Key Points
Question
Are blood donor sex and prior pregnancy associated with mortality of red blood cell transfusion recipients?
Findings
In 3 separately analyzed retrospective cohorts consisting of 34 662, 93 724, and 918 996 transfusion recipients, there were no significant associations between red blood cell transfusions from female donors, previously pregnant donors, or sex-discordant donors and in-hospital mortality (hazard ratios per transfused unit ranging from 0.99 to 1.01).
Meaning
Red blood cell transfusions from female donors, sex-discordant donors, and previously pregnant donors were not significantly associated in a dose-dependent manner with increased mortality among transfusion recipients.
Abstract
Importance
Evidence regarding associations of blood donor sex with mortality among red blood cell transfusion recipients is conflicting.
Objective
To study associations of donor sex and prior pregnancy with mortality of transfusion recipients.
Design, Setting, and Participants
Data from 3 retrospective cohorts of transfusion recipients (the Kaiser Permanente Northern California [KPNC] and Recipient Epidemiology and Donor Evaluation Study-III [REDS-III] databases of data from January 2013 to December 2016 and the Scandinavian Donations and Transfusions [SCANDAT] database with data from January 2003 to December 2012) were analyzed. Final dates of follow-up were December 31, 2016, for the KPNC and REDS-III cohorts and December 31, 2012, for the SCANDAT cohort. Stratified Cox regression models were used to estimate associations between donor exposure groups with risk of mortality, adjusting for the number of red blood cell unit transfusions.
Exposures
The number of transfused red blood cell units from female donors, previously pregnant donors, and sex-discordant donors (male donor and female recipient or female donor and male recipient).
Main Outcomes and Measures
In-hospital mortality.
Results
The study population included 34 662 patients (mean age, 69 years; 18 652 [54%] women) from the KPNC cohort, 93 724 patients (mean age, 61 years; 48 348 [52%] women) from the REDS-III cohort, and 918 996 patients (mean age, 72 years; 522 239 [57%] women) from the SCANDAT cohort. The median number of red blood cell transfusions per patient was 3 in the KPNC cohort, 2 in the REDS-III cohort, and 3 in the SCANDAT cohort. The percentage of transfusions from previously pregnant or parous donors was 9% in the KPNC cohort, 18% in the REDS-III cohort, and 25% in the SCANDAT cohort. The percentage of transfusions in the 3 cohorts from female donors ranged from 39% to 43%, from previously pregnant or parous donors ranged from 9% to 25%, and from sex-discordant donors ranged from 44% to 50%. There were 3217 in-hospital deaths in the KPNC cohort, 8519 in the REDS-III cohort, and 198 537 in the SCANDAT cohort. There were no statistically significant associations between any of the 3 donor exposures and in-hospital mortality in the 3 cohorts. Hazard ratios for in-hospital mortality per transfused unit from female donors were 0.99 (95% CI, 0.96-1.03) for the KPNC cohort, 1.00 (95% CI, 0.99-1.01) for the REDS-III cohort, and 1.00 (95% CI, 0.99-1.00) for the SCANDAT cohort. For units from previously pregnant or parous female donors, hazard ratios were 1.00 (95% CI, 1.00-1.01) for the KPNC cohort, 1.01 (95% CI, 0.98-1.03) for the REDS-III cohort, and 1.00 (95% CI, 1.00-1.01) for the SCANDAT cohort. For units from sex-discordant transfusions, hazard ratios were 1.02 (95% CI, 0.99-1.05) for the KPNC cohort, 0.99 (95% CI, 0.98-1.00) for the REDS-III cohort, and 1.00 (95% CI, 0.99-1.00) for the SCANDAT cohort.
Conclusions and Relevance
Among red blood cell transfusion recipients, transfusions from female, previously pregnant, or sex-discordant donors were not significantly associated with increased mortality.
This study examines the association of blood donor sex or previous pregnancy with mortality of transfusion recipients using data from 3 large databases in the United States and Scandinavia.
Introduction
Studies have examined whether blood donor characteristics, such as sex, age, and pregnancy history, were associated with survival of patients receiving transfusions.1,2,3 Results have varied, with at least 2 studies reporting an association between red blood cell units from female donors and increased risk of death1,3 and a larger study finding no statistically significant association.2 Recognizing that plasma transfusions from female donors have been associated with the occurrence of transfusion-related acute lung injury,4,5 likely through an antibody-mediated mechanism,6 it has been hypothesized that adverse outcomes associated with transfusions from female donors to male recipients could be due to immunologic causes.7
This study used data from 3 large cohorts in the United States and Scandinavia to investigate whether blood donor sex and pregnancy history were associated with mortality of transfusion recipients. Multiple statistical methods were employed to determine whether the statistical method used in analysis yielded different conclusions.
Methods
Data Sources
All study procedures were approved by relevant ethics review and data protection agencies at all contributing sites. Consent was waived by each ethics committee.
Analyses used the following 3 cohorts: the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) database, consisting of data from patients who received transfusions in 12 US hospitals between January 2013 and December 20168; the Kaiser Permanente Northern California (KPNC) health care system, which included data from patients who received transfusions between January 2013 and December 20169; and the Scandinavian Donations and Transfusions (SCANDAT) database, consisting of data from all patients who received transfusions in Sweden and Denmark between January 2003 and December 2012.10 KPNC is an integrated health care delivery system that uses a common electronic health record for inpatient and outpatient encounters, including blood transfusions, across 21 community hospitals and 247 medical offices in northern California. SCANDAT is a nationwide database on blood donation and transfusion with data from all blood service providers in all hospitals in Sweden and Denmark. Health care systems in both of these countries are public, resulting in complete data including all transfusions.10
In all 3 cohorts, links between donors and transfusion recipients permitted analysis of associations between donor characteristics, such as donor sex and prior pregnancy, and survival of transfusion recipients. Transfusion and mortality data were available until the end of follow-up on December 31, 2016, for the REDS-III and KPNC cohorts and on December 31, 2012, for the SCANDAT cohort. Outcome data from the REDS-III database included only in-hospital mortality, whereas both the KPNC and SCANDAT cohorts included data on deaths after hospitalization, allowing analyses of both in-hospital mortality and long-term mortality after hospitalization. In-hospital mortality referred to deaths that occurred during the hospitalization in which transfusion took place. Long-term mortality analyses conducted for the KPNC and SCANDAT cohorts included deaths that occurred after hospital discharge. Details of the 3 cohorts have been published.8,9,10,11 Diagnostic codes from the International Classification of Diseases, Ninth Revision and the International Classification of Diseases, Tenth Revision were used to quantify comorbidity burden using Charlson comorbidity index scores (range, 0-21; a higher score indicates more comorbidities).
