Key Points
Question
What is the fracture incidence rate and growth in boys with Duchenne muscular dystrophy?
Findings
In this analysis of 832 boys with Duchenne muscular dystrophy (2006 to 2015) in the UK NorthStar database, fracture incidence rate was at least 4 times higher than in healthy boys. Boys treated with daily deflazacort had the highest fracture incidence rate, at 1367 per 10 000 person-years, and the greatest degree of linear growth failure.
Meaning
Given the high fracture burden and a marked level of linear growth impairment, clinical trials of bone-protective therapies and strategies to improve linear growth are urgently needed.
Abstract
Importance
Based on studies with relatively small sample size, fragility fractures are commonly reported in glucocorticoid (GC)-treated boys with Duchenne muscular dystrophy (DMD).
Objective
To determine the fracture burden and growth impairment in a large contemporary cohort of boys with DMD in the United Kingdom and in relation to GC regimen.
Design, Setting, and Participants
A retrospective review of fracture morbidity and growth from 832 boys with DMD in the UK NorthStar database (2006-2015), which systematically captures information from 23 participating centers. A total of 564 boys had more than 1 visit. No numbers of boys who refused were collected, but informal data from 2 centers in London and from Scotland show that refusal is very low. Data were analyzed between October 2006 and October 2015.
Main Outcomes and Measures
Fracture incidence rate per 10 000 person-years was determined. Cox regression analysis was used to identify factors associated with first fracture.
Results
Median age at baseline was 6.9 years (interquartile range, 4.9-7.2 years). At baseline, new fractures were reported in 7 of 564 participants (1.2%). During a median follow-up of 4 years (interquartile range, 2.0-6.0 years), incident fractures were reported in 156 of 564 participants (27.7%), corresponding to an overall fracture incidence rate of 682 per 10 000 person-years (95% CI, 579-798). The highest fracture incidence rate was observed in those treated with daily deflazacort at 1367 per 10 000 person-years (95% CI, 796-2188). After adjusting for age at last visit, mean hydrocortisone equivalent dose, mobility status, and bisphosphonate use prior to first fracture, boys treated with daily deflazacort had a 16.0-fold increased risk for first fracture (95% CI, 1.4-180.8; P = .03). Using adjusted regression models, change in height standard deviation scores was −1.6 SD lower (95% CI, −3.0 to −0.1; P = .03) in those treated with daily deflazacort compared with GC-naive boys, whereas there were no statistical differences in the other GC regimen.
Conclusions and Relevance
In this large group of boys with DMD with longitudinal data, we document a high fracture burden. Boys treated with daily deflazacort had the highest fracture incidence rate and the greatest degree of linear growth failure. Clinical trials of primary bone protective therapies and strategies to improve growth in boys with DMD are urgently needed, but stratification based on GC regimen may be necessary.
This study analyzes the fracture burden and growth impairment in a large contemporary cohort of boys with Duchenne muscular dystrophy in the United Kingdom and in relation to glucocorticoid regimen.
Introduction
Duchenne muscular dystrophy (DMD) is a life-limiting condition associated with progressive muscle wasting and weakness, whereby skeletal muscle fibers are replaced by fat and connective tissue. To date, there is no curative therapy; however, glucocorticoids (GC) are the only disease-modifying therapeutic agent shown to improve short-term muscle strength.1 There is an increasing trend for the continuation of GC beyond the loss of ambulation for the preservation of upper limb, cardiorespiratory function, and reduction in risk of scoliosis.2
As life expectancy continues to improve, understanding the long-term adverse effects of GC in this population is increasingly important.3 Glucocorticoids are often commenced at the plateauing phase of motor function, usually between ages 4 and 6 years given in the form of either prednisolone or deflazacort at 0.75 mg/kg/d and 0.9 mg/kg/d, respectively, in daily or intermittent fashion.4 In the United States, prednisone, the prodrug of prednisolone but with the same anti-inflammatory potency, is used instead of prednisolone.5 The optimum GC regimen for disease outcome in DMD is currently unknown. Moreover, there are insufficient data available from randomized clinical trials to determine the influence of GC regimen on fracture risk or linear growth.1 Previous studies have shown that linear growth impairment may be worse in boys treated with deflazacort, while weight gain was greater in those on prednisolone.6,7 To date, reports of fractures in DMD have been limited to relatively small cohorts, and the association of GC regimen with fracture have not been previously evaluated in detail.8,9,10 A 2018 retrospective review of 50 boys with DMD reported higher frequency of vertebral fracture (VF) and greater linear growth impairment in those treated with daily GC compared with those receiving intermittent regimen, although details of GC and the regimen were not reported.11
The primary aim of this study was to evaluate fracture incidence and linear growth impairment from a national cohort of boys with DMD in the United Kingdom. In addition, we aimed to explore the influence of different GC regimens on fracture and growth.
