Abstract
The Focal adhesion kinase (FAK) is a ubiquitous cytoplasmic tyrosine‐kinase promoting tumor progression and metastasis processes by acting in cancer cells and their tumor microenvironment partners. FAK overexpression in primary colon tumors and their metastasis is associated to poor colorectal cancer (CRC) patients’ outcome. Eight FAK mRNA alternative splice variants have been described and contribute to additional level of FAK activity regulation, some of them corresponding to overactivated FAK isoforms. To date, FAK mRNA alternative splice variants expression and implication in CRC processes remain unknown. Here, using different human CRC cells lines displaying differential invasive capacities in an in vivo murine model recapitulating the different steps of CRC development from primary tumors to liver and lung metastasis, we identified three out of the eight mRNA variants (namely FAK0, FAK28 and FAK6) differentially expressed along the CRC process and the tumor sites. Our results highlight an association between FAK0 and FAK6 expressions and the metastatic potential of the most aggressive cell lines HT29 and HCT116, suggesting that FAK0 and FAK6 could represent aggressiveness markers in CRC. Our findings also suggest a more specific role for FAK28 in the interactions between the tumors cells and their microenvironment. In conclusion, targeting FAK0, the common form of FAK, might not be a good strategy based on the numerous roles of this kinase in physiological processes. In contrast, FAK6 or FAK28 splice variants, or their corresponding protein isoforms, may putatively represent future therapeutic target candidates in the development of CRC primary tumors and metastasis.
Keywords: FAK, alternative splicing, colorectal cancer, metastases
Short abstract
What's new?
Overexpression of the focal adhesion kinase (FAK) is associated with poor outcome in patients with colorectal cancer but the role of the eight splice variants of FAK remains unknown. Here the authors correlated FAK splice variant expression in colorectal tumor cell lines with invasiveness in mouse models. FAK0 and FAK6 splice variant expression was associated with higher aggressiveness and metastatic potential, underscoring that distinct FAK splice variants may represent new targets in the development of drugs against colorectal cancer and associated metastasis.
Abbreviations
- AML
acute myeloid leukemia
- APC
adenomatous polyposis coli
- AS
alternative splicing
- BMP
bone morphogenic protein
- Bp
base pair
- CRC
colorectal cancer
- FAK
focal adhesion kinase
- FAT
focal adhesion targeting
- FERM
F for 4.1 protein, E for ezrin, R for radixin and M for moesin
- mRNA
messenger RiboNucleic Acid
- mTOR
mammalian Target Of Rapamycin
- NCAM
neural cell adhesion molecule
- PI3K
phosphatidyl Inositol 3‐Kinase
- PR
proline rich
- qRT‐PCR
quantitative Reverse Transcription‐Polymerase Chain Reaction
- SCID
severe combined immunodeficiency
Introduction
Along development, cells have elaborated Alternative Splicing (AS) of messenger ribonucleic acid (mRNA) in order to increase the fine‐tuning of protein functions. AS is a substitute to the regular constitutive splicing where intron removal and exon ligation are performed in the order of introns and exons within the gene sequence. In AS, due to weak splicing signals, shorter exon or high sequence conservation either sides of orthologous alternative exons, certain exons are bypassed by the spliceosome leading to various forms of mature mRNA.1 Thus, by generating from an initial unique premessenger RNA, different protein isoforms varying in terms of expression, subcellular localization, interactions and activities, AS represents a critical player in protein function regulation in development, physiology and disease.2 Indeed, AS directly influences carcinogenesis and metastasis, and some alternative mRNAs splice variants are proposed as early markers in diagnosis and as therapeutic targets.3 For instance, splice variants of the Focal Adhesion Kinase (FAK) are abnormally expressed in the primitive leukemic cells of patients suffering of acute myeloid leukemia (AML) presenting a poor prognostic,4 and may represent potential biomarkers for FAK‐targeted therapies in non small cell lung cancers.5
FAK is a cytoplasmic tyrosine kinase activated by growth factors and integrins. Alternative splicing of FAK pre‐mRNA allows the inclusion, independently of each other, of the exons 13, 14, 16, and 31, respectively coding for the boxes 28, 6, 7, and Pro‐Trp‐Arg (PWR, characterizing FAK+ which has only been described in neurons)6 (Fig. 1). The most common form FAK0, ubiquitously expressed, contains none of these four exons. The FAK28 splice variant, including exon 13 coding for a peptide fragment of 28 amino acids (box 28), juxtaposed in N‐terminal to the box 67 displays a pattern of progressive increased expression toward adulthood, but the box 28 function in the regulation of FAK remains unknown.6 FAK6 and FAK7 splice variants, respectively including exons 14 or 16, have a peak of expression at the final stages of the embryonic development. From a functional point of view, the inclusion of boxes 6 and/or 7 results in constitutively active FAK protein isoforms8 that could strongly enhance the activity of Src kinases, a proximal signaling partner of FAK, which plays a key role in the metastasis process.9 Thus, depending on the association of specific exons (e.g. FAK6,7,28), the expression of some splice variants can be either ubiquitous or tissue specific, stage specific or detected throughout the lifetime. So far, FAK splice variants have been mainly studied in the brain. However FAK6,7, FAK28,6,7 and FAK28 have been found in the liver, a tissue of endodermal origin like the intestine. Importantly and as previously mentioned, our previous work in leukemia4 and the recent data obtained by Zhou and collaborators in non small cell lung cancer,5 both demonstrated that FAK6 expression is related to the tumor state and correlated to an aggressive tumor phenotype in patients.