Study Design and Participants
All analyses were conducted separately at the 3 sites and there were no pooled analyses. The same analyses were performed in all 3 cohorts and were modeled after previous publications that investigated associations between donor sex and age and transfusion recipient mortality.1,2 Recipients of autologous transfusions were excluded. There were no other exclusions.
The main analyses were based on a fully time-dependent model, allowing exposures to vary over time, following up patients from their first recorded receipt of a red blood cell transfusion to the occurrence of death during follow-up. Sensitivity analyses of the SCANDAT data were restricted to patients who received only 1 red blood cell unit (single transfusion cohort) or only units from donors of 1 of the 3 transfusion donor groups (ie, only units from previously pregnant women, nulligravid women, or men; single donor group cohort) on their first day of transfusion. In both the single transfusion and single donor group cohorts, the reference category was composed of all transfusion recipients from the male donor group. In addition, longitudinal parity data, which were only available in the SCANDAT database, were used to evaluate mortality outcomes of patients who received red blood cell transfusions from female donors whose parity status changed during the study period.
Exposures
The exposures of interest in the main analyses were the number of red blood cell transfusions from female donors, previously pregnant or parous female donors, and sex-discordant donors (ie, male donor to female recipient or female donor to male recipient). For the analysis of sex-discordant transfusions, male and female transfusion recipients were grouped together. The reference group for each donor exposure was an equal number of red blood cell units from all other donor exposures. Exposure definitions differed between the cohorts. In the KPNC and REDS-III cohorts, history of pregnancy was self-reported and parity was not assessed. In the SCANDAT cohort, only parity data were available. Henceforth, this exposure is referred to as “transfusions from previously pregnant or parous donors.”
Outcomes
In-hospital mortality was the primary outcome. Long-term mortality was a secondary outcome and was available only in the KPNC and SCANDAT cohorts. In the analysis of in-hospital mortality, red blood cell recipients contributed multiple hospital episodes if they received transfusions during more than 1 hospital stay. In the analyses of long-term mortality, each patient was included once beginning at the time of first transfusion, with additional transfusion exposures accounted for prospectively until death or the end of follow-up. Transfusion of other blood components (eg, platelets or plasma) was not analyzed or included in the models.
Statistical Analyses
Separate analyses for the 3 exposures (red blood cell units from all female donors, from previously pregnant or parous donors, and from donors of the opposite sex of the recipient) were conducted using stratified Cox proportional hazards regression model with time-dependent exposures, in which time after first transfusion was used as the time scale and stratified on calendar year, hospital, and time-varying total number of red blood cell units transfused. Because the number of transfusions was one of the stratifying variables, comparisons were only done between patients who received the same number of transfusions. The data were analyzed with the Andersen-Gill counting process format using the publicly available Stratify macro.12,13 Analyses were adjusted for recipient age (as a restricted cubic spline with 3 evenly placed knots), recipient sex (as a categorical term), ABO blood type (as a categorical term), and Charlson comorbidity index (categorized as 0, 1-2, 3-4, 5-6, 7-8, 9-10, or >10).14 In the analyses of opposite-sex donor/recipients, an interaction term between time-varying number of red blood cell transfusions (as a restricted cubic spline with knots at 1, 3, 5, 10, 20, and 50 units) and recipient sex was also included.15 In the primary analyses, the 3 donor exposures were fitted as log-linear terms, estimating trends in mortality per additional unit transfused. These exposures were also categorized (as 0, 1-2, 3-4, 5-6, or ≥7 units) and analyzed to evaluate for a dose response. For each exposure category, separate statistical models were fit estimating mortality for the donor exposure controlling for the total number of transfusions. All analyses were performed separately for each cohort.
To be consistent with a previous publication,3 the analyses were stratified by recipient sex and/or age (<50 or ≥50 years). Formal interaction testing was performed by including interaction terms between the main exposure and recipient sex and/or age, with the latter term included as a binary term (ie, aged <50 or ≥50 years). To avoid multiple testing, interaction testing was only performed for the analyses in which the main exposures were fitted as linear terms. Because patients could contribute multiple transfusion episodes, confidence limits for the hazard ratios (HRs) were constructed using robust standard errors.
Missing pregnancy history data in the 2 US cohorts were accounted for using multiple imputation.16 For each red blood cell unit from a female donor with missing pregnancy history, prior pregnancy status was imputed using a logistic regression model fitted with all female red blood cell units transfused from the same cohort, with information on pregnancy history, including categorical terms for year of transfusion, hospital, and between year of transfusion and hospital to allow for variation in donor demographics to differ between hospitals. A total of 100 imputed datasets were created and used to fit the models for in-hospital and long-term mortality.
The proportional hazards assumption was assessed for all non–time-varying factors (age, sex, ABO blood group, and Charlson comorbidity index) by testing for interactions between these factors and log follow-up time. These interactions were statistically significant for all variables for the SCANDAT and REDS-III data, but inclusion of these factors as additional stratification variables did not noticeably change point estimates for the variables of interest. For the KPNC data, only the interaction between the Charlson comorbidity index and follow-up time was significant (P < .001), but accounting for this nonproportionality did not affect estimates for the parameters of interest (eTable 1 in the Supplement). Possible nonproportionality was accounted for by fitting the number of overall transfusions (the main confounding factor) as a stratification variable. Therefore, in the main analyses, only nonproportionality for hospital, calendar year, and number of transfusions were accounted for by incorporating these variables as stratification variables in the models.