Methods
The UK NorthStar database systematically captures information on boys with DMD from 23 participating centers via biannual clinical assessments. The use of this database has been described in detail previously.12,13 The diagnosis of DMD is confirmed by DNA diagnostic technique and/or confirmatory muscle biopsy. Ethics approval was sought from the Sheffield Medical Research and Ethics Committee, and the committee was of the opinion that formal ethical approval for the NorthStar database was not needed. Participants gave consent to enter the database.
Study Design and Participants
We included 832 participants (3925 assessment records) with DMD from 2006 to 2015. Written informed consent was obtained from each family for anonymized data to be used for future research purposes.
New fracture occurrence, back pain, height, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) standard deviation scores (SDS) were reported at each clinic review.14,15 Fractures were recorded as VF or long bone/other fracture (non-VF). Data on VF, non-VF, and back pain were recorded in 3497 (89%), 3607 (92%), and 3527 (90%) assessments, respectively. Height and weight were recorded in 3259 (83%) and 3624 (92%) assessments, respectively. Further details on the NorthStar database, methods on analysis of fracture, and growth data according to age groups and GC regimen are available in the eMethods in the Supplement.
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics Software version 22 (SPSS Inc). Results were presented as mean (standard deviation) or median (interquartile range [IQR]). Differences between paired nonparametric data were analyzed using Wilcoxon rank sum test, and differences in categorical variables were analyzed using χ2 test. Comparison in mean daily hydrocortisone equivalent dose between GC regimen groups was determined by Welch analysis of variance and post hoc analysis with Tukey test. The significance level of P less than .05 was used to denote statistical significance, and P values were 2-sided.
First Fracture Occurrence
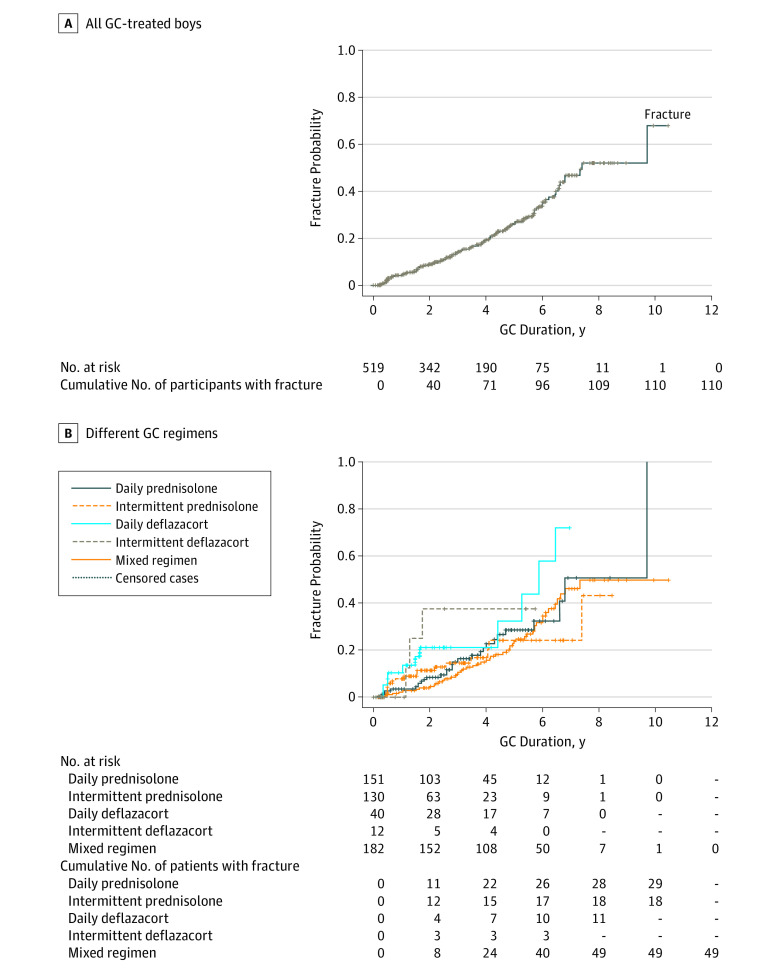
Kaplan-Meier analysis was performed to provide the probability of the first fracture according to age and duration of GC exposure (number at risk = 520) (Figure 1). Participants without fracture were censored at last visit (n = 410), and total GC exposure before the end of observation for these participants was included. Breslow test was used to compare the probability of developing first non-VF and VF and differences between GC duration with the first fracture between the GC regimen. Multivariate Cox regression proportional hazard analysis was performed to investigate the influence of risk factors to time to first fracture (Figure 1). Fracture risk between different groups was expressed by 95% confidence interval of hazard ratio (HR).
