Key Points
Question
Is the use of sodium polystyrene sulfonate associated with a higher risk of hospitalization for adverse gastrointestinal events?
Findings
In this population-level cohort study of 20 020 matched individuals, sodium polystyrene sulfonate use was associated with a 1.9-fold higher risk of hospitalization within 30 days of initial prescription for adverse gastrointestinal events compared with nonuse.
Meaning
The use of sodium polystyrene sulfonate was associated with a high risk of hospitalization for serious adverse gastrointestinal events.
This population-based cohort study assesses the risk of hospitalization or emergency department visit for adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age.
Abstract
Importance
Sodium polystyrene sulfonate is commonly prescribed for the treatment of hyperkalemia. Case reports of intestinal injury after administration of sodium polystyrene sulfonate with sorbitol resulted in a US Food and Drug Administration warning and discontinuation of combined 70% sorbitol–sodium polystyrene sulfonate formulations. There are ongoing concerns about the gastrointestinal (GI) safety of sodium polystyrene sulfonate use.
Objective
To assess the risk of hospitalization for adverse GI events associated with sodium polystyrene sulfonate use in patients of advanced age.
Design, Setting, and Participants
Population-based, retrospective matched cohort study of eligible adults of advanced age (≥66 years) dispensed sodium polystyrene sulfonate from April 1, 2003, to September 30, 2015, in Ontario, Canada, with maximum follow-up to March 31, 2016. Initial data analysis was conducted from August 1, 2018, to October 3, 2018; revision analysis was conducted from February 25, 2019, to April 2, 2019. Cox proportional hazards regression models were used to examine the association of sodium polystyrene sulfonate use with a composite of GI adverse events compared with nonuse that was matched via a high-dimensional propensity score. Additional analyses were limited to a subpopulation with baseline laboratory values of estimated glomerular filtration rate and serum potassium level.
Exposure
Dispensed sodium polystyrene sulfonate in an outpatient setting.
Main Outcomes and Measures
The primary outcome was a composite of adverse GI events (hospitalization or emergency department visit with intestinal ischemia/thrombosis, GI ulceration/perforation, or resection/ostomy) within 30 days of initial sodium polystyrene sulfonate prescription.
Results
From a total of 1 853 866 eligible adults, 27 704 individuals were dispensed sodium polystyrene sulfonate (mean [SD] age, 78.5 [7.7] years; 54.7% male), and 20 020 sodium polystyrene sulfonate users were matched to 20 020 nonusers. Sodium polystyrene sulfonate use compared with nonuse was associated with a higher risk of an adverse GI event over the following 30 days (37 events [0.2%]; incidence rate, 22.97 per 1000 person-years vs 18 events [0.1%]; incidence rate, 11.01 per 1000 person-years) (hazard ratio, 1.94; 95% CI, 1.10-3.41). Results were consistent in additional analyses, including the subpopulation with baseline laboratory values (hazard ratio, 2.91; 95% CI, 1.38-6.12), and intestinal ischemia/thrombosis was the most common type of GI injury.
Conclusions and Relevance
The use of sodium polystyrene sulfonate is associated with a higher risk of hospitalization for serious adverse GI events. These findings require confirmation and suggest caution with the ongoing use of sodium polystyrene sulfonate.
Introduction
Sodium polystyrene sulfonate is a commonly used cation-exchange resin used for the management of hyperkalemia. Originally introduced for use in 1959, it is frequently prescribed, with an estimated 5 million doses administered in the United States per annum.1 As the incidence of recognized hyperkalemia in the population has continued to rise, it is anticipated that there will be a parallel rise in the use of therapeutic agents to safely manage hyperkalemia.2,3,4
Despite its long-standing and widespread use, there have been several case reports of serious and often fatal gastrointestinal (GI) injury associated with sodium polystyrene sulfonate use.5,6 This injury was originally attributed to coadministration of sodium polystyrene sulfonate with 70% sorbitol as a premixed suspension agent, and the US Food and Drug Administration (FDA) issued a black box warning against their concurrent use.7 Nevertheless, case reports of GI injuries, primarily colonic necrosis, have persisted with the use of sodium polystyrene sulfonate as a solo agent.8,9,10,11,12 Clinical trials evaluating its use in a total of 136 patients reported no serious adverse GI events.13,14,15 A single-center observational study10 assessing 2194 inpatients who were administered sodium polystyrene sulfonate reported biopsy-proven colonic necrosis within 30 days to be extremely rare, with only 3 events related to its use (incidence rate, 0.14%). Gastrointestinal injury does not appear to be limited to colonic necrosis because mucosal ulceration has been reported in the esophagus, stomach, and duodenum with sodium polystyrene sulfonate use.6 To date, considerable uncertainty persists regarding the true extent and risk of adverse GI events with sodium polystyrene sulfonate use.1,16
As such, the objective of the present study was to examine the population-level incidence and relative risk of GI injury requiring hospitalization or emergency department visit associated with sodium polystyrene sulfonate use compared with nonuse. The study hypothesis was that sodium polystyrene sulfonate use would not be significantly associated with a higher risk of GI events compared with nonuse.
