Abstract
Aims
After gestational diabetes, many women exhibit behaviours that increase their risk of developing Type 2 diabetes. We aimed to systematically synthesize the literature that focuses on the views of women with a history of gestational diabetes on reducing their risk of developing diabetes postpartum through lifestyle and behaviour changes.
Methods
We identified qualitative studies that examined the views of women with a history of gestational diabetes towards healthy eating and physical activity, Type 2 diabetes risk management or their experience of a diabetes prevention programme, and conducted a thematic synthesis to develop descriptive and then analytical themes. We also evaluated the quality of each study and the confidence that we had in our findings.
Results
We included 21 articles after screening 23 160 citations and 129 full texts. We identified six themes of interacting influences on postpartum behaviour: role as mother and priorities; social support; demands of life; personal preferences and experiences; risk perception and information; and finances and resources (plus preferred format of interventions). These factors inhibited many women from addressing their own health, while they motivated others to persevere. We also developed 20 recommendations, most with high or moderate confidence, for effective promotion of healthy lifestyles in this population.
Conclusions
Many factors hinder healthy lifestyles after gestational diabetes, yet how women interpret them can motivate or prevent changes that reduce diabetes risk. As our recommendations emphasize, women's experiences and needs should be considered when designing strategies to promote healthier lifestyles in this population.
What's new?
After having had gestational diabetes, many women do not adopt healthy lifestyles that would reduce their risk of developing Type 2 diabetes.
We found, in summary, that women identified themselves primarily as mothers who prioritized their family above themselves, and needed resources, time, energy, information and support to encourage healthy diets and levels of activity.
Based on these findings, we developed 20 recommendations for effectively promoting healthy lifestyle in this population. These recommendations highlight the need for interventions to be centred on women's needs and experiences.
What's new?
After having had gestational diabetes, many women do not adopt healthy lifestyles that would reduce their risk of developing Type 2 diabetes.
We found, in summary, that women identified themselves primarily as mothers who prioritized their family above themselves, and needed resources, time, energy, information and support to encourage healthy diets and levels of activity.
Based on these findings, we developed 20 recommendations for effectively promoting healthy lifestyle in this population. These recommendations highlight the need for interventions to be centred on women's needs and experiences.
Introduction
Gestational diabetes (GDM) is a common disorder of pregnancy and the single most important risk factor for the development of Type 2 diabetes 1, 2, 3. It is defined as diabetes with an onset or first diagnosis during pregnancy and increases the risk of adverse pregnancy outcomes for both mother and baby 4. Many find it distressing, with the shock of diagnosis followed by self‐blame and anxiety for the unborn baby or, for some, motivation to take control of their health during pregnancy such as managing GDM by following advised lifestyle changes 5, 6.
Glucose control typically returns to normal after delivery and maternal care tends to focus on regular screening for diabetes 4, 7. Postpartum health behaviours (specifically healthy diet and physical activity) are strongly associated with diabetes risk; however, most women do not attempt behaviour change, instead maintaining lifestyles that increase their risk 8. In the UK, women are managed according to the guidelines for preventing Type 2 diabetes 4. These include referral to weight‐loss or exercise programmes 9. Such programmes were developed for the general population, which tends to be older and not to have young families. Current evidence also shows that interventions to prevent Type 2 diabetes after GDM can have positive, but sometimes limited, effects if engagement is poor 10, 11, 12. Notably, there was ~50% lower incidence of diabetes after GDM in the Diabetes Prevention Programme (DPP) after intensive lifestyle and metformin intervention compared with placebo after 3 years 13, indicating potential benefits of behavioural interventions on diabetes outcomes.
Previous qualitative or mixed methods reviews have explored women's postpartum views on reducing diabetes risk as part of broader investigations into their experience of GDM 6, 14, 15, 16. A wide variety of views and determinants have been presented, including positive attitudes towards behaviour change, particularly when it is understood to reduce diabetes risk and when women have support and self‐efficacy for change. Changes can be prevented by lack of information, support, time and help with childcare. To date, however, no comprehensive review has focused on postpartum lifestyle.
We have systematically synthesized the literature reporting the views of women with a history of GDM on reducing their risk of developing diabetes, including women participating in interventions. These findings help to identify gaps in our understanding of the acceptability, feasibility and practicality of intervening postpartum and will subsequently inform the development or tailoring of effective approaches for this easily identifiable, high‐risk population.
Methods
Details of the review protocol were registered on PROSPERO (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=82049).
Search strategy
We searched MEDLINE, Embase, PsychINFO, CINAHL and the Cochrane Library electronic databases in September 2017 as part of a group of literature reviews concerning GDM using the search strategy shown in Table S1. No language or other restrictions were applied. Reference lists of included studies were screened for citations not identified by this search.
Study selection
Our predefined selection criteria included studies published in peer‐reviewed journals that examined women's experiences of healthy eating and physical activity after GDM, views on diabetes risk management, or experience of a diabetes prevention programme. All qualitative methods were eligible, including mixed methods. Views of healthcare providers and about postpartum diabetes screening were excluded in order to focus on lifestyle.
After deduplication, all titles and abstracts were assessed against the selection criteria by R.D. or R.W. Both authors reviewed ~10% of the citations to ensure agreement. Any differences were discussed, and the selection criteria were refined and elaborated in conjunction with the other authors so that they could be applied consistently. Full‐text articles were then acquired and rechecked against these criteria by R.D. J.U‐S. reviewed all included articles as well as those excluded for reasons other than article type, and agreed with the classification.
Quality assessment
With discussion with the other authors, R.D. assessed the quality of each study's qualitative findings against the Critical Appraisal Skills Programmes (CASP) checklist for qualitative research 17. No studies were excluded based on quality.
Qualitative synthesis
Data were defined as text or tables labelled as ‘Results’ (or equivalent) that arose from qualitative methods. Data were analysed using thematic synthesis, as described by Thomas and Harden 18, with the aid of Nvivo 11. After carefully reading and re‐reading each primary study, we coded the findings, organized these codes into related areas to develop descriptive themes and then developed analytical themes. The first stage was completed in two steps: firstly, data were categorized into anticipated or experienced barriers and facilitators to healthy diet, physical activity and participation in an intervention programme, alongside other information such as perception of diabetes risk. Secondly, codes were developed within these categories. R.D. extracted and coded the data, with J.U‐S. independently coding a subset of papers at multiple stages to check consistency. In the next stage, concepts were translated from one study and category to another by making summaries and comparisons, and new concepts were developed as shown in Fig 1. Themes were discussed with all authors throughout.
Figure 1.
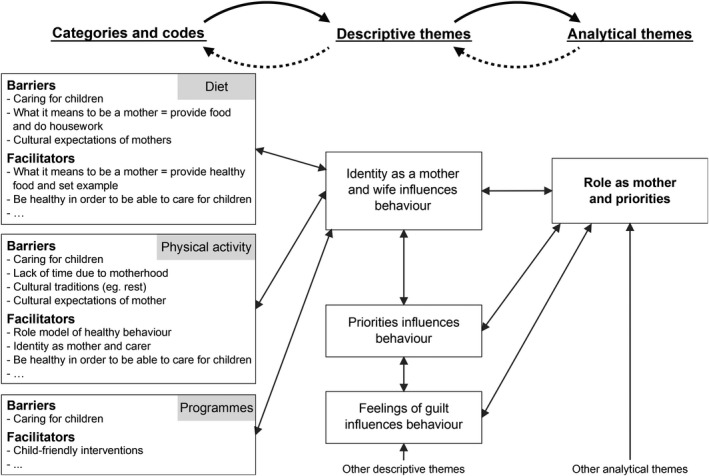
Example of development of the analytical theme ‘Role as mother and priorities’ within the thematic synthesis. Actual and anticipated barriers and facilitators were combined in this diagram and not all codes were presented for simplicity.