Sensitivity Analyses
In addition to the main analyses, a series of sensitivity analyses were performed using the SCANDAT database. First, the single transfusion cohort was identified, composed of patients who received only 1 red blood cell unit on their first day of transfusion. The second analysis was based on a single donor group cohort with patients who received only units from 1 donor group on their first day of transfusion. For both cohorts, patients were followed up until death or 1 year after transfusion, whichever occurred first, but were censored upon receiving additional transfusions. Because there were no patients who received more than 6 transfusions only from nulliparous female donors, the least common donor category, the single donor group cohort was restricted to patients with 6 or fewer transfusions to allow direct comparisons. For the single transfusion and single donor group cohorts, the association between donor characteristics (previously pregnant, nulliparous, and male) and transfusion recipient survival was analyzed using Cox regression, with hospital and year constituting strata, adjusting for patient sex (as a categorical term), age (using a restricted cubic spline with 3 evenly placed knots), and Charlson comorbidity index (categorized as 0, 1-2, 3-4, 5-6, 7-8, 9-10, or >10). In the single donor group cohort analyses, models also included a categorical term for the number of transfusions. Both analyses were conducted overall and then restricted to male transfusion recipients younger than 50 years.
In the third sensitivity analysis, the parity data from the SCANDAT database were used to perform an analysis evaluating mortality outcomes of patients who received red blood cell transfusions from female donors who changed parity status during follow-up. The analyses were performed using stratified Cox regression comparing mortality of multiple recipients of these female donors in relation to the parity of the donors at the time of the blood collection (categorized as nulliparous, 1 delivery, 2 deliveries, or ≥3 deliveries). For additional details, see the eMethods in the Supplement.
Because of the potential for type I error due to multiple comparisons, findings from the secondary analyses should be interpreted as exploratory. All statistical tests were 2-sided and P values less than .05 were considered statistically significant. All data processing and statistical analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
In total, 34 662 transfusion recipients were included in the KPNC cohort, 93 724 in the REDS-III cohort, and 918 996 in the SCANDAT cohort. Multiple imputation was used to account for 13.6% in the REDS-III cohort and 16.2% in the KPNC cohort of transfusions from female donors in whom the pregnancy status was unknown.
Baseline characteristics are presented in Table 1. Transfusion recipients were younger in the REDS-III cohort, with a median (interquartile range [IQR]) age of 64 (51-75) years compared with 71 (60-81) years in the KPNC cohort and 72 (59-82) years in the SCANDAT cohort. The percentage of women was higher in the SCANDAT cohort at 56.8% vs 53.8% in the KPNC cohort and 51.6% in the REDS-III cohort. The median (IQR) number of transfusions per patient was 3 (2-5) in the KPNC cohort, 2 (1-5) in the REDS-II cohort, and 3 (2-5) in the SCANDAT cohort. In the KPNC cohort, the percentage of transfusions from previously pregnant female donors was 9%; female donors, 39%; and sex-discordant donors, 44%. In the REDS-III cohort, the percentage of transfusions from previously pregnant female donors was 18%; female donors, 43%; and sex-discordant donors, 49%. In the SCANDAT cohort, the percentage of transfusions from parous female donors was 25%; female donors, 41%; and sex-discordant donors, 50%.
Table 1. Characteristics of Red Blood Cell Transfusion Recipients in a Study of the Association of Donor Sex and Prior Pregnancy With Mortality Among Transfusion Recipients .
| Outcomes | Study Cohort, No. (%) | ||
|---|---|---|---|
| KPNC | REDS-III | SCANDAT | |
| No. of patients | 34 662 | 93 724 | 918 996 |
| Sex | |||
| Male | 16 010 (46.2) | 45 376 (48.4) | 396 757 (43.2) |
| Female | 18 652 (53.8) | 48 348 (51.6) | 522 239 (56.8) |
| Age at first transfusion, y | |||
| 0-17 | 0 | 3641 (3.9) | 25 695 (2.8) |
| 18-25 | 506 (1.5) | 2973 (3.2) | 16 528 (1.8) |
| 26-50 | 4046 (11.7) | 16 752 (17.9) | 111 696 (12.2) |
| 51-75 | 16 442 (47.4) | 47 773 (51.0) | 379 551 (41.3) |
| >75 | 13 468 (38.9) | 22 585 (24.1) | 385 526 (42.0) |
| Median (IQR), y | 71 (60-81) | 64 (51-75) | 72 (59-82) |
| Charlson comorbidity index at first transfusiona | |||
| 0 | 16 725 (48.3) | 31 023 (33.1) | 519 282 (56.5) |
| 1-2 | 10 786 (31.1) | 43 815 (46.7) | 299 674 (32.6) |
| 3-4 | 6243 (18.0) | 15 223 (16.2) | 73 828 (8.0) |
| 5-6 | 885 (2.6) | 3333 (3.6) | 20 778 (2.3) |
| 7-8 | 23 (0.1) | 319 (0.3) | 4620 (0.5) |
| 9-10 | 0 | 11 (<0.1) | 751 (0.1) |
| >10 | 0 | 0 | 63 |
| No. of transfusions administered during follow-up, U | |||
| 1-2 | 16 433 (47.4) | 48 193 (51.4) | 451 720 (49.2) |
| 3-4 | 8140 (23.5) | 19 886 (21.2) | 228 216 (24.8) |
| 5-9 | 6314 (18.2) | 15 896 (17.0) | 153 599 (16.7) |
| 10-49 | 3569 (10.3) | 9454 (10.1) | 81 139 (8.8) |
| ≥50 | 206 (0.6) | 295 (0.3) | 4322 (0.5) |
| Median (IQR), U | 3 (2-5) | 2 (1-5) | 3 (2-5) |
| No. of red blood cell transfusions from female donors, median (IQR), Ub | 1 (0-2) | 1 (0-2) | 1 (0-2) |
| No. of red blood cell transfusions from previously pregnant or parous female donors, median (IQR), Ub | 0 (0-0) | 0 (0-1) | 1 (0-1) |
| No. of sex-discordant red blood cell transfusions, median (IQR), Ub | 1 (1-2) | 1 (1-3) | 2 (1-3) |
Abbreviations: IQR, interquartile range; KPNC, Kaiser Permanente Northern California; REDS-III, Recipient Epidemiology and Donor Evaluation Study-III; SCANDAT, Scandinavian Donations and Transfusions.
