain. Reductions in middle cerebral artery PI are valid indicators of the brain‐sparing effect and fetal redistribution.15 An indicator of the “brain‐sparing effect” is a raised ratio between the umbilical artery PI and the cerebral artery PI (U/C ratio).5.
Pulmonary outflow flow‐velocity waveforms from the aorta were recorded from the five‐chamber view and the short‐axis view of the fetal heart just above the semilunar valves. Time velocity integral (TVI) during systole was recorded. A higher pulmonary artery TVI indicated higher pulmonary vascular resistance.16.
Reproducibility of ultrasound measurements was adequate with high intraclass correlation coefficient values (>0.80) with corresponding low variation coefficient values (<10%).4 All ultrasound examinations were performed with an ATL‐Philips model HDI 5000 (Seattle, Washington, USA) equipped with a 5.0‐MHz high‐frequency, curved‐array transducer.
2.3. Childhood lung function and asthma
Information about wheezing was obtained by questionnaires until 6 years. We constructed wheezing patterns based on time of onset and subsequent absence or persistence (“never”; “early” [≤3 years only]; “late” [>3‐6 years] and “persistent wheezing”) in children with information on wheezing for at least two time points.17 Children visited the research center at a median age of 9.7 years (range 8.5‐12.0 years), and we performed spirometry: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC. Spirometry was performed according to the American Thoracic Society and European Respiratory Society recommendations.18 All spirometry variables were converted into sex‐, age‐, height‐, and ethnicity‐adjusted z‐scores.19 Current asthma (no; yes) was defined as physician‐diagnosed asthma ever, with either wheezing or the use of (airway) medication in the past 12 months by questionnaires at age 10 years.
2.4. Covariates
We obtained information on maternal educational level, pre‐pregnancy weight, parity, smoking during pregnancy, folic acid use during pregnancy, and history of asthma or atopy, from questionnaires. Maternal height was measured and pre‐pregnancy BMI was calculated (kg/m2). Estimated fetal weight in the third trimester was calculated.20 Information on gestational hypertensive disorders, child's sex, gestational age at birth, and birthweight was obtained by midwife and hospital registries. At age 10 years, information on ever diagnosis of eczema was obtained by questionnaire. During the research center visit at age 10 years, child height and weight were measured using Standard Operating Procedures. We measured the children without shoes and heavy clothing, and BMI was calculated. Allergic sensitization to the most common inhalant allergens (house dust mite, grass, birch, cat, and dog; ALK‐Abelló BV, Almere, The Netherlands) was determined by a skin prick test using the “scanned‐area method.”21.
2.5. Statistical analysis
First, we performed a non‐response analysis by assessing the differences in characteristics of participants included and not included in the study. Second, we assessed the associations of fetal blood flow with wheezing patterns, lung function, and asthma using multivariate logistic or linear regression models. These models were first adjusted for gestational age at third‐trimester fetal blood flow measurement and estimated fetal weight at third‐trimester fetal blood flow measurement or child sex only (basic model), and secondly, additionally adjusted for maternal educational level, pre‐pregnancy BMI, parity, smoking, folic acid use, and gestational hypertensive disorders (adjusted model). We used generalized estimating equations (GEEs) to examine longitudinal effects of fetal blood flow with the risk of overall wheezing until age 6 years. These models take into account the correlations between repeated measurements within the same subject. An unstructured correlation matrix was used. Third, we examined whether any association of fetal blood flow with wheezing patterns, lung function, or asthma was mediated by birthweight or gestational age at birth or child's BMI at age 10 years by adding them additionally in our models (mediation model). Furthermore, we examined whether maternal history of asthma or atopy, child's eczema, or inhalant allergic sensitization modified any association by analyzing the statistical interaction between these variables and the fetal blood flow–related exposures (effect modification model). The percentages of missing covariate values within the population for analysis were lower than 19%. Missing covariate data were imputed using the multiple imputations procedure (n = 5 imputations), and the imputed datasets were analyzed together. All measures of associations are presented with their 95% confidence intervals (CI). Statistical analyses were performed using SPSS version 24.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
3. RESULTS
3.1. Participant characteristics
Table 1 shows the characteristics of the mothers and children included in the current study. Table S1 shows the participant characteristics before multiple imputation. Non‐response analyses showed that mothers of children not included in the analysis were more frequently lower educated, had a higher prevalence of multiparity, smoked more often, and used less often folium acid supplement during pregnancy. Their children were born at a younger gestational age (Table S2).
