Key Points
Question
Do patients with ischemic stroke with large-vessel occlusion in the anterior circulation who were transferred from outside facilities and had penumbral imaging mismatch prior to endovascular thrombectomy have similar outcomes with thrombectomy in the late window as those who were directly admitted to thrombectomy-capable hospitals?
Findings
In this secondary analysis of a randomized clinical trial, transfer and direct patients had comparable rates of functional independence and similar treatment effect with endovascular thrombectomy as well as similar symptomatic intracranial hemorrhage and mortality.
Meaning
Transferring patients for late-window thrombectomy may be associated with substantial clinical benefits and should be encouraged.
Abstract
Importance
Although thrombectomy benefit was maintained in transfer patients with ischemic stroke in early-window trials, overall functional independence rates were lower in thrombectomy and medical management–only groups.
Objective
To evaluate whether the imaging-based selection criteria used in the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) trial would lead to comparable outcome rates and treatment benefits in transfer vs direct-admission patients.
Design, Setting, and Participants
Subgroup analysis of DEFUSE 3, a prospective, randomized, multicenter, blinded–end point trial. Patients were enrolled between May 2016 and May 2017 and were followed up for 90 days. The trial comprised 38 stroke centers in the United States and 182 patients with stroke with a large-vessel anterior circulation occlusion and initial infarct volume of less than 70 mL, mismatch ratio of at least 1.8, and mismatch volume of at least 15 mL, treated within 6 to 16 hours from last known well. Patients were stratified based on whether they presented directly to the study site or were transferred from a primary center. Data were analyzed between July 2018 and October 2018.
Interventions or Exposures
Endovascular thrombectomy plus standard medical therapy vs standard medical therapy alone.
Main Outcomes and Measures
The primary outcome was the distribution of 90-day modified Rankin Scale scores.
Results
Of the 296 patients who consented, 182 patients were randomized (66% were transfer patients and 34% directly presented to a study site). Median age was 71 years (interquartile range [IQR], 60-79 years) vs 70 years (IQR, 59-80 years); 69 transfer patients were women (57%) and 23 of the direct group were women (37%). Transfer patients had longer median times from last known well to study site arrival (9.43 vs 9 hours) and more favorable collateral profiles (based on hypoperfusion intensity ratio): median for transfer, 0.35 (IQR, 0.18-0.47) vs 0.42 (IQR, 0.25-0.56) for direct (P = .05). The primary outcome (90-day modified Rankin Scale score shift) did not differ in the direct vs transfer groups (direct OR, 2.9; 95% CI, 1.2-7.2; P = .01; transfer OR, 2.6; 95% CI, 1.3-4.8; P = .009). The overall functional independence rate (90-day modified Rankin Scale score 0-2) in the thrombectomy group did not differ (direct 44% vs transfer 45%) nor did the treatment effect (direct OR, 2.0; 95% CI, 0.9-4.4 vs transfer OR, 3.1; 95% CI, 1.6-6.1). Thrombectomy reperfusion rates, mortality, and symptomatic intracranial hemorrhage rates did not differ.
Conclusions and Relevance
In late-window patients selected by penumbral mismatch criteria, both the favorable outcome rate and treatment effect did not decline in transfer patients. These results have health care implications indicating transferring potential candidates for late-window thrombectomy is associated with substantial clinical benefits and should be encouraged.
Trial Registration
ClinicalTrials.gov identifier: NCT02586415
This secondary analysis of the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 trial evaluates whether the imaging-based selection criteria used in the trial would lead to comparable outcome rates and treatment benefits in transfer vs direct-admission patients with ischemic stroke.
Introduction
The Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) study demonstrated the efficacy and safety of endovascular thrombectomy (EVT) up to 16 hours after last-known-well time in patients selected by perfusion imaging. However, the timely delivery of the endovascular procedure is still vital for achieving good clinical outcomes.