Methods
Design and Setting
We conducted a population-level, retrospective matched cohort study of eligible adults of advanced age (≥66 years) dispensed sodium polystyrene sulfonate from April 1, 2003, to September 30, 2015, in Ontario, Canada, using linked databases held at the Institute for Clinical Evaluative Sciences (ICES).17 Ontario is Canada’s largest province, with more than 13 million residents, of whom 17% are 65 years or older.18 These data sets were linked using unique encoded identifiers and analyzed at ICES. All citizens have access to universal public health care, and individuals 65 years or older have universal outpatient prescription drug coverage. The use of deidentified data in this project was authorized under §45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board. The reporting of this study follows guidelines for observational studies (eTable 1 in the Supplement).19
Data Sources
We ascertained patient characteristics, medication data, and outcome data from decoded, linked databases housed at ICES.20,21 Demographics and vital status information were obtained from the Ontario Registered Persons Database. Medication information was obtained from the Ontario Drug Benefit claims database, which contains accurate records of all outpatient prescriptions dispensed to patients 65 years or older, with an error rate of less than 1%.22 Diagnostic and procedural information from all hospitalizations was determined using the Canadian Institute for Health Information Discharge Abstract Database. Diagnostic information from emergency department visits was obtained using the National Ambulatory Care Reporting System. Information was also obtained from the Ontario Health Insurance Plan database, which contains all health claims for inpatient and outpatient physician services. Whenever possible, we defined patient characteristics and outcomes using validated codes (eTable 2 in the Supplement). Laboratory information is contained in the Ontario Laboratories Information System, which captures 95% of laboratory tests for individuals in Ontario.23 The databases were complete for all variables used except for rural location, which was missing in less than 0.5% of individuals. The only reason for loss to follow-up was emigration from the province, which occurs in less than 0.5% of residents each year.24
Cohort Definition
All adults 66 years or older with a first outpatient prescription dispensed for sodium polystyrene sulfonate (eTable 3 in the Supplement) from April 1, 2003, to September 30, 2015, were included, with maximum follow-up to March 31, 2016. Patients with a history of the composite outcome of adverse GI events were excluded (look back to 1991 except for resection/ostomy, which was a 5-year look-back window). The eFigure in the Supplement shows the cohort creation. The sodium polystyrene sulfonate prescription date served as the study index date, which was also used as the date of cohort entry and the start of follow-up. We excluded patients with a sodium polystyrene sulfonate prescription in the 180 days before the index date. A random date based on the statistical distribution of index dates for the exposure group was assigned for the unexposed group such that there were no differences in the distributions of index dates between the 2 groups.
Exposure
The study exposure was the first sodium polystyrene sulfonate prescription dispensed in the accrual period. Individuals were followed up until the study outcome, 30 days, emigration from the province, or death. Sodium polystyrene sulfonate users (the exposure group) were matched to nonusers using a high-dimensional propensity score (HDPS). The HDPS is a computer algorithm designed for use in administrative databases that selects and ranks variables based on multiplicative bias testing (ie, an empirical method of variable selection).25 The HDPS is calculated from 200 variables and has improved covariate balance between matched groups, with the potential for less biased treatment estimates compared with traditional propensity score techniques (eTable 4 and eTable 5 and Additional Methodological Details in the Supplement list all covariates included and the HDPS rank).26
Outcomes
The primary study outcome was a hospitalization or emergency department visit for a composite of adverse GI events (intestinal ischemia/thrombosis, GI ulceration/perforation, or resection/ostomy). The secondary outcomes were the 3 individual components of the composite (eTable 2 in the Supplement lists outcome codes used), which reflects the broad spectrum of reported GI injury associated with sodium polystyrene sulfonate use.11,12 Patients were followed up for at least 30 days after the index date (sodium polystyrene sulfonate dispensing). Furthermore, we evaluated the risk of a negative control outcome consisting of a composite GI outcome of cholecystitis, diverticulitis, or appendicitis between sodium polystyrene sulfonate users vs nonusers. The composite of cholecystitis, diverticulitis, or appendicitis was not expected to be associated with sodium polystyrene sulfonate use, and we reasoned that a null association with this outcome would increase the credibility of any observed association with adverse GI events.
Statistical Analysis
We used standardized differences to assess covariate balance before and after HDPS matching between individuals based on sodium polystyrene sulfonate use.27 This measurement assesses differences between group means relative to the pooled standard deviation, with a significant difference considered to be 10% or greater. Individuals with sodium polystyrene sulfonate use were matched (greedy algorithm, without replacement) 1:1 to individuals without use on the logit of the HDPS (±0.2 of the standard deviation) and the following set of investigator-defined variables: age, sex, diabetes, congestive heart failure, prior acute kidney injury (AKI), chronic dialysis, history of hyperkalemia, previous nephrologist visits, medication use (β-blocker or renin-angiotensin-aldosterone system blockade), and index date (within 1 year).28 Variables selected by the HDPS were visually inspected for clinical appropriateness and truncated to the top 200 covariates based on multiplicative bias ranking (eTables 4 and 5 in the Supplement).25 We calculated the incidence rate (defined as the rate per 1000 person-years of follow-up) for the outcomes of interest. We examined the association between sodium polystyrene sulfonate exposure and the study outcomes using Cox proportional hazards regression models. Several sensitivity analyses were conducted as follows: (1) the matching and analysis were repeated in a subpopulation with known baseline categories of estimated glomerular filtration rate (eGFR) (<15, 15-29, 30-44, 45-59, 60-89, or ≥90 mL/min/1.73 m2) (calculated by the Chronic Kidney Disease Epidemiology Collaboration equation) and serum potassium level (<5 or ≥5 mEq/L) (to convert potassium level to millimoles per liter, multiply by 1.0), (2) our models were limited to individuals with a potassium level of at least 5 mEq/L at sodium polystyrene sulfonate dispensing, (3) all patients with a hospitalization or emergency department visit within 30 days of initial sodium polystyrene sulfonate dispensing were excluded, and (4) a negative control outcome (composite of cholecystitis, diverticulitis, or appendicitis) was tested in our matched cohort to examine for residual confounding. In the subpopulation with known baseline eGFR and serum potassium level, individuals with sodium polystyrene sulfonate use were matched (greedy algorithm, without replacement) 1:4 to individuals without use on the logit of the HDPS (±0.2 of the standard deviation) and the following set of investigator-defined variables: eGFR (<15, 15-29, 30-44, 45-59, 60-89, or ≥90 mL/min/1.73 m2), potassium level (<5 or ≥5 mEq/L), age, sex, diabetes, congestive heart failure, prior AKI, chronic dialysis, medication use (renin-angiotensin-aldosterone system blockade or low-molecular-weight heparin), and index date (within 1 year). Both eGFR and serum potassium level were the earliest immediate values before sodium polystyrene sulfonate dispensing (1-year look-back window). Models were also adjusted for place of residence. We tested for an association of several a priori–defined subgroups of interest (eGFR [<15, 15-29, 30-44, 45-59, 60-89, or ≥90 mL/min/1.73 m2], potassium level [<5 or ≥5 mEq/L], history of diabetes or congestive heart failure, prior AKI, use of renin-angiotensin-aldosterone system blockade, and before vs after the 2009 FDA warning against concurrent 70% sorbitol use) with sodium polystyrene sulfonate exposure using interaction terms and the primary study outcome.2,29,30 We conducted all analyses with statistical software (SAS software, SAS Enterprise Guide version 7.1; SAS Institute Inc). On 2-sided testing, confidence intervals that did not overlap with 1 were treated as statistically significant.