Figure 1.

Domain composition of FAK. The protein sequence is indicated without the alternatively spliced exons, which are represented as boxes above the sequence, with the corresponding number of amino acids that they insert (28, 6, 7 or 3). The N‐terminal band FERM domain is indicated in yellow, the catalytic tyrosine kinase domain is shown in red, the Proline‐Rich regions are shown in blue and the FAT domain in green. The positions of the autophosphorylated tyrosine residues (397) and those of other tyrosines phosphorylated by p60‐Src kinases are indicated in orange under the protein sequence.
In addition to its enzymatic activity, FAK is also a scaffold protein allowing the interaction between integrins, components of the focal contacts and the actin network, and thus functions as a biosensor to control cell adhesion and the interaction of the cell with its environment. Consequently, FAK is a major player in cell migration and invasion. Colorectal cancer (CRC) is the third cause of cancer‐related death, mainly due its high metastatic potential. In the colon, this kinase plays critical roles in regeneration and tumorigenesis.10 After the tissue damage in the colon, FAK is overexpressed and is required for stem cell and progenitor proliferation and migration.10 Ashton et al. also reported that the dysregulation of the Wnt/β‐catenin pathway activates c‐myc that, in turn, promotes FAK gene transcription. Importantly, FAK regulates pathways such as APC/β‐catenin, Raf/Ras, PI3K/mTOR and BMP/Smad, which are all dysregulated in CRC. Moreover, FAK overexpression is an independent bad prognostic factor in colorectal cancer.11 Clinical data report that FAK mRNA and protein expression levels are increased in CRC primary tumors and their matched metastases in the liver when compared to normal colorectal mucosa.12 Importantly, in unmatched specimens, FAK protein is overexpressed in metastases compared to primary tumors, whereas in matched samples its expression was equivalent.12 This suggests that primary tumors with higher FAK expression are more efficient to produce liver metastases in line with the known role of FAK in the promotion of cell migration and invasion.13
In order to decipher the putative different involvement of FAK splice variants along CRC processes, we investigated the pattern of expression of FAK spliced mRNA variants at both the primary tumor site and in liver metastasis, using a pathophysiological murine model of primary and metastatic CRC.14
Material and Methods
Human cell lines and tissues
The human colon carcinoma cell lines HT29, HCT116, SW480 and SW620, were purchased from the American Type Culture Collection (ATCC). While HT29, HCT116 and SW480 cell lines were established from primary tumors, SW620 cell line was established from a lymph node metastasis from a colorectal cancer patient (same patient as for SW480). All cell lines were transduced for TdTomato (fluorescence) and luciferase (bioluminescence) expressions and kindly provided by Vectalys® company. These cells are indicated as “cell line name‐ T‐Luc” in the study. Cell lines were maintained at 37°C and 5% CO2 in Dulbecco's Modified Eagle Medium (Gibco, Life Technologies) supplemented with 10% heat‐inactivated fetal calf serum (Gibco, Life Technologies), 2 mM glutamine (Gibco, Life Technologies).
Human colon resection samples were obtained from patients suffering from colorectal cancer and treated at the Toulouse University Hospital. Patients gave informed consent, and tissues samples were included in the registered COLIC collection (DC‐2015‐2443). Tissues used in our study were harvested in healthy normal zones, at least 10 cm away from the tumor.
Mice
SCID Beige were purchased from Charles River Laboratoire (France CRLF) and maintained in Toulouse‐Purpan animal facilities. Female mice at 7 weeks of age were used. For the guidelines on animal welfare, we followed the European directive 2010/63/EU. For administrations, including routes and volumes, we followed the good practice guide published in 2001. The ethics committee has approved our protocol (2015‐U1043‐CD‐20).
In vitro imaging of cell lines
Cell lines were plated at 1x105 cells per well in black 96‐well plate overnight. Luciferase substrate, d‐luciferin, was added at 0.3 mg/well in phosphate saline buffer (PBS), 5 min before imaging with a highly sensitive cooled charge‐coupled device camera (IVIS Spectrum Lumina II; Perkin Elmer). Wells were then rinsed with PBS and fluorescent acquisition was performed for Tomato fluorescence emission (excitation: 535 nm, emission: 580 nm) with the IVIS camera (Supporting Information Fig. 1). Quantity of bioluminescent or fluorescent photons emitted by cells was measured in well‐surrounding region of interest (ROI) in plates, using Living Image® 4.4 software (Perkin Elmer). Bioluminescence was quantified in photons (ph)/sec(s)/cm2/steradian (sr) and fluorescence was quantified in [ph/cm2/s/sr]/[υW/cm2] using the Living Images version 4.5.5. (Perkin Elmer).
Transplantation and growth of human tumors in mice
Orthotopic colon tumors were established by injecting 1 × 106 colon carcinoma cells subsereously in the caecum (IC) in 20 μL of PBS as previously described15. Progression of luciferase‐expressing tumors was monitored using bioluminescent imaging performed with IVIS camera (IVIS Spectrum Lumina II; Perkin Elmer) and intraperitoneal (IP) injection of 100 μL d‐luciferin (OZ Biosciences) in 30 mg/mL of PBS.15 Mice were sacrificed as overt signs of pain and stress. Primary caecum tumors, livers and lungs were then harvested and placed in 6‐well plates. Organs were imaged for their Tomato fluorescence emission with IVIS camera. Quantity of bioluminescent or fluorescent photons emitted by tumors was measured in total mice region of interest (ROI) for mice analysis and in well‐surrounding ROI for plates organs analysis, using Living Image® 4.4 software (Perkin Elmer). Bioluminescence was quantified using the Living Images version 4.5.5. (Perkin Elmer) in photons (ph)/sec(s)/cm2/sr and fluorescence was quantified in [ph/cm2/s/sr]/[υW/cm2].