Stroke treatment paradigms focus on transporting patients to the closest hospital with intravenous thrombolysis capability1 that often are not thrombectomy capable. Patients with large-vessel occlusion (LVO) are subsequently transferred for consideration of thrombectomy. Prior studies suggested that patients who present first to facilities without EVT capability and are then transferred to EVT-capable hospitals have worse outcomes compared with those who present directly to EVT-capable centers.2,3
Most transferred patients present to thrombectomy centers beyond 6 hours owing to interfacility delays.2,3,4 The extended treatment time window shown in the DEFUSE 3 trial provides opportunities for more patients with LVO to be transferred to EVT-capable centers and potentially treated with thrombectomy.
Because the late-window trials5,6 used perfusion and/or diffusion imaging to identify patients with salvageable brain tissue as part of the inclusion criteria, we hypothesized that transfer patients who are selected based on the presence of penumbral tissue prior to EVT will have similar rates of good outcomes compared with patients who present directly to an endovascular center. We assessed the efficacy and safety of EVT in patients who were transferred from outside hospitals to EVT-capable study sites in the DEFUSE 3 trial.
Methods
Trial Design and Participants
The DEFUSE 3 trial was a prospective, randomized, multicenter, phase III, blinded–end point trial. Patients with ischemic stroke from 38 centers across the United States were randomized 1 to 1 to EVT vs medical therapy based on clinical features and perfusion imaging selection criteria using the RAPID software (iSchemaView). We stratified patients based on their initial presentation status into direct, those who presented to an EVT-capable study site directly, and transfer, those who presented to an outside facility and then transferred to a study site for EVT. Enrolled patients or their surrogates provided written informed consent, and the trial was approved by the institutional review board at Stanford University. This analysis design and plan were not prespecified in the trial protocol but were preplanned before any of the DEFUSE3 data were analyzed or any results were reached. The qualifying imaging was acquired after the transfer at the EVT-capable center and prior to thrombectomy; therefore, patients who had no mismatch or a large ischemic core on arrival were excluded. The full methods of DEFUSE 3 have been reported7 as well as the primary results.5 The formal trial protocols can be found in the Supplement.
Outcomes
The primary outcome was an ordinal analysis of the distribution of scores on the 90-day modified Rankin Scale score (mRS). The secondary outcome was functional independence (90-day mRS, 0-2). Additional outcomes included the median 24-hour and discharge National Institutes of Health Stroke Scale score.
Key imaging outcomes were infarct volume at 24 hours (±6 hours); lesion growth (increase in infarct volume from baseline to 24 hours); reperfusion, defined as a greater than 90% reduction in the region of perfusion delay (time to peak of the residual function [Tmax] >6 seconds) between baseline and 24 hours; and complete recanalization at 24 hours on computed tomography angiography or magnetic resonance angiography and collateral status based on the hypoperfusion intensity ratio (ratio of Tmax >10 seconds volume to Tmax >6 seconds showed).8,9
Safety outcomes were death within 90 days, the incidence of symptomatic intracranial hemorrhage within 36 hours, neurologic deterioration, and parenchymal hematoma type 2.
Statistical Analysis
We compared clinical and imaging outcomes in transfer patients who received thrombectomy vs medical therapy with the results in patients who presented directly to the study sites. We evaluated whether time to treatment was associated with the treatment effect by comparing thrombectomy vs medical therapy in the 3 prespecified DEFUSE 3 treatment windows (6-9 hours, 9-12 hours, and 12-16 hours). We also assessed whether the thrombectomy treatment effect in transferred patients was affected by the ischemic core volume (volume of tissue with regional cerebral blood flow <30% or apparent diffusion coefficient <620 × 10−3 mm2/s) or Alberta Stroke Program Early Computed Tomography score (ASPECTS) at the study center by stratifying ischemic core volumes (<30 mL vs ≥30 mL) and ASPECTS (8-10 vs 5-7).
We compared continuous and ordinal variables using Mann-Whitney U test and assessed differences in the proportions and rates with χ2 or Fisher exact tests. Breslow-Day test was used to assess homogeneity of the odds ratios between transfer and direct patients and between other time and imaging subgroups. All P value comparisons were 2-sided, and P less than .05 was considered significant.