Results
From a total 1 853 866 eligible patients, 27 704 individuals (1.5%) (mean [SD] age, 78.5 [7.7] years; 54.7% male) were dispensed sodium polystyrene sulfonate, and 20 020 (72.3%) were retained after matching, for a total of 40 040 matched patients. Baseline characteristics of the total, unmatched, and matched cohorts are listed in Table 1. After matching, the median patient age was 78 years, and 54.4% were male. There was no detectable imbalance between baseline characteristics of the matched groups except for residence in long-term care and/or in a rural area. Just over half of patients had a history of hypertension or diabetes. Most were taking an angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, or aldosterone receptor antagonist, and more than half were taking a nonpotassium-sparing diuretic. Subsets of 7557 patients with known eGFR and serum potassium level at sodium polystyrene sulfonate dispensation were further matched to 18 492 controls. For this cohort, 77.1% had an eGFR less than 60 mL/min/1.73 m2, and the baseline serum potassium level differed between the sodium polystyrene sulfonate cases (mean [SD], 5.6 [0.7] mEq/L) and controls (mean [SD], 5.1 [0.3] mEq/L) (eTable 6 in the Supplement).
Table 1. Baseline Characteristics of Sodium Polystyrene Sulfonate Users and Nonusers Before and After Matching.
| Variable | Total | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|---|
| Use | Nonuse | Standardized Difference, % | Use | Nonuse | Standardized Difference, % | ||
| Total No. | 1 853 866 | 27 704 | 1 826 162 | NA | 20 020 | 20 020 | NA |
| Demographics | |||||||
| Age, median (IQR), y | 74 (69-81) | 78 (72-84) | 74 (69-81) | NA | 78 (72-84) | 78 (72-84) | NA |
| Female, No. (%) | 1 028 487 (55.5) | 12 554 (45.3) | 1 015 933 (55.6) | 21a | 9134 (45.6) | 9134 (45.6) | 0 |
| Long-term care, No. (%) | 206 983 (11.2) | 4588 (16.6) | 202 395 (11.1) | 16a | 3212 (16.0) | 4646 (23.2) | 18.0a |
| Reside in a rural area, No. (%) | 244 567 (13.2) | 4523 (16.3) | 240 044 (13.2) | 9 | 3402 (17.0) | 2405 (12.0) | 14.2a |
| Comorbidities, No. (%) | |||||||
| Hypertension | 717 899 (38.7) | 15 973 (57.7) | 701 926 (38.4) | 39a | 10 830 (54.1) | 11 395 (56.9) | 5.7 |
| Diabetes | 394 896 (21.3) | 15 358 (55.4) | 379 538 (20.8) | 76a | 10 583 (52.9) | 10 583 (52.9) | 0 |
| Stroke/transient ischemic attack | 80 466 (4.3) | 1933 (7.0) | 78 533 (4.3) | 12a | 1339 (6.7) | 1436 (7.2) | 1.9 |
| Atrial fibrillation | 98 562 (5.3) | 3321 (12.0) | 95 241 (5.2) | 24a | 1972 (9.9) | 2381 (11.9) | 6.6 |
| Peripheral vascular disease | 10 984 (0.6) | 776 (2.8) | 10 208 (0.6) | 18a | 419 (2.1) | 384 (1.9) | 1.2 |
| Congestive heart failure | 70 543 (3.8) | 6020 (21.7) | 64 523 (3.5) | 57a | 3065 (15.3) | 3065 (15.3) | 0 |
| Coronary artery disease | 187 475 (10.1) | 6781 (24.5) | 180 694 (9.9) | 39a | 4355 (21.8) | 4760 (23.8) | 4.8 |
| Angina | 37 437 (2.0) | 1448 (5.2) | 35 989 (2.0) | 18a | 869 (4.3) | 1049 (5.2) | 4.2a |
| Prior acute kidney injury | 50 214 (2.7) | 5640 (20.4) | 44 574 (2.4) | 59a | 2139 (10.7) | 2139 (10.7) | 0 |
| Chronic dialysis | 3209 (0.2) | 938 (3.4) | 2271 (0.1) | 25a | 220 (1.1) | 220 (1.1) | 0 |