Illustrative quotations from the original studies are reported alongside analytical themes to allow appreciation of the primary data. We considered our perspectives on the findings as clinical or non‐clinical researchers in the UK throughout this process. S.G. and J.U‐S. are general practitioners with qualitative research experience, R.W. is an academic general practice registrar and R.D. has undertaken postgraduate training in public health and completed this research as part of her doctoral studies.
Recommendations for promoting behaviour change
We developed 20 recommendations for promoting healthy postpartum lifestyle based on our results, and considered which behaviour change techniques could be used to implement them in line with the behaviour change technique taxonomy (v.1) 19. Our confidence in each recommendation was assessed using the Grading of Recommendations Assessment, Development and Evaluation‐Confidence in Evidence from Reviews of Qualitative Research (GRADE‐CERQual) approach 20 and discussed in order to inform the final interpretation.
Results
We screened 23 160 citations and reviewed 129 full texts. As shown in Fig. 2 articles were included. Table 1 shows the characteristics of these studies, which together represent the views of 926 postpartum women [median (interquartile range) 17 (11–26) participants per study]. Most included face‐to‐face interviews of women in high‐income countries. Of the 17 studies that specified the timing of data collection, 12 were conducted ≥1 year after the affected pregnancy. The study populations had similar characteristics: women in their mid‐30s who tended to be overweight and have more than one child. Where reported, more than half of the population were employed, married and had higher than secondary education.
Figure 2.
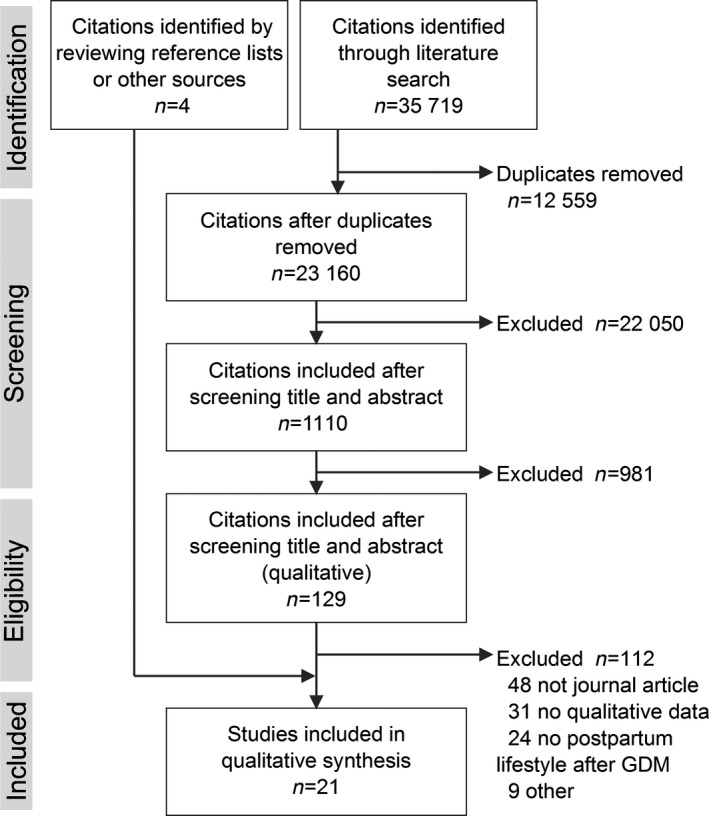
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram showing number of studies included at each stage of the literature review. GDM, gestational diabetes.
Table 1.
Characteristics of the studies included in the qualitative synthesis
| First author and year | Sample size | Setting (country) | Study aim(s) relevant to this analysis | Recruitment strategy | Key inclusion/exclusion criteria | Method of data collection | Time of data collection* | Quality rating (CASP checklist) |
|---|---|---|---|---|---|---|---|---|
| Graco 2009 24 | 10 | Australia | To explore perceptions of PA among women with previous GDM, in context of Type 2 diabetes prevention | Purposive sampling (adverts at maternal and child health centres) | hGDM, English‐speaking, age ≥18 years, residence in selected area, not pregnant or since developed Type 2 diabetes | Interviews (not specified) | NR | 8.0 |
| Doran 2010 53 | 11 | Tonga | To explore how GDM diagnosis influenced change in diet and PA, influencing factors and support of sustained change | Purposive sampling (hospital records) | hGDM within 1 year, delivered baby at the recruiting hospital | Interviews (face‐to‐face) | Within 1 year | 7.0 |
| Evans 2010 37 | 16 | Canada | To determine perceived health status and experiences in establishing and maintaining healthy lifestyle changes | Purposive sampling (GDM clinic) | hGDM, English‐speaking, in the final trimester of pregnancy, telephone access | Interviews (not specified) | At 6 weeks, 3 and 6 months, and 1 year | 8.5 |
| Lindmark 2010 36 | 10 | Sweden | To investigate perceptions about lifestyle | Recruited from outpatient endocrinology hospital clinic by mailout | hGDM within 1 year, Swedish‐speaking, age 30–40 years, no other known diseases | Interviews (face‐to‐face) | At 1 year | 8.5 |
| Razee 2010 30 | 57 | Australia | To explore beliefs, attitudes, social support, environmental influences etc. on diabetes risk behaviours; preferred forms of programme delivery to inform health promotion | Purposive sampling (GDM hospital clinic databases via letter) | hGDM within 6–36 months, Cantonese‐, Mandarin‐, Arabic‐ or English‐speaking, not pregnant or since developed Type 2 diabetes | Interviews (telephone) | Between 6 months and 3 years | 8.0 |
| Bandyopad‐hyay 2011 34 | 17 | Australia | To explore understanding of Type 2 diabetes risk, risk reduction, management strategies, and attitudes and behaviour | Immigrant South Asian women recruited from GDM clinic after diagnosis | hGDM, age ≥18 years, Hindi‐, Bengali‐ or English‐speaking | Interviews (face‐to‐face) | At 6 weeks† | 8.0 |
| Nicklas 2011 28 | 25 | US | To identify barriers and facilitators to healthy lifestyle changes, and approaches to facilitate participation in interventions | Recruited through flyers and internet postings | hGDM within 7 years, age 18–50 years, English‐speaking, not since developed Type 2 diabetes | Interviews (telephone) and focus groups | Within 7 years | 8.5 |
| Gaudreau 2012 40 | 7 | Canada | To understand cultural factors contributing to maintenance of health behaviours encouraged during GDM pregnancy | Recruited by general informants contacts | hGDM within 2–10 years, age ≥18 years, Algonquin peoples, GDM/healthcare in Algonquin community, not breastfeeding or pregnant | Ethnography (observations and interviews) | Between 2 and 10 years | 8.5 |
| Hjelm 2012 21 | 14 | Sweden | To explore beliefs about health, illness and healthcare and study their influence on self‐care and care seeking | Consecutive sampling (women born in the Middle East living in Sweden recruited by staff at hospital‐based specialist clinic) | hGDM, age ≥16 years | Interviews (face‐to‐face) | At 3 and 14 months† | 9.5 |
| Jones 2012 35 | 17 | US | To describe knowledge, perceptions and self‐efficacy beliefs related to preventing cardiometabolic disease | Purposeful and snowball sampling (through fliers distributed by tribal health system care staff) | hGDM, self‐identify as American Indian, age 19–45 years, not pregnant or within 6 weeks postpartum (including 3 with Type 2 diabetes) | Interviews (not specified) | NR | 8.0 |
| Dasgupta 2013 22 | 29 | Canada | To identify factors that could enhance participation and engagement in a Type 2 diabetes prevention program | Recruited from GDM clinic via letter from physician (structured recruitment strategy) | hGDM, English‐ or French‐speaking, not pregnant or since developed Type 2 diabetes | Focus groups | Within 5 years | 9.0 |