Results
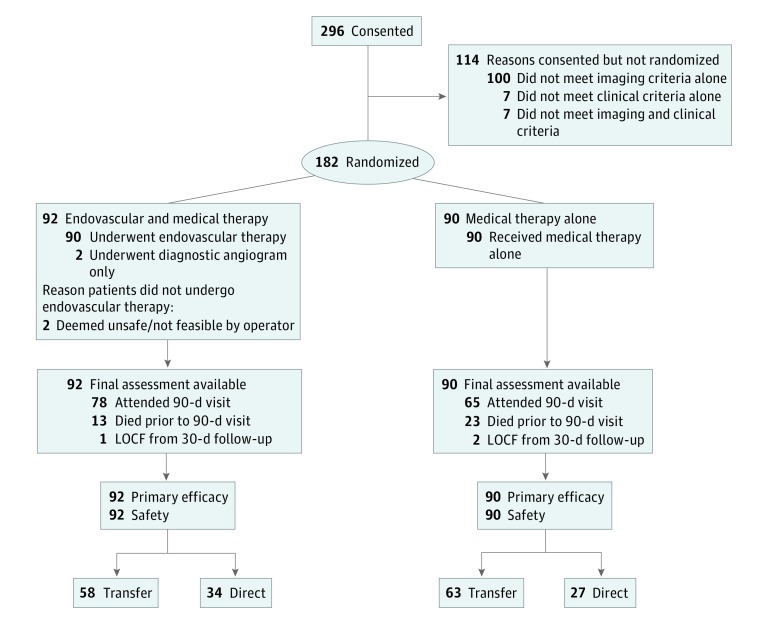
Between May 2016 and May 2017, 182 patients were enrolled in DEFUSE 3, of whom 121 were transferred (58 EVT and 63 medical therapy) and 61 presented directly to the study site (34 EVT and 27 medical management [MM]) (Figure 1). The direct vs transfer groups had similar age, National Institutes of Health Stroke Scale score, and other baseline characteristics as detailed in Table 1.10,11 The median infarct core volume at enrollment for transfer patients (EVT, 9.4 mL; MM, 10.0 mL) was similar to those who presented directly to a study site (EVT, 12.2 mL; MM, 10.1 mL). Transfer patients had more favorable collateral profiles on perfusion imaging based on the hypoperfusion intensity ratio (percentage of Tmax >6-second lesion volume that has Tmax >10 seconds); median for transfer was 0.35 (interquartile range [IQR], 0.18-0.47) vs 0.42 (IQR, 0.25-0.56) for direct; P = .05. The transfer group had median times from stroke onset to arrival at the study site that were about 30 minutes longer (EVT group, 9.45 hours and MM, 9.43 hours) compared with those presenting directly (EVT group, 9 hours and MM, 9.07 hours). Intravenous tissue plasminogen activator rates were higher in the transfer patients (9 of 58 patients receiving EVT [16%] and 8 of 63 patients receiving MM [13%]) than the directly presenting patients (1 of 34 [3%] and 0 of 27 [0%], respectively).
Figure 1. Study Flowchart.
LOCF indicates last observation carried forward.