| History of hyperkalemia | 16 876 (0.9) | 4771 (17.2) | 12 105 (0.6) | 61a | 1037 (5.2) | 1037 (5.2) | 0 |
| Gastrointestinal surgery | 6142 (0.3) | 326 (1.2) | 5816 (0.3) | 10a | 157 (0.8) | 201 (1.0) | 2.1 |
| Access to Care, % Using the Health Service ≥1 Episode | |||||||
| Emergency department visits | 56.9 | 80.2 | 56.5 | 53a | 77.0 | 76.2 | 2.2 |
| Hospitalizations | 10.6 | 41.9 | 10.1 | 78a | 32.0 | 32.9 | 1.9 |
| Gastroenterologist visits | 6.4 | 15.7 | 6.3 | 31a | 12.5 | 14.7 | 6.6 |
| Nephrologist visits | 4.6 | 41.6 | 4.0 | 100a | 34.1 | 34.1 | 0 |
| Cardiology visits | 28.7 | 58.0 | 28.2 | 63a | 52.8 | 56.9 | 7.6 |
| Medications, No. (%) | |||||||
| Angiotensin-converting enzyme inhibitor | 464 260 (25.0) | 13 596 (49.1) | 450 664 (24.7) | 52a | 10 082 (50.4) | 9643 (48.2) | 4.4 |
| Nonpotassium-sparing diuretic | 523 427 (28.2) | 15 886 (57.3) | 507 541 (27.8) | 63a | 10 747 (53.7) | 11 201 (56.0) | 4.6 |
| Potassium-sparing diuretic | 68 790 (3.7) | 4436 (16.0) | 64 354 (3.5) | 43a | 2381 (11.9) | 2381 (11.9) | 0 |
| β-Blocker | 411 883 (22.2) | 13 961 (50.4) | 397 922 (21.8) | 62a | 9567 (47.8) | 9567 (47.8) | 0 |
| Angiotensin II receptor blocker | 281 326 (15.2) | 8218 (29.7) | 273 108 (15.0) | 36a | 6115 (30.5) | 5913 (29.5) | 2.2 |
| Aldosterone receptor antagonist | 31 289 (1.7) | 3850 (13.9) | 27 439 (1.5) | 48a | 2034 (10.2) | 1872 (9.5) | 2.7 |
| Nonsteroidal anti-inflammatory drug | 266 304 (14.4) | 5319 (19.2) | 260 985 (14.3) | 13a | 4037 (20.2) | 3666 (18.3) | 4.7 |
| Low-molecular-weight heparin | 4923 (0.3) | 431 (1.6) | 4492 (0.3) | 14a | 268 (1.3) | 145 (0.7) | 6.1 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Standardized differences exceeding 10% are statistically significant.
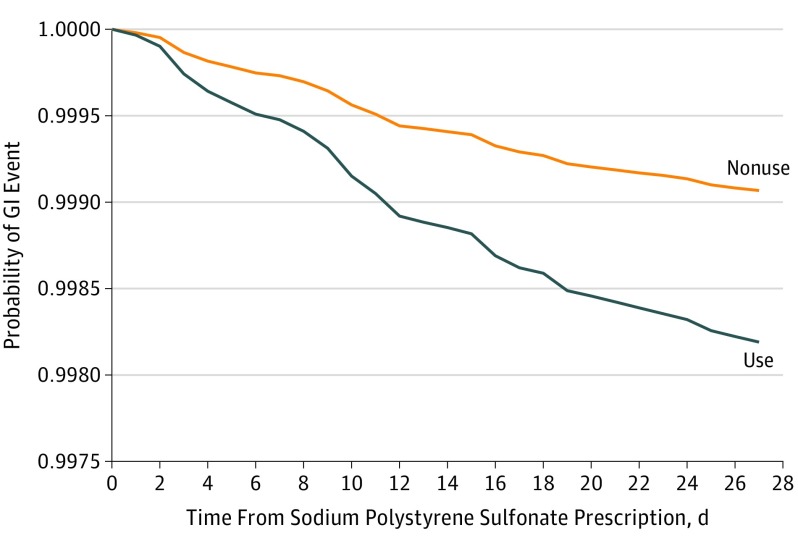
The primary study outcome is summarized in Table 2 and the Figure. In the total study cohort, there were 63 events (0.2%) in the sodium polystyrene sulfonate use group and 756 events (0.04%) events in the nonuse group. In the matched cohort, there were 37 events (0.2%) in the sodium polystyrene sulfonate use group and 18 events (0.1%) in the nonuse group. A higher risk for an adverse GI event was observed with sodium polystyrene sulfonate use vs nonuse in the matched cohort (37 [0.2%]; incidence rate, 22.97 per 1000 person-years vs 18 [0.1%]; incidence rate, 11.01 per 1000 person-years) (hazard ratio [HR], 1.94; 95% CI, 1.10-3.41), with the risk of an adverse event detected early (within 10 days of sodium polystyrene sulfonate dispensing) and persisting during the entire follow-up period (Figure). When limited to patients with laboratory values and matched for eGFR and serum potassium level, the association persisted (HR, 2.91; 95% CI, 1.38-6.12), albeit in a smaller number of events (15 events in the sodium polystyrene sulfonate use group vs 13 events in the nonuse group). Similarly, the association was consistent when the cohort was limited to patients with a serum potassium level of at least 5 mEq/L (HR, 2.59; 95% CI, 1.10-6.09) and when we excluded all patients with a hospitalization or emergency department visit during the 30 days preceding sodium polystyrene sulfonate dispensing (HR, 3.11; 95% CI, 1.31-7.38).
