Key Points
Question
Are there rare variants associated with Alzheimer disease among individuals who possess or lack the APOE ε4 allele?
Findings
This case-control, whole-exome sequencing study of 10 441 individuals identified a possibly novel association with a GPAA1 variant among those who lacked the APOE ε4 allele, a finding that was replicated in independent data sets and supported by analyses of whole-genome and RNA sequencing data derived from human brain tissue. Novel associations were identified among individuals with the APOE ε4 allele for variants in ISYNA1, OR8G5, IGHV3-7, and SLC24A3.
Meaning
This study supports the apparent involvement of genes in Alzheimer disease whose effects are dependent on APOE genotype.
Abstract
Importance
Previous genome-wide association studies of common variants identified associations for Alzheimer disease (AD) loci evident only among individuals with particular APOE alleles.
Objective
To identify APOE genotype-dependent associations with infrequent and rare variants using whole-exome sequencing.
Design, Setting, and Participants
The discovery stage included 10 441 non-Hispanic white participants in the Alzheimer Disease Sequencing Project. Replication was sought in 2 independent, whole-exome sequencing data sets (1766 patients with AD, 2906 without AD [controls]) and a chip-based genotype imputation data set (8728 patients with AD, 9808 controls). Bioinformatics and functional analyses were conducted using clinical, cognitive, neuropathologic, whole-exome sequencing, and gene expression data obtained from a longitudinal cohort sample including 402 patients with AD and 647 controls. Data were analyzed between March 2017 and September 2018.
Main Outcomes and Measures
Score, Firth, and sequence kernel association tests were used to test the association of AD risk with individual variants and genes in subgroups of APOE ε4 carriers and noncarriers. Results with P ≤ 1 × 10−5 were further evaluated in the replication data sets and combined by meta-analysis.
Results
Among 3145 patients with AD and 4213 controls lacking ε4 (mean [SD] age, 83.4 [7.6] years; 4363 [59.3.%] women), novel genome-wide significant associations were obtained in the discovery sample with rs536940594 in AC099552 (odds ratio [OR], 88.0; 95% CI, 9.08-852.0; P = 2.22 × 10−7) and rs138412600 in GPAA1 (OR, 1.78; 95% CI, 1.44-2.2; meta-P = 7.81 × 10−8). GPAA1 was also associated with expression in the brain of GPAA1 (β = −0.08; P = .03) and its repressive transcription factor, FOXG1 (β = 0.13; P = .003), and global cognition function (β = −0.53; P = .009). Significant gene-wide associations (threshold P ≤ 6.35 × 10−7) were observed for OR8G5 (P = 4.67 × 10−7), IGHV3-7 (P = 9.75 × 10−16), and SLC24A3 (P = 2.67 × 10−12) in 2377 patients with AD and 706 controls with ε4 (mean [SD] age, 75.2 [9.6] years; 1668 [54.1%] women).
Conclusions and Relevance
The study identified multiple possible novel associations for AD with individual and aggregated rare variants in groups of individuals with and without APOE ε4 alleles that reinforce known and suggest additional pathways leading to AD.
This case-control study evaluates APOE associations with variants, using whole-exome sequencing, in participants with and without APOE ε4 alleles.
Introduction
The APOE (348 Entrez Gene) ε4 allele is consistently identified as the strongest common genetic factor contributing to the risk of late-onset Alzheimer disease (AD).1,2,3 However, drugs targeting APOE have proven to be ineffective,4 suggesting that APOE genotype might act as a proxy or biomarker5 for the causal mechanism. Aleternatively, the influence of APOE on AD pathogenesis may have a role in multiple pathways leading to AD,6 or is dependent on other genetic or nongenetic factors.7 More than 30 additional AD loci have been identified by genome-wide association studies and bioinformatics approaches,3,7,8,9,10,11,12,13 but their individual contributions to the total heritability of AD are comparatively small,3 suggesting that many loci have escaped detection even in analyses of very large data sets. A previous study by the Alzheimer’s Disease Genetics Consortium identified among individuals lacking the ε4 allele genome-wide significant association of AD with single-nucleotide variants (SNVs) in the region of MAPT (4137 Entrez Gene),7 the gene encoding tau protein, which is central to AD hallmark abnormalities.14 This finding suggests that other genes may exist whose effects on AD risk are masked by or dependent on particular APOE alleles. We applied this APOE genotype stratification analysis strategy in a study aimed at identifying novel associations with common and rare variants using a large whole-exome sequence (WES) data set from the Alzheimer Disease Sequencing Project.