The Charlson comorbidity index is composed of 22 conditions and scores on each are summed to create an overall score with a range from 0 to 41. A higher score implies a higher comorbidity burden.
Differences in the distributions of different types of transfused red blood cell units were largely driven by the ability of men to donate more frequently than women because of hemoglobin eligibility requirements and the physiology of iron recovery after donation.
There were 3217 in-hospital deaths in the KPNC cohort, 8519 in the REDS-III cohort, and 198 537 in the SCANDAT cohort (Table 2). No significant associations between the number of transfusions from a previously pregnant female donor, any female donor, or sex-discordant donor and the risk of in-hospital mortality were observed (Table 2).The HR estimates for each additional transfusion from a previously pregnant or parous donor were 1.00 (95% CI, 1.00-1.01) for the KPNC cohort, 1.01 (95% CI, 0.98-1.03) for the REDS-III cohort, and 1.00 (95% CI, 1.00-1.01) for the SCANDAT cohort. There were no significant associations between receipt of at least 7 red blood cell units from a previously pregnant or parous female donor with increased mortality compared with patients who received the same number of transfusions but no units from a previously pregnant female or parous donor (ie, patients who only received units from male donors or female donors who were nulliparous or not previously pregnant), with HRs of 1.14 (95% CI, 0.92-1.42) in the KPNC cohort, 1.27 (95% CI, 0.94-1.72) in the REDS-III cohort, and 1.01 (95% CI, 0.96-1.06) in the SCANDAT cohort. Corresponding unadjusted deaths per 1000 person-years in patients who received at least 7 transfusions from previously pregnant or parous female donors vs patients who received no transfusions from previously pregnant or parous female donors were 8565 vs 3385 in the KPNC cohort, 6581 vs 2642 in the REDS-III cohort, and 704 vs 473 in the SCANDAT cohort (eTable 2 in the Supplement). Absolute in-hospital mortality rates were high given the short follow-up of most patients. With the exception of a statistically significant higher mortality rate in the KPNC cohort in recipients of 3 to 4 sex-discordant transfusions (HR, 1.22 [95% CI, 1.05-1.41]) or 5 to 6 (HR, 1.31; [95% CI, 1.03-1.66]), there were no other statistically significant associations in the analyses in which the number of units was categorized (Table 2).
Table 2. Hazard Ratios of In-Hospital Death in Relation to the Number of Transfused Units From Female, Previously Pregnant, and Sex-Discordant Donorsa.
| Donor Group | HR per Unit Transfused (95% CI)b | Units of Each Exposure Categoryc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1-2 | 3-4 | 5-6 | ≥7 | ||||||||||||
| Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | ||
| KPNC (n = 34 662) | ||||||||||||||||
| Female | 0.99 (0.96-1.03) | 870 | 293 | 1 [Reference] |
1554 | 478 | 1.03 (0.94-1.13) | 405 | 90 | 1.07 (0.93-1.25) | 154 | 26 | 1.17 (0.92-1.49) | 234 | 26 | 0.86 (0.64-1.16) |
| Previously pregnantd | 1.00 (1.00-1.01) | 2430 | 718 | 1 [Reference] |
668 | 175 | 1.01 (0.90-1.14) | 75 | 16 | 1.03 (0.86-1.23) | 16 | 3 | 0.85 (0.63-1.17) | 28 | 3 | 1.14 (0.92-1.42) |
| Sex-discordant | 1.02 (0.99-1.05) | 762 | 265 | 1 [Reference] |
1580 | 490 | 1.10 (1.00-1.21) | 461 | 99 | 1.22 (1.05-1.41) | 170 | 31 | 1.31 (1.03-1.66) | 244 | 30 | 0.87 (0.66-1.14) |
| REDS-III (n = 93 724) | ||||||||||||||||
| Female | 1.00 (0.99-1.01) | 2 036 | 854 | 1 [Reference] |
3960 | 1558 | 1.02 (0.96-1.08) | 1224 | 425 | 0.99 (0.90-1.09) | 519 | 155 | 0.92 (0.80-1.05) | 780 | 174 | 0.95 (0.79-1.14) |
| Previously pregnantd | 1.01 (0.98-1.03) | 4388 | 1661 | 1 [Reference] |
3122 | 1113 | 1.02 (0.97-1.08) | 584 | 173 | 0.91 (0.82-1.02) | 188 | 50 | 0.91 (0.74-1.11) | 237 | 36 | 1.27 (0.94-1.72) |
| Sex-discordant | 0.99 (0.98-1.00) | 1334 | 718 | 1 [Reference] |
3988 | 1604 | 0.99 (0.92-1.05) | 1447 | 466 | 1.05 (0.95-1.16) | 612 | 181 | 1.02 (0.89-1.18) | 1138 | 211 | 1.07 (0.90-1.28) |
| SCANDAT (n = 918 996) | ||||||||||||||||
| Female | 1.00 (0.99-1.00) | 38 475 | 78 538 | 1 [Reference] |
90 551 | 200 837 | 1.00 (0.99-1.01) | 31 856 | 64 575 | 0.99 (0.97-1.01) | 14 300 | 25 287 | 1.01 (0.98-1.04) | 23 355 | 35 553 | 0.98 (0.94-1.02) |
| Parous | 1.00 (1.00-1.01) | 67 388 | 142 537 | 1 [Reference] |
87 195 | 187 922 | 1.01 (0.99-1.02) | 23 236 | 43 430 | 0.99 (0.97-1.01) | 9086 | 14 368 | 1.04 (1.00-1.08) | 11 632 | 16 533 | 1.01 (0.96-1.06) |
| Sex-discordant | 1.00 (0.99-1.00) | 28 509 | 59 539 | 1 [Reference] |
91 124 | 202 536 | 1.01 (0.99-1.02) | 35 461 | 72 282 | 1.02 (1.00-1.04) | 16 061 | 28 787 | 1.02 (0.99-1.05) | 27 382 | 41 647 | 1.00 (0.96-1.04) |
Abbreviations: HR, hazard ratio; KPNC, Kaiser Permanente Northern California; REDS-III, Recipient Epidemiology and Donor Evaluation Study-III; SCANDAT, Scandinavian Donations and Transfusions.