Polymerase chain reaction (PCR) and capillary electrophoresis
For in vitro cell lines analysis, the four different cell lines were harvested from tissue culture, rinsed in PBS and lysed. For in vivo tumors analysis, primary‐caecum tumors, livers and lungs were excised from mice at various time points, snap‐froze, crushed and lysed. RNA was extracted from all tissues using the NucleoSpin® RNA/Protein kit (Macherey‐Nagel) following the manufacturer instruction. RNA (3 μg) was reverse transcribed using Maxima First Strand cDNA synthesis kit for RT‐PCR (ThermoFisher Scientific). The resulting cDNA was used for amplification. PCR were performed using Fast cycling PCR kit (Qiagen) in a reaction volume 20 μL using 3 μL of cDNA (1/5 dilution), 10 μL of 2X PCR Buffer and 0.5 μM of primers specific for FAK mRNA alternatively spliced variants. The following primers were used: FAK0/ FAK7 human (h) primers (forward 3 (F3): 5′‐TCTCTGTGTCAGAAACAGATGATT3′‐5‐Carboxyfluorescine (FAM)‐coupled reverse 2 (R2): 5′CTGACGCATTGTTAAGGCTTC3 ′), FAK28/ FAK7,28 hprimers (F4: 5′CTCCTTCTACGGAAACAGATGATT3′‐ FAM‐R2), FAK6/ FAK6,7 hprimers (F5: 5′TCTCTGTGTCAGATGAAATTAGTGG3′‐FAM‐R2) and FAK6,28/ FAK6,7,28 hprimers (F6: 5′CTCCTTCTACGGATGAAATTAGTG3′‐FAM‐R2). Fast cycling PCR amplification consists in initial denaturation step (95°C for 5 s) followed by 35 cycles of amplification (96°C 5 s, 58°C 5 s and 68°C 9 s) and a final extension of 1 min at 72°C.
PCR products were analyzed in a genetic analyzer ABI 3130xl (Applied Biosystems, Saint Aubin, France). Briefly, 1 μL of PCR product (pure, 1/10 or 1/20 dilutions) was mixed with 8.7 μL of Hi‐Di formamide (Applied Biosystems) and 0.3 μL of GS‐500‐ROX (DNA Size Marker with fragments ranging from 35 to 500 bp used to size PCR products, ThermoFisher Scientific). Analysis were performed with Peak Scanner 2® software. FAK mRNA variants were independently detected as single peak at the corresponding product size (see Supporting Information Fig. 2 as an example): for F3‐FAMR2: FAK0 = 178 bp and FAK7 = 199 bp, F4‐FAMR2: FAK28 = 178 bp and FAK7,28 = 199 bp, F5‐FAMR2: FAK6 = 196 bp and FAK6,7 = 217 bp, F6‐FAMR2: FAK6,28 = 196 bp and FAK 6,7,28 = 217 bp.
Quantitative real‐time PCR
Quantitative (q)RT‐PCR was performed on the capillary electrophoresis‐detected FAK mRNA variants using LightCycler 480 SYBR Green I Master (Roche, Boulogne‐Billancourt, France). Briefly, per sample 45 ng of cDNA was mixed with 5 μL of Syber Green Mix and 0.5 μM of the following primers: hF3‐R2 (for FAK0), hF4‐R2 (5′ATTTCTCTCTCACGCTGTCC3′) (for FAK28), hF5‐R2 (for FAK6) and forward hHPRT primer (CCTGGCGTCGTGATTAGTGA) and reverse hHPRT primer (CGAGCAAGACGTTCAGTCCT) (for housekeeping gene). hHPRT has been validated as specific for Homo sapiens that does not cross‐react with Mus musculus (data not shown). qPCR reaction was performed for 45 cycles in a LightCycler 480 (Roche) and analyzed using LightCycler 480 SW1.5.1 software.
Statistical analysis
Results are expressed as the mean ± S.E.M. The difference in tumor growth and qPCR were analyzed by one‐way or two‐way ANOVA. When two columns of data have been compared, the Student t test has been used. A p value <0.05 was considered significant.
Results
CRC tumor development after SW480, SW620, HT29 and HCT116 cell lines orthotopic implantation in SCID beige mice
The four CRC human cell lines (SW480, SW620, HT29 and HCT116) are known for their differential growth and invasive capacities following intracaecal (IC) xenografting in immunodeficient nude mice.16, 17 However, the immunodeficient mouse models previously used displayed a limited metastatic potential of the tumor cells due to the presence of natural killer (NK) cells. In order to favor the metastatic process by eliminating NK antitumor functions, we used here SCID beige mice. The four cell lines, emitting similar levels of either luminescent or fluorescent signals in vitro (Supporting Information Fig. 1), were injected IC and tumor development was monitored over time using an in vivo imaging system allowing noninvasive monitoring of the disease progression in living animals.
