Abstract
1. Condition‐dependent traits can act as honest signals of mate quality, with fitter individuals being able to display preferred phenotypes. Nutrition is known to be an important determinant of individual condition, with diet known to affect many secondary sexual traits.
2. In Heliconius butterflies, male chemical signalling plays an important role in female mate choice. Potential male sex pheromone components have been identified previously, although it is unclear what information they convey to the female.
3. In the present study, the effect of diet on androconial and genital compound production is tested in male Heliconius melpomene rosina. To manipulate larval diet, larvae are reared on three different Passiflora host plants: Passiflora menispermifolia, the preferred host plant, Passiflora vitifolia and Passiflora platyloba. To manipulate adult diet, adult butterflies are reared with and without access to pollen, a key component of their diet.
4. No evidence is found to suggest that adult pollen consumption affects compound production in the first 10 days after eclosion. There is also a strong overlap in the chemical profiles of individuals reared on different larval host plants. The most abundant compounds produced by the butterflies do not differ between host plant groups. However, some compounds found in small amounts differ both qualitatively and quantitatively. Some of these compounds are predicted to be of plant origin and the others synthesised by the butterfly. Further electrophysiological and behavioural experiments will be needed to determine the biological significance of these differences.
Keywords: Chemical signalling, effect of diet, host plant, Lepidoptera, mate choice, pollen feeding, sexual selection
Introduction
Sexual ornaments often act as an indicator of mate quality and evolve in response to sexual selection imposed by female preferences (Zahavi, 1975, 1977; Andersson, 1986). Male ‘quality’ can reflect both direct and indirect benefits gained by the female (Andersson, 1994). Direct benefits might include resources that increase female lifetime reproductive success, such as food, shelter, parental care or protection from predators. Indirect benefits, on the other hand, are those that increase genetic quality of a female's offspring. In this case, sexually selected traits reflect the ability of males to provide genes that increase the survivorship or mating success of offspring (Andersson, 1986, 1994). These traits may be an honest signal of quality if they are condition‐dependent, where only the best quality males are able to display the phenotype.
Male pheromones are a good candidate as an honest signal. Diet‐mediated changes can enforce signal reliability (Henneken et al., 2017) and compounds can be costly to produce (Johansson et al., 2005; Harari et al., 2011). Nutritional condition affects male pheromone production in Tenebrio beetles (Rantala et al., 2003), cockroaches (Clark et al., 1997) and burying beetles (Chemnitz et al., 2015). Diet manipulation studies show that diet not only affects pheromone production, but also that these changes affect female mate choice (e.g. cockroaches: South et al., 2011; fruit flies: Liedo et al., 2013).
In addition to overall diet quality, specific diet components can be important. Compounds sequestered in the diet can act directly as sex pheromones or as pheromone precursors that are then metabolised further (Landolt & Phillips, 1997). One well‐studied example of this is the sequestration of pyrrolizidine alkaloids (PAs) to make hydroxydanaidal by males of the moth Utethesia ornatrix (Conner et al., 1981; Eisner & Meinwald, 2003). Some PAs are transferred to the female during mating and chemically protect the eggs, with the male pheromone signalling a direct benefit (Dussourd et al., 1988, 1991; Eisner & Meinwald, 1995; Iyengar et al., 2001). In many cases, it is probable that both overall nutrient condition and the consumption of specific compounds are important, such as in the oriental fruit fly, where both overall protein intake and the intake of a specific precursor, methyl eugenol, affect mating success (Shelly et al., 2007).
Diet shifts can provide species with new ecological and evolutionary opportunities. Being unique among butterflies, Heliconius are able to feed on pollen (Gilbert, 1972). They collect pollen from flowers and masticate it on their proboscis to extract amino acids. Heliconius have a long lifespan in the wild, facilitated by pollen feeding, which is important for oviposition and viability (Dunlap‐Pianka et al., 1977). The lack of dependence upon larval resources for reproduction may have facilitated a greater investment in defensive compounds during the larval stage (Cardoso & Gilbert, 2013). As larvae, Heliconius caterpillars feed on the cyanogenic leaves of Passiflora. Heliconius butterflies produce cyanogenic compounds de novo, making them unpalatable, and some are also able to sequester compounds directly from Passiflora plants (Engler‐Chaouat & Gilbert, 2006). Both larval and adult diet play important roles in Heliconius biology, affecting reproductive lifespan and palatability.
The importance of diet for chemical signalling in Heliconius is unclear. The role of chemical signalling in mate choice has been best studied in Heliconius melpomene rosina Boisduval (Nymphalidae), a subspecies of Heliconius melpomene found in central Panama. Potential male sex pheromone components have been described in the wing overlap region of sexually mature males (Darragh et al., 2017). The morphology of this wing region is sexually dimorphic, with specialised androconial scales only found on male wings. A bouquet of compounds that include octadecanal as a main component is found in males but not females (Mérot et al., 2015; Darragh et al., 2017; Mann et al., 2017). These chemical cues are important for mating, with females strongly discriminating against males that have their androconia experimentally blocked (Darragh et al., 2017). However, it is still unclear what information (e.g. age or male quality) is being conveyed to females by these cues.
By contrast to androconial compounds, which are assumed to be aphrodisiac in nature, Heliconius males also store anti‐aphrodisiac compounds in genital scent glands (Gilbert, 1976; Schulz et al., 2007, 2008; Estrada et al., 2011). These are transferred to the female and repel males, delaying re‐mating (Gilbert, 1976; Schulz et al., 2008). In H. melpomene, (E)‐β‐ocimene acts as an anti‐aphrodisiac (Schulz et al., 2008). Reduced harassment by other males is considered to be beneficial to the female, and so these compounds could lead to a direct benefit to females. Longer‐term, there may be conflict over the timing of re‐mating, as supported by the rapid evolution of genital chemical composition (Andersson et al., 2000, 2004; Estrada et al., 2011). Despite this clear role of genital compounds in male deterrence, the role of these same compounds in female choice remains unknown. Females may benefit from choosing males that have a lower amount of (E)‐β‐ocimene, allowing them to re‐mate again sooner. Although the dynamics of the costs and benefits of this are unclear, it is quite likely that the genital compounds are involved in female choice.