Methods
Subjects
The discovery sample included 10 441 unrelated non-Hispanic white individuals (5522 with AD, 4919 cognitively normal controls) in the Alzheimer’s Disease Sequencing Project case-control WES data set. The details of the study design, sequencing, and data quality control procedures were described previously.15,16 In brief, participants were selected on the basis of a risk score that considers age, sex, and APOE genotype in a manner that maximized power for detection of novel AD risk and protective variants, but this ascertainment scheme yielded groups of patients with AD and controls that were not well balanced with respect to age and APOE genotype. An independent sample including WES data sets from the Alzheimer’s Disease Exome Sequencing–France Project (subset of 1174 patients with late-onset AD, 1101 controls), the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium (592 patients with AD, 1805 controls), and the Alzheimer’s Disease Genetics Consortium by genome-wide association studies data set (8728 patients with AD, 9808 controls) imputed to the Haplotype Reference Consortium panel was used for replication. Variants with imputation quality values (R2) of 0.4 or less were excluded. Additional details of the replication data sets are reported elsewhere.15 Functional tests of the top variants were conducted using whole-genome sequencing data obtained from participants (402 patients with AD, 647 controls) of the Religious Orders Study (ROS) or the Rush Memory and Aging Project (MAP). Details of the study design, as well as derivation of phenotype, genotype, and gene expression data, were described previously17,18 and are briefly stated in the eMethods in the Supplement. Salient characteristics of the discovery and replication data sets are summarized in eTable 1 in the Supplement. Data were analyzed between March 2017 and September 2018. Written informed consent was obtained from all participants or their legal guardians. This study was approved by the Boston University Institutional Review Board.
Statistical Analysis
Genome-wide association analyses in the discovery sample were conducted separately in groups of individuals with and without an APOE ε4 allele (ie, ε4+ and ε4−). To have sufficient statistical power to detect associations with rare variants and minimize the number of tests, we limited our analyses of individual biallelic variants or short indels to those with a minor allele count of 10 or more, yielding 87 405 variants in the ε4+ group and 123 178 variants in the ε4− group (eFigure 1 in the Supplement). Based on a total of 210 583 tests, the Bonferroni-corrected threshold for study-wise significance (SWS) was 2.37 × 10−7. The association of each variant with AD risk was evaluated using 2 regression models. By design, there was a substantial difference in mean age between patients with AD and controls, so model 1 included only the first 10 ancestry principal components (PCs) to account for population substructure. Covariates for age, sex, and sequencing center, in addition to 10 PCs, were included in model 2 to account for dependencies on age, sex, and batch effects.15 Analyses of single variants were conducted using the score test that was designed for extremely rare variants19 implemented in the EPACTS software.20
Because the score test may overestimate the significance of some results, associations of the top-ranked variants were reevaluated using the Firth test, which better controls for type I error than the score test.21 Association of single variants yielding score test P values ≤ 1 × 10−5 in the discovery sample (a post hoc cutoff level that limits the number of tests in the replication stage and may yield a potentially SWS result after combining data from the discovery and replication samples) was evaluated in each replication data set using a generalized linear model implemented with the UGA tool’s glm function22 to obtain the logistic regression coefficients and SEs that were input into the meta-analysis using the fixed-effects, inverse-weighted method in METAL.23
Gene-based tests were performed by aggregating variants with a minor allele frequency less than 5% (except for singletons) that were annotated as having high or moderate influence on the encoded protein as previously described.15 In brief, variants with high influence are those classified by variant effect predictor or single-nucleotide polymorphism (SNP) effect as splice acceptor, splice donor, stop gained, frameshift, stop lost, start lost, or transcript amplification, and variants with moderate influence are those annotated as inframe insertion, inframe deletion, missense variant, or protein altering.24,25 Genes with a cumulative minor allele count of 10 or more were included in the analysis, yielding a total of 78 779 tests across 6 groups with variants aggregated by ε4 status and functional influence (all, high + moderate, high) and a corresponding Bonferroni-corrected SWS threshold of 6.35 × 10−7. The combined association of multiple variants in each gene with the risk of AD was evaluated using the optimal sequence kernel association test26 with default rho settings implemented in the seqMeta R package.27 Gene-based association results with P values < 1 × 10−5 in the discovery sample were further evaluated in each replication data set using the same optimal sequence kernel association test settings as in the discovery analysis. Because not all replication data sets were analyzed using seqMeta or EPACTS, gene-based P values were combined across data sets using the z-score approach in METAL.
Potential functional significance of genome-wide significant variants was investigated by applying multiple analytical approaches using data on gene expression in brain tissue, cognitive performance, and AD-related neuropathologic changes obtained from autopsied patients in the ROSMAP Project.18 First, we tested the association of a variant on the cell-type gene expression modules–adjusted residuals of expression in brain that were adjusted for source of sample (ROS or MAP studies), postmortem interval, 3 PCs of ancestry, age at death, sex, RNA integrity number, number of ribosomal bases, and number of aligned reads in a manner described previously using a general linear regression model.18 Next, we evaluated the influence of the variant on a test of global cognition function and neuropathologic measures of neuritic plaques and neurofibrillary tangles adjusted for source of sample, postmortem interval, PCs, age at death, and sex. The association of GPAA1 variants with transcriptional and posttranscriptional mechanisms was explored using genomic information in the Regulatory Build of Ensembl (Human GRCh38.p12).28,29 Significance was determined using a 2-tailed unpaired analysis model and a significance threshold of P < .05.