Table 1. Baseline Characteristics of the Patients and Features of Thrombectomy.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Direct (n = 61) | Transfer (n = 121) | |||
| Endovascular Therapy (n = 34) | Medical Therapy (n = 27) | Endovascular Therapy (n = 58) | Medical Therapy (n = 63) | |
| Age, median (IQR), y | 71.5 (59-79) | 67 (59-81) | 69.5 (60-78) | 72 (59-80) |
| Female | 13 (38) | 10 (37) | 33 (57) | 36 (57) |
| NIHSS score, median (IQR) | 16.5 (11-21) | 15 (12-20) | 16 (10-20) | 17 (12-22) |
| Glucose level, median (IQR), mg/dL | 137.5 (113-191)a | 122 (105-159) | 118.5 (106-139)a | 126 (111-152) |
| Medical history | ||||
| Hypertension | 24 (71) | 23 (85) | 47 (81) | 14 (78) |
| Myocardial infarction | 8 (24)a | 0b | 4 (7)a | 11 (17)b |
| Atrial fibrillation | 16 (47) | 8 (30) | 18 (31) | 20 (32) |
| Diabetes | 13 (38) | 9 (33) | 15 (26) | 18 (29) |
| Dyslipidemia | 20 (59) | 12 (44) | 29 (50) | 22 (35) |
| Previous stroke | 7 (21) | 5 (19) | 6 (10) | 7 (11) |
| Stroke onset witnessed | ||||
| Yes | 8 (24) | 8 (30) | 23 (40) | 27 (43) |
| No | ||||
| Symptoms were present on awakening | 22 (65) | 13 (48) | 27 (47) | 29 (46) |
| Symptoms began during wakefulness | 4 (12) | 6 (22) | 8 (14) | 7 (11) |
| Treatment with intravenous tPA | 1 (3) | 0 | 9 (16) | 8 (13) |
| Imaging characteristic | ||||
| Qualifying imaging | ||||
| CT perfusion imaging | 26 (76) | 21 (78) | 43 (74) | 43 (68) |
| Diffusion and perfusion MRI | 8 (24) | 6 (22) | 15 (26) | 20 (32) |
| Volume of ischemic core, median (IQR), mLa | 12.2 (2.4-37.6) | 10.1 (0-30.1) | 9.4 (2.2-17.1) | 10.0 (4.1-23.9) |
| Volume of perfusion lesion (Tmax >6 s), median (IQR), mL | 122.3 (70.8-185.0) | 120.9 (80.7-156.9) | 104.2 (82.6-136.4) | 110.3 (64.2-158.5) |
| Hypoperfusion intensity ratio | 0.44 (0.23-0.58) | 0.38 (0.21-0.59) | 0.33 (0.18-0.44) | 0.35 (0.23-0.52) |
| Occlusion site on baseline CTA or MRA | ||||
| Internal carotid artery | 9 (26)b | 15 (56)c | 23 (40)b | 21 (33)c |
| Middle cerebral artery | 25 (74)b | 12 (44)c | 35 (60)b | 42 (67)c |
| ASPECTS on baseline CT, median (IQR) | 8 (7-9) | 8 (7-9) | 8 (7-9) | 8 (7-9) |
| CTA collateral score | ||||
| 0 | 0 | NA | 0 | NA |
| 1 | 1 (3) | NA | 2 (4) | NA |
| 2 | 4 (12) | NA | 9 (16) | NA |
| 3 | 7 (21) | NA | 7 (12) | NA |
| 4 | 1 (3) | NA | 3 (5) | NA |
| NA | 21 (62) | NA | 36 (63) | NA |
| Maas system10 score | ||||
| No vessel opacification (1) | 1 (3) | 0 (0) | 3 (7) | 3 (6) |
| Collaterals visible less than on contralateral side (2) | 18 (56) | 14 (56) | 25 (58) | 20 (41) |
| Collaterals visible equal to those on contralateral side (3) | 10 (31) | 7 (28) | 14 (33) | 18 (37) |
| Collaterals visible more than those on contralateral side (4) | 3 (9) | 4 (16) | 1 (2) | 7 (14) |
| Exuberant collaterals (5) | 0 | 0 | 0 | 1 (2) |
| Tan scale11 score | ||||
| Collaterals in <50% of the MCA territory | 11 (34) | 7 (28) | 11 (26) | 14 (29) |
| Collaterals in ≥50% of the MCA territory | 21 (66) | 18 (72) | 31 (74) | 35 (71) |
| Process measures, median (IQR), h:min | ||||
| Time from stroke onset to arrival to non-EVT capable hospital | NA | NA | 6:28 (4:05-8:25) | 6:26 (3:18-9:34) |
| Time from stroke onset to arrival to EVT-capable hospital | 9:00 (6:59-9:48) | 9:04 (7:39-9:39) | 9:27 (7:05-11:18) | 9:26 (6:56-12:23) |
| Time from stroke onset to qualifying imaging | 10:38 (8:20-11:44) | 9:52 (8:53-11:30) | 10:23 (7:23-11:36) | 9:58 (7:39-13:23) |
| Time from stroke onset to randomization | 11:09 (9:17-12:20) | 10:55 (10:09-12:35) | 10:48 (7:54-12:23) | 10:19 (8:19-13:38) |
| Time from qualifying imaging to femoral puncture | 1:04 (0:45-1:29) | NA | 0:53 (0:39-1:20) | NA |
| Time from femoral puncture to reperfusion | 0:38 (0:24-1:00) | NA | 0:40 (0:28-0:59) | NA |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography score; CTA, computed tomography angiography; ICA, internal carotid artery; IQR, interquartile range; MCA, middle cerebral artery; MRA, magnetic resonance angiography; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale score; Tmax, bolus-shape-independent estimate of time delay for blood delivery between a main feeding artery and tissue at a given spatial location; tPA, tissue plasminogen activator (alteplase).