All analyses were based on the full cohorts of patients who received at least 1 red blood cell transfusion. Each row in the table represents a separate statistical model. Patients who received no red blood cell units from female donors thus implicitly received at least 1 red blood cell unit from a male donor; patients who received at least 1 unit from female donors may also have received male red blood cell units. Absolute rates for categorized estimates are available in eTable 2 in the Supplement. The number of deaths exceeds the person-years of follow-up because the hospital length of stay was typically short (less than 1 year).
The HRs per unit transfused were computed by fitting models where each risk variable was included as a log-linear term. Absolute rates are not possible to compute for these estimates because hazard rates represent averages across a range of units.
For each exposure variable, comparisons should be done across so that, for example, in the KPNC cohort, the risk of death increased by 0.99 for each additional female donor unit, and that recipients of at least 7 units from a female donor had an HR of 0.86 (95% CI, 0.64-1.16) compared with recipients of no units from female donors.
Due to missing data on donor pregnancy history in the KPNC and REDS-III cohorts, analyses for the association between the number of units from previously pregnant female donors and risk of death were conducted using multiple imputation. Multiple imputation was used to account for 16.2% of transfusions from female donors in whom the pregnancy status was unknown in the KPNC cohort and 13.6% in the REDS-III cohort.
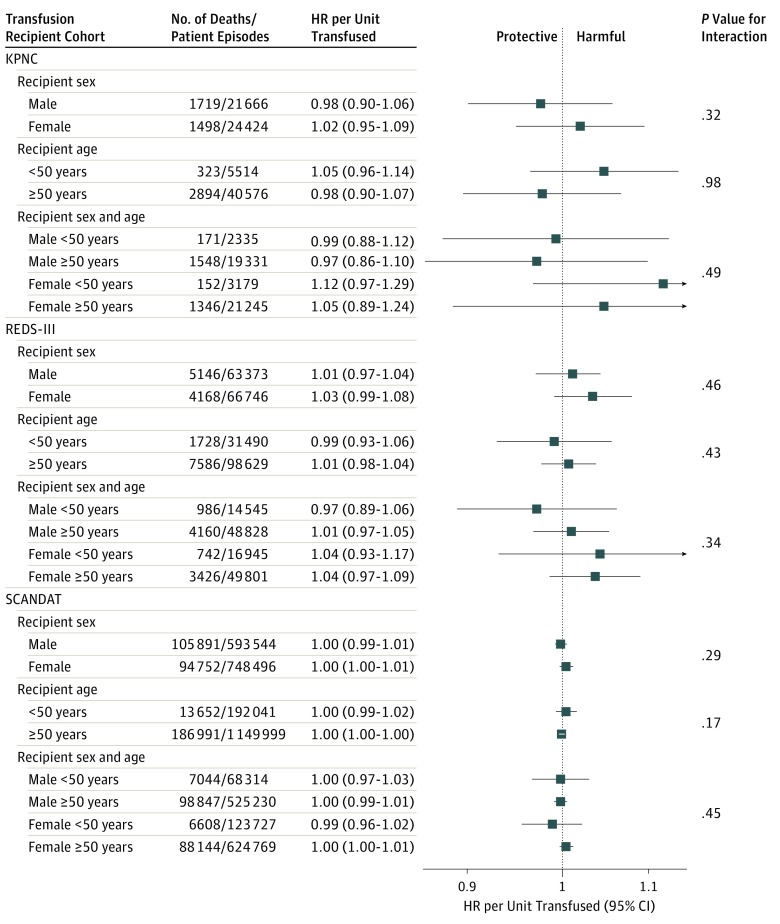
The Figure shows the association between the number of transfusions from a previously pregnant or parous female donor and in-hospital mortality, per additional unit transfused, stratified by recipient sex, recipient age (<50 years vs ≥50 years), and the combination of sex and age. There was no statistically significant association between the number of units transfused from a previously pregnant or parous donor and risk of death in any of these subgroups. Similarly, no statistically significant associations were observed when the same analyses were conducted for the number of units transfused from female donors (eFigure 1 in the Supplement) or the number of units transfused from sex-discordant donors (eFigure 2 in the Supplement).
Figure. Subgroup Analysis of Hazard Ratios (HRs) of Death in Relation to the Number of Red Blood Cell Transfusions From Previously Pregnant or Parous Female Donors, Presented Separately for Each Cohort, Stratified by Recipient Sex and Age.