| Lie 2013 32 | 35 | UK | To explore views on postnatal lifestyle change to prevent Type 2 diabetes to inform development of intervention approaches | Purposive then theoretical sampling (diabetes obstetric service contacted by clinic staff while attending appointments or from hospital records) | hGDM within 2 years, English‐speaking, age ≥16 years, successful pregnancy outcome, received antenatal care at specified sites, able to consent | Interviews (face‐to‐face) | Within 2 years then between 12 and 18 months later | 8.5 |
| Abraham 2014 33 | 10 | US | To explore lived experiences of women in rural communities with GDM | Purposive and snowball sampling (via obstetric and healthcare providers) | hGDM within 5 years, age ≥18 years, residence in a county eligible for rural community grants, not since developed Type 2 diabetes | Interviews (face‐to‐face and telephone) | Between 2 and 5 years | 8.0 |
| Morrison 2014 39 | 393 | Australia | To describe reflections on the experience of GDM‐pregnancy | Australian women recruited from the NDSS database for cross sectional survey by mailout | hGDM within 3 years, age ≥18 years at time of registration, not residing in a Queensland postcode‡ | Open‐ended survey | At 3 years | 7.0 |
| Jones 2015 23 | 26 | USA | To elicit women's perspectives on cardiometabolic risk reduction behaviours to inform the development of a postpartum lifestyle modification intervention | Contact study team after advertising study through fliers and business card distribution at the CNDH | hGDM within 10 years, self‐identify as American Indian, age 19–45 years, healthcare through CNDH | Interviews (face‐to‐face and telephone) and focus groups | Within 10 years (1 or 2 interviews) | 8.5 |
| O'Dea 2015 31 | 17 | Ireland | To evaluate a lifestyle intervention programme (give context to quantitative findings) | Women identified from the Atlantic DIP research database and hospital pregnancy service contacted by letters and telephone | hGDM within 1–3 years, English‐speaking, not pregnant or since developed Type 2 diabetes (randomized to the trial intervention arm) | Interviews (face‐to‐face) | Between 1 and 3 years | 7.5 |
| Tang 2014 26 | 23 | USA | To explore Type 2 diabetes risk perception and motivators and barriers to preventive health behaviours, to inform intervention approaches | Purposive sampling (African American, Hispanic, non‐Hispanic White women recruited from hospital‐affiliated academic clinics via telephone call from researcher or response to flyer) | hGDM within 1 year, English‐ or Spanish‐speaking, no pre‐existing diabetes or since developed Type 2 diabetes | Interviews (face‐to‐face) | Within 1 year | 8.5 |
| Lim 2017 27 | 165 | Australia | To explore the acceptability of a diabetes prevention programme and compare the characteristics associated with programme engagement | Women enrolled in the MAGDA trial | hGDM in most recent pregnancy, English‐speaking, not pregnant, with pre‐existing Type 2 diabetes or other severe illness | Interviews (face‐to‐face and telephone) | NR (1 or 2 interviews) | 8.5 |
| Pennington 2017 38 | 16 | Australia | To investigate factors influencing engagement with diabetes preventative care (barriers and enablers), the GP's role in care | Purposive sampling (approached or advertisements at general practices and MCHN centres) | hGDM | Interviews (face‐to‐face and telephone) | NR | 8.5 |
| Svensson 2017 25 | 5 | Denmark | To examine the experience of transition from a GDM‐affected pregnancy to postpartum | Random sampling (sent invitation letters via the hospital patient registry and telephoned) | hGDM, recently delivered at the hospital | Interviews (face‐to‐face) | Between 3 and 5 months | 8.0 |
| Zulfiqar 2017 29 | 23 | Australia | To explore barriers and facilitators to following long‐term healthy lifestyle recommendations, and whether there were differences between overseas‐born‐ and Australian‐born‐women | Women managed by a hospital DIP Service who attended a GDM‐related health education programme | hGDM, English‐speaking, live singleton delivery, not pregnant or since developed Type 2 diabetes | Interviews (face‐to‐face) | More than 3 years | 8.5 |
CASP, Critical Appraisal Skills Programme (score out of 10); CNDH, Chickasaw Nation Department of Health; DIP, Diabetes in Pregnancy; GDM, gestational diabetes; GP, general practitioner; hGDM, history of gestational diabetes; MAGDA, Mothers After Gestational Diabetes in Australia, MHCN, maternal and child health nurse centres; NDSS, National Diabetes Service Scheme; NR, not reported; PA, physical activity.
* reference to/since gestational diabetes‐affected pregnancy (studies collected data once postpartum unless otherwise specified); †Plus 1 during pregnancy; ‡Due to a concurrent study.
If reported, healthier diets usually involved trying to consume more fruit and vegetables, and less sugar, fat and processed foods by making substitutions: for example, ‘…I take light milk… We have changed… so it's low‐fat…’ 21. Walking was most frequently mentioned because it ‘…is the easiest exercise you can do’ 22, and several participants mentioned running.
We found all of the studies to be good quality (mean CASP score 8.0/10; Table S2). All were appropriate for qualitative methods, with clear aims, results and implications. Generally, data collection was suitable, although sometimes important details were missing: authors rarely commented on their relationship with participants or their implementation of ethical procedures, even though approval had been granted. Mixed methods studies scored lower because qualitative aspects were less well reported or fitted around quantitative methods.
Actual and anticipated barriers to and facilitators of healthy postpartum lifestyle were translated into six themes that are described below and summarized in Table 2, alongside a seventh theme covering views on practical aspects of interventions. The studies contributing to each theme are shown in Table S3. We did not include a theme regarding culture but discussed it in context.
Table 2.