Both larval and adult diet could be important for the production of androconial and genital compounds because they are not present in freshly‐eclosed males (Schulz et al., 2008; Darragh et al., 2017). Feeding experiments with chemically labelled precursors showed that the anti‐aphrodisiac compound, (E)‐β‐ocimene, can be synthesised by adult H. melpomene via the terpene biosynthetic pathway (Schulz et al., 2008). Pollen intake could be important in providing an energy source for production. Host plant use could affect the chemical bouquet if larval sequestration of specific compounds, or compound precursors, from the host plant is necessary. Heliconius raised on their preferred host plant may have a higher quality diet (Smiley, 1978) and so compound production could also be increased as a result of higher overall quality of the individual.
In the present study, we investigated how larval and adult diet affect the chemical profile of male H. melpomene rosina from central Panama. In Panama, H. melpomene rosina females oviposit almost exclusively on Passiflora menispermifolia (Merrill et al., 2013). We reared larvae on three different Passiflora species: Passiflora menispermifolia, the preferred host plant, Passiflora vitifolia and Passiflora platyloba. The latter two species are not used by H. melpomene in the wild in Panama but are found within the range of H. melpomene rosina. These species are therefore potential hosts and larvae survive well on both (Merrill et al., 2013). Heliconius melpomene reared on its preferred host plant may have increased energy sources to dedicate to compound production during adult life. We predict the existence of both qualitative and quantitative differences in the chemical bouquets of adults reared on different host plants. In a second experiment, we maintained adult male H. melpomene rosina with and without access to pollen. We predict that males reared without pollen demonstrate reduced compound production. In both experiments, we analysed chemical extracts from both the androconial and genital regions of sexually mature male butterflies.
Materials and methods
Butterfly stocks
Heliconius melpomene rosina were reared under ambient conditions at the Smithsonian Tropical Research Institute facilities in Gamboa, Panama. Outbred stocks were established from wild individuals collected in Gamboa (9°7.4′N, 79°42.2′W, elevation 60 m) in the nearby Soberania National Park and in San Lorenzo National Park (9°17′N, 79°58′W; elevation 130 m). Individuals for the study were reared between February 2016 and April 2017.
Effects of larval diet
Larvae were reared on either P. platyloba, P. vitifolia or P. menispermifolia (preferred host plant). Adult butterflies were kept in cages with other males and were provided with an approximately 20% sucrose solution containing bee pollen (Apiarios Malivern, Panama) and with Psychotria poeppigiana, Gurania eriantha, Psiguiria triphylla and Psiguria warscewiczii as pollen sources. We collected 42 androconial samples from adult butterflies: 19 reared on P. platyloba, 11 on P. menispermifolia and 12 on P. vitifolia. We also collected 43 genital samples from adult butterflies: 17 reared on P. platyloba, 13 on P. menispermifolia and 13 on P. vitifolia. We aimed for a minimum of 10 individuals in each group. Variance between groups was a result of the availability of host plants during the experiment and rearing difficulties on different host plants. Variance between the number of androconial and genital samples within each group is a result of issues with contamination of samples, which were then unsuitable for analysis. The 19 androconial samples from larvae reared on P. platyloba have been described previously (Darragh et al., 2017).
To account for a potential difference in growth rate of individuals reared on different host plants, we measured the forewing length of adult butterflies for the host plant experiments. Before cutting the wings for chemical analysis, we photographed wings beside a ruler. We used imagej (NIH, Bethseda, Maryland) to calculate forewing length, calibrating the size using the ruler (Schneider et al., 2012).
Effects of adult diet
All larvae were reared on P. platyloba because this was the plant that was available in the largest quantities. Adults were then randomly divided into two groups. The first was provided with an approximately 20% sugar solution containing bee pollen (Apiarios Malivern, Panama) and P. poeppigiana, G. eriantha, P. triphylla and P. warscewiczii as pollen sources; the second group was only provided with an approximately 20% sugar solution and no pollen source. We analysed the androconia of 20 individuals reared with pollen and 33 without, as well as the genitals of 20 individuals reared with pollen and 27 without.
Extraction and chemical analysis of tissues
Chemical extractions were carried out on androconial and genital tissue of mature male individuals (10–12 days post‐eclosion). The individuals raised on P. platyloba for the host plant experiment are previously reported samples (Darragh et al., 2017). Genitals were removed using forceps. The wings of the individual were then removed. The hindwing androconial region of the wing, previously described as the grey–brown overlapping region of the wing (Darragh et al., 2017), was dissected from the wings for analysis. To extract compounds, the tissue was soaked, immediately after dissection, in 200 µl of dichloromethane containing 200 ng of 2‐tetradecyl acetate (internal standard) in 2‐ml glass vials with polytetrafluoroethylene‐coated caps (Agilent, Santa Clara, California) for 1 h. The solvent was then transferred to new vials and stored at −20 °C. Samples were evaporated in the laboratory at room temperature prior to analysis.
Chemical extracts were analysed by gas chomatography‐mass spectrometry (GC‐MS) using an Agilent (model 5977) mass‐selective detector connected to an Agilent GC (model 7890B). This was equipped with an Agilent ALS 7693 autosampler and an HP‐5MS fused silica capillary column (Agilent) (length 30 m, inner diameter 0.25 mm, film thickness 0.25 μm). Injection was performed in splitless mode (injector temperature 250 °C) with helium as the carrier gas (constant flow of 1.2 ml min−1). The temperature programme started at 50 °C, was held for 5 min, and then rose at a rate of 5 °C min−1 to 320 °C, before being held at 320 °C for 5 min. Components were identified by comparison of mass spectra and gas chromatographic retention indices with those of authentic reference samples and also by analysis of mass spectra. Components were quantified using 2‐tetradecyl acetate as an internal standard. Only compounds eluting earlier than hexacosane were analysed in androconial samples (Darragh et al., 2017). Later compounds were identified as cuticular hydrocarbons, 2,5‐dialkyltetrahydrofurans, cholesterol and artefacts (e.g. phthalates or adipates). The variability in the late eluting cuticular hydrocarbons was low and did not show characteristic differences between samples.