Volume of ischemic core indicates relative cerebral blood flow less than 30% on CT perfusion and apparent diffusion coefficient less than 620 × 10 −3 mm2/s on magnetic resonance imaging.
Variable demonstrating statistically significant (P < .05) difference within EVT group between transfer vs direct patients.
Variable demonstrating statistically significant (P < .05) difference within medical therapy only group between transfer vs direct patients.
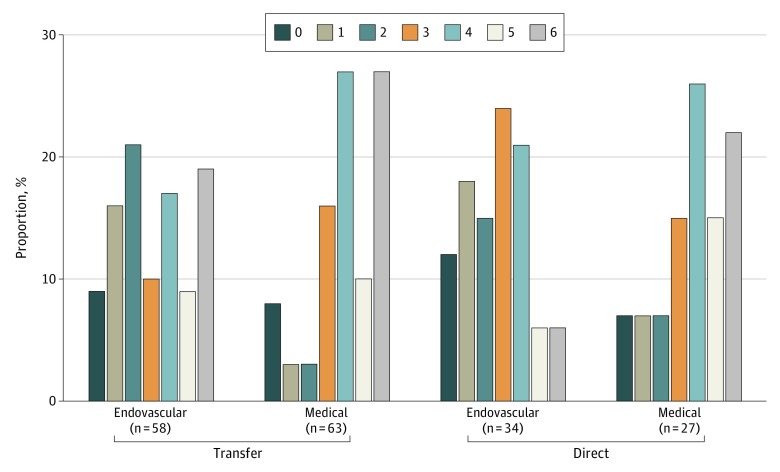
Endovascular thrombectomy was associated with a more favorable distribution of functional outcome scores on the mRS at 3 months compared with MM in both the direct and transfer groups as shown in Figure 2 (OR, 2.9; 95% CI, 1.2-7.2; P = .01 for direct and OR, 2.6; 95% CI, 1.3-4.8; P = .009 for transfer).
Figure 2. 90-Day Modified Rankin Scale Score Distribution Stratified by Transfer vs Direct.
Patients who received EVT had a higher rate of functional recovery (mRS, 0-2 at 90 days) compared with those who received MM in both the direct (15 of 34 patients receiving EVT [44%] vs 6 of 27 patients receiving MM [22%]; OR, 2.0; 95% CI, 0.9-4.4; P = .07) and transfer group (26 of 58 patients receiving EVT [45%] vs 9 of 63 patients receiving MM [14%]; OR, 3.1; 95% CI, 1.6-6.1; P < .001) (Figure 2). There was no heterogeneity for the EVT treatment effect based on direct vs transfer status. Median 24 hours and discharge NIHSS scores were lower and statistically different in thrombectomy treated patients in both groups (Table 2).