Summary of themes developed in the qualitative synthesis
| Theme | Description | Consequences for healthy lifestyle | Illustrative quotations |
|---|---|---|---|
| Role as mother and priorities | Women's identity was as a mother, requiring them to prioritize their family; most guilt was felt for not doing this | This was a barrier when giving families what they wanted and not having time for themselves, or a facilitator when health was recognized as important for their family |
‘[My child] already goes to occasional care on Friday mornings… but that's mainly so I can do the housework… the thought of putting him in care so I can do exercise, yeah, that's a big guilt on me’ 24
‘I don't [change my eating habits] so much for protecting me from getting diabetes; I do it so that my son, as he is learning to eat, he learns to eat healthier’ 26 |
| Support from family and friends | Family could provide support by reducing burdens and, particularly affecting diet, providing information and being involved. Friends could offer encouragement for exercise and make it more pleasant. Societal/cultural norms influenced ability to have a healthy diet | Having support facilitated healthfulness; absence of support was identified as barrier |
‘Maybe [you need] help from your significant other because it's hard when they are eating cake and ice cream, all the stuff you can't have, and maybe just don't even have it in the house’ 33
‘If the other women can do it so can I. If others with three children can exercise, I with one can also change’ 27 |
| Demands of life | Lack of time and energy, busyness and work influenced lifestyle choices, as did how convenient and easy to integrate into daily life it was | This was mainly a barrier to healthy lifestyle, although sometimes healthy options became part of daily life and saved time |
‘I was exhausted and already feeling so guilty for being away from my child while I was working, so I did not exercise’ 28
Meal planning ‘to reduce the number of trips per week to grocery stores’ 22 |
| Personal preferences and experiences | Food played an important role in women's personal and social lives. Both diet and exercise affected emotions | Behaviour was determined by whether women had positive experiences or benefitted from healthy/unhealthy lifestyles |
‘Everything's back to normal so I've sort of been making up for lost time a little bit with all the chocolate I couldn't have’ 32
‘…If I'm not active then I find I don't cope as well with things’ 24 |
| Diabetes risk perception and information | Women learned about diet during their GDM‐affected pregnancy; knowledge included risk of Type 2 diabetes, how to prevent it, repetition of messages and the need for culturally relevant information | Relevant information facilitated healthfulness; absence of information was identified as a barrier |
‘The women felt neglected by healthcare providers and were left with unanswered questions about what to do next’ 37
‘…So the plan is to try and live healthy, get rid of the extra pregnancy kilos and return to my normal weight again, and then to be physically active’ 25 |
| Finances and resources | Resources were needed to help women sustain a healthy lifestyle, and their lifestyle affected the family's finances | Women thought that more resources would help them to be more healthy |
‘…[Healthy foods] are not the cheap items; they're a kind of more in the pricy end. It could be a bit irritating to prioritise your money in that way…’ 25
‘I didn't eat out as often. It became less expensive to eat out because I cut down on my portions’ 40 |
Italic highlights key components of the themes (subthemes). GDM, gestational diabetes.
Role as mother and priorities
Prioritizing children and trying to be what the women perceived to be a good mother had one of the greatest influences on their views of healthy postpartum behaviour; preventing diabetes was rarely the primary motivation.
Many women's principle identity was as a mother and partner (‘matriarch’ 23), which meant responsibility for childcare, housework and food, and they wanted to do a ‘good job’. Specifically, many found it difficult to exercise while with a child because they needed or wanted to care for them: ‘[My child] already goes to occasional care on Friday mornings… but that's mainly so I can do the housework… the thought of putting him in care so I can do exercise, yeah, that's a big guilt on me’ 24. Healthy lifestyle could become less important after pregnancy because it was ‘no longer seen as having a direct impact on the child’ 25. Conversely, others thought they should role model healthy behaviour, provide healthy food and maintain their own health in order to care for their children: ‘I don't [change my eating habits] so much for protecting me from getting diabetes; I do it so that my son, as he is learning to eat, he learns to eat healthier’ 26.
Similarly, mothers often prioritized their family's preferences or finances. Some experienced objection when they cooked healthy foods or thought that not eating their traditional diet jeopardized family identity (‘…chang[ed] the culture of the food…’ 22), although in some cases the whole family's diet changed to prioritize children's health. Some even thought that it was ‘inappropriate’ to consider exercise while caring for a small child: ‘All my time is devoted to them now…’ 24. Conversely, some participants in the study by Lim et al. 27 planned how to overcome challenges and prioritized attendance at a diabetes prevention programme: ‘I gave up working on Thursdays to come’. For these reasons, many wanted to include their families and children in healthier lifestyles or programmes.
Resulting from this strong identity, guilt was common across several themes. Some felt ‘a moral tug’ 23 if they left children or housework in order to exercise or attend a programme, and did not see these as legitimate reasons to use external childcare. They also felt guilty for inconveniencing their wider family when they believed they should do childcare, even if help was offered. In contrast, others felt guilty for not exercising when they thought they should.
Support from family and friends
Support was an important facilitator to healthy behaviour whereas its absence was a barrier, considering the support‐giver's own knowledge and diabetes risk perception. Family could help with childcare or housework to reduce busyness and tiredness, and encourage exercise: ‘[The partner needs to consider that] if I don't help with this then [the mother] might be too tired to actually get out for the run she actually would like to go for…’ 25. In particular, family could be a source of information about healthy diet such as the nutritional content of food. They needed to support and join in eating healthily: ‘They'll tease you about how you can't eat this food, and they put it in front of you… try to get you to eat it’ 23 and ‘[I would need] family on board because I can't make two separate meals’ 28. Additionally, more support was expected if partners attended part of the intervention: ‘…I can explain to him really what's going on but if he would hear it from elsewhere, maybe, it'll be different’ 22.
Peer support encouraged exercise, which became an opportunity for socializing: ‘I like having a buddy system. I've never liked to do exercise on my own…’ 22. General lack of support, particularly in migrant populations, could result in isolation, depression and abandonment because women avoided social eating or dropped their diets in certain situations 29. Arabic‐speaking women ‘felt duty bound to eat whatever was offered to them when they visited their family or friends. Such cultural expectations “created more problems” even when the family or friends’ intention was to be helpful’ 30.
Women valued social support from programmes. They motivated and shared experiences with fellow participants ‘…because we're all in that group together’ 23, and clinicians or programme facilitators provided further accountability. Some continued this supportive relationship beyond the programme. O'Dea et al. 31 reported that childcare was the biggest barrier to attending lifestyle interventions, women without a partner could not attend, and the partner needed to support attendance.
Demands of life
Affected by the maternal role and limited support, lack of time and energy were key barriers to healthy behaviour. Specifically, these were barriers to thinking about, preparing for and doing exercise, and planning and cooking healthy meals: ‘You're so busy and so tired and the last thing you want to be bothered thinking about is whether you're eating properly and exercising enough’ 32 and changes could go ‘by the wayside’ when ‘you get busy’ 33. In particular, physical activity was frequently viewed as distinct from the other parenting demands: for many it required ‘set[ting] aside time’ 34 and ‘taking time out for themselves’ 24 away from children and housework (their priorities). They needed to preserve energy, not use it on exercise. Alternatively, physical activity became more sustainable when it became a ‘daily habit’ 31, such as a mother ‘always walk[ing] upstairs to change her baby's diaper’ 28. Similar views were held when considering attending a programme, particularly if the woman needed to travel or the time was inconvenient.
Although shopping with children was difficult, healthy diet did not have as big an impact on time because the role of a mother already included cooking. Furthermore, some reported saving time through meal planning, such as ‘to reduce the number of trips per week to grocery stores’ 22.
Similarly, work increased busyness and created opportunities for unhealthy eating, such as canteens and because ‘[sweets] are often available at work. Meetings have danishes and muffins, cheese plate’ 28. Work also took women away from their children, exaggerating the feelings of guilt and the desire not to access childcare in order to exercise.
Finally, a healthier lifestyle was thought to be hard because of the possibility of saving time and inconvenience through unhealthy options. Using the car was easier than walking, and unhealthy ready meals were easily available.
Personal preferences and experiences
Behaviours were also influenced by personal perspectives and previous experiences. Food was considered as an important part of life. Acting as a barrier to healthy eating, food was a key aspect of many get‐togethers and celebrations: the ‘…highlight of any kind of social gathering is that you've got to have food to celebrate’ 35. Furthermore, some women viewed unhealthy food as a pleasure, reward or comfort. For example, home cooking made a South Asian woman living in Australia ‘…feel closer to your home and that you still have this power and that you're still free to choose…’ 29. Some considered it their right to eat what they wanted. Breastfeeding led to additional hunger and eating more; some craved food such as chocolate. Conversely, other participants enjoyed feeling healthier on certain diets.