Results
After quality control, there remained 2377 AD cases and 706 controls (mean [SD] age, 75.2 [9.6] years; 1668 [54.1%] women) who had the APOE ε4 allele (ε4+) and 3145 AD cases and 4213 controls (mean 83.4 [7.6]; years; 4363 [59.3%] women) who lacked the ε4 allele (ε4−). The quantile-quantile plots indicated modest inflation of P values for the regression model, including covariates for PCs (model 1) in both the APOE ε4+ (λ = 1.083) and ε4− (λ = 1.062) groups (eFigure 1 in the Supplement), whereas there was no evidence of inflation for the model with additional adjustment for age, sex, and sequencing center (model 2) in either the APOE ε4+ (λ = 0.994) or ε4− (λ = 0.998) groups. A total of 22 variants (12 in the ε4+ group, 10 in the ε4− group) showed suggestive evidence of association (P ≤ 1 × 10−5) in at least 1 model, and these variants were further evaluated in the replication data sets (eFigure 2, eTable 2, and eTable 3 in the Supplement).
Meta-analysis of the results from the discovery and replication cohorts showed that the association of AD risk with several SNVs in 1 novel locus, ISYNA1, under model 1 was nearly SWS in the APOE ε4+ group (top SNV rs2303697, P = 4.61 × 10−7) (eTable 3, eFigure 3B in the Supplement). The rs2303697 minor (T) allele was protective in all cohorts except for CHARGE (eTable 3, eFigure 3B in the Supplement). In the discovery sample, the interaction between rs2303697 and APOE ε4 status was significant (interaction P = 3.88 × 10−5) (eTable 2 in the Supplement), and the T allele showed a significant protective effect in APOE ε4+ participants (odds ratio [OR], 0.73; 95% CI, 0.64-0.84; P = 3.49 × 10−6), but it was not associated with AD risk in ε4− participants (OR, 1.00; 95% CI, 0.93-1.04; P = .98) (eFigure 3A in the Supplement).
In the APOE ε4− group, SWS associations were observed with variants in the established AD loci TREM2 (54209 Entrez Gene) (top SNV rs75932628: OR, 3.66; 95% CI, 2.36-5.68; P = 6.84 × 10−9) and MAPT (top SNV rs62063857: OR, 1.17; 95% CI, 1.1-1.23; P = 1.59 × 10−7) (Table 1). The associations for rs75932628 and rs62063857 were only nominally significant in the APOE ε4+ group (eTable 2, eFigure 4 and eFigure 5 in the Supplement). The SWS associations were also identified with variants in novel loci including NSF (4905 Entrez Gene) (top SNV rs199533, OR, 0.86; 95% CI, 0.82-0.91; P = 1.66 × 10−7), GPAA1 (8733 Entrez Gene) (top SNV rs138412600: OR, 1.78; 95% CI, 1.44-2.2; P = 7.81 × 10−8), and AC099552 (18873975 Entrez Gene) (top SNV rs536940594: OR, 88.0; 95% CI, 9.08-852.0; P = 2.22 × 10−7) (Table 1; eTable 2 and eFigures 5, 6, and 7 in the Supplement), noting that the MAPT and NSF SNPs are in high linkage disequilibrium (r2 = 0.85) (eFigure 5 in the Supplement) and results for rs536940594 were not available in the replication data sets. Among these 4 variants, only GPAA1 rs138412600 showed a significant interaction with APOE ε4 status (interaction P = 8.12 × 10−4; OR, 3.01; 95% CI, 1.58-5.72); the minor A allele was significantly associated with increased AD risk in the ε4− group (OR, 2.13; 95% CI, 1.59-2.87; P = 3.91 × 10−7) but had a nonsignificant protective effect in the ε4+ group (OR, 0.64; 95% CI, 0.31-1.35; P = .24) (eTable 2 in the Supplement). The deleterious effect of the A allele in the ε4− group was consistently observed in all replication cohorts (Figure, B).
Table 1. Study-Wide Significant (P ≤ 2.37 × 10−7) Associations With Individual Variants in the APOE ε4− Group.
| Chr | Positiona | rsID | EA | Gene | GnomAD MAF (%) | Discovery | Replicationb | Discovery + Replicationb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAC | MAF (%) | OR (95% CI)b | P Valuec | P Valueb | OR (95% CI) | P Value | OR (95% CI) | P Value | ||||||
| 6 | 41,129,252 | rs75932628d | T | TREM2 | 0.2 | 68 | 0.50 | 4.85 (2.74-8.60) | 2.12 × 10−9 | 4.59 × 10−8 | 2.40 (1.20-4.81) | .01 | 3.66 (2.36-5.68) | 6.84 × 10−9 |
| 7 | 154,988,675 | rs536940594e | A | AC099552 | 0 | 10 | 0.10 | 88.0 (9.08-852.0) | 2.22 × 10−7 | 9.47 × 10−5 | NA | NA | NA | NA |
| 8 | 145,138,063 | rs138412600e | A | GPAA1 | 2 | 239 | 2.00 | 2.13 (1.59-2.87) | 2.70 × 10−7 | 3.91 × 10−7 | 1.47 (1.08-1.98) | .01 | 1.78 (1.44-2.2) | 7.81 × 10−8 |
| 17 | 44,076,665 | rs62063857d | A | MAPT | 18.8 | 2063 | 16.33 | 1.25 (1.14-1.37) | 1.83 × 10−6 | 4.23 × 10−6 | 1.12 (1.04-1.2) | 2.03 × 10−3 | 1.17 (1.1-1.23) | 1.59 × 10−7 |
| 17 | 448,28,931 | rs199533d | A | NSF | 17.8 | 2983 | 20.35 | 0.82 (0.76-0.89) | 3.97 × 10−6 | 4.28 × 10−6 | 0.9 (0.83-0.96) | 3.38 × 10−3 | 0.86 (0.82-0.91) | 1.66 × 10−7 |
Abbreviations: AD, Alzheimer disease; Chr, chromosome; EA, effect allele; GnomAD, Genome Aggregation Database; MAC, minor allele count; MAF, minor allele frequency; NA, not available; OR, odds ratio; rsID, rs number.