Table 2. Association of Sodium Polystyrene Sulfonate Use vs Nonuse With Hospitalization or Emergency Department Visit for an Adverse Gastrointestinal Event Within 30 Days.
| Variable | No. of Events (% Total) | Time to Event, Median (IQR), d | Incidence Rate (95% CI) per 1000 Person-Years | HR (95% CI) |
|---|---|---|---|---|
| Total Cohort (27 704 With Use and 1 826 162 With Nonuse) | ||||
| Use | 63 (0.2) | 12 (6.2) | 28.33 (21.33-35.32) | 5.61 (4.34-7.26) |
| Nonuse | 756 (0.04) | 16 (9.2) | 5.05 (4.69-5.41) | |
| Matched Cohort (20 020 With Use and 20 020 With Nonuse)a | ||||
| Use | 37 (0.2) | 12 (8.2) | 22.97 (15.57-30.37) | 1.94 (1.10-3.41) |
| Nonuse | 18 (0.1) | 14 (3.2) | 11.01 (5.92-16.09) | |
| Matched With Laboratory Values (7557 With Use and 18 492 With Nonuse)b | ||||
| Use | 15 (0.2) | 12 (6.2) | 24.54 (12.11-36.95) | 2.91 (1.38-6.12) |
| Nonuse | 13 (0.1) | 8 (4.2) | 8.59 (3.92-13.26) | |
Abbreviations: HR, hazard ratio; IQR, interquartile range.
SI conversion factor: To convert potassium level to millimoles per liter, multiply by 1.0.
Matched (greedy algorithm, without replacement) 1:1 to individuals without use on the logit of the high-dimensional propensity score (±0.2 of the standard deviation) and the following: age, sex, diabetes, congestive heart failure, prior acute kidney injury, chronic dialysis, history of hyperkalemia, previous nephrologist visit, medication use (β-blocker or renin-angiotensin-aldosterone system blockade), and index date (within 1 year). Additional adjustment was for place of residence.
Matched (greedy algorithm, without replacement) 1:4 to individuals without use on the logit of the high-dimensional propensity score (±0.2 of the standard deviation) and the following: baseline estimated glomerular filtration rate (<15, 15-29, 30-44, 45-59, 60-89, or ≥90 mL/min/1.73 m2), baseline serum potassium level (<5 or ≥5 mEq/L), age, sex, diabetes, congestive heart failure, prior acute kidney injury, chronic dialysis, medication use (renin-angiotensin-aldosterone system blockade or low-molecular weight heparin), and index date (within 1 year). Additional adjustment was for place of residence.
Figure. 30-Day Probability of Gastrointestinal (GI) Injury Requiring Hospitalization or Emergency Department Visit Associated With Sodium Polystyrene Sulfonate Use Compared With Nonuse.
Results presented are for the matched analysis of sodium polystyrene sulfonate users to nonusers on the logit of the high-dimensional propensity score (±0.2 of the standard deviation) and the following: age, sex, diabetes, congestive heart failure, prior acute kidney injury, chronic dialysis, history of hyperkalemia, previous nephrologist visit, medication use (β-blocker or renin-angiotensin-aldosterone system blockade), and index date (within 1 year). Additional adjustment was for place of residence.
When examining the subtypes of GI injury, the hazard ratio was highest for intestinal ischemia/thrombosis (HR, 4.92; 95% CI, 1.09-22.25), whereas no association was detected for GI ulceration/perforation (HR, 1.75; 95% CI, 0.70-4.41) or resection/ostomy (HR, 1.34; 95% CI, 0.59-3.02). These results are summarized in Table 3.
Table 3. Association of Sodium Polystyrene Sulfonate Use vs Nonuse With Hospitalization or Emergency Department Visit by Type of Gastrointestinal (GI) Event Within 30 Daysa.
| Variable | Use | Nonuse | HR (95% CI) | ||
|---|---|---|---|---|---|
| No. of Events (% Total)b | Incidence Rate (95% CI) per 1000 Person-Years | No. of Events (% Total) | Incidence Rate (95% CI) per 1000 Person-Years | ||
| Intestinal ischemia/thrombosis | 11 (0.1) | 6.82 (3.78-12.32) | <5c | 1.22 (0.31-4.89) | 4.92 (1.09-22.25) |
| GI ulceration/perforation | 13 (0.1) | 8.07 (4.68-13.89) | 7 (0) | 4.28 (2.04-8.98) | 1.75 (0.70-4.41) |
| Resection/ostomy | 14 | 8.69 (5.15-14.67) | 10 (0.1) | 6.11 (3.29-11.36) | 1.34 (0.59-3.02) |
Abbreviation: HR, hazard ratio.
Matched (greedy algorithm, without replacement) 1:1 to individuals without use on the logit of the high-dimensional propensity score (±0.2 of the standard deviation) and the following: age, sex, diabetes, congestive heart failure, prior acute kidney injury, chronic dialysis, history of hyperkalemia, previous nephrologist visit, medication use (β-blocker or renin-angiotensin-aldosterone system blockade), and index date (within 1 year). Additional adjustment was for place of residence.
Total number of events is 38 because 1 patient had 2 events on the same day.
Number of events is suppressed when less than 5 as per the Institute for Clinical Evaluative Sciences privacy policy.
We examined several a priori–defined subgroups of interest, including those with baseline eGFR and serum potassium level, diabetes, congestive heart failure, AKI, chronic dialysis, renin-angiotensin-aldosterone system blockade, and before vs after the 2009 FDA warning against concurrent 70% sorbitol use. There were no statistically significant differences in risk between any of the subgroups examined (Table 4).