Table 2. Clinical and Imaging Outcomes.
| Outcome | Median (IQR) | Odds Ratio or Relative Risk (95% CI) Within Direct and Transfer Groups | P Value | ||||
|---|---|---|---|---|---|---|---|
| Direct (n = 61) | Transfer (n = 121) | ||||||
| Endovascular Therapy (n = 34) | Medical Therapy (n = 27) | Endovascular Therapy (n = 58) | Medical Therapy (n = 63) | Within Direct and Transfer Groups | For Difference of Treatment Effect in Direct vs Transfer | ||
| Primary efficacy outcome: score on (mRS) at 90 d | 3 (1-4) | 4 (3-5) | 3 (2-5) | 4 (3-6) | 2.9 (1.2-7.2) vs 2.6 (1.3-4.8) | .01 vs .009 | .80 |
| Secondary efficacy outcome: functional independence at 90 d, No. (%) | 15 (44) | 6 (22) | 26 (45) | 9 (14) | 2.0 (0.9-4.4) vs 3.1 (1.6-6.1) | .07 vs <.001 | .44 |
| 24-h NIHSS | 7.5 (5-15) | 15 (9-20) | 9 (5-20)d | 17 (12-21) | .01 vs .003 | .33 and .35 | |
| Discharge NIHSS | 4 (2-9) | 15 (4-20) | 5 (3-12) | 15 (8-21) | .005 vs <.001 | .28 and .50 | |
| Discharge mRS | 4 (2-4) | 5 (4-5) | 4 (2-5) | 4 (4-5) | .009 vs .007 | .74 and .88 | |
| Safety outcomes, No. (%) | |||||||
| Death at 90 d | 2 (6) | 6 (22) | 11 (19) | 17 (27) | 0.26 (0.02-1.16) vs 0.70 (0.36-1.37) | .12 vs .30 | .39 |
| Symptomatic intracranial hemorrhage | 1 (3) | 0 (0) | 5 (9) | 4 (6) | 2.4 (0.1-56.7) vs 1.36 (0.34-5.95) | >.99 vs .74 | >.99 |
| Early neurologic deterioration | 3 (9) | 3 (11) | 5 (9) | 9 (14) | 0.79 (0.14-4.59) vs 0.60 (0.21-1.70) | >.99 vs .33 | >.99 |
| Parenchymal hematoma type 2 | 3 (9) | 0 (0) | 5 (9) | 3 (5) | 5.6 (0.30-104.0) vs 1.8 (0.40-15.8) | .25 vs .48 | .53 |
| Imaging outcomes | |||||||
| Infarct volume at 24 h, mL | 38.0 (12.9-80.0) | 64.3 (14.8-159.4) | 32.2 (22.8-87.2) | 39.0 (28.1-89.0) | NA | .28 vs .33 | .76 and .63 |
| Infarct growth at 24 h, mL | 23.6 (5.8-55.3) | 41.9 (14.8-125.5) | 23.2 (11.6-76.8) | 30.1 (19.6-58.7) | NA | .12 vs .30 | .61 and .33 |
| Reperfusion >90% at 24 h, No. (%) | 22 (76) | 2 (9) | 37 (80) | 10 (22) | 8.3 (2.5-96.4) vs 3.7 (2.1-6.5) | <.001 vs <.001 | .65 |
| Complete recanalization at 24 h, No. (%) | 23 (74) | 2 (10) | 42 (81)d | 12 (21) | 7.4 (2.3-81.9) vs 3.8 (2.3-6.5) | <.001 vs <.001 | >.99 |
| TICI score of 2b or 3. No. (%) | 26 (76) | NA | 44 (76) | NA | NA | NA | .95 |
| Length of stay | |||||||
| Enrolling hospital only | 6.4 (4.4-10.5) | 9.3 (8.2-13.6) | 6.6 (3.2-8.9) | 8.7 (5.9-14.5) | NA | .03 vs .003 | .28 and .32 |
| Combined transfer and enrolling hospitals | NA | NA | 6.8 (3.3-8.9) | 9.1 (6.0-15.1) | NA | .03 vs .002 | .36 and .48 |
Abbreviations: IQR, interquartile range; mRS, Modified Rankin Scale score; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale score; TICI, modified thrombolysis in cerebral infarction.