Figure 1. CONSORT Diagram.
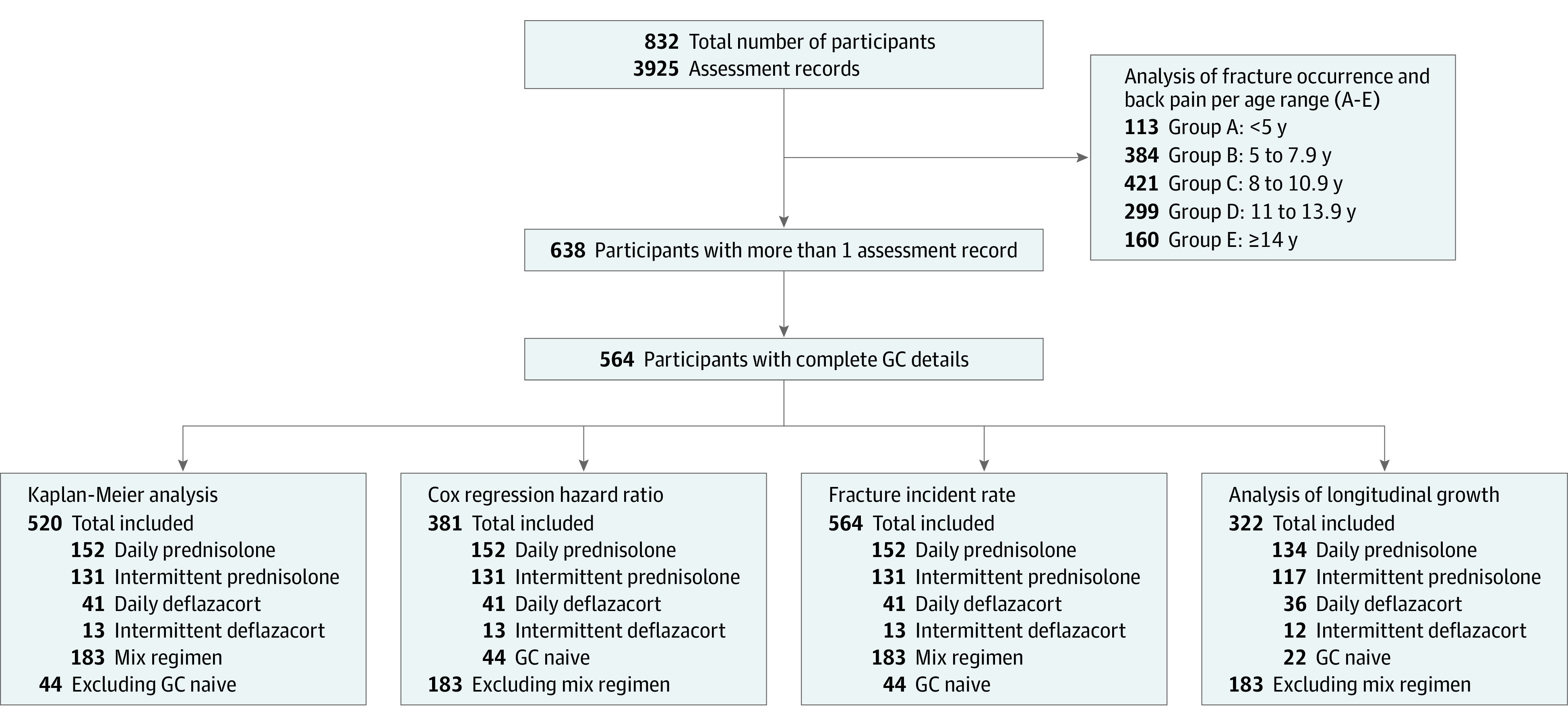
Flow diagram of participant inclusion in fracture and growth analysis. Data from 832 participants were used to assess new fracture occurrence and growth according to age groups. A total of 564 participants had more than 1 visit and complete clinical data. These participants were used for analysis of fracture incidence and longitudinal growth. Numbers included in each analysis described in detail. Participants included in the longitudinal growth analysis had complete data for height and weight in addition to clinical data. GC indicates glucocorticoid.