All the four implanted CRC cell lines produced solid growing tumors, as detected by the measure of the luminescence emissions at the indicated days post cells engraftment (Fig. 2 a), but with different growth rates while the same number of cells was originally engrafted in the caecum (Fig. 2 b). Both SW480‐ and SW620‐induced tumors grew at slow rate during the full 35 days of follow‐up. HT29‐induced tumors grew at a similar slow rate until approximatively days 25–28 but then displayed a rapid growth until the end‐point of the experiment. HCT116‐induced tumor grew faster than the ones generated by the other cell lines but the growth rate remained stable passed day 20/25. The decrease in the luminescence signal in HCT116 tumors might be due to the development of hypoxic area in tumors associated with their rapid growth. Indeed, the Firefly luciferase activity is highly oxygen‐dependent since it uses both oxygen and adenosine triphosphate to catalyze the emission of light from luciferin. Since solid tumors are known to develop hypoxic area while growing, the availability of the oxygen for the luciferase is limited, reducing the bioluminescence emission and leading to an underestimation of the number of alive luciferase‐expressing tumor cells in vivo (Fig. 2 b).
Figure 2.
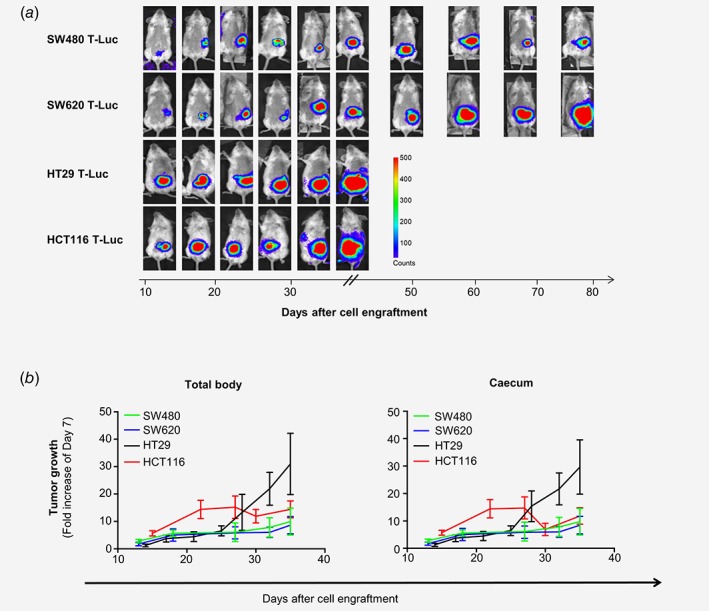
Comparative tumor growth of four human colon carcinoma cell lines after orthotopic implantation in SCID beige mice. SCID Beige mice were implanted intracaecally (IC) with 1 × 106 SW480 T‐Luc, SW620 T‐Luc, HT29 T‐Luc or HCT116 T‐Luc cells. Bioluminescent emission imaging allowed the monitoring of tumor development along the time. (a) General mice status: Representative bioluminescent imaging of one mouse per group from day 10 after implantation. Imaging was followed up until day 80 for SW480 T‐Luc and SW620 T‐Luc cell lines. Setting is applied in counts. (b) Comparative time‐course of tumor growth: Bioluminescence emitted by tumors was quantified in total body regions of interest (ROI) and caecum ROI and then normalized as fold increase of emission at day 7 after implantation. n = 5–7 mice per group, average ± S.E.M.
In order to discriminate between the primary tumor and the metastasis capacities of the different cell lines, we performed an ex vivo analysis of the TdTomato fluorescence of the different organs. In the caecum, this analysis confirmed the previous observations of tumor progression obtained by bioluminescence emission analyses (Fig. 3). Concerning liver and lung metastases development, the four CRC cell lines behaved differently. No TdTomato fluorescence was detected in the liver and lungs of the SW480‐IC‐injected mice. In the SW620‐IC‐engrafted mice, liver metastases were detected from day 80. In HT29‐IC‐engrafted mice, TdTomato fluorescence was detected from day 35 in the liver but only traces of metastases could be detected in the lungs from day 45. Finally, regarding HCT116 cells, liver and lung metastases were clearly detected as early as day 35 highlighting the high aggressiveness capacities of this CRC cell line. By comparison with HCT116, only a small amount of liver metastases was detected at days 45 and 80 for HT29 and SW620, respectively (Fig. 3).
Figure 3.