Statistical analysis
We visualised the data using a non‐metric multidimensional scaling (NMDS) ordination, based on a Bray–Curtis similarity matrix, in three dimensions. This was carried out using the metaMDS function in the r‐package vegan, version 2.5‐1 (Oksanen et al., 2017), with visualisation using the r‐package ade4 (Dray & Dufour, 2007).
After visualisation of the data, we used multivariate statistical techniques to investigate differences between the groups. To identify differences in variance between groups, we used the betadisper and permutest functions to test for homogeneity of dispersion. To compare overall chemical composition between groups, we carried out permutational multivariate analysis of variance (permanova) testing. This was performed using a Bray–Curtis similarity matrix, with 1000 permutations, using the adonis2 function in the package vegan (Oksanen et al., 2017). The ‘margin’ option in adonis2 was used to determine the effect of each term in the model, including all other variables, aiming to avoid sequential effects. The dependent variable in the model was the matrix of compounds, with different explanatory variables in the two experiments. In the larval diet experiments, we included wing length and plant species as explanatory variables. In the adult diet experiment, we tested the explanatory variable of presence or absence of pollen. We repeated the multivariate analysis using relative rather than absolute amounts of compounds.
We followed up the multivariate statistical analysis with univariate analysis to test for differences in amounts of individual compounds between groups. We tested each compound individually using non‐parametric Kruskal–Wallis tests. To correct for multiple‐testing, we used the p.adjust function in r, with false detection rate correction, which controls for the proportion of false positives. We repeated the univariate analysis using relative rather than absolute amounts of compounds.
To test for differences in forewing length for individuals reared on different host plants we carried out an anova. For post hoc analysis, Tukey's honestly significant difference test was used to determine which groups were significantly different from each other. All statistical analyses were performed with r, version 3.3.1 (R Core Team, 2016).
Results
Effect of host plant on wing size
Larval host plant affected forewing length (anova, F 2,42 = 3.755, P = 0.032) (Fig. 1). Mean ± SD forewing length was 3.54 ± 0.27 cm for adults reared as larvae on P. menispermifolia (the preferred host plant of H. melpomene rosina), 3.52 ± 0.15 cm for those reared on P. platyloba and 3.36 ± 0.10 cm for those reared on P. vitifolia. However, post hoc Tukey comparisons did not find any pairwise significant difference between groups.
Figure 1.

Larval host plant affects forewing length of Heliconius melpomene male adults (anova, d.f. = 2, F = 3.755, P = 0.032). Post hoc testing using Tukey's honestly significant difference found no significant pairwise differences between groups.
Chemical compounds in androconia and genitals of H. melpomene
We initially analysed 19 androconia samples and 18 genital samples of H. melpomene reared on P. platyloba. This is a reanalysis of the 19 androconial samples that have been described previously (Darragh et al., 2017). The most abundant compounds found in the androconia are syringaldehyde, octadecanal, octadecan‐1‐ol, (Z)‐11‐icosenal and (Z)‐11‐icosenol, as reported previously (see Supporting information, Figure S1 and Table S1) (Darragh et al., 2017). These compounds are present with a mean of greater than 100 ng per individual, with octadecanal being found in the highest amounts (mean 740 ng).
The genital region is dominated by one main compound, (E)‐β‐ocimene, which is found in 20‐fold greater amounts than any other genital compound (mean 34789 ng). This was previously reported in other samples of H. melpomene (Schulz et al., 2008). It is found alongside a bouquet of other terpenes, alcohols, aromatic compounds, macrolides, esters and alkanes (see Supporting information, Figure S2 and Table S2) (Schulz et al., 2008).
There is little overlap in compounds found between the two body regions, with only 10 out of 117 compounds being found in both. The genital region contains higher amounts of compounds and more compounds overall, with 80 in the genitals compared with 47 in the androconia. The most abundant genital compound, (E)‐β‐ocimene, is more volatile (has a higher vapour pressure) than the main compounds found in the androconial region.
Effects of larval diet
Wing size was not a significant factor influencing chemical composition of H. melpomene genitals (permanova, F 1,39 = 0.577, P = 0.606) but did influence androconial chemical bouquets (permanova, F 1,38 = 3.033, P = 0.038), accounting for approximately 7% of the variation.
Our experiments revealed that H. melpomene reared on P. platyloba, P. menispermifolia or P. vitifolia did not differ significantly in their overall androconial bouquet (permanova, F 2,38 = 1.791, P = 0.080) (Fig. 2a; see also Supporting information, Table S1) and did not differ in dispersion between groups (permutation test of homogeneity of dispersion, F 2,39 = 1.335, P = 0.275). However, when we look at the individual compounds in each treatment, more than one‐quarter (12/47) are present in significantly different amounts between groups (Table 1). These same compounds were also found to be significantly different between groups using relative amounts (for further analysis of relative amounts, see Supporting information).
Figure 2.
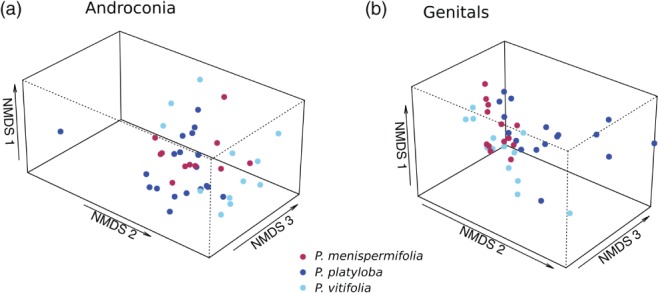
Non‐metric multidimensional scaling (NMDS) plot illustrating in three dimensions the overlapping variation in chemical compounds of male Heliconius melpomene raised on three different Passiflora species. Passiflora menispermifolia is the preferred host plant of this species. (a) Androconial compound bouquets do not differ significantly after 10 days. Stress = 0.140. (b) Genital compound bouquets do not differ significantly after 10 days. Stress = 0.098. [Colour figure can be viewed at wileyonlinelibrary.com].