KPNC indicates Kaiser Permanente Northern California; REDS-III, Recipient Epidemiology and Donor Evaluation Study-III; SCANDAT, Scandinavian Donations and Transfusions
In the long-term mortality analyses, a total of 10 942 deaths occurred in 60 922 person-years in the KPNC cohort and 475 249 deaths occurred in 3 078 677 person-years in the SCANDAT cohort (Table 3). Results were similar to the in-hospital mortality analyses. For the number of transfusions from a previously pregnant or parous donor, the HR was 1.01 (95% CI, 1.00-1.01) per unit transfused in the KPNC cohort and 1.00 (95% CI, 1.00-1.01) per unit transfused in the SCANDAT cohort. Transfusion recipients who received at least 7 units from a previously pregnant or parous female donor did not have a higher mortality rate than patients who received the same number of transfusions from male donors or female donors who were nulliparous or not previously pregnant, with HRs of 1.28 (95% CI, 0.91-1.81) in the KPNC cohort and 1.00 (95% CI, 0.95-1.04) in the SCANDAT cohort. Corresponding mortality rates were 317 vs 169 deaths per 1000 person-years in the KPNC cohort and 397 vs 121 deaths per 1000 person-years in the SCANDAT cohort (eTable 3 in the Supplement). The only statistically significant finding was observed in the KPNC cohort in which increased risk of death was observed in patients who received 1 to 2 sex-discordant transfusions (HR, 1.08 [95% CI, 1.03-1.14]) or 5 to 6 (HR, 1.14 [95% CI, 1.01-1.29]) compared with patients who received no sex-discordant transfusions.
Table 3. Hazard Ratios of Death in Relation to the Number of Transfused Units From Female, Previously Pregnant, and Sex-Discordant Donorsa.
| Outcomes | HR per Unit Transfused (95% CI)b | Units of Each Exposure Categoryc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1-2 | 3-4 | 5-6 | ≥7 | ||||||||||||
| Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | Deaths | Person-Years | HR (95% CI) | ||
| KPNC (n = 34 662) | ||||||||||||||||
| Female | 0.99 (0.98-1.01) | 2932 | 18 431 | 1 [Reference] |
5359 | 32 878 | 1.02 (0.97-1.07) | 1453 | 6377 | 1.01 (0.93-1.10) | 546 | 1723 | 1.02 (0.90-1.16) | 652 | 1511 | 0.92 (0.78-1.07) |
| No. of transfusions from previously pregnant female donorsd | 1.01 (1.00-1.01) | 8281 | 48 857 | 1 [Reference] |
2215 | 10 671 | 1.10 (0.95-1.28) | 302 | 973 | 1.16 (0.92-1.46) | 84 | 230 | 1.02 (0.66-1.55) | 60 | 189 | 1.28 (0.91-1.81) |
| No. of sex-discordant transfusions | 1.01 (1.00-1.02) | 2532 | 16 480 | 1 [Reference] |
5497 | 33 563 | 1.08 (1.03-1.14) | 1544 | 7056 | 1.05 (0.97-1.14) | 607 | 2032 | 1.14 (1.01-1.29) | 762 | 1790 | 1.02 (0.88-1.19) |
| SCANDAT (n = 918 996) | ||||||||||||||||
| No. of transfusions from female donors | 1.00 (0.99-1.00) | 89 197 | 771 834 | 1 [Reference] |
214 660 | 1 596 273 | 0.99 (0.99-1.00) | 81 949 | 415 685 | 0.99 (0.97-1.00) | 35 928 | 138 516 | 0.99 (0.97-1.01) | 53 515 | 156 369 | 0.98 (0.95-1.01) |
| No. of transfusions from parous female donors | 1.00 (1.00-1.01) | 159 509 | 1 316 102 | 1 [Reference] |
210 706 | 1 386 682 | 1.00 (0.99-1.01) | 58 258 | 243 202 | 1.00 (0.98-1.01) | 21 611 | 69 294 | 1.03 (1.00-1.06) | 25 165 | 63 397 | 1.00 (0.95-1.04) |
| No. of sex-discordant transfusions | 1.00 (1.00-1.01) | 59 604 | 484 952 | 1 [Reference] |
210 369 | 658 873 | 1.00 (0.99-1.01) | 93 278 | 533 747 | 1.00 (0.99-1.02) | 43 509 | 186 742 | 1.02 (1.00-1.04) | 68 489 | 214 363 | 1.00 (0.98-1.03) |
Abbreviations: HR, hazard ratio; KPNC, Kaiser Permanente Northern California; REDS-III, Recipient Epidemiology and Donor Evaluation Study-III; SCANDAT, Scandinavian Donations and Transfusions.
All analyses were based on the full cohorts of patients who had all received at least 1 red blood cell transfusion. Each row in the table represents a separate statistical model. Patients who received no red blood cell units from female donors thus implicitly received at least 1 red blood cell unit from a male donor; patients who received at least 1 unit from a female donor may also have received red blood cell units from a male donor. Absolute rates for categorized estimates are available in eTable 2 in the Supplement.
The HRs per unit transfused were computed by fitting models where each risk variable was included as a log-linear term.
For each exposure variable, comparisons should be done across so that, for example, in the KPNC cohort, the risk of death increased with 0.99 for each additional unit from a female donor, and that recipients of ≥7 units from a female donor had an HR of 0.92 (95% CI, 0.78-1.07) compared with to recipients of no units from female donors.
Due to missing data on donor pregnancy in the KPNC and REDS-III cohorts, analyses for the association between the number of units from previously pregnant female donors and risk of death were conducted using multiple imputation. Multiple imputation was used to account for 16.2% of transfusions from female donors in whom the pregnancy status was unknown in the KPNC cohort and 13.6% in the REDS-III cohort.