Regarding imaging outcomes, both 24-hour median infarct volume and 24-hour lesion infarct growth were numerically smaller in patients receiving thrombectomy than those receiving medical therapy regardless of their presentation status, but these differences did not reach statistical significance. Two key imaging outcomes reached statistical significance: reperfusion of greater than 90% of the lesion at 24 hours (direct: 22 of 34 patients receiving EVT [76%] vs 2 of 27 patients receiving MM [9%]; transfer: 37 of 58 patients receiving EVT [80%] vs 10 of 63 patients receiving MM [22%]; P < .001 for both) and complete recanalization on computed tomography angiography or magnetic resonance angiography (direct: 23 of 34 patients receiving EVT [74%] vs 2 of 27 patients receiving MM [10%]; transfer: 42 of 58 patients receiving EVT [81%] vs 12 of 63 patients receiving MM [21%]; P < .001 for both) as depicted in Table 2. There was no difference in treatment effect for all imaging outcomes based on direct vs transfer status.
Successful angiographic reperfusion (modified thrombolysis in cerebral infarction ≥2b) was identical (76%) in the direct and transfer groups. There was no interaction between time-to-treatment and the treatment benefit in either the direct or transfer groups. Furthermore, there was no difference in the likelihood of good outcome with thrombectomy in the 3 prespecified DEFUSE 3 treatment windows (6-9 hours, 9-12 hours, and 12-16 hours).
Similarly, the thrombectomy treatment effect in transferred patients was not modified when stratified by ischemic core volume on computed tomography perfusion images (regional cerebral blood flow <30%) or diffusion-weighted imaging (apparent diffusion coefficient <620 × 10−3 mm2/s). Also, there was no heterogeneity in the treatment effect when stratified by ASPECTS (8-10 vs 5-7).
In regards to safety outcomes, the rates of death within 90 days were lower but did not reach statistical significance in patients receiving thrombectomy, irrespective of whether they were transferred or presented directly to the study site. The incidence of neurologic deterioration, symptomatic intracranial hemorrhage, and parenchymal hematoma type 2 were similar between direct vs transfer patients in both patients receiving EVT and patients receiving MM. There was no difference between transferred and direct patients in regards to any safety outcome.
Discussion
Endovascular thrombectomy is safe and effective in appropriately selected patients up to 24 hours.5,6 However, this treatment is not available in all hospitals and many patients are transferred for the intervention. These transfers are associated with delays,2,3,12 and prior studies reported worse outcomes in those who are transferred compared with those presenting directly to thrombectomy centers.2,3
Most previous studies did not have a medical therapy arm for comparison, thus, there was no estimation of the treatment effect in transferred patients. A post hoc analysis of Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment (SWIFT PRIME)13 showed that the rate of good outcome among patients receiving EVT who were transferred was lower than those who presented directly to EVT centers; however, the difference did not reach statistical significance (mRS of 0 to 2 in 49% of transfer patients vs 66% in those who presented directly to the study site; P = .34). However, the study included patients who had earlier presentation compared with DEFUSE 3 (0-4.5 hours), and all of those patients received intravenous tissue plasminogen activator. Most patients in SWIFT PRIME also had a favorable perfusion imaging profile.
Two-thirds of DEFUSE 3 patients were transfers. Our analysis showed similar outcomes in transferred patients and a similar treatment effect. All key outcomes reached statistical significance in both groups without any evidence of heterogeneity based on transfer vs direct presentation to the study site. Also, there was no heterogeneity in the treatment efficacy based on the ischemic core volume or ASPECT score prior to thrombectomy in the direct vs transfer patients. Furthermore, there was no difference in any of the safety outcomes.