Some women reported positive experiences that helped them to maintain exercise: it relaxed and energized them, reduced stress, and helped them to eat a healthy diet. Conversely, others did not enjoy exercise (‘I find it so boring’ 36) or struggled to exercise in bad weather.
Diabetes risk perception and information
Perception of diabetes risk varied. Women in most studies were aware of the link between GDM and Type 2 diabetes but many did not recognize their personal risk 21, 25, 26, 28, 29, 32, 33, 36, 37, 38, thinking that they could go ‘back to normal’ 29 rather than make lifestyle changes. Some demonstrated a lack of understanding (‘I am confident. Nobody in my family ever had it… [explains her lifestyle]’ 26) or had misleading information that the diabetes risk was in the past.
Conversely, others were worried about developing diabetes, which they viewed as inevitable (‘…there's not a great lot more I can do’ 32) or motivated attempts to delay (rather than prevent) it through lifestyle changes, particularly if women were familiar with diabetes through affected friends or family. Nevertheless, strong risk perception influenced willingness rather than ability to make changes. In this case, the risk tended to be considered in the future (‘I feel like I still have time to make changes down the track’ 39); others were continuously aware (‘The risk of getting diabetes is in the back of your mind, you think about what to eat and to exercise, struggling to reduce weight. It is really that simple but also so hard’ 36), even if risk perception declined over time as life ‘moved on’ 37.
Lack of information was reported in most studies. After the intense monitoring of pregnancy, women felt ‘abandoned’ 25, 37, 39, ‘…left high and dry’ 32, and were ‘neglected by healthcare providers and were left with unanswered questions about what to do next’ 37. Some could not remember the health messages after delivery, while a few of those that heard the same information again found it either annoying or said ‘…even if it is old knowledge it is good to hear it once more’ 36. Some women focused on diet to lose weight to prevent diabetes, and used dietary advice from their GDM‐affected pregnancy postpartum. Diabetes prevention programmes were considered useful for learning about diabetes, exercise, diet and weight loss.
Women appreciated information that was relevant to them, particularly information that was culturally appropriate. Algonquin women benefitted from help to adapt their traditional diet by switching cooking oil or using alternative meats. Furthermore, the information was delivered appropriately because ‘…they intervened immediately, adapting to a culture‐specific concept of time described by the general informants as “now or never” 40. Irrelevant information was not useful; for instance, women then became torn between healthier diets and maintaining cultural identity.
Finances and resources
Lack of resources and the need to prioritize finances were frequently cited as barriers to healthy behaviour. Healthy lifestyles were perceived as more expensive than unhealthy ones: healthy food was more expensive than junk food and going to the gym was more expensive than not exercising (particularly when external childcare was needed). Gyms, if available, were also seen to take up time and keep them away from children; none reported being able to use them. Access to cheaper or free healthy food and facilities, and resources such as recipes and home exercise equipment or DVDs were expected to increase healthiness. Gaudreau and Michaud 40 found that women could sustain a healthier diet because they found that it was cheaper: ‘I didn't eat out as often. It became less expensive to eat out because I cut down on my portions’.
Format of interventions
Finally, ‘social support and promot[ing] family participation’ 23 were more important than how diabetes prevention programmes or interventions were delivered. Web‐based interventions were flexible, which could help with time and childcare barriers and allow provision of support and encouragement; however, some wanted face‐to‐face contact or not to spend more time on computers. Telephone call interventions were not popular, despite women in the study by Lim et al. 27 finding that they were personal and flexible, but one population preferred text messages 23. The greatest appeal of face‐to‐face interventions was that they could provide social support, including accountability, motivation and fulfilling social needs. However, mental health could be a barrier to group settings: one woman found it awkward to discuss and another did not attend because she had depression 27. Mixed interventions were suggested to obtain the benefits of multiple approaches: ‘a peer group in‐person to start, to get to know each other, then use chat rooms/email to access at all times of the night’ 28.
Little was discussed about the preferred timing for intervention. Participants in the studies by Dasgupta et al. 22 and Jones et al. 2015 23 reported that the intervention should start during pregnancy or immediately postpartum to address feeling unsupported after pregnancy. Conversely, Lie et al. 32 concluded that the weaning period provided a ‘window of opportunity’.
Several considered that lifestyle coaches, trainers or counsellors could provide support, while medical staff were seen as a trustworthy source of knowledge, but the studies did not discuss who should deliver a programme.
Recommendations for promoting behaviour change
In light of our findings, we developed 20 recommendations for promoting healthier lifestyles after GDM (Table 3) and mapped these onto the behaviour change technique taxonomy to suggest a range of behaviour change techniques that could be included in future interventions, if appropriate to the setting. To illustrate, recommendation 7 (‘provide guidance about how to buy and prepare healthy, tasty food efficiently’) is a ‘10.6 Non‐specific incentive’ in itself that incentivizes women to save time and money through dietary changes. The physical activities suggested in recommendation 17 could be implemented through ‘1.1 Goal setting (behaviour)’ by helping women to create personal daily walking targets or playing with their children at the park four times a week rather than sitting and watching.
Table 3.
Twenty recommendations for promoting healthier lifestyles after gestational diabetes, and our confidence in each recommendation made using the GRADE‐CERQual approach
| Recommendation | Behaviour change techniques relevant to recommendation 19 | Confidence in evidence and explanation |
|---|---|---|
| Role as mother and priorities | ||
|
5.1 Information about health consequences, 5.3 Information about social and environmental consequences, 10.5 Social incentive, 10.7 Self‐incentive, 13.1 Identification of self as role model |
Moderate: Women directly or indirectly reported that their children were their incentive for change; whether it is appropriate for all should be considered |
|
12.2 Restructuring the social environment, 14.1 Behaviour cost |
Moderate: Few studies contributed to this recommendation but some directly suggested it; it is supported by general concern about children/childcare |
| Support from family and friends | ||
|
7.3 Reduce prompts/cues, 12.2 Restructuring the social environment |
Moderate: It is clear that women need support for a healthy diet but few studies clearly discussed family and friends exercising |
|
3.2 Social support (practical), 3.3 Social support (emotional) |
High: Many studies explained the benefits of or need for support for lifestyle change |
|
3.2 Social support (practical), 3.3 Social support (emotional) |
Moderate: Inadequate data reduced our confidence that this recommendation would be useful to postpartum women |
|
3.3 Social support (emotional) | Moderate: This recommendation was developed from the general need for support, plus a few studies that specifically addressed it |
| Demands of life | ||
|
1.2 Problem solving, 4.1 Instruction on how to perform a behaviour, 10.6 Non‐specific incentive |
High: Many women reported the lack of and need for more guidance for having a healthy diet |
|
4.1 Instruction on how to perform a behaviour, 8.3 Habit formation, 10.6 Non‐specific incentive |
Moderate: It is clear, and stated, that women need help to increase exercise; however, there are some contradictory suggestions about the best form(s) of exercise to promote and how |
| Personal preferences and experiences | ||
|
1.2 Problem solving, 1.4 Action planning, 4.2 Information about antecedents |
Low: Certain situations affect women's ability to maintain healthy diets; the best way to address this is unclear |
|
9.2 Pros and cons, 9.3 Comparative imagining of future outcomes, 13.2 Framing/reframing |
High: Women had identified many benefits of adopting healthier lifestyles that helped them to maintain them (perhaps after awareness of diabetes risk declined over time) |
| Diabetes risk perception and information | ||
|
4.1 Instruction on how to perform a behaviour, 5.1 Information about health consequences, 5.2 Salience of consequences |
High: This recommendation resulted from many studies that were in agreement, with few exceptions |
|
13.2 Framing/reframing, 13.5 Identity associated with changed behaviour |
High: It was clear that women wanted culturally relevant interventions and that they were beneficial to those who received it |
|
5.1 Information about health consequences, 5.2 Salience of consequences |
Low: This recommendation is a step on from women's attitudes towards behaviour change and their clinician |
|
5.1 Information about health consequences | Moderate: Paucity of data reduced our confidence in this recommendation |
| Finances and resources | ||
|
4.1 Instruction on how to perform the behaviour | High: There was agreement across studies but this was not reported in detail |
| Format of intervention and other | ||
|
1.1 Goal‐setting (behaviour), 1.4 Action planning |
Moderate: Several studies briefly reported women being able to makes these changes |
|
1.1 Goal‐setting (behaviour), 1.4 Action planning |
High: Women across several studies reported how and why they did these types of exercises |
|
6.2 Social comparison | Low: There was no agreement across studies; this recommendation attempted to consider what women wanted but also what was most practical |
|
9.1 Credible source | Low: Preferred source of the intervention was not discussed; however women reported benefits from their interactions with various professionals |
|
2.2 Feedback on behaviour, 2.3 Self‐monitoring of behaviour, 2.4 Self‐monitoring of outcome of behaviour, 3.2 Social support (practical) |
High: Accountability facilitates behaviour change, but the best way to promote this remains uncertain |
Recommendations frequently result from findings within multiple themes but have been presented under the primary contributing theme.