Position based on genome build GRCh37.
Odds ratios, 95% CIs, and P values are based on generalized linear model.
P value for score test.
All statistics and estimates based on model 1, which adjusted only for principal components.
All statistics and estimates based on model 2, which adjusted for age, sex, and principal components.
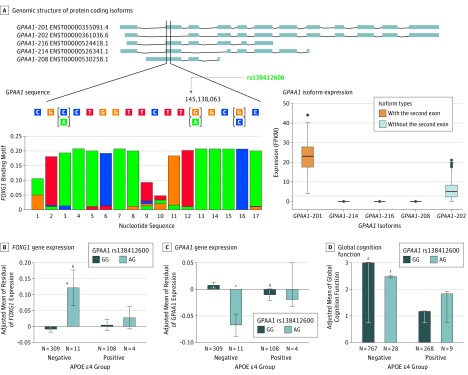
Figure. Functional Analysis of GPAA1 rs138412600.
The top panel shows the genomic structure of 5 protein coding isoforms with wild-type full length on the top and the alternative ones below (A). The expression levels of these 5 isoforms in postmortem dorsolateral prefrontal cortex (DLPFC) samples obtained from 423 ROSMAP participants aged 70 years or older (mean age at death, 88.5 years, and 38% patients with AD) are presented in the adjacent box plot. The rs138412600 single-nucleotide polymorphism is located in a 17-nucleotide motif sequence (chr8: 145 138 051-145,138,067) for the DNA binding site of transcription factor FOXG1. The reference sequence for this region is presented with genetic variants shown in brackets and highlighted with color according to their functional influence (yellow, missense; green, synonymous). Alternative alleles are shown below the wild-type alleles. The sequence of the corresponding FOXG1 binding motif was downloaded from the Ensembl website and is presented under the GPAA1 sequence using color-coding for the 4 nucleotides (green, A; red, T; blue, C; and orange, G). The x-axis indicates the nucleotide position in the motif and the y-axis indicates the bit score that represents the certainty of the enrichment of the nucleotide at each location. APOE ε4-dependent effects of rs138412600 on expression of FOXG1 (B) and GPAA1 (C), as well as on global cognition function (D) measured in ROSMAP participants. Homozygotes of the major allele (GG) and heterozygotes (AG) are shown with dark blue and light blue bars, respectively.
aP < .01 compared with negative GG.
bP < .01 compared with positive GG.
cP < .05 compared with negative GG.
dP < .05 compared with positive GG.
eP < .01 compared with negative AG.
fP < .05 compared with positive AG.
The rs138412600 variant is located within the promoter region of GPAA1, which is expressed in many cell types. Bioinformatic analysis revealed that the A allele (37th nucleotide within exon 2) binds exclusively to the 13th nucleotide in the motif of the DNA binding site for its repressive transcription factor, FOXG1 (2290 Entrez Gene), and inclusion of exon 2 in the transcript is necessary but not sufficient for high expression of GPAA1 (Figure, A). Functional analysis of whole-genome sequencing data from ROSMAP showed that, particularly among APOE ε4− participants, the A allele was significantly associated with higher expression of FOXG1 (β = 0.13, P = .003) (Figure, B) and lower expression of GPAA1 (β = −0.08, P = .03) (Figure, C), but not associated with expression of the GPAA1-202 isoform, which lacks the second exon. The A allele was associated with lower global cognition function (β = − 0.53, P = .009) (Figure, D) in the APOE ε4− group, but not in the APOE ε4+ group (β = 0.70, P = .12).
Gene-based analysis conducted in the discovery data set yielded 4 SWS significant (P ≤ 2.37 × 10−7) loci in the ε4+ group, including OR8G5 (219865 Entrez Gene; ε4+ P = 4.67 × 10−7; ε4− P = .93), IGHV3-7 (28452 Entrez Gene; ε4+ P = 9.75 × 10−16; ε4− P = .46), and SLC24A3 (57419 Entrez Gene; ε4+ P = 2.67 × 10−12; ε4− P = .15) (Table 2). Gene-based test results for these loci in the replication sample were not significant in the ε4+ group; however, inspection of the counts for each variant included in these tests revealed that the sentinel variants, which primarily accounted for the significance in the discovery sample, were not observed in the replication data sets (eTable 4 in the Supplement). TREM2 was the only SWS gene in the ε4− group in both the discovery (P = 2.75 × 10−8) and replication (P = 2.25 × 10−4) samples.