Fracture Incidence Rate
The total person-years (PY) were assessed by the sum of all the number of years from the date of the first and last assessment. Fracture rate is associated with the number of fractures during the cumulative observation time. Fracture rate was reported as fracture incidence per 10 000 PY (Figure 1). Byar method was used to compute fracture incidence per 10 000 PY with 95% CI.16,17
Longitudinal Growth
Wilcoxon test was used to compare height SDS (HtSDS) and BMI SDS at baseline and last follow-up (Figure 1). The association of GC regimen with change in HtSDS and BMISDS was evaluated using multiple linear regression after adjusting for baseline HtSDS or baseline BMI SDS, baseline age, and duration of follow-up. Each GC regimen was compared against the GC-naive group as reference category. Multiple linear regression was performed to evaluate the association of hydrocortisone equivalent with change in HtSDS and BMISDS, adjusting for baseline HtSDS or baseline BMISDS, baseline age, and duration of follow-up.
Results
Clinical Characteristics
There were 832 participants at baseline, and 638 participants had more than 1 assessment record (Figure 1). Median age at baseline was 6.9 years (IQR, 4.9-7.2) and at last visit was 10.9 years (IQR, 8.1-13.8), with a median duration of follow-up of 4.0 years (IQR, 2.0-6.0). At baseline, 638 of 832 (76.7%) were receiving GC, whereas at last visit, this was observed in 527 of 638 (82.6%) (P < .001). At baseline, 13 of 832 (1.6%) were receiving bisphosphonate, whereas at last visit, this was observed in 47 of 638 (7.4%) (P < .001). At baseline, 7 of 832 (0.8%) were nonambulant, whereas at last visit, this was observed in 154 of 638 (24.1%) (P < .001).
Bone Morbidity in DMD
Fracture Occurrence
Of the 832 participants, a total of 178 fractures (non-VF and VF) were reported in 148 participants (17.7%). A total of 118 non-VFs were reported in 112 of 832 participants (13.5%). Of the 112 participants with non-VF fractures, multiple non-VFs were reported in 5 participants (5%), ranging from 2 to 3 episodes. A total of 60 episodes of new VFs were reported in 52 of 832 participants (6%). Of the 832 participants, 96 (12%) had non-VF only. Of the 832 participants, 36 (4%) had VF only. Of the 832 participants, 16 (2%) had both VF and non-VF.
The proportion of participants with new non-VF and VF appeared to increase from group A to E, with 31 of 160 new fractures (19%) reported in group E (≥14 years) (eFigure 1A in the Supplement). Back pain was not reported in participants in group A (<5 years). However, it was increasingly common in the other groups, with the highest proportion in group E (≥14 years), reported in 35 of 160 (22%) (eFigure 1B in the Supplement). New VFs were reported most commonly in group E (≥14 years) in 14 of 160 (9%). New VF without reported back pain at the clinic visits were observed in all 5 age groups (eFigure 1C in the Supplement).
First Fracture Probability
On Kaplan-Meier analysis, the probability of first fracture (non-VF and VF) was 50% by 11.0 years (95% CI, 10.6-11.4). The probability of sustaining a non-VF was 50% by 10.9 years (95% CI, 10.3-11.6), whereas the probability of sustaining a VF was 50% by 12.0 years (95% CI, 10.5-13.5). The probability of first fracture (VF and non-VF) was 50% by 7.4 years (95% CI, 6.1-8.7) of GC exposure (Figure 2A). The difference between the probabilities of first fracture (VF and non-VF) was statistically significant between the different GC regimens (P =.03) (Figure 2B). Mean GC exposure to fracture was shortest in the daily deflazacort group at 5.9 years (95% CI, 4.5 to 7.3).
Figure 2. Probability of First Fracture in Relation to Duration of Glucocorticoid (GC) Exposure.
A, Probability of first fracture in all GC-treated boys (n = 520) in relation to duration of GC exposure using Kaplan-Meier analysis. Those who did not sustain fracture by last visit were included and marked as censored cases (vertical lines on Kaplan-Meier curve). B, Probability of first fracture in different GC regimen in relation to duration of GC exposure using Kaplan-Meier analysis. Those who did not sustain fracture were included and marked as censored cases (vertical lines on Kaplain-Meier curves).