Table 1.
Androconial compounds that significantly differed between Heliconius melpomene reared on different host plants.
| Chemical | RI | Passiflora platyloba | Passiflora menispermifolia | Passiflora vitifolia | H test statistic | P‐value |
|---|---|---|---|---|---|---|
| Methyl salicylate | 1189 | 0.59 ± 1.97 | 0.98 ± 3.13 | 2.32 ± 1.95 | 10.23 | 0.026 |
| Unknown compound | 1353 | 0 ± 0 | 0.40 ± 1.31 | 3.30 ± 3.85 | 16.68 | 0.006 |
| 1‐(3,5‐Dimethoxy‐4‐hydroxybenzyl)ethanone | 1735 | 0.99 ± 1.58 | 0.67 ± 0.76 | 0.01 ± 0.04 | 10.71 | 0.022 |
| Benzyl benzoate | 1766 | 0.25 ± 0.57 | 0.13 ± 0.44 | 1.27 ± 1.40 | 10.78 | 0.022 |
| 1‐(4‐Hydroxy‐3,5‐dimethoxyphenyl)‐2‐propen‐1‐one | 1807 | 1.48 ± 1.93 | 1.15 ± 1.32 | 0 ± 0 | 12.06 | 0.016 |
| Syringaldehyde derivative | 1891 | 0 ± 0 | 3.05 ± 2.74 | 0 ± 0 | 19.11 | 0.003 |
| Unknown hydrocarbon | 1962 | 1.32 ± 1.14 | 1.80 ± 1.41 | 0.28 ± 0.97 | 11.19 | 0.022 |
| Ethyl 4‐hydroxy‐3,5‐ dimethoxybenzoate | 2057 | 32.61 ± 55.79 | 11.60 ± 13.95 | 0.14 ± 0.37 | 13.52 | 0.011 |
| Henicosadiene | 2065 | 1.36 ± 4.28 | 0.22 ± 0.74 | 2.97 ± 3.91 | 9.75 | 0.030 |
| Methyloctadecanal | 2077 | 30.77 ± 15.99 | 15.37 ± 8.54 | 10.94 ± 13.15 | 13.92 | 0.011 |
| Icosanal | 2224 | 23.21 ± 11.46 | 16.08 ± 14.41 | 8.41 ± 7.59 | 14.92 | 0.009 |
| Fatty acid amide | 2325 | 0.27 ± 1.20 | 0 ± 0 | 1.09 ± 1.66 | 12.53 | 0.015 |
The gas chromatographic retention index (RI) is reported for each compound. Mean ± SD amounts (ng), as well as Kruskal–Wallis non‐parametric test P‐values (false detection rate corrected) are provided. Compounds shown in bold are predicted to be plant‐derived.
Genital compounds of H. melpomene reared on P. platyloba, P. menispermifolia or P. vitifolia did not differ overall between host plant treatments (permanova, F2,39 = 1.184, P = 0.308) (Fig. 2b; see also Supporting information, Table S2). The dispersion of individuals between treatments did differ (permutation test of homogeneity of dispersion, F 2,40 = 3.668, P = 0.034), with pairwise permutation analysis revealing that the dispersion of individuals raised on P. menispermifolia is different from both P. vitifolia and P. platyloba, which do not differ from each other (see Supporting information, Table S3). NMDS visualisation reveals less variation between individuals reared on the preferred host P. menispermifolia (Fig. 2b). Furthermore, seven out of 80 individual compounds differ between groups (Table 2). These compounds were also found to be significantly different between groups using relative amounts, excluding an unknown compound (gas chromatographic retention index, RI = 1396), which is no longer significant (for further analysis of relative amounts, see Supporting information).
Table 2.
Genital compounds that differed significantly between Heliconius melpomene reared on different host plants.
| Chemical | RI | Passiflora platyloba | Passiflora menispermifolia | Passiflora vitifolia | H test statistic | P‐value |
|---|---|---|---|---|---|---|
| 7‐β‐(H)‐Silphiperfol‐5‐ene | 1345 | 9.75 ± 12.23 | 0 ± 0 | 0 ± 0 | 21.46 | < 0.001 |
| Unknown sesquiterpene | 1378 | 2.87 ± 4.20 | 0 ± 0 | 0 ± 0 | 19.02 | 0.001 |
| Unknown sesquiterpene | 1384 | 20.90 ± 21.99 | 0 ± 0 | 0 ± 0 | 32.40 | < 0.001 |
| Unknown compound | 1396 | 39.76 ± 26.86 | 26.83 ± 30.96 | 8.23 ± 6.87 | 16.03 | 0.004 |
| β‐Caryophyllene | 1417 | 18.97 ± 22.09 | 0 ± 0 | 0 ± 0 | 32.40 | < 0.001 |
| Unknown compound | 2250 | 0 ± 0 | 1.48 ± 5.32 | 1.33 ± 2.09 | 11.37 | 0.039 |
| Cholestadiene | 2744 | 0 ± 0 | 9.56 ± 34.48 | 18.18 ± 24.10 | 19.96 | < 0.001 |
The gas chromatographic retention index (RI) is reported for each compound. Mean ± SD amounts (ng), as well as Kruskal–Wallis non‐parametric test P‐values (false detection rate corrected) are provided. Compounds shown in bold are predicted to be plant‐derived.