Table 2. Study-Wide Significant Gene-Based Test Results .
| APOE ε4 Group | Chr | Start | End | Gene | SNV Influence | Modela | No. Variants | cMAC | Sentinel Variant | No. | P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| With ε4 | 11 | 124,134,751 | 124,135,752 | OR8G5 | High + moderate | 2 | 3 | 267 | rs200328143 | 3082 | 4.67 × 10−7 |
| 14 | 106,518,415 | 106,518,824 | IGHV3-7 | All | 2 | 4 | 11 | rs188349361 | 2870 | 9.75 × 10−16 | |
| 20 | 19,261,606 | 19,261,724 | SLC24A3 | All | 2 | 2 | 68 | rs3790174 | 3082 | 2.67 × 10−12 | |
| Without ε4 | 6 | 41,126,505 | 41,130,815 | TREM2 | High + moderate | 1 | 14 | 310 | rs75932628 | 6656 | 2.75 × 10−8 |
Abbreviations: Chr, chromosome; cMAC, cumulative minor allele count; SNV, single-nucleotide variant.
Model 1: adjusted only for principal components; model 2, adjusted for age, sex, and principal components.
Discussion
Our WES study of more than 16 000 patients with AD and 17 500 controls confirmed the association of AD risk with variants in the MAPT region among participants lacking ε4, which had been established previously by analysis of common variants.7 We also identified SWS associations with the well-established AD risk variant TREM2 R47H30,31 and variants in several novel loci, including GPAA1 and NSF among ε4− participants. We also showed a possible association of AD with a variant in another novel locus, ISYNA1 (P = 4.61 × 10−7) that approached the SWS threshold (P < 2.37 × 10−7) among participants with ε4+ in the combined discovery and replication samples. Analysis of aggregated rare variants identified possibly novel associations with OR8G5, IGHV3-7, and SLC24A3 among ε4+ individuals in the discovery sample, but the replication data sets were not suitable for replication testing because the sentinel variants accounting for the associations were either not present or not well imputed in these samples. Thus, these gene-based findings should be considered tentative. This limitation of gene-based tests of rare variants was previously recognized15 and indicates the need for much larger replication samples or collating and simultaneous recalling variants in deep-sequencing data sets.
GPAA1 encodes glycosylphosphatidylinositol (GPI) anchor attachment 1 protein, which is a subunit of the protein complex of GPI transamidase. GPI transamidase supports the GPI translational modifications of GPI-anchored proteins.32 The associated variant, rs138412600, encodes amino acid 37 of the protein, which is located within exon 2 that encodes a portion of the functional luminal loop (from start to amino acid 282).33,34 Although our analyses did not indicate that this variant is associated with expression of the alternative GPAA1 isoform resulting from splicing of this exon, we demonstrated that the minor allele A is significantly associated with increased expression of the transcription factor FOXG1, which binds to GPAA1. FOXG1 is a repressive transcription factor with restricted expression in neuronal cells and strong expression in the developing dentate gyrus and hippocampus.35 FOXG1 mutations cause the congenital form of Rett syndrome,36 a severe neurodevelopmental disorder. Our results also suggest that rs138412600 is associated with global cognition function. Expression of GPAA1 in hippocampus was reported to be upregulated after spatial training in a calcium/calmodulin kinase β mutant mouse model.37 Studies of this model suggested that calcium/calmodulin kinase β has a male-specific function in hippocampal memory formation.38
Our association finding in the total sample with the ISYNA1 variant, rs2303697, in the APOE ε4+ group is also noteworthy. ISYNA1 encodes inositol-3-phosphate synthase 1, a rate-limiting enzyme that catalyzes the conversion from glucose-6-phosphate to myoinositol (MI) 1-phosphate,39 which is a component of plasma membrane phospholipids and functions as a cell signaling molecule. Glucose is the major energy source for brain and the reduction of brain glucose metabolism is a prominent feature of AD.40 The level of brain MI detected by magnetic resonance spectroscopy in patients with AD has been shown to be positively correlated with total and phosphorylated tau, but not Aβ in cerebrospinal fluid.41 A 7-year, longitudinal study of individuals aged 69 to 89 years who were cognitively normal at baseline found that the ratio of N-acetyl aspartate to myoinositol in the posterior cingulate cortex was significantly decreased in individuals who subsequently developed AD, mild cognitive impairment, and dementia with Lewy bodies compared with those who remained cognitively normal.42 This study also showed that the N-acetyl aspartate/MI ratio was significantly lower among individuals with vs without APOE ε4. Evidence for a connection between myoinositol and APOE genotype is also suggested by a cross-sectional study showing that the ratio of MI to creatine was significantly higher in an elderly group of ε4+ compared with ε4− participants who had normal cerebrospinal fluid Aβ42 levels.43
The association of AD with the NSF rs199533 variant, which was SWS among ε4− individuals, may not be an independent signal because of the high linkage disequilibrium between rs199533 and the MAPT rs62063857 variant (r2 = 0.85). Nonetheless, the protein encoded by NSF (N-ethylmaleimide–sensitive factor) may be functionally related to AD because it is an adenosine triphosphatase that is involved in cellular membrane fusion events, including vesicle-mediated protein transport, exocytosis of neurotransmitters, and reassembly of the Golgi apparatus during mitosis.44 It has been shown that proteins in the soluble N-ethylmaleimide-sensitive factor attachment protein receptors complex are essential for neuronal Aβ release at presynaptic terminals.45 The explanation for the stronger association of rs199533 with AD risk among persons lacking ε4 is unclear, but warrants further study.