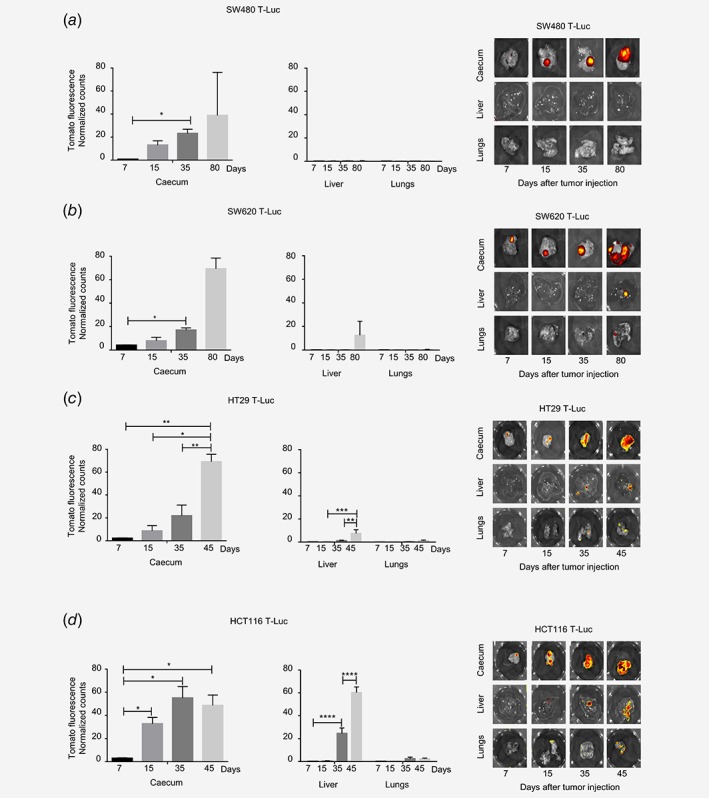
Different metastatic potential of the four human colon carcinoma cell lines implanted in SCID beige mice. Primary tumors (caecum) and organs with metastases (liver, lungs) were harvested from SW480 T‐Luc (a), SW620 T‐Luc (b), HT29 T‐Luc (c) and HCT116 T‐Luc (d) tumor‐bearing mice at various cancer development time‐points. Counts of TdTomato fluorescence were normalized on in vitro quantification for each cell line (n = 3–4 mice, average ± S.E.M.). Representative fluorescence imaging of one mouse per group is shown. ****p < 0.0001, ***p < 0.0005, **p < 0.005, *p < 0.05.
Thus, our data obtained from the orthotopic CRC model in SCID beige mice demonstrate that HCT116 and HT29 cells are more aggressive, with higher proliferative and invasive properties, compared to SW620 and SW480 cells.
Expression of FAK alternative splice variants mRNA in SW480, SW620, HT29 and HCT116 CRC cell lines
Folllowing the characterization of the metastatic potential of the four human CRC cell lines in our model, we first investigated the expression of FAK splice variants in those cells in vitro, before their engraftment in mice. Our aim was to characterize the in vitro basal intrinsic regulation of the FAK mRNA splice variants expression independently from an expression regulation potentially controlled by the microenvironment in vivo.
The RT‐PCR products for the following human FAK mRNA splice variants: FAK0, FAK6, FAK7, FAK28, FAK6,7, FAK6,28, FAK7,28, FAK6,7,28 were analyzed for the four CRC cell lines using capillary electrophoresis, since this technique is highly sensitive and allows the specific discrimination of the RT‐PCR products based on the base pair sizes. As shown in Table 1, FAK0, FAK6 and FAK28 were the only three mRNA variants detected in the four tested CRC cell lines.
Table 1.
Analysis by capillary electrophoresis of the pattern of FAK alternative splice variant mRNAs expression in the four human CRC cell lines (+: Variant detected, −: Variant not detected)
| FAK variants CRC cell lines | 0 | 7 | 28 | 7,28 | 6 | 6,7 | 6,28 | 6,7,28 |
|---|---|---|---|---|---|---|---|---|
| SW480 T‐Luc | + | − | + | − | + | − | − | − |
| SW620 T‐Luc | + | − | + | − | + | − | − | − |
| HT29 T‐Luc | + | − | + | − | + | − | − | − |
| HCT116 T‐Luc | + | − | + | − | + | − | − | − |
The quantification of FAK0, FAK6 and FAK28 mRNA was then performed by qRT‐PCR. Considering that the SW480 cell line presents the lowest aggressiveness capacity, it was chosen as reference of the basal level of FAK splice variant expression for the comparison with the three other cell lines (Figs. 4 and 5).
Figure 4.
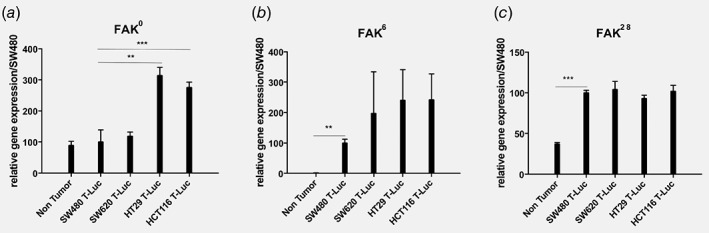
Quantitative analyses for FAK0, FAK6 and FAK28 mRNA variants expression in vitro in normal epithelial cells and the four‐tumor colon cell lines used in our study. qRT‐PCR was performed to quantify the expression of (a) FAK0 (b) FAK6 and (c) FAK28 mRNA in non tumoral colon epithelial cells isolated from tumor‐bearing patients (n = 5, average ± S.E.M.) and in SW480 T‐Luc, SW620 T‐Luc, HT29 T‐Luc and HCT116 T‐Luc tumor cell lines from in vitro tissue culture (n = 6, average ± S.E.M.). Relative Expression of all mRNA has been normalized on the relative expression of SW480: ***p < 0.001, **p < 0.01.
Figure 5.