None of the individual compounds found to differ between groups were the most abundant compounds. These compounds are not found in an average amount higher than 40 ng per individual, which is much smaller amount than the most abundant compound in androconia (740 ng) or genitals (34 789 ng). We found both qualitative (presence or absence of compound) and quantitative (difference in amount of compound) differences between butterflies reared on different host plants. For example, in the androconial samples, a syringaldehyde derivative was only found in individuals reared on P. menispermifolia. Icosanal, in contrast, was found in butterflies reared on all three host plant species but in significantly different amounts (Table 1).
Effects of adult diet
Heliconius melpomene butterflies reared with or without pollen for 10 days do not differ in either androconial (permanova, F 1,51 = 1.653, P = 0.145) or genital (permanova, F 1,45 = 1.259, P = 0.260) chemical bouquets (Fig. 3; see also Supporting information, Tables S4 and S5). False detection rate corrected Kruskal–Wallis testing found no compounds in significantly different amounts between the groups.
Figure 3.
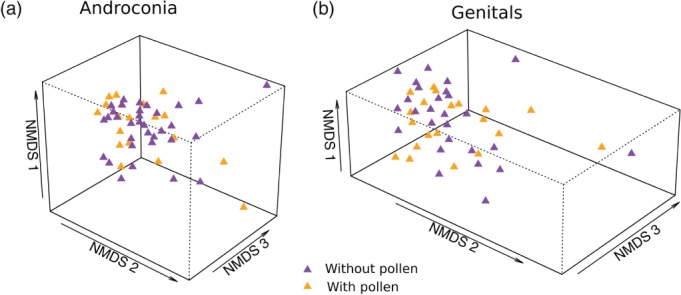
Non‐metric multidimensional scaling (NMDS) plot illustrating in three dimensions the overlapping variation in chemical compounds of male Heliconius melpomene raised with or without pollen. (a) Androconial compound bouquets do not differ significantly after 10 days. Stress = 0.131. (b) Genital compound bouquets do not differ significantly after 10 days. Stress = 0.108. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
Chemical signalling is known to be important for intraspecific female mate choice in Heliconius (Darragh et al., 2017). The information conveyed by these compounds, such as age, species identity or mate quality, remains unclear. We find no evidence that adult pollen consumption affects compound production in the first 10 days of adult life. By contrast, individual androconial and genital compounds were found in different amounts between larval host plant treatment groups, and dispersion varied between host plant treatments for genitals.
The most abundant compounds identified in the androconia and genitals of H. melpomene are the same as those identified in previously published studies (Schulz et al., 2008; Estrada et al., 2011; Mérot et al., 2015; Darragh et al., 2017; Mann et al., 2017). We did not identify all of the compounds found previously in genitals (Schulz et al., 2008). This is likely a result of variation in sample collection because the list of previous compounds was derived from pooled samples, allowing for a higher detection threshold. In the androconia, we did not detect ethyl palmitate, ethyl oleate or ethyl stearate, previously reported compounds (Mann et al., 2017). We did detect ethyl oleate in the genitals, suggesting that previous reports in the androconia may be a result of contamination from genital contact. We also found many more compounds, probably because of improved GC‐MS detection thresholds that are more sensitive to compounds found only in low levels.
We did not find a difference in the ability of males to produce compounds when reared with and without pollen in this experiment. This finding was somewhat unexpected because pollen is an important resource for adult Heliconius. This result could mean that chemical signalling is not nutritionally dependent. Heliconius comprise one of the most long‐lived butterflies, with adults being known to live more than 8 months in the wild (Gilbert, 1972), and pollen limitation might play a more important role over longer time scales. In females, the effects of pollen for oviposition and viability are evident after approximately 1 month (Dunlap‐Pianka et al., 1977), suggesting that, until that point, larval reserves are sufficient. This could be the same for males and therefore we cannot rule out the possibility that adult nutrition might influence pheromone production only later in life.
Pollen‐feeding is considered to be important for another aspect of reproduction in Heliconius: the donation of a spermatophore to the female during mating. The spermatophore is protein‐rich, and so the amino acids obtained by pollen‐feeding are probably needed to make new spermatophores after each mating (Cardoso & Silva, 2015), as supported by the finding that males with more lifetime matings collect more pollen overall (Boggs, 1990). This is beneficial for the females because its reduces the need for females to forage for pollen (Boggs & Gilbert, 1979; Boggs, 1981, 1990). It has been proposed that females may determine spermatophore quality using cues (e.g. chemicals) that indicate direct benefits for the female (Cardoso & Silva, 2015). Alternatively, females could benefit indirectly through a ‘good genes’ mechanism (Andersson & Simmons, 2006); for example, through inheritance of foraging ability (Karino et al., 2005). Further experiments will be required to determine whether chemical signalling in older males can indicate spermatophore production and male quality.
Chemical profiles produced by adult butterflies reared on the three larval host plants are largely overlapping. Heliconius melpomene is able to produce the majority of compounds found in both androconial and genital bouquets when reared on all three Passiflora species. The most abundant compounds are not found in significantly different amounts. However, we find less variation in genital compounds produced by individuals reared on P. menispermifolia, the preferred host plant of H. melpomene rosina, compared with P. vitifolia and P. platyloba, perhaps suggesting some level of chemical or digestive specialisation.
Despite an overall similarity between butterflies reared on different host plants, there are differences in some specific androconial and genital compounds. These differences are both qualitative and quantitative in nature. Over one‐quarter of androconial compounds are found in significantly different amounts between the three groups, along with almost one‐tenth of genital compounds. One‐third of these significant androconial compounds are considered to come from the phenylpropanoid pathway, active in plants (Boerjan et al., 2003). This pathway forms aromatic compounds with an alkyl sidechain of three carbons that serve as building blocks for lignin and lignans. Oxidative degradation of lignin or by‐products of this biosynthetic pathway are the source of the aromatic compounds syringaldehyde, 1‐(3,5‐dimethoxy‐4‐hydroxybenzyl)ethanone or ethyl 4‐hydroxy‐3,5‐dimethoxybenzoate. They are not currently known to act as pheromones in insects, although closely‐related compounds, lacking one methoxy group, are reported as fruit fly and moth pheromones (Francke & Schulz, 2010).