The most significant finding was observed with IGHV3-7 among participants with ε4+ in a gene-based test including 4 aggregated variants (P = 9.75 × 10−16). IGHV3-7 encodes one of the immunoglobulin heavy variable chains and is a good candidate given its functional similarity to IGHG3 (3502 Entrez Gene) and IGHJ6 (28475 Entrez Gene), 2 of the top associated genes in the Alzheimer Disease Sequencing Project WES sample including participants with and without ε4,15,46 and to IGHV1-67 (28463 Entrez Gene), which was identified as an AD locus in a large genome-wide association study performed by the International Genomics of Alzheimer Project,11 as well as evidence that antibodies to IgG cross-react with fibril and oligomer amyloid-β aggregates.47
Limitations
Our study has several limitations. First, the WES discovery sample for the present study (n = 10 441) is more than 5 times smaller than that for a previous chip-based APOE ε4-stratified analysis (n = 53 771).7 This disparity is particularly acute for ε4+ controls (n = 706 vs n = 9207). However, the present study based on WES was more uniquely suited than the previous study of imputed genotypes for detecting associations with rare variants, particularly in gene-based tests. Reduced power was also exacerbated by the stratification into APOE genotype subgroups. However, this concern is mitigated by the increased ability to detect association with variants whose effects are dependent on interaction with or could be diluted in a sample including individuals with the APOE ε4 allele. In addition, the greater than 2-fold sample size for the ε4− group compared with the ε4+ group may account for the paucity of significant and replicable findings among the ε4+ group. This idea is exemplified by the TREM2 R47H variant, which had nearly identical ORs in both groups but was 6 orders of magnitude more significant in the ε4− group. Another concern is that the comparatively small size of the follow-up WES data sets and unreliable imputation of very rare variants in imputed samples limited our ability to replicate findings, especially from gene-based tests, as exemplified by a previous study of this data set without stratification by APOE genotype.15 These limitations underscore the need to replicate our findings in other data sets.
Conclusions
We identified multiple possibly novel associations for AD with individual and aggregated rare variants in groups of individuals with and without APOE ε4. Bioinformatics and functional studies of the GPAA1 rs138412600 variant, which was the most robust novel association signal, demonstrated that it may also be associated with global cognition function and expression in brain of GPAA1 and its repressive transcription factor, FOXG1.
eMethods. Dataset Used for Functional Genomic Analysis
eTable 1. Characteristics of the Discovery and Replication Samples
eTable 2. Results for SNVs and Indels in the Discovery Sample
eTable 3. Meta-analysis Results for SNVs and Indels in the Discovery and Replication Samples
eTable 4. Results of Gene-Based Tests in the Discovery and Replication Samples
eFigure 1. QQ Plots for Association by Analysis Model and APOE Genotype in the Discovery Sample
eFigure 2. Manhattan Plots for Association by Analysis Model and APOE Genotype in the Discovery Sample
eFigure 3. Association of Alzheimer Disease With Variants in the ISYNA1 Region
eFigure 4. Association of Alzheimer Disease With Variants in the TREM2 Region
eFigure 5. Association of Alzheimer Disease With Variants in the AC099552 Region
eFigure 6. Association of Alzheimer Disease With Variants in the GPAA1 Region
eFigure 7. Association of Alzheimer’s disease with variants in the region including MAPT and NSF
eReferences.