In the single transfusion cohort analyses, which included 229 684 transfusion recipients, mortality was not associated with receipt of red blood cell units from nulliparous female donors (mortality rate, 286 deaths/1000 person-years; HR, 0.96 [95% CI, 0.93-1.00]) or parous female donors (mortality rate, 281 deaths/1000 person-years; HR 0.97 [95% CI, 0.94-1.01]) compared with receipt of units from male donors (mortality rate, 293 deaths/1000 person-years) (eTable 4 in the Supplement). Findings were similar when analyses were restricted to male transfusion recipients younger than 50 years. Among male red blood cell recipients younger than 50 years, there was no significant association between receiving red blood cell units from a parous female donor (mortality rate, 103 deaths/1000 person-years; HR, 0.95 [95% CI, 0.74-1.23]) or from a nulliparous female donor (mortality rate, 89 deaths/1000 person-years; HR, 0.85 [95% CI, 0.64-1.14]) and mortality compared with recipients of transfusion of red blood cell units from male donors (mortality rate, 78 deaths/1000 person-years) (eTable 4 in the Supplement).
Similarly, there was no significant association with mortality in analyses restricted to patients who received units from 1 donor exposure group on their first day of transfusion only (units from nulliparous female donors: mortality rate, 275 deaths/1000 person-years; HR, 0.98 [95% CI, 0.95-1.01]; units from parous female donors: mortality rate, 284 deaths/1000 person-years; HR 0.99 [95% CI, 0.97-1.01]), compared with recipients of transfusions from male donors (mortality rate, 270 deaths/1000 person-years) (eTable 5 in the Supplement). In analyses restricted to male transfusion recipients younger than 50 years, receipt of units only from parous female donors (mortality rate, 102 deaths/1000 person-years; HR, 1.00 [95% CI, 0.81-1.23]) or nulliparous female donors (mortality rate, 89 deaths/1000 person-years; HR, 0.92 [95% CI, 0.72-1.17]) was not statistically significantly associated with mortality compared with recipients of units from male donors (mortality rate, 85 deaths/1000 person-years) (eTable 5 in the Supplement). The SCANDAT analysis of recipients of transfusions from female donors with changes in parity during the study period was based on data for 110 996 unique patients who received at least 1 red blood cell unit from one of the 18 748 female donors who donated blood before delivering their first child and who continued donating after delivering a child. Analyses were based on 141 344 patient observations in whom 57 938 deaths were observed. There was no statistically significant association between donor parity history and transfusion recipient mortality when comparing recipients of parous donors vs nulliparous donors (HR, 1.01 [95% CI, 0.99-1.02]) or when considering the number of deliveries (Table 4).
Table 4. Results From Analysis Using the SCANDAT Database, Comparing Survival of Patients Transfused Before and After Female Donors Deliver Their First Child.
| Donor Parity | ||||
|---|---|---|---|---|
| Nulliparous | 1 Delivery | 2 Deliveries | ≥3 Deliveries | |
| Donor recipientsa | 78 594 | 39 383 | 19 275 | 4092 |
| Person-years | 54 542 | 26 437 | 12 558 | 2577 |
| Deaths | 32 219 | 16 278 | 7801 | 1640 |
| HR (95% CI)b | 1 [Reference] | 1.01 (0.99-1.03) | 1.01 (0.97-1.04) | 1.01 (0.94-1.08) |
Abbreviations: HR, hazard ratio; SCANDAT, Scandinavian Donations and Transfusions.
A total of 110 996 unique patients were included in this analysis, but because some patients received blood from more than 1 female donor who changed parity status, the sum is higher at 141 344.
HRs were calculated using a 2-step process, first adjusting for calendar year, hospital, patient age, patient sex, as well as patient Charlson comorbidity index and then considering the association of donor parity using a stratified Cox model, with the aforementioned adjustment included as an offset.
Discussion
In these analyses from 3 large population-based cohorts in the United States and Scandinavia, there were no consistent statistically significant associations between the number of red blood cell transfusions from female donors, previously pregnant female donors, or sex-discordant donors and mortality of both male and female transfusion recipients. With few exceptions, results were consistent in the 3 cohorts, despite the variability of patient characteristics across the cohorts.
Furthermore, in exploratory analyses, results did not appear to differ significantly between male and female recipients or between younger and older recipients as previously proposed.3 No significant mortality differences were identified related to red blood cell units from female donors whose parity status changed during the study period.
Some statistically significant associations were observed in the analyses of sex-discordant transfusions in the KPNC cohort, in which transfusions of 3 to 4 and 5 to 6 units were associated with increased mortality compared with recipients of the same number of sex-concordant units. However, these findings are not likely related to true biologic effects. First, similar associations were not observed in either of the 2 larger cohorts. Second, the findings from the KPNC data did not follow a clear dose-response pattern, in that the highest category (ie, receipt of 7 or more units from sex-discordant donors) was not associated with an increased risk and no statistically significant association was seen in the log-linear analyses.
The findings in this study differ from the results reported by Caram-Deelder et al3 in which receipt of a transfusion from an ever-pregnant female donor was associated with a statistically significant increase in all-cause mortality among male recipients of red blood cell transfusions but not among female recipients. Possible reasons for the discrepancies in study findings may include differences in donor and blood bank practices or methodological differences encountered in conducting studies examining the relationship between transfusion and mortality, such as the use of statistical models with different assumptions.
Limitations
This study has several limitations. First, the study design was observational and retrospective. Second, there may have been confounding if red blood cell units from previously pregnant female donors were preferentially allocated to patients who were less ill. However, blood donor age, sex, and pregnancy history are not typically used to select units for transfusion. Third, while there were no significant associations between any of the donor exposures and mortality, it is possible that there may have been an association with other adverse outcomes, such as hospital length of stay or specific causes of mortality. Fourth, red blood cell units from female donors contain fewer erythrocytes compared with red blood cell units from male donors. It is possible that patients who preferentially received units from female donors would need more transfusions, in which case a bidirectional association would exist in which donor sex could affect the total number of transfusions (ie, the main confounding factor) and vice versa. This may have biased the risk estimates in an unpredictable way, depending on the strengths of the respective associations and of the confounding effect. Fifth, the Charlson comorbidity index has not been validated for use in children. Sixth, the study did not account for blood components other than red blood cells, which may have influenced the results. Seventh, it is possible that the results are affected by errors in data processing or study design. To mitigate this possibility, 3 different independent analytical approaches were used.