Over one‐half of the genital compounds, specifically sesquiterpenes, are also considered to originate from plant sources. These include the specific compounds 7‐β‐(H)‐silphiperfol‐5‐ene and β‐caryophyllene, as well as some unknown other sesquiterpenes. The genome currently does not show any indication of a required sesquiterpene cyclase in H. melpomene, thus making de novo synthesis of sesquiterpenes by the butterflies unlikely. These data suggest that differences in plant biochemistry affect the chemicals released from both androconial and genital regions of the adult butterfly.
We do not know which components of the androconial bouquet are biologically important in H. melpomene. It cannot be assumed that the most abundant compounds are necessarily the most important because minor compounds can often play important roles in attraction (D'Alessandro et al., 2009; McCormick et al., 2014). Furthermore, the response to pheromonal cues is blend‐specific in other Lepidopteran systems (Yildizhan et al., 2009; Larsdotter‐Mellström et al., 2016). Despite the overlap in overall chemical composition between host plant treatments, those compounds that are significantly different could possibly drive a change in female response. This is particularly likely for the androconial bouquet, where more than one‐quarter of compounds are found in different amounts between larval host plant treatments. However, it is important to note that direct tissue extraction of chemicals may not accurately reflect the chemical amounts emitted by live butterflies (Visser et al., 2018). Electrophysiological and behavioural experiments will be required to determine whether these differences are biologically relevant.
Our results contribute to an understanding of why some populations of H. melpomene are host specialists when their larvae can successfully feed on a wider variety of host plants. Heliconius melpomene larval growth rate is similar on different host plants under laboratory conditions (Smiley, 1978), although there are slight differences in survival in the wild, perhaps as a result of ant attendance or parasitism (Merrill et al., 2013). In particular, H. melpomene fed on P. vitifolia show a somewhat lower survival rate compared with the natural host plant P. menispermifolia (Merrill et al., 2013), which may be related to our finding that host plant affects size in Heliconius melpomene. If host specialisation is not a result of physiological adaptation in the larvae, an alternative is that it could be explained by sexual selection. Female choice on diet‐derived pheromones could potentially drive host plant specialisation (Quental et al., 2007). In this case, we would not expect larval host plant specialisation because selection is acting on the adult stage. To determine whether this could be a plausible mechanism to explain host plant preference in Heliconius, we would need to determine whether the compounds that change with host plant use are also important for female choice.
We might expect to find intraspecific differences in chemistry between Heliconius races. Across their geographical range, Heliconius butterflies use different host plants and can vary in their extent of host plant specialisation (Benson et al., 1975; Benson, 1978). Based on our results, we predict that this will lead to differences in chemical bouquets between populations as a result of differing host plant use. We might also expect to find more intraspecific differences in chemistry in populations that are more generalist. Future investigations using different geographical races will help us understand the role of diet in the chemistry of Heliconius butterflies, as well as its link to host plant specialisation.
Supporting information
Figure S1. Total ion chromatogram of extract from androconial region of Heliconius melpomene.
Figure S2. Total ion chromatogram of extract from genital region of Heliconius melpomene.
Table S1. Androconial compounds identified in Heliconius melpomene rosina males reared on Passiflora platyloba, Passiflora menispermifolia or Passiflora vitifolia.
Table S2. Genital compounds identified in Heliconius melpomene rosina males reared on Passiflora platyloba, Passiflora menispermifolia or Passiflora vitifolia.
Table S3. Pairwise comparisons of dispersion of Heliconius melpomene genital compounds when reared on different plants.
Table S4. Androconial compounds identified in Heliconius melpomene rosina males fed as adults with or without pollen.
Table S5. Genital compounds identified in Heliconius melpomene rosina males fed as adults with or without pollen.
Acknowledgements
We thank our team at the insectaries in Panama, including Oscar Paneso, Sylvia Fernanda Garza Reyes and Diana Abondano. We also acknowledge the support and advice of William Wcislo. We thank three anonymous reviewers who provided helpful comments on the manuscript. KD was supported by a Natural Research Council Doctoral Training Partnership and a Smithsonian Tropical Research Institute Short Term Fellowship. KJRPB and CDJ were supported by a European Research Council grant number 339873 SpeciationGenetics. WOM was supported by the Smithsonian Tropical Research Institute and National Science Foundation (NSF) grant DEB 1257689. SS thanks the Deutsche Forschungsgemeinschaft (DFG) for support through grant Schu984/12‐1. The authors declare that they have no conflicts of interest.