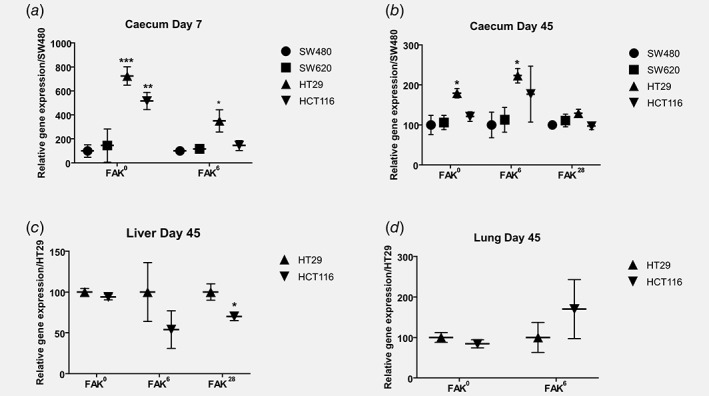
Quantitative analyses for FAK0, FAK6 and FAK28 mRNA variants expression at early and late steps of human colon tumors development in an in vivo mouse model. qRT‐PCR was performed to quantify the expression of FAK0, FAK6 and FAK28 mRNA variants from caecum primary tumors, livers and lungs metastases samples harvested early at day 7 (caecum‐a) or late at days 45 (caecum‐b; liver‐c and lungs‐d) after the orthotopic implantation of tumor cell lines as described in Material and Methods. Relative Expression of all mRNA has been normalized and statistics have been calculated in comparison to the relative expression of mRNAs in SW480 (a and b) or mRNAs in HT29 (c and d): ***p < 0.0005, **p < 0.01, *p < 0.05.
The mRNA of FAK0, the common form of FAK, was highly expressed in the aggressive cell lines HT29 and HCT116 compared to the less invasive cell lines SW620 and SW480, and to primary non tumoral colon cells isolated from normal tissue distant from the primary tumor site in CRC patients colon resections (Fig. 4). FAK6 and FAK28 mRNA expressions did not significantly differ between the four cell lines but were highly expressed in the four CRC cell lines compared to non tumoral cells which expressed low level of FAK28 and only traces of FAK6 (Fig. 4).
Altogether, these data show that CRC cell lines are characterized by a high level of FAK6 and FAK28 transcripts compared to normal (non tumoral) colon cells. Moreover, the CRC cell lines with the highest invasive potential display high levels of FAK0 associated with FAK6 and FAK28 transcripts.
Expression of FAK0, FAK6 and FAK28 mRNA splice variants in CRC primary tumors and in distant metastases after orthotopic implantation of CRC cell lines
After the intracaecal orthotopic implantation of SW480, SW620, HT29 and HCT116 CRC cell lines, human FAK mRNA spliced variants FAK0, FAK6 and FAK28 were quantified by qRT‐PCR within caecum, liver and lung samples. Importantly, as observed for the in vitro cell lines, only FAK0, FAK6 and FAK28 were expressed in vivo as assessed using PCR followed by capillary electrophoresis performed on caecum, liver and lungs extracted at various time‐points after HT29 and HCT116 engraftment (Supporting Information Table I). Here we decided to perform the analysis only on the most aggressive cell lines, which displayed the highest levels of expression for these splice variants.
In line with our results on the expression measured in the cell lines in vitro, FAK0 expression level in primary tumors (caecum) was higher in HT29 and HCT116 cells compared to SW480 and SW620 cells at day 7 post‐engraftment (Fig. 5 a). At day 45, the FAK0 transcript expression level in caecum‐tumors was only significantly higher in HT29 compared to the other cell lines (Fig. 5 b). Regarding FAK6 transcript in the primary tumors, at day 7 after engraftment, the expression was increased in HT29 cells compared to other cell lines (Fig. 5 a). At day 45, a stage where liver metastases from the HCT116 and HT29 cell lines were well established (Fig. 3), FAK6 expression level was significantly increased in tumor samples obtained from HT29 cells, and tends to be upregulated in HCT116 cells compared to non‐invasive SW480 and less‐invasive SW620 cells (Fig. 5 b). FAK28 mRNA from caecum samples was only measurable at day 45 using qRT‐PCR (despite earlier traces detected by capillary electrophoresis, Supporting Information Table I) with no significant differences between the tumor samples obtained from the different CRC cell lines (Fig. 5 b).
At day 45, metastases were only detected in mice grafted with HT29 or HCT116 cells (Fig. 3), and FAK0 and FAK6 transcripts are measured in both liver and lungs metastases while the expression of FAK28 was only found in liver metastases (Figs. 5 c and 5d).
Altogether, these results confirmed an association between FAK0 and FAK6 expressions and the aggressiveness of HT29 and HCT116 cells.
Discussion
The non receptor tyrosine kinase FAK is now well described to play a crucial role in several steps of cancer processes, driving either tumor growth or metastasis.18 FAK involvement in metastasis process implies the regulation of tumor cell motility and invasion via the control of focal adhesion, cytoskeletal dynamics or the modulation of matrix metalloproteinase expression and thus, the interaction of the tumor cells with their microenvironment.18 Moreover, FAK also regulates cell survival as well as cancer‐stem cell renewal, two crucial events for the development of secondary tumors.18
During the 1990s, a new level of FAK regulation has been found with the identification of several FAK splice variants generated after alternative splicing, as described above.7, 19 In our study, the expression of FAK mRNA splice variants comprising exons 13 (FAK28), 14 (FAK6) or none (FAK0) was investigated in four human colorectal cancer cell lines with different invasive properties, in vitro and in vivo after orthotopic xeno‐engraftment of these cells in the caecum of SCID beige mice. The combination of qRT‐PCR and capillary electrophoresis approaches allowed us to obtain data with high sensitivity and specificity, and to exclude other FAK mRNA splice variants (FAK7, FAK6,7, FAK6,28, FAK7,28, FAK6,7,28) from our analyses.