Table 1.
Characteristics of children and their mothers after multiple imputation (n = 903)
| Study population | |
|---|---|
| Maternal characteristics | |
| Education (%) | |
| Low (no, primary, secondary education) | 32.9 (297) |
| High (higher education) | 67.1 (606) |
| Pre‐pregnancy body mass index (kg/m2) | 23.6 (3.9) |
| Parity (%) | |
| Nullipara | 62.8 (567) |
| Multipara | 37.2 (336) |
| History of asthma or atopy (%) | |
| No | 61.7 (557) |
| Yes | 38.2 (346) |
| Smoking during pregnancy (%) | |
| No smoking throughout pregnancy | 79.1 (714) |
| Yes | 20.9 (189) |
| Folic acid supplement use (%) | |
| No use | 8.2 (74) |
| Start within the first 10 wk of pregnancy | 29.3 (265) |
| Preconceptional start | 62.5 (564) |
| Pregnancy‐induced complications (gestational hypertension/preeclampsia) (%) | |
| No | 91.7 (828) |
| Yes | 8.3 (75) |
| Third‐trimester fetal characteristics | |
| Gestational age at measurement, wk | 30.4 (28.5‐32.7) |
| Estimated fetal weight, grams | 1632 (266) |
| Umbilical artery PI | 0.97 (0.16) |
| Middle cerebral artery PI | 1.97 (0.33) |
| Umbilical/middle cerebral artery ratio | 0.50 (0.11) |
| Pulmonary artery time velocity integral | 12.04 (1.83) |
| Birth characteristics | |
| Gestational age at birth, wk | 40.3 (36.7‐42.4) |
| Birthweight, g | 3528 (509) |
| Sex | |
| Male | 50.6 (457) |
| Female | 49.4 (446) |
| Childhood characteristics | |
| Wheezing patterns until 6 y (%) | |
| Never | 45.8 (414) |
| Early | 23.0 (208) |
| Late/persistent | 13.3 (120) |
| Missing | 17.8 (161) |
| Age at follow‐up, y | 9.8 (9.1‐10.5) |
| Body mass index at age 10 y (kg/m2) | 17.1 (2.2) |
| Ever eczema at age 10 y (%) | |
| No | 73.9 (667) |
| Yes | 26.1 (236) |
| Inhalant allergic sensitization at age 10 y (%) | |
| No | 67.7 (611) |
| Yes | 32.3 (292) |
| Current asthma (%) | |
| No | 88.7 (801) |
| Yes | 3.7 (33) |
| Missing | 7.6 (69) |
| FEV1 (L/s) | 2.05 (0.29) |
| FVC (L) | 2.39 (0.35) |
| FEV1/FVC | 0.86 (0.06) |
Values are means (standard deviation), medians (95% range), or valid percentages (absolute numbers). Data were not imputed for the third trimester, birth characteristics, and respiratory outcomes. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NI, not imputed; PI, pulsatility index.
3.2. Fetal blood flow and wheezing patterns until age 6 years
Table 2 shows, in the adjusted models, a non‐significant relationship between a higher umbilical artery PI with higher risks for early and late/persistent wheezing in children (odds ratio [OR] 2.07 [95% CI, 0.70, 6.10] and 1.68 [0.34, 6.50] per unit increase in umbilical artery PI, respectively). Similarly, a higher U/C ratio, which indicates redistribution of blood flow in favor of the fetal brain, tended to be associated with a higher risk for early wheezing (OR, 2.74 [0.60, 12.62] per unit increase in U/C ratio). None of these associations were statistically significant. Middle cerebral artery PI was not associated with wheezing patterns. A higher pulmonary artery TVI, which indicates higher pulmonary vascular resistance, was associated with a higher risk of late or persistent wheezing (OR, 1.14 [1.01, 1.29] per unit increase in pulmonary artery TVI). The effect estimates for the associations of fetal umbilical, cerebral, and pulmonary blood flow adaptations with wheezing patterns adjusted for gestational age at third trimester, estimated fetal weight, and child sex only (basic model) were similar as for the adjusted models, and are presented in Table S3.