As explained in Table S4, we had high confidence in 8 recommendations, moderate confidence in another 8 recommendations and low confidence in 4 recommendations in the GRADE‐CERQual evaluation. The recommendations were based on many good‐quality, relevant studies; confidence was therefore largely influenced by coherence and agreement between studies and richness of the data. We tended to have greater confidence about information that women wanted and the need for support and accountability, but less confidence in recommendations about equipping women in situations such as at work, the behaviour of friends and family (other than offering support) and interactions with professionals because continued contact is not common. We felt that it was important to adapt interventions to the target population and facilitate family‐friendly changes because the mother's own diabetes risk was unlikely to motivate change without her perceiving benefits for her children. Some of the most beneficial aspects of groups (such as forming supportive relationships) mean that they are impractical for most to commit to in the long term. Consequently, a combination of approaches could be most appropriate; for example, online information, target‐setting and accountability, plus options to arrange video calls with dietitians and connections with local mothers’ groups.
Discussion
Adopting a healthy lifestyle after a pregnancy affected by GDM is complex. Their identity as a mother who prioritized family above themselves influenced many women's ability to care for their own health, as did the need for resources, time, energy, information and support. Taking into consideration the significant impact that having new children has, these barriers frequently appeared to outweigh the perceived benefits of behaviour change by those maintaining established unhealthy behaviours, particularly when a negative effect on the family was anticipated.
Influences on behaviours were similar, although a diet could be adapted because meal preparation and eating were already necessary, whereas exercise was an additional task. Some influences were both positively and negatively reported; for example, lack of culturally specific information inhibited healthy diet (information as a barrier), while guidance about adapting traditional foods helped women to make changes (information as a facilitator). In contrast, some facilitators were only anticipated; for example, women suggested giving gym passes to increase exercise, but none reported regularly using gyms.
Strengths and limitations
Strengths of the present study include the fact that it is the first comprehensive qualitative synthesis to focus on the views of women with a history of GDM on having a healthy lifestyle, and to make clear recommendations for implementing the findings. As a multidisciplinary team, we conducted a comprehensive literature search and thematic synthesis to identify repeated themes across studies and to recognize those that may have previously been overlooked 18. Our concurrent comparison of positive and negative influences and different behaviours permitted a more representative understanding. We observed diverse perspectives and variety between and within study populations (such as ethnicity, social norms, other children and family members). Congruence between high‐quality studies increased our confidence in our recommendations, which were transparently evaluated using GRADE‐CERQual and linked to standard behaviour change techniques.
The study also has some limitations. We did not distinguish between time points but collated studies that collected data from 6 weeks to 10 years postpartum so we could not synthesize changes over time as reported by Hjelm et al. 21. Furthermore, we were not able to investigate specifically how experience of pregnancy, such as struggling to manage diabetes through lifestyle modifications or feeling guilty for having GDM 5, influenced postpartum behaviour based on the included studies. Most data were from educated or employed women recruited from medical settings in developed countries, meaning that we missed some experiences of motherhood (although the populations were quite different, as discussed). Although it is possible that participants felt that mental health did not influence behaviour, it is also possible that they avoided this topic and that women experiencing mental health difficulties did not participate in these studies. We did not access the primary data therefore were reliant on how the studies’ authors interpreted and reported their data, nor did we examine quantitative literature. Barriers made the greatest contribution to analytical themes, perhaps because they were emphasized by researchers or respondents. Fewer studies reported experiences of diabetes prevention programmes, but they were consistent with other themes.
Although the studies were good quality, quality did affect the results of the synthesis and recommendations. Authors rarely adequately considered their role as researchers, which could have led to bias in the formation and evaluation of research questions and social desirability bias among respondents. Furthermore, although we did not influence the participants or original analyses, our analysis was inevitably affected by our own preconceptions. In recognition of this, we developed the coding frame from the study findings in order not to impose a framework from our review question, used structured CASP and GRADE‐CERQual checklists, and all authors discussed the themes and findings.
Comparison to other studies
Whilst our findings are broadly consistent with previous literature reviews, we have added more studies, data and detail. In 2014, a meta‐synthesis found that, in the context of preventing diabetes in the future, women prioritized children and families and listed barriers and facilitators 6. The authors of that paper noted that few studies contributed to this, whereas we identified 11 more studies published since their search. Two other reviews, which had a greater focus on healthcare seeking, commented that many women have knowledge regarding diabetes prevention that affects their desire to live healthily 14, 16. They also list numerous barriers, including some that we found less emphasis on, such as poor body image and an unsuitable neighbourhood. Consistent with our findings, a discussion of a recent symposium concluded that postpartum behaviour is affected by women's beliefs about their susceptibility to diabetes, and is considered at the cost of their family, and that healthcare systems gave disjointed care so women lacked information 41.
Postpartum mothers in the general population also report barriers to physical activity including lack of energy, time for housework and the responsibility of childcare 42, 43. In the study by Graco et al. 24, women with GDM did not want to be seen as a separate group but to attend classes with mothers who had had a normoglycaemic pregnancy. This raises the question of whether interventions should be specifically targeted at women with previous GDM or mothers seeking healthy lifestyles in general. Our results also broadly agree with the determinants of healthy behaviour in the wider adult population, although we think that there is a different emphasis: mothers with previous GDM appear to weigh relational factors (such as the possible impact of their behaviour on others) higher than other populations and place less emphasis on environmental factors and personal health benefits 44.
Moreover, our recommendations are similar to those identified in the development of the STAR MAMA intervention 45. In that study, focus groups (including overweight women or those with GDM), alongside experts, were used to adapt the DPP to Latina women through the behaviour change wheel framework. In the adapted programme, techniques such as modelling narratives and role‐playing were used to help participants with a history of GDM overcome barriers to behaviour change through automated weekly telephone calls and coaching. The initial evaluation of the intervention was positive 46.