References
- 1.Farrer LA, Cupples LA, Haines JL, et al. ; APOE and Alzheimer Disease Meta Analysis Consortium . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- 2.Sherva R, Farrer LA. Power and pitfalls of the genome-wide association study approach to identify genes for Alzheimer’s disease. Curr Psychiatry Rep. 2011;13(2):138-146. doi: 10.1007/s11920-011-0184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452-1458. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umar T, Hoda N. Alzheimer’s disease: a systemic review of substantial therapeutic targets and the leading multi-functional molecules. Curr Top Med Chem. 2017;17(31):3370-3389. doi: 10.2174/1568026618666180112161024 [DOI] [PubMed] [Google Scholar]
- 5.Ruthirakuhan M, Lanctôt KL, Di Scipio M, Ahmed M, Herrmann N. Biomarkers of agitation and aggression in Alzheimer’s disease: A systematic review. Alzheimers Dement. 2018;14(10):1344-1376. doi: 10.1016/j.jalz.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 6.Lee LC, Goh MQL, Koo EH. Transcriptional regulation of APP by apoE: to boldly go where no isoform has gone before: ApoE, APP transcription and AD—hypothesised mechanisms and existing knowledge gaps. Bioessays. 2017;39(9). doi: 10.1002/bies.201700062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jun G, Ibrahim-Verbaas CA, Vronskaya M, et al. ; IGAP Consortium . A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21(1):108-117. doi: 10.1038/mp.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun GR, Chung J, Mez J, et al. ; Alzheimer’s Disease Genetics Consortium . Transethnic genome-wide scan identifies novel Alzheimer’s disease loci. Alzheimers Dement. 2017;13(7):727-738. doi: 10.1016/j.jalz.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mez J, Chung J, Jun G, et al. ; Alzheimer’s Disease Genetics Consortium . Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 2017;13(2):119-129. doi: 10.1016/j.jalz.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sims R, van der Lee SJ, Naj AC, et al. ; ARUK Consortium; GERAD/PERADES, CHARGE, ADGC, EADI . Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49(9):1373-1384. doi: 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escott-Price V, Bellenguez C, Wang LS, et al. ; United Kingdom Brain Expression Consortium; Cardiovascular Health Study (CHS) . Gene-wide analysis detects two new susceptibility genes for Alzheimer’s disease. PLoS One. 2014;9(6):e94661. doi: 10.1371/journal.pone.0094661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancour D, Naj A, Mayeux R, et al. One for all and all for one: improving replication of genetic studies through network diffusion. PLoS Genet. 2018;14(4):e1007306. doi: 10.1371/journal.pgen.1007306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desikan RS, Schork AJ, Wang Y, et al. ; ADNI, ADGC, GERAD, CHARGE and IPDGC Investigators . Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry. 2015;20(12):1588-1595. doi: 10.1038/mp.2015.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9(1):25-34. doi: 10.1038/nrneurol.2012.236 [DOI] [PubMed] [Google Scholar]
- 15.Bis JC, Jian X, Kunkle BW, et al. ; Alzheimer’s Disease Sequencing Project . Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation. Mol Psychiatry. In press. doi: 10.1038/s41380-018-0112-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beecham GW, Bis JC, Martin ER, et al. The Alzheimer’s Disease Sequencing Project: study design and sample selection. Neurol Genet. 2017;3(5):e194. doi: 10.1212/NXG.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Jager PL, Ma Y, McCabe C, et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci Data. 2018;5:180142. doi: 10.1038/sdata.2018.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostafavi S, Gaiteri C, Sullivan SE, et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat Neurosci. 2018;21(6):811-819. doi: 10.1038/s41593-018-0154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin DY, Tang ZZ. A general framework for detecting disease associations with rare variants in sequencing studies. Am J Hum Genet. 2011;89(3):354-367. doi: 10.1016/j.ajhg.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center for Statistical Genetics EPACTS. https://genome.sph.umich.edu/wiki/EPACTS. Accessed September 15, 2015.
- 21.Ma C, Blackwell T, Boehnke M, Scott LJ; GoT2D investigators . Recommended joint and meta-analysis strategies for case-control association testing of single low-count variants. Genet Epidemiol. 2013;37(6):539-550. doi: 10.1002/gepi.21742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GitHub https://github.com/rmkoesterer/uga. Accessed February 21, 2016.
- 23.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP effect predictor. Bioinformatics. 2010;26(16):2069-2070. doi: 10.1093/bioinformatics/btq330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cingolani P, Platts A, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80-92. doi: 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Wu MC, Lin X. Optimal tests for rare variant effects in sequencing association studies. Biostatistics. 2012;13(4):762-775. doi: 10.1093/biostatistics/kxs014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.seqMeta: Meta-Analysis of Region-Based Tests of Rare DNA Variants. https://cran.r-project.org/web/packages/seqMeta/index.html. Accessed February 21, 2016.
- 28.Zerbino DR, Wilder SP, Johnson N, Juettemann T, Flicek PR. The ensembl regulatory build. Genome Biol. 2015;16:56. doi: 10.1186/s13059-015-0621-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ensambl. Gene regulation data in Ensembl. https://useast.ensembl.org/info/genome/funcgen/index.html. Accessed March 29, 2018.