Associate Editor: Sofia Gripenberg
References
- Andersson, J. , Borg‐Karlson, A.‐K. & Wiklund, C. (2000) Sexual cooperation and conflict in butterflies: a male‐transferred anti‐aphrodisiac reduces harassment of recently mated females. Proceedings of the Royal Society of London B: Biological Sciences, 267, 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. , Borg‐Karlson, A.‐K. & Wiklund, C. (2004) Sexual conflict and anti‐aphrodisiac titre in a polyandrous butterfly: male ejaculate tailoring and absence of female control. Proceedings of the Royal Society B: Biological Sciences, 271, 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, M. (1986) Evolution of condition‐dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution, 40, 804–816. [DOI] [PubMed] [Google Scholar]
- Andersson, M. & Simmons, L.W. (2006) Sexual selection and mate choice. Trends in Ecology & Evolution, 21, 296–302. [DOI] [PubMed] [Google Scholar]
- Andersson, M.B. (1994) Sexual Selection. Princeton University Press, Princeton, New Jersey. [Google Scholar]
- Benson, W.W. (1978) Resource partitioning in passion vine butterflies. Evolution; International Journal of Organic Evolution, 32, 493–518. [DOI] [PubMed] [Google Scholar]
- Benson, W.W. , Brown, K.S. & Gilbert, L.E. (1975) Coevolution of plants and herbivores: passion flower butterflies. Evolution; International Journal of Organic Evolution, 29, 659–680. [DOI] [PubMed] [Google Scholar]
- Boerjan, W. , Ralph, J. & Baucher, M. (2003) Lignin biosynthesis. Annual Review of Plant Biology, 54, 519–546. [DOI] [PubMed] [Google Scholar]
- Boggs, C.L. (1981) Selection pressures affecting male nutrient investment at mating in heliconiine butterflies. Evolution, 35, 931–940. [DOI] [PubMed] [Google Scholar]
- Boggs, C.L. (1990) A general model of the role of male‐donated nutrients in female insects' reproduction. American Naturalist, 136, 598–617. [Google Scholar]
- Boggs, C.L. & Gilbert, L.E. (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science (New York, NY), 206, 83–84. [DOI] [PubMed] [Google Scholar]
- Cardoso, M.Z. & Gilbert, L.E. (2013) Pollen feeding, resource allocation and the evolution of chemical defence in passion vine butterflies. Journal of Evolutionary Biology, 26, 1254–1260. [DOI] [PubMed] [Google Scholar]
- Cardoso, M.Z. & Silva, E.S. (2015) Spermatophore quality and production in two Heliconius butterflies with contrasting mating systems. Journal of Insect Behavior, 28, 693–703. [Google Scholar]
- Chemnitz, J. , Jentschke, P.C. , Ayasse, M. & Steiger, S. (2015) Beyond species recognition: somatic state affects long‐distance sex pheromone communication. Proceedings of the Royal Society of London B: Biological Sciences, 282, 20150832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, D.C. , DeBano, S.J. & Moore, A.J. (1997) The influence of environmental quality on sexual selection in Nauphoeta cinerea (Dictyoptera: Blaberidae). Behavioral Ecology, 8, 46–53. [Google Scholar]
- Conner, W.E. , Eisner, T. , Meer, R.K.V. , Guerrero, A. & Meinwald, J. (1981) Precopulatory sexual interaction in an arctiid moth Utetheisa ornatrix: role of a pheromone derived from dietary alkaloids. Behavioral Ecology and Sociobiology, 9, 227–235. [Google Scholar]
- D'Alessandro, M. , Brunner, V. , von Mérey, G. & Turlings, T.C.J. (2009) Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. Journal of Chemical Ecology, 35, 999–1008. [DOI] [PubMed] [Google Scholar]
- Darragh, K. , Vanjari, S. , Mann, F. , Gonzalez‐Rojas, M.F. , Morrison, C.R. , Salazar, C. et al (2017) Male sex pheromone components in Heliconius butterflies released by the androconia affect female choice. PeerJ, 5, e3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray, S. & Dufour, A.B. (2007) The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software, 22, 1–20. [Google Scholar]
- Dunlap‐Pianka, H. , Boggs, C.L. & Gilbert, L.E. (1977) Ovarian dynamics in heliconiine butterflies: programmed senescence versus eternal youth. Science, 197, 487–490. [DOI] [PubMed] [Google Scholar]
- Dussourd, D.E. , Harvis, C.A. , Meinwald, J. & Eisner, T. (1991) Pheromonal advertisement of a nuptial gift by a male moth (Utetheisa ornatrix). Proceedings of the National Academy of Sciences of the United States of America, 88, 9224–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussourd, D.E. , Ubik, K. , Harvis, C. , Resch, J. , Meinwald, J. & Eisner, T. (1988) Biparental defensive endowment of eggs with acquired plant alkaloid in the moth Utetheisa ornatrix . Proceedings of the National Academy of Sciences of the United States of America, 85, 5992–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner, T. & Meinwald, J. (1995) The chemistry of sexual selection. Proceedings of the National Academy of Sciences of the United States of America, 92, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner, T. & Meinwald, J. (2003) Alkaloid‐derived pheromones and sexual selection in Lepidoptera Insect Pheromone Biochemistry and Molecular Biology (ed. by Blomquist G. J. and Vogt R. G.), pp. 341–368. Elsevier/Academic Press, Amsterdam, Netherlands; Boston, Massachusetts. [Google Scholar]
- Engler‐Chaouat, H.S. & Gilbert, L.E. (2006) De novo synthesis vs. sequestration: negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. Journal of Chemical Ecology, 33, 25–42. [DOI] [PubMed] [Google Scholar]
- Estrada, C. , Schulz, S. , Yildizhan, S. & Gilbert, L.E. (2011) Sexual selection drives the evolution of antiaphrodisiac pheromones in butterflies. Evolution; International Journal of Organic Evolution, 65, 2843–2854. [DOI] [PubMed] [Google Scholar]
- Francke, W. & Schulz, S. (2010) Pheromones of terrestrial invertebrates Comprehensive Natural Products II (ed. by Liu H.‐W. and Mander L.), p. 153 Elsevier, Oxford, U.K. [Google Scholar]
- Gilbert, L.E. (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Sciences of the United States of America, 69, 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, L.E. (1976) Postmating female odor in Heliconius butterflies: a male‐contributed antiaphrodisiac? Science, 193, 419–420. [DOI] [PubMed] [Google Scholar]