Correlates of First Fracture: Multivariate Analysis
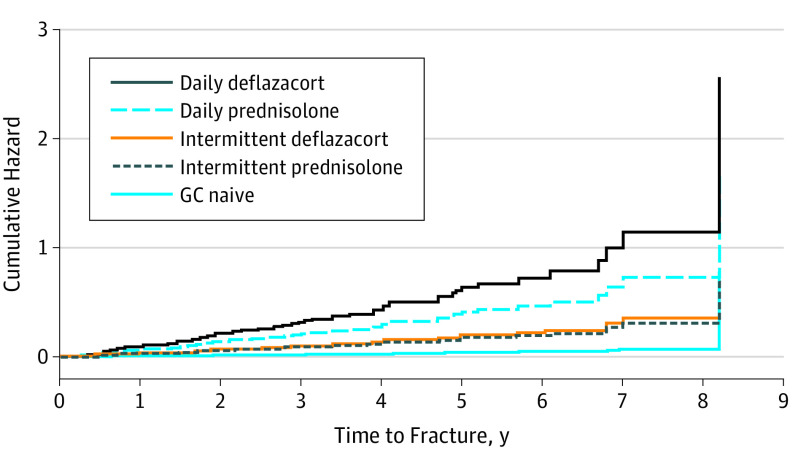
Glucocorticoid regimen and ambulant status were both independently associated with an increase in HR for first fracture (non-VF and VF). Hazard ratio was increased by 16.0-fold (95% CI, 1.4-180.8, P = .03) in participants receiving daily deflazacort after adjusting for age at last assessment, mean hydrocortisone equivalent dose (in milligrams per meters squared per day), mobility status, and bisphosphonate use prior to first fracture (Figure 3). Hazard ratio was not increased in participants receiving the other GC regimen after adjusting for similar covariates. Hazard for first fracture was increased by 3.7-fold (95% CI, 1.5-9.2; P = .005) in ambulant participants, after adjusting for age at last assessment, GC regimen, mean hydrocortisone equivalent dose, and bisphosphonate use. Bisphosphonate use prior to first fracture was not associated with significant reduction in HR, 0.5 (95% CI, 0.2-1.5; P = .23) after adjusting for age at last assessment, GC regimen, mean hydrocortisone equivalent dose, and mobility status. Similar results were obtained in adjustment models including mean hydrocortisone equivalent does (in milligrams per kilograms per day) or cumulative hydrocortisone equivalent dose (in milligrams per kilograms) (eFigure 2A and B in the Supplement).
Figure 3. Multivariate Cox Regression Analysis of Cumulative Hazard According to Glucocorticoid (GC) Regimen.
Hazard ratio of sustaining the first fracture was analyzed using multivariate Cox regression adjusting for age at last assessment, average hydrocortisone equivalent (milligrams per meters squared per day), mobility status, and bisphosphonate use prior to the first fracture.
Fracture Incident Rate
Of the 638 participants with more than 1 assessment record, 564 had complete GC regimen and dose data (Figure 1 and Table 1). Mean (SD) daily hydrocortisone equivalent dose for surface area during the observation period (in milligrams per meters squared per day) in daily deflazacort, daily prednisolone, intermittent deflazacort, and intermittent prednisolone regimen were 46.5 (12.8) mg/m2/d , 57.5 (19.4) mg/m2/d, 20.6 (5.4) mg/m2/d, and 30.6 (10.7) mg/m2/d , respectively. Mean (SD) prescribed drug dose in daily deflazacort, daily prednisolone, intermittent deflazacort, and intermittent prednisolone regimen during the observation period, taking into account days of drug exposure, were 0.6 (0.2) mg/kg/d, 0.5 (0.1) mg/kg/d, 0.3 (0.1) mg/kg/d, and 0.3 (0.1) mg/kg/d, respectively.
Table 1. Group Characteristics and Growth According to GC Regimen.