- 30.Guerreiro R, Wojtas A, Bras J, et al. ; Alzheimer Genetic Analysis Group . TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117-127. doi: 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368(2):107-116. doi: 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhaber B, Eisenhaber S, Kwang TY, Grüber G, Eisenhaber F. Transamidase subunit GAA1/GPAA1 is a M28 family metallo-peptide-synthetase that catalyzes the peptide bond formation between the substrate protein’s omega-site and the GPI lipid anchor’s phosphoethanolamine. Cell Cycle. 2014;13(12):1912-1917. doi: 10.4161/cc.28761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vainauskas S, Maeda Y, Kurniawan H, Kinoshita T, Menon AK. Structural requirements for the recruitment of Gaa1 into a functional glycosylphosphatidylinositol transamidase complex. J Biol Chem. 2002;277(34):30535-30542. doi: 10.1074/jbc.M205402200 [DOI] [PubMed] [Google Scholar]
- 34.Vainauskas S, Menon AK. Endoplasmic reticulum localization of Gaa1 and PIG-T, subunits of the glycosylphosphatidylinositol transamidase complex. J Biol Chem. 2005;280(16):16402-16409. doi: 10.1074/jbc.M414253200 [DOI] [PubMed] [Google Scholar]
- 35.Murphy DB, Wiese S, Burfeind P, et al. Human brain factor 1, a new member of the fork head gene family. Genomics. 1994;21(3):551-557. doi: 10.1006/geno.1994.1313 [DOI] [PubMed] [Google Scholar]
- 36.Mitter D, Pringsheim M, Kaulisch M, et al. FOXG1 syndrome: genotype-phenotype association in 83 patients with FOXG1 variants. Genet Med. 2018;20(1):98-108. doi: 10.1038/gim.2017.75 [DOI] [PubMed] [Google Scholar]
- 37.Mizuno K, Antunes-Martins A, Ris L, Peters M, Godaux E, Giese KP. Calcium/calmodulin kinase kinase beta has a male-specific role in memory formation. Neuroscience. 2007;145(2):393-402. doi: 10.1016/j.neuroscience.2006.11.056 [DOI] [PubMed] [Google Scholar]
- 38.Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25(41):9413-9417. doi: 10.1523/JNEUROSCI.3190-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seelan RS, Parthasarathy LK, Parthasarathy RN. E2F1 regulation of the human myo-inositol 1-phosphate synthase (ISYNA1) gene promoter. Arch Biochem Biophys. 2004;431(1):95-106. doi: 10.1016/j.abb.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Calsolaro V, Edison P. Alterations in glucose metabolism in Alzheimer’s Disease. Recent Pat Endocr Metab Immune Drug Discov. 2016;10(1):31-39. doi: 10.2174/1872214810666160615102809 [DOI] [PubMed] [Google Scholar]
- 41.Mullins R, Reiter D, Kapogiannis D. Magnetic resonance spectroscopy reveals abnormalities of glucose metabolism in the Alzheimer’s brain. Ann Clin Transl Neurol. 2018;5(3):262-272. doi: 10.1002/acn3.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waragai M, Moriya M, Nojo T. Decreased N-acetyl aspartate/myo-inositol ratio in the posterior cingulate cortex shown by magnetic resonance spectroscopy may be one of the risk markers of preclinical alzheimer’s disease: a 7-year follow-up study. J Alzheimers Dis. 2017;60(4):1411-1427. doi: 10.3233/JAD-170450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voevodskaya O, Sundgren PC, Strandberg O, et al. ; Swedish BioFINDER study group . Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology. 2016;86(19):1754-1761. doi: 10.1212/WNL.0000000000002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pountney DL, Raftery MJ, Chegini F, Blumbergs PC, Gai WP. NSF, Unc-18-1, dynamin-1 and HSP90 are inclusion body components in neuronal intranuclear inclusion disease identified by anti-SUMO-1-immunocapture. Acta Neuropathol. 2008;116(6):603-614. doi: 10.1007/s00401-008-0437-4 [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Luo F, Zhang Z, et al. SNARE proteins synaptobrevin, SNAP-25, and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep. 2013;3(5):1414-1421. doi: 10.1016/j.celrep.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Zhu C, Beecham G, et al. ; Alzheimer’s Disease Sequencing Project . A rare missense variant of CASP7 is associated with familial late-onset Alzheimer’s disease. Alzheimers Dement. 2019;15(3):441-452. doi: 10.1016/j.jalz.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Nuallain B, Acero L, Williams AD, et al. Human plasma contains cross-reactive Abeta conformer-specific IgG antibodies. Biochemistry. 2008;47(47):12254-12256. doi: 10.1021/bi801767k [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Dataset Used for Functional Genomic Analysis
eTable 1. Characteristics of the Discovery and Replication Samples
eTable 2. Results for SNVs and Indels in the Discovery Sample
eTable 3. Meta-analysis Results for SNVs and Indels in the Discovery and Replication Samples
eTable 4. Results of Gene-Based Tests in the Discovery and Replication Samples
eFigure 1. QQ Plots for Association by Analysis Model and APOE Genotype in the Discovery Sample
eFigure 2. Manhattan Plots for Association by Analysis Model and APOE Genotype in the Discovery Sample
eFigure 3. Association of Alzheimer Disease With Variants in the ISYNA1 Region
eFigure 4. Association of Alzheimer Disease With Variants in the TREM2 Region
eFigure 5. Association of Alzheimer Disease With Variants in the AC099552 Region
eFigure 6. Association of Alzheimer Disease With Variants in the GPAA1 Region
eFigure 7. Association of Alzheimer’s disease with variants in the region including MAPT and NSF
eReferences.