Table 4. Risk of Adverse Gastrointestinal Injury Comparing Matched Sodium Polystyrene Sulfonate Users to Nonusers in Subgroups of Interest.
| Variable | Use | Nonuse | HR (95% CI)a | Interaction P Value | ||
|---|---|---|---|---|---|---|
| No. of Events (%) | Incidence Rate (95% CI) per 1000 Person-Years | No. of Events (%) | Incidence Rate (95% CI) per 1000 Person-Years | |||
| Baseline Serum Potassium Level, mEq/Lb | ||||||
| <5 | 11 (0.2) | 36.75 (13.79-97.93) | 10 (0.1) | 8.77 (2.93-27.18) | 4.45 (0.99-19.94) | .54 |
| ≥5 | NR | 21.89 (12.12-39.52) | NR | 8.54 (4.59-15.87) | 2.59 (1.10-6.09) | |
| Diabetes | ||||||
| Yes | 18 (0.2) | 21.00 (13.23-33.32) | 8 (0.1) | 9.24 (4.62-18.48) | 2.12 (0.92-4.87) | .78 |
| No | 19 (0.2) | 25.22 (16.09-39.54) | 10 (0.1) | 12.99 (6.99-24.14) | 1.80 (0.83-3.87) | |
| Congestive Heart Failure | ||||||
| Yes | 7 (0.2) | 28.78 (13.72-60.38) | NR | 8.02 (2.01-32.06) | 3.35 (0.70-16.14) | .46 |
| No | 30 (0.2) | 21.94 (15.34-31.38) | 16 (0.1) | 11.55 (7.07-18.84) | 1.76 (0.96-3.24) | |
| Prior Acute Kidney Injury | ||||||
| Yes | NR | NA | NR | NA | NA | NA |
| No | 33 (0.2) | NA | 18 (0.1) | NA | 1.74 (0.98-3.10) | |
| Chronic Dialysis | ||||||
| Yes | NR | NA | NR | NA | NA | NA |
| No | 37 (0.2) | NA | 17 (0.1) | NA | 2.05 (1.15-3.65) | |
| Renin-Angiotensin-Aldosterone System Blockade | ||||||
| Yes | 30 (0.2) | 24.12 (16.86-34.50) | 12 (0.1) | 9.52 (5.41-16.76) | 2.36 (1.21-4.62) | .24 |
| No | 7 (0.2) | 19.08 (9.10-40.02) | 6 (0.1) | 16.01 (7.19-35.64) | 1.10 (0.37-3.27) | |
| Before vs After the 2009 FDA Warning Against Concurrent 70% Sorbitol Use | ||||||
| Before | 15 (0.2) | 20.13 (12.13-33.39) | 9 (0.1) | 11.91 (6.19-22.87) | 1.52 (0.66-3.48) | .44 |
| After | 22 (0.2) | 25.42 (16.74-38.61) | 9 (0.1) | 10.24 (5.33-19.68) | 2.37 (1.09-5.16) | |
Abbreviations: FDA, US Food and Drug Administration; HR, hazard ratio; NA, not applicable; NR, not reportable (number of events is suppressed when <5 as per the Institute for Clinical Evaluative Sciences privacy policy).
SI conversion factor: To convert potassium level to millimoles per liter, multiply by 1.0.
Comparing users vs nonusers (reference).
Calculated from matched cohort with laboratory values (7557 with use and 18 492 with nonuse). Baseline estimated glomerular filtration rate subgroups had few events and are not presented.
When examining a composite negative GI outcome (unrelated to sodium polystyrene sulfonate use), there was no statistically significant association between sodium polystyrene sulfonate use and the risk of cholecystitis, diverticulitis, or appendicitis (153 events; HR, 1.22; 95% CI, 0.89-1.69). These results are summarized in eTable 7 in the Supplement.
Discussion
In this population-based, retrospective matched cohort study, individuals who received sodium polystyrene sulfonate were at an elevated risk for a serious adverse GI event. The heightened risk was independent of baseline eGFR and serum potassium level and was detected within 30 days of sodium polystyrene sulfonate being dispensed. Consistent with previous reports,5 the most common type of GI injury was intestinal ischemia/thrombosis. The higher risk did not differ according to baseline eGFR or serum potassium level, diabetes, congestive heart failure, AKI, chronic dialysis, renin-angiotensin-aldosterone system blockade, and before vs after the 2009 FDA warning against concurrent 70% sorbitol use.
To our knowledge, this is the first population-based study of sodium polystyrene sulfonate use and serious adverse GI events. Previous studies5,10,13,14,15,31 are based on case reports, single-center cohort studies, or small trials, leaving considerable uncertainty regarding the true association of sodium polystyrene sulfonate exposure and GI injury. Harel et al5 identified 58 cases of adverse GI events across 30 case reports associated with sodium polystyrene sulfonate use (17 without sorbitol) in a systematic review. Moreover, symptoms were reported shortly after sodium polystyrene sulfonate use (median, 2 days), lesions were reported throughout the GI tract, and subsequent mortality was high (33%). Using a retrospective cohort study design at a single tertiary care center, Watson et al16 reported an overall low risk of sodium polystyrene sulfonate–associated GI injury, with a 2-fold increase in the crude incidence with sodium polystyrene sulfonate use vs nonuse (0.14% vs 0.07%). Pathological confirmation was required for identification, and only colonic GI injuries were included. In a small randomized clinical trial (RCT) of sodium polystyrene sulfonate use (n = 33) associated with mild hyperkalemia, Lepage et al15 reported no increase in any GI symptoms or adverse events with sodium polystyrene sulfonate exposure vs placebo. Similar small RCTs13,14 reported no serious adverse GI events associated with sodium polystyrene sulfonate use.
Using a broader definition of GI injury, we observed a consistent and concerning risk of serious injury shortly after sodium polystyrene sulfonate administration. Our findings allow an estimation of the incidence of serious GI injury at a population level associated with outpatient sodium polystyrene sulfonate use at roughly 23 cases per 1000 person-years of exposure (Table 2). It remains unclear whether the risk is similar with chronic, repeat sodium polystyrene sulfonate use.