Implications
As outlined in Table 3, the present qualitative review can inform approaches to promoting healthier lifestyles. These recommendations could be used to develop new interventions or adapt existing ones. For example, the effective DPP intensive lifestyle intervention focused on repeated face‐to‐face meetings with a case manager 13; given our findings, this could make it hard for many women to commit to (the DPP has already been adapted for the STAR MAMA intervention 46). Total diet replacement and stepped food reintroduction in a population with diabetes (DiRECT trial) resulted in diabetes remission in half of their participants 47, but a diet that is so controlled and different from that of the rest of the family may not be attractive to mothers. Web‐based interventions with additional face‐to‐face or remote support from a nurse (POWeR+ trial) have led to weight loss in the general population 48, and could be adapted to meet the specific requirements of this population.
We have also identified areas that need further research. Despite including a number of recent studies, we were not able to examine the use of technologies such as smartphone applications and social media, which are growing across the world. In a study that was published after we conducted our literature search, participants suggested that more support should be provided via online forums and information on general practice websites 49. The authors of that study reported that technology could provide information, enable personalized self‐management and meet social needs, with flexibility noted as a benefit. Additionally, we were unsure whether promoting change in the wider family would specifically facilitate mothers to be healthier based on this review; however, the risk of diabetes is higher in partners and children of mothers with GDM 50, 51 and maternal behaviour strongly correlates with childhood obesity 52, therefore, it should be carefully considered.
Furthermore, careful attention should be given to how best to apply these recommendations. For example, interventions could be tailored to working and single mothers or those experiencing postpartum mental health disorders, and the appropriateness of using additional behaviour change techniques (such as ‘14. Scheduled consequences’ 19).
Conclusion
In conclusion, many factors make it difficult to adopt healthy lifestyles after GDM, yet how women interpret these factors can motivate or prevent changes that reduce their diabetes risk. Women's needs and experiences should be considered when designing strategies to promote healthier lifestyles. We have made key recommendations based on a synthesis of qualitative data that will inform the development of feasible interventions, or adaption of existing ones, to educate and support women in achieving and maintaining a healthy postpartum lifestyle in order to reduce their risk of developing Type 2 diabetes.
Funding sources
R.A.D. is funded by a PhD studentship from the National Institute for Health Research (NIHR) School for Primary Care Research (SPCR). This paper presents independent research funded by the NIHR SPCR. The views expressed are those of the author(s) and not necessarily those of the NIHR, the NHS or the Department of Health. R.J.W. is funded by an NIHR Academic Clinical Fellowship. S.J.G. is supported by the Medical Research Council (MC_UU_12015/4). The University of Cambridge has received salary support in respect of S.J.G. from the NHS in the East of England through the Clinical Academic Reserve. J.U‐S. is funded by a Cancer Research UK Cancer Prevention Fellowship (C55650/A21464).
Competing interests
None declared.
Supporting information
Table S1. Medline search strategy.
Table S2. Findings from the Critical Skills Appraisal Programme (CASP) checklist.
Table S3. Studies contributing to each theme.
Table S4. CERQual qualitative evidence profile of recommendations for promoting healthy lifestyles after gestational diabetes.
Acknowledgements
The authors thank Isla Kuhn, Medical Librarian, University of Cambridge Medical Library, for her help developing the search strategy.
Diabet. Med. 36: 702–717 (2019)
References
- 1. Bellamy L, Casas J‐P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta‐analysis. Lancet. 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 2. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002; 25: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr Diab Rep 2016; 16: 702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence guideline . Diabetes in pregnancy: Management from preconception to the postnatal period. 2015;13:2–65. Available at https://www.nice.org.uk/guidance/ng3 Last accessed 18 October 2018 [PubMed] [Google Scholar]
- 5. Devsam BU, Bogossian FE, Peacock AS. An interpretive review of women's experiences of gestational diabetes mellitus: Proposing a framework to enhance midwifery assessment. Women Birth 2013;26:e69–76. [DOI] [PubMed] [Google Scholar]
- 6. Parsons J, Ismail K, Amiel S, Forbes A. Perceptions among women with gestational diabetes. Qual Health Res 2014; 24: 575–585. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care 2003; 26(Suppl. 1): S103–105. [DOI] [PubMed] [Google Scholar]
- 8. Kieffer EC, Sinco B, Kim C. Health behaviors among women of reproductive age with and without a history of gestational diabetes mellitus. Diabetes Care 2006; 29: 1788–1793. [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Health and Care Excellence . Type 2 diabetes: Prevention in people at high risk. Guidance and guidelines, 2017. Available at https://www.nice.org.uk/guidance/ph38 Last accessed 18 October 2018.
- 10. Pedersen ALW, Terkildsen Maindal H, Juul L. How to prevent type 2 diabetes in women with previous gestational diabetes? A systematic review of behavioural interventions. Prim Care Diabetes 2017; 11: 403–413. [DOI] [PubMed] [Google Scholar]
- 11. Chasan‐Taber L. Lifestyle interventions to reduce risk of diabetes among women with prior gestational diabetes mellitus. Best Pract Res Clin Obstet Gynaecol 2015; 29: 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morton S, Kirkwood S, Thangaratinam S. Interventions to modify the progression to type 2 diabetes mellitus in women with gestational diabetes. Curr Opin Obstet Gynecol 2014; 26: 476–486. [DOI] [PubMed] [Google Scholar]
- 13. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi‐Sunyer X et al. Prevention of diabetes in women with a history of gestational diabetes: Effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008; 93: 4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Ryswyk E, Middleton P, Shute E, Hague W, Crowther C. Women's views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: A systematic review of qualitative/survey studies. Diabetes Res Clin Pract 2015; 110: 109–122. [DOI] [PubMed] [Google Scholar]
- 15. Jones EJ, Roche CC, Appel SJ. A review of the health beliefs and lifestyle behaviors of women with previous gestational diabetes. J Obstet Gynecol Neonatal Nurs 2009; 38: 516–526. [DOI] [PubMed] [Google Scholar]
- 16. Nielsen KK, Kapur A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow‐up ‐ The determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy Childbirth 2014; 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Critical Appraisal Skills Program (Qualitative Research Checklist) 2017. Available at https://casp-uk.net/casp-tools-checklists. Last accessed 30 October 2017.