Conclusions
Among red blood cell transfusion recipients, transfusions from female, previously pregnant, or sex-discordant donors were not significantly associated with increased mortality.
eMethods.
eTable 1. Effect of Association Between Donor Parameters on Recipient Mortality Upon Adding Additional Parameters As Stratification Variables in the Cox Model, to Account for Possible Non-Proportional Hazards
eTable 2. Unadjusted Mortality Rates, in Relation to Number of Transfused Sex-Discordant Units, Units From Female Donors, and Units From Previously Pregnant Donors, Estimated From In-Hospital Mortality Analyses
eTable 3. Unadjusted Mortality Rates, in Relation to Number of Transfused Sex-Discordant Units, Units From Female Donors, and Units From Previously Pregnant Donors, Estimated From Long-Term Mortality Analyses
eTable 4. Results From Analyses Investigating the Effect of Donor Sex and Parity on Patient Survival Based on Single Unit Cohort
eTable 5. Results From Analyses Investigating the Effect of Donor Sex and Parity on Patient Survival Based on Discrete Exposure Group Cohort
eTable 6. Comparison of Standard Cox Model and Fine-Gray Model for Analyses of In-Hospital Mortality
eFigure 1. Subgroup Analyses for Number of Red-Cell Units From Female Donors
eFigure 2. Subgroup Analyses for Number of Sex-Discordant Red-Cell Units
References
- 1.Chassé M, Tinmouth A, English SW, et al. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med. 2016;176(9):1307-1314. doi: 10.1001/jamainternmed.2016.3324 [DOI] [PubMed] [Google Scholar]
- 2.Edgren G, Ullum H, Rostgaard K, et al. Association of donor age and sex with survival of patients receiving transfusions. JAMA Intern Med. 2017;177(6):854-860. doi: 10.1001/jamainternmed.2017.0890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caram-Deelder C, Kreuger AL, Evers D, et al. Association of blood transfusion from female donors with and without a history of pregnancy with mortality among male and female transfusion recipients. JAMA. 2017;318(15):1471-1478. doi: 10.1001/jama.2017.14825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews AT, Zmijewski CM, Bowman HS, Reihart JK. Transfusion reaction with pulmonary infiltration associated with HL-A-specific leukocyte antibodies. Am J Clin Pathol. 1976;66(3):483-487. doi: 10.1093/ajcp/66.3.483 [DOI] [PubMed] [Google Scholar]
- 5.Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet. 2013;382(9896):984-994. doi: 10.1016/S0140-6736(12)62197-7 [DOI] [PubMed] [Google Scholar]
- 6.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825-1835. doi: 10.1111/j.1537-2995.2009.02206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cable RG, Edgren G. Blood transfusions from previously pregnant women and mortality: interpreting the evidence. JAMA. 2017;318(15):1445-1447. doi: 10.1001/jama.2017.15095 [DOI] [PubMed] [Google Scholar]
- 8.Karafin MS, Bruhn R, Westlake M, et al. ; National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) . Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion. 2017;57(12):2903-2913. doi: 10.1111/trf.14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roubinian NH, Murphy EL, Swain BE, Gardner MN, Liu V, Escobar GJ; NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III); Northern California Kaiser Permanente DOR Systems Research Initiative . Predicting red blood cell transfusion in hospitalized patients: role of hemoglobin level, comorbidities, and illness severity. BMC Health Serv Res. 2014;14:213. doi: 10.1186/1472-6963-14-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgren G, Rostgaard K, Vasan SK, et al. The new Scandinavian Donations and Transfusions database (SCANDAT2): a blood safety resource with added versatility. Transfusion. 2015;55(7):1600-1606. doi: 10.1111/trf.12986 [DOI] [PubMed] [Google Scholar]
- 11.Roubinian NH, Escobar GJ, Liu V, et al. ; NHLBI Recipient Epidemiology and Donor Evaluation Study (REDS-III) . Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678-2686. doi: 10.1111/trf.12825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostgaard K. Methods for stratification of person-time and events: a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov . 2008;5:7. doi: 10.1186/1742-5573-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10(4):1100-1120. doi: 10.1214/aos/1176345976 [DOI] [Google Scholar]
- 14.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288-1294. doi: 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Holzmann MJ, Sartipy U, Olsson ML, Dickman P, Edgren G. Sex-discordant blood transfusions and survival after cardiac surgery: a nationwide cohort study. Circulation. 2016;134(21):1692-1694. doi: 10.1161/CIRCULATIONAHA.116.025087 [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473-489. doi: 10.1080/01621459.1996.10476908 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Effect of Association Between Donor Parameters on Recipient Mortality Upon Adding Additional Parameters As Stratification Variables in the Cox Model, to Account for Possible Non-Proportional Hazards
eTable 2. Unadjusted Mortality Rates, in Relation to Number of Transfused Sex-Discordant Units, Units From Female Donors, and Units From Previously Pregnant Donors, Estimated From In-Hospital Mortality Analyses
eTable 3. Unadjusted Mortality Rates, in Relation to Number of Transfused Sex-Discordant Units, Units From Female Donors, and Units From Previously Pregnant Donors, Estimated From Long-Term Mortality Analyses
eTable 4. Results From Analyses Investigating the Effect of Donor Sex and Parity on Patient Survival Based on Single Unit Cohort
eTable 5. Results From Analyses Investigating the Effect of Donor Sex and Parity on Patient Survival Based on Discrete Exposure Group Cohort
eTable 6. Comparison of Standard Cox Model and Fine-Gray Model for Analyses of In-Hospital Mortality
eFigure 1. Subgroup Analyses for Number of Red-Cell Units From Female Donors
eFigure 2. Subgroup Analyses for Number of Sex-Discordant Red-Cell Units