Table 2.
Associations of third‐trimester fetal blood flow with wheezing patterns until the age of 6 y (adjusted model)
|
Never wheezing Odds ratio (95% CI) (n = 414) |
Early wheezing Odds ratio (95% CI) (n = 208) |
Late or persistent wheezing Odds ratio (95% CI) (n = 120) |
Overall wheezing Odds ratio (95% CI) (n = 386) |
|
|---|---|---|---|---|
| Umbilical artery PI (n = 884) | Reference | 2.07 (0.70, 6.10) | 1.68 (0.34, 6.50) | 1.61 (0.92, 2,83) |
| Middle cerebral artery PI (n = 877) | Reference | 0.77 (0.46, 1.31) | 1.08 (0.56, 2.05) | 1.06 (0.81, 1.39) |
| Umbilical/middle cerebral artery ratio (n = 858) | Reference | 2.74 (0.60, 12.62) | 0.96 (0.14, 6.80) | 1.07 (0.48, 2.41) |
| Pulmonary artery time velocity integral (n = 788) | Reference | 1.01 (0.91, 1.12) | 1.14 (1.01, 1.29)* | 1.03 (0.98, 1.08) |
Values are odds ratios (95% confidence intervals) from logistic regression models and generalized estimating equation models (overall wheezing; wheezing on at least one time point). “n” represents number of cases. Models were adjusted for maternal educational level, parity, body mass index, smoking, folic acid use, pregnancy complications and gestational age at third trimester, estimated fetal weight, and child sex. PI, pulsatility index.
*P < 0.05.
3.3. Fetal blood flow and lung function and asthma at age 10 years
Table 3 shows that in the adjusted models, a higher third‐trimester fetal middle cerebral artery PI was associated with a higher FEV1/FVC only (Z‐score, 0.21 [95% CI, 0.01, 0.42] per unit increase middle cerebral artery PI). We observed no consistent associations of fetal blood flow with other lung function measures. Results from the models focused on these associations adjusted for gestational age at third trimester, estimated fetal weight, and child's sex only (basic model) showed no associations of fetal umbilical, cerebral, and pulmonary blood flow with lung function or asthma at age 10 years (Table S4).
Table 3.
Associations of third‐trimester fetal blood flow with lung function and asthma at age 10 y (adjusted model)
|
FEV1
Z‐score (95% CI) (n = 785) |
FVC Z‐score (95% CI) (n = 785) |
FEV1/FVC Z‐score (95% CI) (n = 785) |
Current asthma Odds ratio (95% CI) (n = 834) |
|
|---|---|---|---|---|
| Umbilical artery PI (n = 884) | −0.06 (−0.46, 0.33) | −0.23 (−0.61, 0.14) | 0.32 (−0.09, 0.74) | 2.54 (0.26, 24.59) |
| Middle cerebral artery PI (n = 877) | 0.01 (−0.18, 0.20) | −0.10 (−0.28, 0.08) | 0.21 (0.01, 0.42)* | 0.83 (0.28, 2.44) |
| Umbilical/middle cerebral artery ratio (n = 858) | −0.16 (−0.73, 0.41) | −0.20 (−0.74, 0.34) | 0.09 (−0.52, 0.69) | 0.84 (0.03, 23.28) |
| Pulmonary artery time velocity integral (n = 788) | 0.01 (−0.03, 0.05) | 0.02 (−0.01, 0.06) | −0.04 (−0.08, 0.01) | 1.08 (0.88, 1.34) |
Values are z‐score differences or odds ratios (95% confidence intervals) and reflect the change in lung function or risk for asthma per change in fetal blood flow. Lung function variables were converted into sex‐, height‐, age‐, and ethnicity‐adjusted z‐scores. Models were adjusted for maternal educational level, parity, body mass index, smoking, folic acid use, pregnancy complications and gestational age at third trimester, estimated fetal weight, and child sex. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PI, pulsatility index.
*P < 0.05.
Results of the associations of fetal umbilical, cerebral, and pulmonary blood flow with wheezing patterns, lung function, or asthma did not materially change after adding the mediators birthweight, gestational age at birth, or BMI at age 10 years (mediation model, data not shown). The associations were not modified by maternal history of asthma or atopy or child's eczema or inhalant allergic sensitization (effect modification model, all P‐values for interaction >0.05).