When examining the subtypes of GI injury, the highest risk associated with sodium polystyrene sulfonate use was intestinal ischemia/thrombosis, which is consistent with previous studies.5 Of note, the requirement for pathological confirmation may significantly underestimate the true risk of injury because it may not be routinely performed unless GI adverse events associated with sodium polystyrene sulfonate exposure are clinically suspected. Therefore, our broader definition of GI injury (including resection/ostomy) may more reliably capture the clinical consequences of sodium polystyrene sulfonate–associated injury. Furthermore, upper GI tract injuries are likely underrecognized and were previously reported in a small case series describing sodium polystyrene sulfonate crystallization in the esophagus, stomach, and duodenum.6 Of concern, 9 of 11 patients demonstrated concurrent endoscopic or histopathological mucosal ulceration or erosion.6 Herein, we detected numerically but not statistically significant elevations in the risk of upper GI tract or surgical complications associated with sodium polystyrene sulfonate use (likely related to the small number of events). Further studies should specifically test for a more diverse range of adverse GI risk associated with sodium polystyrene sulfonate exposure to add clarity to the issue.
Overcoming many of the limitations of the existing studies, we discovered a remarkably consistent higher risk of sodium polystyrene sulfonate use associated with adverse GI events. We used a broad definition of GI injury that would capture the spectrum of previously reported sodium polystyrene sulfonate–related injury.8,12 Our findings remained consistent through multiple sensitivity analyses, which included matching patients according to baseline eGFR and serum potassium level and limiting our analysis to those without hospitalization or emergency department visits in the preceding 30 days. Our use of the HDPS matching technique theoretically reduced measured and unmeasured residual confounding. This tactic is supported by the lack of any association being detected between sodium polystyrene sulfonate exposure and an unrelated negative control outcome. Because this research was a population-level study, we were able to capture all relevant, outpatient sodium polystyrene sulfonate prescriptions and outcomes in our region, thus allowing for a more accurate estimate of the true incidence of adverse events.
We examined multiple, relevant subgroups suspected of having a heightened risk of sodium polystyrene sulfonate–associated GI injury. There was no significant difference in the increased risk of adverse GI events in groups of patients with comorbidities (diabetes, congestive heart failure, or prior AKI), reduced kidney function, history of hyperkalemia, or medication use (renin-angiotensin-aldosterone system blockade). Furthermore, the risk remained consistent before and after 2009, the year when the FDA issued a warning regarding concurrent administration of sodium polystyrene sulfonate and sorbitol, suggesting that sorbitol alone is not driving the risk of GI injury.7 Our inability to detect risk differences across subgroups may reflect that there is a consistent risk associated with sodium polystyrene sulfonate use or, alternatively, that risk is due to sample size limitations of the subgroups.
Because sodium polystyrene sulfonate is commonly used for the treatment of hyperkalemia, our study has important clinical implications. The heightened risk warrants careful consideration before sodium polystyrene sulfonate prescription, especially when alternative treatment options exist, such as nonpotassium-sparing diuretics or other GI-based cation binders.4 Because we were unable to identify specific subgroups at higher risk, all individuals being considered for sodium polystyrene sulfonate administration should be assumed to be at risk and be informed of the potential risks and treatment alternatives.
Limitations
Our study has some important limitations. First, we did not have information on the dose or route of sodium polystyrene sulfonate administration in our cohort. As such, we could not test for a dose-response association. In previous studies,5,32 it has been suggested that there is a dose-dependent link with intestinal injury and necrosis, with low-dose sodium polystyrene sulfonate being less likely to cause GI injury. Some reports suggest that rectal, as opposed to oral, administration of sodium polystyrene sulfonate is primarily associated with adverse GI events.1 Second, sodium polystyrene sulfonate exposure was defined at the time of dispensing and may not reflect its actual use or length of treatment. Third, despite our large data set, there remained a limited number of events in certain subgroups. Fourth, we were unable to determine if sorbitol was concurrently administered with sodium polystyrene sulfonate, but we anticipate its use to be limited, especially after 2009. Fifth, our study outcomes were not validated, and there is a possibility for misclassification. Sixth, despite our use of sophisticated matching in cohorts with and without laboratory values, patients who received sodium polystyrene sulfonate had a higher proportion of potassium levels exceeding 6 mEq/L. Seventh, we lacked any pathological data that could suggest a causal association between sodium polystyrene sulfonate use and adverse GI events. However, the presence or absence of sodium polystyrene sulfonate crystals and their pathophysiological role in GI injury remain unclear.11
Conclusions
Sodium polystyrene sulfonate is a widely used, generically available, cation-exchange resin used to treat an increasingly common condition of hyperkalemia. Our large, population-level retrospective matched cohort study investigating GI outcomes in outpatients receiving sodium polystyrene sulfonate found a significant and consistent association of serious adverse GI events with use of the drug. These findings require confirmation and suggest that clinicians should exercise caution in prescribing sodium polystyrene sulfonate.
eTable 1. Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement Checklist
eTable 2. Administrative Data Definitions
eTable 3. Ontario Drug Benefit Program Drug Identification Numbers Used to Define Exposure and Baseline Variables
eTable 4. Covariates Included in High Dimensional Propensity Score (HDPS)
eTable 5. Covariates Included in HDPS Score for Matched Subcohort With Known Baseline eGFR and Serum Potassium Values
eTable 6. Baseline Characteristics of Sodium Polystyrene Sulfonate (SPS) and Non-SPS Users Post-Matching in the Subcohort With Known Baseline eGFR and Serum Potassium Values
eTable 7. Association Between Sodium Polystyrene Sulfonate (SPS) Use and a Negative Control (Composite of Hospitalization or Emergency Room Visit for Cholecystitis, Diverticulitis or Appendicitis With 30-Day Follow-up)
eFigure. Cohort Creation Flow Diagram
eMethods.