Prior studies reported stroke evolution, as measured by the decay in the ASPECTS scores, during transfer as a predictor of poor outcomes after thrombectomy.14,15 Patients in DEFUSE 3 were selected based on the presence of penumbral tissue, which may explain the similar outcomes in both groups. Enrichment of the study population with slow progressors may also explain the similar outcomes in transfer patients compared with patients directly presenting to EVT-capable centers.16
Limitations
Similar to prior studies, assessment of collaterals status by hypoperfusion intensity ratio showed that patients with better collaterals were more likely to benefit from thrombectomy.15,17 Of interest, patients in the transfer group had slightly more favorable collaterals, as assessed by perfusion imaging, than those who presented directly. It is likely that some of the patients who originally presented to the outside hospital with a favorable imaging profile extended their infarct during transfer and therefore were not eligible for enrollment in DEFUSE 3. However, because penumbral imaging was typically not available at the outside hospitals, we do not have data on how frequently this occurred. This concept is supported by a 2018 study17 in which endovascular therapy candidates with a favorable collaterals on computed tomography perfusion at the referring hospital had minimal infarct core growth during transfer compared with substantial growth in patients with unfavorable collaterals.17 These findings suggest that repeated imaging after transfer can potentially be limited to selected patients.
Now that thrombectomy is known to be safe and efficacious in appropriately selected patients, efforts are in place to improve patients’ access to EVT. Still, many patients present to non-EVT centers and require transfer for the procedure. Our study suggests that transferred late-window patients selected by penumbral imaging criteria at arrival to EVT centers achieve similar outcomes to those presenting directly to EVT centers and strongly support the practice of transferring potential EVT patients for consideration of treatment in the late window.
Conclusions
In late-window patients selected by computed tomography perfusion and/or magnetic resonance diffuse-weighted imaging and perfusion images with penumbral mismatch criteria at arrival, EVT outcomes were similar between those presenting directly to EVT-capable hospitals and those who were transferred. Both direct and transfer patients who received EVT had significantly better outcomes than patients receiving MM, with similar safety profiles. The results have health care implications indicating that transferring patients for late-window thrombectomy is associated with substantial clinical benefits and should be strongly encouraged.
Trial Protocol.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.Froehler MT, Saver JL, Zaidat OO, et al. ; STRATIS Investigators . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke). Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun CH, Nogueira RG, Glenn BA, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127(10):1139-1148. doi: 10.1161/CIRCULATIONAHA.112.000506 [DOI] [PubMed] [Google Scholar]
- 4.Rinaldo L, Brinjikji W, McCutcheon BA, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg. 2017;9(12):1166-1172. doi: 10.1136/neurintsurg-2016-012824 [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke. 2017;12(8):896-905. doi: 10.1177/1747493017701147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivot JM, Mlynash M, Inoue M, et al. ; DEFUSE 2 Investigators . Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45(4):1018-1023. doi: 10.1161/STROKEAHA.113.003857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenillas JF, Cortijo E, García-Bermejo P, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab. 2018;38(10):1839-1847. doi: 10.1177/0271678X17740293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40(9):3001-3005. doi: 10.1161/STROKEAHA.109.552513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525-531. doi: 10.3174/ajnr.A1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asaithambi G, Castle AL, Stein LJ, et al. Real-world treatment of large vessel occlusions: combined outcomes of directly presenting and transferred-in patients to a stroke center. Neurol Res. 2018;40(8):637-643. doi: 10.1080/01616412.2018.1460700 [DOI] [PubMed] [Google Scholar]
- 13.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 14.Boulouis G, Lauer A, Siddiqui AK, et al. Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol. 2017;74(11):1361-1367. doi: 10.1001/jamaneurol.2017.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun CH, Connelly K, Nogueira RG, et al. ASPECTS decay during inter-facility transfer predicts patient outcomes in endovascular reperfusion for ischemic stroke: a unique assessment of dynamic physiologic change over time. J Neurointerv Surg. 2015;7(1):22-26. doi: 10.1136/neurintsurg-2013-011048 [DOI] [PubMed] [Google Scholar]
- 16.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48(9):2621-2627. doi: 10.1161/STROKEAHA.117.017673 [DOI] [PubMed] [Google Scholar]
- 17.Guenego A, Mlynash M, Christensen S, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol. 2018;84(4):616-620. doi: 10.1002/ana.25320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.