| Characteristic | Median (IQR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Daily Deflazacort (n = 41) | Intermittent Deflazacort (n = 13) | Daily Prednisolone (n = 152) | Intermittent Prednisolone (n = 131) | GC Naive (n = 44) | ||||||
| Baseline | Last Visit | Baseline | Last Visit | Baseline | Last Visit | Baseline | Last Visit | Baseline | Last Visit | |
| Age, y | 7.4 (5.8 to 10.5) | 10.1 (9.0 to 14.0) | 6.8 (5.4 to 9.0) | 11.4 (9.2 to 13.4) | 7.0 (5.3 to 8.3) | 10.0 (7.8 to 13.0) | 5.6 (6.7 to 10.7) | 10.7 (9.3 to 13.2) | 5.3 (4.0 to 6.5) | 6.8 (4.9 to 8.0) |
| Duration of follow-up, y | 2.5 (1.6 to 5.2) | 4.0 (2.2 to 5.7) | 2.8 (1.5 to 4.6) | 5.1 (2.6 to 5.4) | 1.7 (0.9 to 2.1) | |||||
| Nonambulant, No./total No. (%) | 1/41 (2) | 10/41 (24) | 0/152 (0) | 38/152 (25) | 1/131 (1) | 21/131 (16) | 0/13 (0) | 6/13 (46) | 0/44 (0) | 1/44 (2) |
| SDS | ||||||||||
| Height | −1.4 (−2.9 to −0.6) | −2.6 (−3.2 to −1.6)a | −1.0 (−1.9 to −0.4) | −1.4 (−2.3 to 0.4)a | −1.2 (−1.9 to −0.5) | −1.3 (−2.2 to 0.2) | −0.9 (−1.3 to −0.4) | −1.3 (−1.7 to −0.5) | −0.8 (−1.7 to −0.4) | −0.9 (−1.6 to −0.3) |
| Change in height | −0.6 (−1.5 to −0.1) | −0.4 (−1.1 to 0.3) | 0.0 (−0.5 to 0.3) | −0.4 (−0.8 to −0.1) | −0.1 (−0.4 to 0.4) | |||||
| Body mass indexb | 1.8 (1.2 to 2.9) | 2.3 (0.8 to 3.2) | 1.2 (0.4 to 1.7) | 1.9 (1.0 to 2.6)a | 1.1 (0.3 to 1.8) | 1.7 (0.6 to 2.3)a | 1.7 (0.9 to 2.0) | 1.6 (1.0 to 2.3) | 1.1 (0.3 to 1.5) | 1.0 (−0.5 to 1.5) |
| Change in body mass index | 0.1 (−0.4 to 0.5) | 0.6 (0.0 to 1.3) | 0.3 (−0.2 to 1.0) | 0.1 (−0.1 to 0.3) | 0.0 (−0.3 to 0.3) | |||||
Abbreviations: GC, glucocorticoid; IQR, interquartile range; SDS, standard deviation score.
P < .001 (last visit vs baseline).
Calculated as weight in kilograms divided by height in meters squared.
At baseline, new fractures were reported in 7 of 564 participants (1%), all of which were non-VFs. Over a total observation time of 2288 PY, incident fractures occurred in 156 of 564 (28%) (Table 2). Incident non-VFs were reported in 107 of 564 participants (19%), whereas incident VFs were reported in 49 of 564 participants (9%). Overall, fracture incidence rate for DMD was 682 per 10 000 PY (95% CI, 579-798) (Table 2). Non-VF incidence rate was 468 per 10 000 PY (95% CI, 383-565), and VF incident rate was 214 per 10 000 PY (95% CI, 159-283). Fracture incidence rate for GC-naive cases was 254 per 10 000 PY (95% CI, 30-887). Fracture incidence rate was highest in the daily deflazacort group at 1366.6 per 10 000 PY (95% CI, 796.1-2188.0) (Table 2).
Table 2. Fracture Incident Rate According to Glucocorticoid Regimen.
| GC Regimen | Bisphosphonate Therapy Over the Observation Period | No./ Total No. (%) | Type of Fractures | Incidence per 10 000 Person-Years (95% CI) |
|---|---|---|---|---|
| Daily deflazacort | Oral and IV | 26/41 (63) | VF and non-VF | 1367 (796-2188) |
| Oral | 18/26 (69) | VF | 322 (88-823) | |
| IV | 8/26 (31) | Non-VF | 1045 (556-1787) | |
| Intermittent deflazacort | Oral and IV | 2/13 (15) | VF and non-VF | 577 (119-1686) |
| Oral | 2/2 (100) | VF | 192 (5-1072) | |
| IV | 0 | Non-VF | 385 (47-1389) | |
| Daily prednisolone | Oral and IV | 31/152 (20) | VF and non-VF | 748 (550-995) |
| Oral | 21/31 (68) | VF | 223 (122-374) | |
| IV | 10/31 (32) | Non-VF | 525 (362-738) | |
| Intermittent prednisolone | Oral and IV | 13/131 (16) | VF and non-VF | 512 (32-776) |
| Oral | 7/13 (54) | VF | 186 (80-367) | |
| IV | 6/13 (46) | Non-VF | 326 (178-547) | |
| Mixed regimen | Oral and IV | 40/183 (22) | VF and non-VF | 669 (516-852) |
| Oral | 28/40 (70) | VF | 226 (142-343) | |
| IV | 12/40 (30) | Non-VF | 442 (320-596) | |
| GC naive | Oral and IV | 0 | VF and non-VF | 254 (30-887) |
| Oral | 0 | VF | 0 | |
| IV | 0 | Non-VF | 254 (30-887) |
Abbreviations: GC, glucocorticoid; IV, intravenous; non-VF, nonvertebral fracture; VF, vertebral fracture.