4. DISCUSSION
We observed a non‐significant relationship between a higher umbilical artery PI and U/C ratio with higher risk of early and late/persistent wheezing. A higher pulmonary artery TVI was associated with a higher risk of late/persistent wheezing. We found that a higher middle cerebral artery PI was associated with a higher FEV1/FVC. Associations were not explained by birth parameters, current BMI, or allergic predisposition. No other consistent associations of changes in fetal umbilical, cerebral, or pulmonary blood flow with wheezing patterns until age 6 years, or lung function and asthma at age 10 years, were found.
4.1. Interpretation and comparison with previous studies
An adverse intrauterine environment in the developing fetus leads to fetal blood flow adaptations. These adaptations may be beneficial for short‐term survival but may lead to a lower delivery of oxygen and nutrients to the trunk.6 Fetal blood flow adaptations can be detected by umbilical vein blood flow. A compensatory increase in ductus venous diameter increases the blood flow to the heart.22 This is eventually followed by a higher umbilical artery blood flow resistance and a decrease in cerebral artery resistance.23 Subsequently, changes in the pulmonary arteries can be observed, such as a higher pulmonary TVI.24 For the current study, we hypothesized that fetal umbilical, cerebral, and pulmonary blood flow adaptations may affect growth and maturation of the airways and lungs, which predispose individuals to lung disease.8, 9
Fetal blood flow adaptations are related to fetal growth restriction or low birthweight with further consequences for childhood respiratory health.12 Umbilical placental embolization in sheep was associated with structural alterations in the lungs, such as fewer but larger alveoli and a 10% reduction in the internal surface area.9 Another animal study did not find differences in lung growth after umbilical placental embolization, but did find higher pulmonary deoxyribonucleic acid and plasma cortisol levels suggesting that the offspring lungs remain underdeveloped during life.25 Children born with a very low birthweight or preterm are at higher risks of severe chronic respiratory diseases, such as bronchopulmonary dysplasia (BPD).26 Intrauterine conditions such as abnormal placental flow or suboptimal development of the placenta may lead to an increased expression of angiogenic factors, such as soluble fms‐like tyrosine kinase‐1 and vascular endothelial growth factor, which increases the risk of chronic respiratory diseases in childhood.27, 28 Also, an elevated inflammation status, found in growth restricted fetuses, could lead to reduced lung function later in life.29 Impaired vasculogenesis and angiogenesis, found in children with intrauterine growth restriction, might be other mechanisms than structural lung growth alterations leading to a suboptimal lung development.30 Children with fetal growth restriction might be vulnerable to more adaptive processes and have increased risk of respiratory morbidity in later life. Furthermore, maternal hypertensive disorders during pregnancy could have an effect on respiratory morbidity through multiple underlying mechanisms such as a disturbed placental blood flow and an altered angiogenic status. Previously published studies have shown that hypertensive disorders in pregnancy might be related to lower lung function in newborn infants or increased risk of wheezing.31, 32 A recent study from the same cohort as the current study reported associations for blood pressure across the full range in different trimesters with asthma‐related outcomes in childhood, but not for maternal hypertensive disorders with these outcomes.34 The role of fetal blood flow patterns for these associations is not clear. Our observed associations were not mediated through birthweight or gestational age at birth, but a possible role of the placenta or inflammatory status of the newborn warrants further studies.
Our study resulted mainly in negative findings, with two exceptions. First, our results showed that an increase in TVI was related to late/persistent wheezing. An increased TVI might be a sign of underdevelopment of the fetal airways, such as fewer but larger alveoli and impaired growth of the airways and lungs.8, 9 This finding might suggest that pulmonary blood flow in fetal life might be related to an increased risk of late/persistent wheezing in later life. A higher middle cerebral artery PI was associated with a higher FEV1/FVC. Our results showed a non‐significant inverse relation of the middle cerebral artery PI with FVC, but no association was observed for FEV1. We speculate that flow patterns related to fetal brain sparing might have consequences for the growth of the lungs. However, as no other effects of fetal blood flow measures on lung function or asthma in childhood were found, the observed association might be a chance finding.