FAK0, coding for the common FAK isoform, was initially known to be the most abundant form of the kinase in tissues, including the colon. In the present study, before (in vitro) and after (in vivo) the cells engraftment, FAK0 mRNA was highly expressed in CRC tumor cells and was specifically increased in the two more aggressive cell lines, namely HT29 and HCT116. The elevated growth and invasion capacities of these two cell lines have been demonstrated by others16, 17 and were confirmed in our study.
Regarding FAK6 and FAK28, both isoforms were found overexpressed in the SW480, SW620, HT29 and HCT116 cell lines compared to human normal epithelial cells, independently of their histological grade and genetic mutations. The leading cause of death among CRC patients is distant metastases whose formation can be promoted by FAK and its signaling partner p60‐Src kinase through an epithelial–mesenchymal transition.20 FAK6 splice variant differs from FAK0 by the alternative addition of spliced exons near the exon 15 coding for the autophosphorylation site Tyrosine 397. Interestingly, FAK6 has been described with increased phosphorylation of this tyrosine that represents a binding site for Src kinases.7 One month after their orthotopic engraftment in mice caecum, HCT116 and HT29 cells generated metastases in liver and lungs, in line with the pathophysiological spreading of CRC in human. At day 45, both FAK0 and FAK6 mRNA were found overexpressed in HCT116 and HT29 primary tumors compared to nonaggressive cell lines, and were also detected in their liver and lung metastases. These data suggest a putative implication of FAK0 and FAK6 in the CRC metastatic process and corroborate the association between those isoforms and the tumor aggressiveness demonstrated in other tumor models such as acute myeloid leukemia or nonsmall cell lung cancer.4, 5 As demonstrated for these two cancers, we could hypothesize that FAK0 and FAK6 may represent potential aggressiveness markers also in CRC.
Regarding FAK28, while its expression was detected in vitro, once in vivo, this splice variant is not found anymore in the cells forming the primary tumor at early stage (day 7). However, its expression is again detected in late primary tumors (Day 45) and subsequent liver metastasis only originating from the most aggressive cell lines. The fact that FAK28 is no more detected at the very early stage of the tumor process suggests that this isoform is not crucial for the initial tumor mass development. However, its detection in in vitro cell tissue‐culture on plastic plates, where cells display strong physical interactions with their support, as well as in advanced primary tumors and their associated metastasis, when cells must develop new interaction capacities with their environment in order to migrate and invade the tissues. This suggests a putative more specific role of this FAK isoform in the interaction of the cell with its support. Interestingly, the box 28 coded by exon 13, maintained in the FAK28 mRNA variant, exhibits an inversed consensus sequence for calmodulin, which could favor the binding of FAK to cell adhesion molecules.21 These data suggest an eventual role of this splice variant in the regulation of cells with their direct environment by the modulation of the functions of cell adhesion molecules. Compared to other cell lines, in HT29 cells, FAK6 mRNA is increased shortly after engraftment in the caecum. On the other hand, FAK28 mRNA is measured in liver metastases and not detected in lung metastases. Further studies would be needed to elucidate putative differential role of the FAK mRNA splice variants depending on microenvironmental niche of tumor cells. Furthermore, beside its potential role in the migration of tumor cells to the liver, a possible association of FAK28 with cell adhesion molecules might be involved in the immune evasion. Indeed, it has been demonstrated that in response to stimulation of the adhesion molecule NCAM, a FAK N‐terminal fragment together with a C‐terminal fragment of the adhesion molecule cotranslocate into the nucleus.22 Moreover a nuclear FAK activity has been described to regulate chemokine and cytokine expressions in tumor cells leading to a control of tumor Treg levels as well as antitumor immune evasion.23 So far, the regulation processes and functional roles of the FAK28 variant are unknown and our work highlights for the first time the potential significance of FAK28 in cancer.
In conclusion, although targeting the common form of FAK, aka FAK0, might not be a good strategy based on the numerous role of this kinase in physiological processes, FAK6 or FAK28 could represent interesting therapeutic targets in CRC and justify to be further investigated in vitro and in vivo, possibly with new tools that would specifically block each isoforms.
Supporting information
Supplementary Figure 1 Comparison of the bioluminescent and fluorescent signals emitted by the four human colon carcinoma cell lines in vitro. Bioluminescence (A) was quantified in photons (ph)/sec(s)/cm2/sr (sr) and fluorescence (B) was quantified in [ph/cm2/s/sr]/[υW/cm2], from in vitro culture of the four cell lines expressing Luciferase and TdTomato. (n = 5, average mean ± S.E.M.).
Supplementary Figure 2 Example of capillary electrophoresis analysis for FAK mRNA variants expression in HT29‐TLuc cells. RT‐PCR (using F3‐R2FAM primers for FAK0) and electrophoresis capillary have been performed on HT29 T‐luc cells from in vitro tissue culture as described in Material and Methods. Representative graph of analysis, using Peak Scanner 2® software, shows the corresponding fluorescent peak for FAK0 PCR product.
Supplementary Table 1 Analysis by capillary electrophoresis of the expression of FAK mRNA alternative splice variants in tumors, after IC‐engraftment of HT29 T‐Luc and HCT116 T‐Luc cells (+: Variant detected, −: Variant not detected).