Growth in DMD
Height SDS According to Age Groups
Median HtSDS in groups A to E were −1.1 (IQR, −1.6 to −0.2), −1.2 (IQR, −1.9 to −0.5), −1.3 (IQR, −2.2 to −0.5), −1.5 (IQR, −2.5 to −0.4), and −1.8 (IQR, −3.3 to −0.8), respectively. The proportions of participants with HtSDS less than −2.0 in groups A to E were 16 of 83 (19%), 72 of 346 (21%), 114 of 354 (32%), 71 of 184 (43%), and 41 of 86 (48%), respectively.
Height SDS With Follow-up
Height SDS at last visit was significantly lower than HtSDS at baseline in the daily deflazacort and daily prednisolone groups (Figure 1 and Table 1). After adjusting for baseline HtSDS, baseline age, and duration of follow-up, change in HtSDS (HtSDS last visit minus HtSDS baseline) was significantly lower only in the daily deflazacort group compared with GC-naive participants (95% CI, −1.20 to −0.17; P = .01) (eTable in the Supplement). Hydrocortisone equivalent dose (in milligrams per kilogram per day) was associated with change in HtSDS (95% CI, −0.38 to −0.01; P = .04) after adjusting for baseline HtSDS, baseline age, and duration of follow-up (model R2 = 0.12).
Body Mass Index SDS According to Age Groups
Median BMI SDS in groups A to E were 1.0 (IQR, 0.3-1.7), 1.2 (IQR, 0.2-1.9), 1.9 (IQR, 0.9-2.6), 2.0 (IQR, 1.1-2.5), and 1.8 (IQR, 0.8-2.5), respectively. The proportion of participants with BMI SDS greater than 2.5 in groups A to E were 5 of 82 (6%), 44 of 342 (13%), 100 of 346 (29%), 47 of 182 (26%), and 21 of 80 (26%), respectively.
Body Mass Index SDS With Follow-up
Body mass index SDS at last visit was significantly higher than BMI SDS at baseline in the daily prednisolone and pulsed prednisolone group (Figure 1; eTable in the Supplement). After adjusting for baseline BMI SDS, baseline age, and duration of follow-up, change in BMI SDS (BMI SDS last visit minus BMI SDS baseline) was significantly higher only in the daily prednisolone group compared with GC-naive participants (95% CI, 0.14-1.15; P = .01) (eTable in the Supplement). Hydrocortisone equivalent dose (in milligrams per kilograms per day) was not associated with change in BMI SDS (95% CI, −0.12 to 0.26; P = .46) after adjusting for baseline BMI SDS, baseline age, and duration of follow-up (model R2 = 0.08).
Discussion
To our knowledge, this is the first study to comprehensively evaluate fracture burden and linear growth impairment in a large group of participants with DMD. The use of the NorthStar database allowed a thorough examination of the association of these findings with GC regimen. While the retrospective analysis poses limitations, such as lack of systematic monitoring of important covariates such as puberty, the data are sufficiently powerful to draw important conclusions. Our results demonstrated that boys treated with daily deflazacort had the shortest time to first fracture, highest fracture incidence rate, and most profound linear growth failure. Weight gain was greatest in those treated with daily prednisolone, consistent with findings from previous randomized trials.18,19
The overall fracture incidence rate of 682 per 10 000 PY in this cohort is 4 times higher than healthy UK boys.20 Fracture incidence is normally age dependent and peaks at age 14 to 15 years at 325 per 10 000 PY.20 The lower limits of the 95% CI of fracture incidence in the current cohort, mostly in early to midchildhood, is approximately 1.8 times higher than the peak fracture incidence of healthy boys. Given that there was no systematic screening of VF, it is likely that the overall observed frequency is an underestimation of the fracture burden in DMD. Despite this, the incidence rate for VF of 214 per 10 000 PY is already 535 times higher than the incidence rate of VF in growing boys.19 In this cohort of 832 boys, only 18% had sustained a fracture. In other previous published studies in DMD, fracture frequency has been reported to vary between 21% and 75%.8,9,10 These studies included older boys, with more than half nonambulant in comparison with just more than 20% at last visit in our study, which we believe may be an explanation of our finding of increased HR for fractures in ambulant boys in our study.