4.2. Strengths and limitations
The main strength of this study was the large population‐based cohort examined from fetal life onward. To our knowledge, this is the first study to examine the effects of fetal blood flow on respiratory outcomes. The population‐based setting enabled us to assess the fetal blood flow across the full range, rather than only in fetuses with growth restriction or other complications. Follow‐up measurements at the age of 10 years were available in 74% of the children. Missing information about wheezing, lung function, or asthma could lead to selection bias and loss of power. Our results would be biased if the associations between fetal blood flow and wheezing, lung function, or asthma differed between those included and those not included in the study. Although this seems unlikely, it cannot be excluded. In the present study, we evaluated multiple associations. However, because of the correlations between the outcome measures, we did not correct for multiple testing. Our results were inconsistent and the associations might be a chance finding. In this study, there might have occurred some bias toward a more affluent and healthy population due to differences in characteristics between those lost to follow‐up and included in the study.13 Information on wheezing patterns, asthma, and eczema was obtained by questionnaires, adapted from the ISAAC‐Core questionnaires. These have been validated and shown adequate for epidemiological studies.35 However, misclassification due to under‐ or overreporting cannot be excluded. Finally, although we had information about a large number of confounders, the influence of residual confounding should be considered, as in any observational study.
4.3. Conclusion and perspectives
The results of our study are important from an etiological perspective. Our findings suggest that adaptations in fetal blood flow might contribute to the risk of wheezing and lung function in childhood. However, the observed effects were small or non‐significant and may reflect subclinical changes only. These findings should be considered as hypothesis generating and need further replication.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
MK, EM, and LD contributed to the conception and design and acquisition, analyses, and interpretation of the data; drafted the article; revised it critically for important intellectual content; and gave final approval of the version to be published. ES, IR, JJ, and VJ contributed to the conception and design and acquisition of data; revised the drafted manuscript critically for important intellectual content; and gave final approval of the version to be published.
Supporting information
Kooijman MN, van Meel ER, Steegers EAP, et al. Fetal umbilical, cerebral and pulmonary blood flow patterns in relation to lung function and asthma in childhood. The Generation R Study. Pediatr Allergy Immunol. 2019;30:443–450. 10.1111/pai.13044
Funding information
The Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam; the Erasmus University Rotterdam; and the Netherlands Organisation for Health Research and Development. VWVJ received an additional grant from the Netherlands Organisation for Health Research and Development (VIDI 016.136.361) and a Consolidator Grant from the European Research Council (ERC‐2014‐CoG‐648916). Dr Liesbeth Duijts received funding from the European Union's Horizon 2020 cofunded program ERA‐Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project [No. 696295; 2017], ZonMW The Netherlands [No. 529051014; 2017]). The study was supported by funding from the European Union's Horizon 2020 research and innovation program (733206, LifeCycle Project). The researchers are independent from the funders. The study sponsors had no role in the study design, analysis and interpretation of data, or writing of this report.
Edited by: Jon Genuneit
REFERENCES
- 1. Maritz GS, Morley CJ, Harding R. Early developmental origins of impaired lung structure and function. Early Hum Dev. 2005;81(9):763‐771. [DOI] [PubMed] [Google Scholar]
- 2. Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728‐742. [DOI] [PubMed] [Google Scholar]
- 3. Degani S. Fetal cerebrovascular circulation: a review of prenatal ultrasound assessment. Gynecol Obstet Invest. 2008;66(3):184‐196. [DOI] [PubMed] [Google Scholar]
- 4. Verburg BO, Jaddoe VW, Wladimiroff JW, Hofman A, Witteman JC, Steegers EA. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation. 2008;117(5):649‐659. [DOI] [PubMed] [Google Scholar]
- 5. Scherjon SA, Kok JH, Oosting H, Wolf H, Zondervan HA. Fetal and neonatal cerebral circulation: a pulsed Doppler study. J Perinat Med. 1992;20(1):79‐82. [DOI] [PubMed] [Google Scholar]