- 18. Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013; 46: 81–95. [DOI] [PubMed] [Google Scholar]
- 20. Lewin S, Glenton C, Munthe‐Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M et al. Using qualitative evidence in decision making for health and social interventions: An approach to assess confidence in findings from qualitative evidence syntheses (GRADE‐CERQual). PLoS Med 2015; 12: 702–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hjelm K, Bard K, Apelqvist J. Gestational diabetes: Prospective interview‐study of the developing beliefs about health, illness and health care in migrant women. J Clin Nurs 2012; 21: 3244–3256. [DOI] [PubMed] [Google Scholar]
- 22. Dasgupta K, Da Costa D, Pillay S, De Civita M, Gougeon R, Leong A et al. Strategies to optimize participation in diabetes prevention programs following gestational diabetes: A focus group study. PLoS One 2013; 8: e67878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones EJ, Peercy M, Woods JC, Parker SP, Jackson T, Mata SA et al. Identifying postpartum intervention approaches to reduce cardiometabolic risk among American Indian women with prior gestational diabetes, Oklahoma, 2012‐2013. Prev Chronic Dis 2015; 12: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graco M, Garrard J, Jasper AE. Participation in physical activity: Perceptions of women with a previous history of gestational diabetes mellitus. Heal Promot J Aust 2009; 20: 20–25. [DOI] [PubMed] [Google Scholar]
- 25. Svensson L, Nielsen KK, Maindal HT, Kragelund Nielsen K, Terkildsen Maindal H, Nielsen KK et al. What is the postpartum experience of Danish women following gestational diabetes? A qualitative exploration. Scand J Caring Sci 2017; 32: 756–764. [DOI] [PubMed] [Google Scholar]
- 26. Tang JW, Foster KE, Pumarino J, Ackermann RT, Peaceman AM, Cameron KA. Perspectives on prevention of type 2 diabetes after gestational diabetes: A qualitative study of Hispanic, African‐American and White women. Matern Child Health J 2014; 19: 1526–1534. [DOI] [PubMed] [Google Scholar]
- 27. Lim S, Dunbar JA, Versace VL, Janus E, Wildey C, Skinner T et al. Comparing a telephone‐ and a group‐delivered diabetes prevention program: Characteristics of engaged and non‐engaged postpartum mothers with a history of gestational diabetes. Diabetes Res Clin Pract 2017; 126: 254–262. [DOI] [PubMed] [Google Scholar]
- 28. Nicklas JM, Zera CA, Seely EW, Abdul‐Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth 2011; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zulfiqar T, Lithander FE, Banwell C, Young R, Boisseau L, Ingle M et al. Barriers to a healthy lifestyle post gestational‐diabetes: An Australian qualitative study. Women Birth 2017; 30: 319–324. [DOI] [PubMed] [Google Scholar]
- 30. Razee H, van der Ploeg HP, Blignault I, Smith BJ, Bauman AE, McLean M et al. Beliefs, barriers, social support, and environmental influences related to diabetes risk behaviours among women with a history of gestational diabetes. Health Promot J Austr 2010; 21: 130–137. [DOI] [PubMed] [Google Scholar]
- 31. O'Dea A, Tierney M, McGuire BE, Newell J, Glynn LG, Gibson I et al. Can the onset of type 2 diabetes be delayed by a group‐based lifestyle intervention in women with prediabetes following gestational diabetes mellitus (GDM)? Findings from a randomized control mixed methods trial. J Diabetes Res 2015; 2015: 798460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lie MLS, Hayes L, Lewis‐Barned NJ, May C, White M, Bell R. Preventing type 2 diabetes after gestational diabetes: Women's experiences and implications for diabetes prevention interventions. Diabet Med 2013; 30: 986–993. [DOI] [PubMed] [Google Scholar]
- 33. Abraham K, Wilk N. Living with gestational diabetes in a rural community. Am J Matern Nurs 2014; 39: 239–245. [DOI] [PubMed] [Google Scholar]
- 34. Bandyopadhyay M, Small R, Davey M‐A, Oats JJN, Forster DA, Aylward A. Lived experience of gestational diabetes mellitus among immigrant South Asian women in Australia. Aust New Zeal J Obstet Gynaecol 2011; 51: 360–364. [DOI] [PubMed] [Google Scholar]
- 35. Jones EJ, Appel SJ, Eaves YD, Moneyham L, Oster RA, Ovalle F. Cardiometabolic risk, knowledge, risk perception, and self‐efficacy among American Indian women with previous gestational diabetes. J Obstet Gynecol Neonatal Nurs 2012; 41: 246–257. [DOI] [PubMed] [Google Scholar]
- 36. Lindmark A, Smide B, Leksell J. Perception of healthy lifestyle information in women with gestational diabetes: A pilot study before and after delivery. Eur Diabetes Nurs 2010; 7: 16–20. [Google Scholar]
- 37. Evans MK, Patrick LJ, Wellington CM. Health behaviours of postpartum women with a history of gestational diabetes. Can J Diabetes 2010; 34: 227–232. [Google Scholar]
- 38. Pennington AVRR, O'Reilly SL, Young D, Dunbar JA. Improving follow‐up care for women with a history of gestational diabetes: Perspectives of GPs and patients. Aust J Prim Health 2017; 23: 66–74. [DOI] [PubMed] [Google Scholar]
- 39. Morrison MK, Lowe JM, Collins CE. Australian women's experiences of living with gestational diabetes. Women Birth 2014; 27: 52–57. [DOI] [PubMed] [Google Scholar]
- 40. Gaudreau S, Michaud C. Cultural factors related to the maintenance of health behaviours in Algonquin women with a history of gestational diabetes. Chronic Dis Inj Can 2012; 32: 140–148. [PubMed] [Google Scholar]
- 41. Nielsen KK, Grunnet LG, Maindal HT. Prevention of type 2 diabetes after gestational diabetes directed at the family context: A narrative review from the Danish Diabetes Academy symposium. Diabet Med 2018; 35: 714–720. [DOI] [PubMed] [Google Scholar]
- 42. Shelton SL, Lee S‐YS. Women's self‐reported factors that influence their postpartum exercise levels. Nurs Womens Health 2018;22:148–157. [DOI] [PubMed] [Google Scholar]
- 43. Saligheh M, McNamara B, Rooney R. Perceived barriers and enablers of physical activity in postpartum women: A qualitative approach. BMC Pregnancy Childbirth 2016; 16: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kelly S, Martin S, Kuhn I, Cowan A, Brayne C, Lafortune L. Barriers and facilitators to the uptake and maintenance of healthy behaviours by people at mid‐Life: A rapid systematic review. PLoS One 2016; 11: e0145074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Handley MA, Harleman E, Gonzalez‐Mendez E, Stotland NE, Althavale P, Fisher L et al. Applying the COM‐B model to creation of an IT‐enabled health coaching and resource linkage program for low‐income Latina moms with recent gestational diabetes: the STAR MAMA program. Implement Sci 2016; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Athavale P, Thomas M, Delgadillo‐Duenas AT, Leong K, Najmabadi A, Harleman E et al. Linking high risk postpartum women with a technology enabled health coaching program to reduce diabetes risk and improve wellbeing: Program description, case studies, and recommendations for community health coaching programs. J Diabetes Res 2016; 2016: 4353956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): An open‐label, cluster‐randomised trial. Lancet 2018; 391: 541–551. [DOI] [PubMed] [Google Scholar]
- 48. Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ et al. An internet‐based intervention with brief nurse support to manage obesity in primary care (POWeR+): A pragmatic, parallel‐group, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 821–828. [DOI] [PubMed] [Google Scholar]
- 49. McMillan B, Easton K, Goyder E, Delaney B, Madhuvrata P, Abdelgalil R et al. Reducing risk of type 2 diabetes after gestational diabetes: A qualitative study to explore the potential of technology in primary care. Br J Gen Pract. 2018; 68: e260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J et al. High prevalence of type 2 diabetes and pre‐diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: The role of intrauterine hyperglycemia. Diabetes Care 2008; 31: 340–346. [DOI] [PubMed] [Google Scholar]
- 51. Dasgupta K, Ross N, Meltzer S, Da Costa D, Nakhla M, Habel Y et al. Gestational diabetes mellitus in mothers as a diabetes predictor in fathers: A retrospective cohort analysis. Diabetes Care 2015; 38: e130–131. [DOI] [PubMed] [Google Scholar]
- 52. Dhana K, Haines J, Liu G, Zhang C, Wang X, Field AE et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: Results from two prospective cohort studies of mother‐child pairs in the United States. BMJ 2018;k2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doran F, Davis K. Gestational diabetes mellitus in Tonga: Insights from healthcare professionals and women who experienced gestational diabetes mellitus. N Z Med J. 2010; 123: 59–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Medline search strategy.
Table S2. Findings from the Critical Skills Appraisal Programme (CASP) checklist.
Table S3. Studies contributing to each theme.
Table S4. CERQual qualitative evidence profile of recommendations for promoting healthy lifestyles after gestational diabetes.