- Harari, A.R. , Zahavi, T. & Thiéry, D. (2011) Fitness cost of pheromone production in signaling female moths. Evolution, 65, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Henneken, J. , Goodger, J.Q.D. , Jones, T.M. & Elgar, M.A. (2017) Diet‐mediated pheromones and signature mixtures can enforce signal reliability. Frontiers in Ecology and Evolution, 4, 145. [Google Scholar]
- Iyengar, V.K. , Rossini, C. & Eisner, T. (2001) Precopulatory assessment of male quality in an arctiid moth Utetheisa ornatrix: hydroxydanaidal is the only criterion of choice. Behavioral Ecology and Sociobiology, 49, 283–288. [Google Scholar]
- Johansson, B.G. , Jones, T.M. & Widemo, F. (2005) Cost of pheromone production in a lekking Drosophila . Animal Behaviour, 69, 851–858. [Google Scholar]
- Karino, K. , Utagawa, T. & Shinjo, S. (2005) Heritability of the algal‐foraging ability: an indirect benefit of female mate preference for males' carotenoid‐based coloration in the guppy, Poecilia reticulata . Behavioral Ecology and Sociobiology, 59, 1–5. [Google Scholar]
- Landolt, P.J. & Phillips, T.W. (1997) Host plant influences on sex pheromone behavior of phytophagous insects. Annual Review of Entomology, 42, 371–391. [DOI] [PubMed] [Google Scholar]
- Larsdotter‐Mellström, H. , Eriksson, K. , Liblikas, I.I. , Wiklund, C. , Borg‐Karlson, A.K. , Nylin, S. et al (2016) It's all in the mix: blend‐specific behavioral response to a sexual pheromone in a butterfly. Frontiers in Physiology, 7, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedo, P. , Orozco, D. , Cruz‐López, L. , Quintero, J.L. , Becerra‐Pérez, C. , Hernández, M.d.R. et al (2013) Effect of post‐teneral diets on the performance of sterile Anastrepha ludens and Anastrepha obliqua fruit flies. Journal of Applied Entomology, 137, 49–60. [Google Scholar]
- Mann, F. , Vanjari, S. , Rosser, N. , Mann, S. , Dasmahapatra, K.K. , Corbin, C. et al (2017) The scent chemistry of Heliconius wing androconia. Journal of Chemical Ecology, 43, 1–15. [DOI] [PubMed] [Google Scholar]
- McCormick, A.C. , Gershenzon, J. & Unsicker, S.B. (2014) Little peaks with big effects: establishing the role of minor plant volatiles in plant–insect interactions. Plant, Cell & Environment, 37, 1836–1844. [DOI] [PubMed] [Google Scholar]
- Mérot, C. , Frérot, B. , Leppik, E. & Joron, M. (2015) Beyond magic traits: multimodal mating cues in Heliconius butterflies. Evolution, 69, 2891–2904. [DOI] [PubMed] [Google Scholar]
- Merrill, R.M. , Naisbit, R.E. , Mallet, J. & Jiggins, C.D. (2013) Ecological and genetic factors influencing the transition between host‐use strategies in sympatric Heliconius butterflies. Journal of Evolutionary Biology, 26, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Oksanen, J. , Guillaume Blanchet, F. , Friendly, M. , Kindt, R. , Legendre, P. , McGlinn, D. , et al (2017) vegan: Community Ecology Package. R package, version 2.4‐2.
- Quental, T.B. , Patten, M.M. & Pierce, N.E. (2007) Host plant specialization driven by sexual selection. The American Naturalist, 169, 830–836. [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rantala, M.J. , Kortet, R. , Kotiaho, J.S. , Vainikka, A. & Suhonen, J. (2003) Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor . Functional Ecology, 17, 534–540. [Google Scholar]
- Schneider, C.A. , Rasband, W.S. & Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, S. , Estrada, C. , Yildizham, S. , Boppré, M. & Gilbert, L.E. (2008) An antiaphrodisiac in Heliconius melpomene butterflies. Journal of Chemical Ecology, 34, 82–93. [DOI] [PubMed] [Google Scholar]
- Schulz, S. , Yildizhan, S. , Stritzke, K. , Estrada, C. & Gilbert, L.E. (2007) Macrolides from the scent glands of the tropical butterflies Heliconius cydno and Heliconius pachinus . Organic & Biomolecular Chemistry, 5, 3434–3441. [DOI] [PubMed] [Google Scholar]
- Shelly, T.E. , Edu, J. & Pahio, E. (2007) Condition‐dependent mating success in male fruit flies: ingestion of a pheromone precursor compensates for a low‐quality diet. Journal of Insect Behavior, 20, 347–365. [Google Scholar]
- Smiley, J. (1978) Plant chemistry and the evolution of host specificity: new evidence from Heliconius and Passiflora . Science, 201, 745–747. [DOI] [PubMed] [Google Scholar]
- South, S.H. , House, C.M. , Moore, A.J. , Simpson, S.J. & Hunt, J. (2011) Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: implications for the study of condition dependence. Evolution, 65, 1594–1606. [DOI] [PubMed] [Google Scholar]
- Visser, B. , Dublon, I.A.N. , Heuskin, S. , Laval, F. , Bacquet, P. , Lognay, G. et al (2018) Common practice solvent extraction does not reflect actual emission of a sex pheromone during butterfly courtship. Frontiers in Ecology and Evolution, 6, 154. [Google Scholar]
- Yildizhan, S. , van Loon, J. , Sramkova, A. , Ayasse, M. , Arsene, C. , ten Broeke, C. et al (2009) Aphrodisiac pheromones from the wings of the small cabbage white and large cabbage white butterflies, Pieris rapae and Pieris brassicae . Chembiochem, 10, 1666–1677. [DOI] [PubMed] [Google Scholar]
- Zahavi, A. (1975) Mate selection – a selection for a handicap. Journal of Theoretical Biology, 53, 205–214. [DOI] [PubMed] [Google Scholar]
- Zahavi, A. (1977) The cost of honesty: further remarks on the handicap principle. Journal of Theoretical Biology, 67, 603–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Total ion chromatogram of extract from androconial region of Heliconius melpomene.
Figure S2. Total ion chromatogram of extract from genital region of Heliconius melpomene.
Table S1. Androconial compounds identified in Heliconius melpomene rosina males reared on Passiflora platyloba, Passiflora menispermifolia or Passiflora vitifolia.
Table S2. Genital compounds identified in Heliconius melpomene rosina males reared on Passiflora platyloba, Passiflora menispermifolia or Passiflora vitifolia.
Table S3. Pairwise comparisons of dispersion of Heliconius melpomene genital compounds when reared on different plants.
Table S4. Androconial compounds identified in Heliconius melpomene rosina males fed as adults with or without pollen.
Table S5. Genital compounds identified in Heliconius melpomene rosina males fed as adults with or without pollen.