References
- 1.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. doi: 10.1681/ASN.2010010079 [DOI] [PubMed] [Google Scholar]
- 2.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loutradis C, Tolika P, Skodra A, Avdelidou A, Sarafidis PA. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested case-control study. Am J Nephrol. 2015;42(5):351-360. doi: 10.1159/000442393 [DOI] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Appel LJ, Grams ME, et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the national kidney foundation and the american society of hypertension. Am J Kidney Dis. 2017;70(6):844-858. doi: 10.1053/j.ajkd.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 5.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e9-264.e24. doi: 10.1016/j.amjmed.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 6.McGowan CE, Saha S, Chu G, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493-497. doi: 10.1097/SMJ.0b013e31819e8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Kayexelate: sodium polystyrene sulfonate, USP, cation-exchange resin. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/011287s022lbl.pdf. Published 2009. Accessed September 20, 2018.
- 8.Albeldawi M, Gaur V, Weber L. Kayexalate-induced colonic ulcer. Gastroenterol Rep (Oxf). 2014;2(3):235-236. doi: 10.1093/gastro/gou011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Cejas MD, de-Torres-Ramírez I, Alonso-Cotoner C. Colonic necrosis due to calcium polystyrene sulfonate (Kalimate) not suspended in sorbitol. Rev Esp Enferm Dig. 2013;105(4):232-234. doi: 10.4321/S1130-01082013000400010 [DOI] [PubMed] [Google Scholar]
- 10.Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. doi: 10.1053/j.ajkd.2012.04.023 [DOI] [PubMed] [Google Scholar]
- 11.Hajjar R, Sebajang H, Schwenter F, Mercier F. Sodium polystyrene sulfonate crystals in the gastric wall of a patient with upper gastrointestinal bleeding and gastric perforation: an incidental finding or a pathogenic factor? J Surg Case Rep. 2018;2018(6):rjy138. doi: 10.1093/jscr/rjy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham SC, Bhagavan BS, Lee LA, Rashid A, Wu TT. Upper gastrointestinal tract injury in patients receiving Kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol. 2001;25(5):637-644. doi: 10.1097/00000478-200105000-00011 [DOI] [PubMed] [Google Scholar]
- 13.Nasir K, Ahmad A. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad. 2014;26(4):455-458. [PubMed] [Google Scholar]
- 14.Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998;9(10):1924-1930. [DOI] [PubMed] [Google Scholar]
- 15.Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. doi: 10.2215/CJN.03640415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5(10):1723-1726. doi: 10.2215/CJN.03700410 [DOI] [PubMed] [Google Scholar]
- 17.Institute for Clinical Evaluative Sciences ICES. https://www.ices.on.ca/. Accessed September 5, 2018.
- 18.Statistics Canada Population by sex and age group, by province and territory (number, both sexes). https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Published 2016. Accessed May 1, 2018.
- 19.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha P, Deboer D, Sykora K, Naylor CD. Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population-based comparison. J Am Coll Cardiol. 1996;27(6):1335-1342. doi: 10.1016/0735-1097(96)00018-6 [DOI] [PubMed] [Google Scholar]
- 21.Sood MM, Bota SE, McArthur E, et al. The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Can J Kidney Health Dis. 2014;1(21):21. doi: 10.1186/s40697-014-0021-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67-71. [PubMed] [Google Scholar]
- 23.Government of Ontario Ontario Laboratories Information System (OLIS): information for providers. AOHC EMR Implementation Toolkit. https://www.ehealthontario.on.ca/en/standards/view/ontario-laboratories-information-system-standard. Published 2018. Accessed June 26, 2018.
- 24.Statistics Canada From east to west: 140 years of interprovincial migration. https://www150.statcan.gc.ca/n1/pub/11-630-x/11-630-x2017002-eng.htm. Published 2018. Accessed October 1, 2018.
- 25.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512-522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guertin JR, Rahme E, Dormuth CR, LeLorier J. Head to head comparison of the propensity score and the high-dimensional propensity score matching methods. BMC Med Res Methodol. 2016;16(1):22. doi: 10.1186/s12874-016-0119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 28.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151-161. doi: 10.1162/003465302317331982 [DOI] [Google Scholar]
- 29.Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(11):e008912. doi: 10.1161/118.008912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg JM, Appel LJ, Bakris G, et al. ; African American Study of Hypertension and Kidney Disease Collaborative Research Group . Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med. 2009;169(17):1587-1594. doi: 10.1001/archinternmed.2009.284 [DOI] [PubMed] [Google Scholar]
- 31.Mistry M, Shea A, Giguère P, Nguyen ML. Evaluation of sodium polystyrene sulfonate dosing strategies in the inpatient management of hyperkalemia. Ann Pharmacother. 2016;50(6):455-462. doi: 10.1177/1060028016641427 [DOI] [PubMed] [Google Scholar]
- 32.Georgianos PI, Liampas I, Kyriakou A, et al. Evaluation of the tolerability and efficacy of sodium polystyrene sulfonate for long-term management of hyperkalemia in patients with chronic kidney disease. Int Urol Nephrol. 2017;49(12):2217-2221. doi: 10.1007/s11255-017-1717-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement Checklist
eTable 2. Administrative Data Definitions
eTable 3. Ontario Drug Benefit Program Drug Identification Numbers Used to Define Exposure and Baseline Variables
eTable 4. Covariates Included in High Dimensional Propensity Score (HDPS)
eTable 5. Covariates Included in HDPS Score for Matched Subcohort With Known Baseline eGFR and Serum Potassium Values
eTable 6. Baseline Characteristics of Sodium Polystyrene Sulfonate (SPS) and Non-SPS Users Post-Matching in the Subcohort With Known Baseline eGFR and Serum Potassium Values
eTable 7. Association Between Sodium Polystyrene Sulfonate (SPS) Use and a Negative Control (Composite of Hospitalization or Emergency Room Visit for Cholecystitis, Diverticulitis or Appendicitis With 30-Day Follow-up)
eFigure. Cohort Creation Flow Diagram
eMethods.