Acknowledgements
The authors thank the patients who agree on supporting our research by giving their consent, Jean‐Antoine Girault and Gilles Dietrich for helpful discussion and advices on the study. This study was supported by European Research Council funds (ERC‐310973 PIPE to NV), The Ligue régionale contre le cancer (Grant to CRS), and The Région Midi‐Pyrénées/Occitanie and Urosphère supported the post‐doctoral salary of CD (Grant to AF). IVIS spectrum used in our study was supported by Equipex “Investissements d'avenir” funds (ANR‐11‐EQPX‐0003).
This manuscript is dedicated to Alix Vignolle‐Vidoni, who passed away during the reviewing process.
References
- 1. Zheng CL, Fu XD, Gribskov M. Characteristics and regulatory elements defining constitutive splicing and different modes of alternative splicing in human and mouse. RNA 2005;11:1777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelemen O, Convertini P, Zhang Z, et al. Function of alternative splicing. Gene 2013;514:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jyotsana N, Heuser M. Exploiting differential RNA splicing patterns: a potential new group of therapeutic targets in cancer. Expert Opin Ther Targets 2018;22:107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Despeaux M, Chicanne G, Rouer E, et al. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells 2012;30:1597–610. [DOI] [PubMed] [Google Scholar]
- 5. Zhou B, Wang GZ, Wen ZS, et al. Somatic mutations and splicing variants of focal adhesion kinase in non‐small cell lung cancer. J Natl Cancer Inst 2018;110. doi: 10.1093/jnci/djx157. [DOI] [PubMed] [Google Scholar]
- 6. Corsi JM, Rouer E, Girault JA, et al. Organization and post‐transcriptional processing of focal adhesion kinase gene. BMC Genomics 2006;7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgaya F, Toutant M, Studler JM, et al. Alternatively spliced focal adhesion kinase in rat brain with increased autophosphorylation activity. J Biol Chem 1997;272:28720–5. [DOI] [PubMed] [Google Scholar]
- 8. Toutant M, Costa A, Studler JM, et al. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol Cell Biol 2002;22:7731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cordero JB, Ridgway RA, Valeri N, et al. C‐Src drives intestinal regeneration and transformation. EMBO J 2014;33:1474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashton GH, Morton JP, Myant K, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c‐Myc signaling. Dev Cell 2010;19:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garouniatis A, Zizi‐Sermpetzoglou A, Rizos S, et al. FAK, CD44v6, c‐met and EGFR in colorectal cancer parameters: tumour progression, metastasis, patient survival and receptor crosstalk. Int J Colorectal Dis 2013;28:9–18. [DOI] [PubMed] [Google Scholar]
- 12. Lark AL, Livasy CA, Calvo B, et al. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real‐time PCR analyses. Clin Cancer Res 2003;9:215–22. [PubMed] [Google Scholar]
- 13. Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed) 2014;19:687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pocard M, Tsukui H, Salmon RJ, et al. Efficiency of orthotopic xenograft models for human colon cancers. In Vivo 1996;10:463–9. [PubMed] [Google Scholar]
- 15. Devaud C, Bilhere E, Loizon S, et al. Antitumor activity of gammadelta T cells reactive against cytomegalovirus‐infected cells in a mouse xenograft tumor model. Cancer Res 2009;69:3971–8. [DOI] [PubMed] [Google Scholar]
- 16. de Vries JE, Dinjens WN, De Bruyne GK, et al. In vivo and in vitro invasion in relation to phenotypic characteristics of human colorectal carcinoma cells. Br J Cancer 1995;71:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajput A, Dominguez San Martin I, Rose R, et al. Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res 2008;147:276–81. [DOI] [PubMed] [Google Scholar]
- 18. Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derkinderen P, Toutant M, Burgaya F, et al. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science 1996;273:1719–22. [DOI] [PubMed] [Google Scholar]
- 20. Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial‐to‐mesenchymal transition. Curr Opin Cell Biol 2005;17:542–7. [DOI] [PubMed] [Google Scholar]
- 21. Armendariz BG, Masdeu Mdel M, Soriano E, et al. The diverse roles and multiple forms of focal adhesion kinase in brain. Eur J Neurosci 2014;40:3573–90. [DOI] [PubMed] [Google Scholar]
- 22. Kleene R, Mzoughi M, Joshi G, et al. NCAM‐induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J Neurosci 2010;30:10784–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serrels A, Frame MC. FAK goes nuclear to control antitumor immunity‐a new target in cancer immuno‐therapy. Oncoimmunology 2016;5:e1119356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Comparison of the bioluminescent and fluorescent signals emitted by the four human colon carcinoma cell lines in vitro. Bioluminescence (A) was quantified in photons (ph)/sec(s)/cm2/sr (sr) and fluorescence (B) was quantified in [ph/cm2/s/sr]/[υW/cm2], from in vitro culture of the four cell lines expressing Luciferase and TdTomato. (n = 5, average mean ± S.E.M.).
Supplementary Figure 2 Example of capillary electrophoresis analysis for FAK mRNA variants expression in HT29‐TLuc cells. RT‐PCR (using F3‐R2FAM primers for FAK0) and electrophoresis capillary have been performed on HT29 T‐luc cells from in vitro tissue culture as described in Material and Methods. Representative graph of analysis, using Peak Scanner 2® software, shows the corresponding fluorescent peak for FAK0 PCR product.
Supplementary Table 1 Analysis by capillary electrophoresis of the expression of FAK mRNA alternative splice variants in tumors, after IC‐engraftment of HT29 T‐Luc and HCT116 T‐Luc cells (+: Variant detected, −: Variant not detected).