- 6. Zhang S, Regnault T, Barker P, et al. Placental adaptations in growth restriction. Nutrients. 2015;7(1):360‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haworth SG, Hislop AA. Lung development‐the effects of chronic hypoxia. Semin Neonatol. 2003;8(1):443‐450. [DOI] [PubMed] [Google Scholar]
- 8. Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27(1):5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long‐term effects on postnatal lung structure in sheep. Pediatr Res. 2004;55(2):287‐295. [DOI] [PubMed] [Google Scholar]
- 10. Pike K, Jane Pillow J, Lucas JS. Long term respiratory consequences of intrauterine growth restriction. Semin Fetal Neonatal Med. 2012;17(2):92‐98. [DOI] [PubMed] [Google Scholar]
- 11. Turner S, Prabhu N, Danielian P, et al. First‐ and second‐trimester fetal size and asthma outcomes at age 10 years. Am J Resp Crit Care. 2011;184(4):407‐413. [DOI] [PubMed] [Google Scholar]
- 12. den Dekker HT, Sonnenschein‐van der Voort AM, de Jongste JC et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta‐analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026‐1035. [DOI] [PubMed] [Google Scholar]
- 13. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28(1):67‐80. [DOI] [PubMed] [Google Scholar]
- 15. Wladimiroff JW, vd Wijngaard JA, Degani S, et al. Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth‐retarded pregnancies. Obstet Gynecol. 1987;69(5):705‐709. [PubMed] [Google Scholar]
- 16. Roule V, Labombarda F, Pellissier A, et al. Echocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133‐138. [DOI] [PubMed] [Google Scholar]
- 18. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 19. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150(2):535‐540. [DOI] [PubMed] [Google Scholar]
- 21. van der Valk JP, Gerth van Wijk R, Hoorn E, et al. Measurement and interpretation of skin prick test results. Clin Transl. Allergy. 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiserud T, Kessler J, Ebbing C, Rasmussen S. Ductus venosus shunting in growth‐restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet Gynecol. 2006;28(2):143‐149. [DOI] [PubMed] [Google Scholar]
- 23. Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral‐umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992;79(3):416‐420. [DOI] [PubMed] [Google Scholar]
- 24. Mielke G, Benda N. Blood flow velocity waveforms of the fetal pulmonary artery and the ductus arteriosus: reference ranges from 13 weeks to term. Ultrasound Obstet Gynecol. 2000;15(3):213‐218. [DOI] [PubMed] [Google Scholar]
- 25. Cock ML, Albuquerque CA, Joyce BJ, Hooper SB, Harding R. Effects of intrauterine growth restriction on lung liquid dynamics and lung development in fetal sheep. Am J Obstet Gynecol. 2001;184(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 26. Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet. 2003;269(1):40‐44. [DOI] [PubMed] [Google Scholar]
- 27. Wallace B, Peisl A, Seedorf G, et al. Anti‐sFlt‐1 therapy preserves lung alveolar and vascular growth in antenatal models of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2018;197(6):776‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins J, Tibboel D, de Kleer IM, Reiss I, Rottier RJ. The future of bronchopulmonary dysplasia: emerging pathophysiological concepts and potential new avenues of treatment. Front Med (Lausanne). 2017;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McElrath TF, Allred EN, Van Marter L, Fichorova RN, Leviton A; Investigators ES . Perinatal systemic inflammatory responses of growth‐restricted preterm newborns. Acta Paediatr. 2013;102(10):e439‐e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bolehovska P, Sehnal B, Driak D, et al. Changes in placental angiogenesis and their correlation with foetal intrauterine restriction. Ceska Gynekol. 2015;80(2):144‐150. [PubMed] [Google Scholar]
- 31. Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348(9034):1060‐1064. [DOI] [PubMed] [Google Scholar]
- 32. Zugna D, Galassi C, Annesi‐Maesano I, et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44(1):199‐208. [DOI] [PubMed] [Google Scholar]
- 33. Shaheen SO, Macdonald‐Wallis C, Lawlor DA, Henderson AJ. Hypertensive disorders of pregnancy, respiratory outcomes and atopy in childhood. Eur Respir J. 2016;47(1):156‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilmink FA, den Dekker HT, de Jongste JC, et al. Maternal blood pressure and hypertensive disorders during pregnancy and childhood respiratory morbidity: the Generation R Study . Eur Respir J. 2018;52(5):1800378. [DOI] [PubMed] [Google Scholar]
- 35. Silverberg JI, Patel N, Immaneni S, et al. Assessment of atopic dermatitis using self‐report and caregiver report: a multicentre validation study. Br J Dermatol. 2015;173(6):1400‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