While VF without reported back pain was observed, it is likely that these were participants who presented clinically with painful VF and received treatment prior to attendance at their clinic visit because none of the centers incorporated routine lateral spine imaging to screen for VF during the study period. We acknowledge that it is possible that some may have been identified from imaging for monitoring of scoliosis, although antero-posterior spine radiographs (instead of lateral spine radiographs) are generally performed for scoliosis. The discordance between reported back pain and VF may be explained by undiagnosed VF. However, there are many other reasons for back pain in DMD including musculoskeletal fatigue, contractures, and mechanical issues relating to positioning and posture.21 In other models of GC-associated osteoporosis, such as childhood leukemia, the likelihood of VF was increased by almost 5-fold in those with back pain.22
The starting dose of deflazacort in DMD is recommended at a 1.2 to 1 ratio compared with prednisolone for equipotent anti-inflammatory effect.4 The equipotency of different GCs for anti-inflammatory effects may not apply to the other observed effects of GC.5 Deflazacort is associated with less cushingoid appearance, appetite increase, behavioral changes, and gastric symptoms.18,23 Experimental studies suggest that deflazacort may have bone-sparing effects via its effects on bone formation in comparison with dexamethasone or cortisol.24,25,26 Deflazacort and dexamethasone have similar effects on stimulating osteoclast formation.26 Evidence of the relative bone sparing in children is based on reports in nephrotic syndrome and rheumatologic conditions where nonsignificant differences in densitometry results were reported.27,28,29 Owing to its improved tolerability, deflazacort is often maintained at higher doses.7 However, in our present cohort, the mean daily prescribed dose of daily deflazacort and prednisolone was 0.6 mg/kg and 0.5 mg/kg, respectively, which was only approximately 70% of the recommended daily dose of deflazacort (0.9 mg/kg) and prednisolone (0.75 mg/kg). This is a finding similar to an earlier report from the NorthStar database.12 We believe that our findings are unlikely to be owing solely to the fact that weight gain in those receiving prednisolone limited dose increase, given that participants in both groups only achieved about 70% of the recommended dose for weight during the observation period, and that hydrocortisone equivalence (in milligrams per meters squared per day and milligrams per kilograms per day) were similar in both groups.
Non-VF may occur more frequently in a group with greater number of falls. To our knowledge, there is no systematic method to assess risk of falls in DMD and is likely to be associated with the underlying muscle function. While the effect of GC regimen in DMD is currently unclear, preliminary evidence suggests that those treated with daily deflazacort may have better muscle outcomes, including 6-minute walk test, 4-stair climb, and time to loss of ambulation, compared with those treated with prednisolone or prednisone.7,30 Therefore, falls or poorer muscle function in those treated with daily deflazacort are unlikely to explain the increased risk of fracture observed in our study.
A greater degree of growth failure observed in those treated with deflazacort has been previously reported.6,7 Our finding of a significant independent association between hydrocortisone equivalent dose and change in HtSDS provides support for a dose effect on linear growth impairment. Similarly, our observation of a greater increase in BMI in those treated with daily prednisolone is consistent with previous reports.18,19 In a previous randomized clinical trial, 12 weeks’ treatment with daily deflazacort (0.9 mg/kg/d) led to a mean weight gain of 1.7 kg in boys with DMD, whereas daily prednisolone (0.75 mg/kg/d) was associated with a mean gain of 3.2 kg.19 Given that linear growth is poorer in those treated with daily deflazacort, our results provide robust evidence that weight gain is indeed greater in those treated with daily prednisolone. The underlying reason for the increased propensity for weight gain in those treated with daily prednisolone may be associated with GC class differences in their effects of adipogenesis, given that we did not observe an association between hydrocortisone equivalent dose and change in BMI SDS.31
Bisphosphonate use appeared to be highest in the daily deflazacort group, with the highest fracture incidence, and we believe this is likely to be owing to introduction of therapy following fracture occurrence. The role of bisphosphonates as prophylactic therapy in childhood GC-associated osteoporosis, including boys with DMD, remains unclear.32,33 Our analysis did not demonstrate a significant protective effect of bisphosphonate therapy on risk for first fracture in DMD. The underlying bone defect in chronic conditions treated with GC, such as DMD, may involve a reduction in both bone formation and resorption.34,35 Therefore, it is possible that the use of bisphosphonates alone may not be appropriate in such conditions with a low bone turnover state, and combining treatment with bone protective therapy that promotes bone formation requires future exploration.36
Limitations
Our study has several limitations. First, this is an observational study based on information collected as part of a national registry. While data on fracture and growth were recorded in about 90% of the visits, the accuracy of such information is unclear. Vertebral fractures are likely to be missed, especially in older participants and those with longer GC exposure, because routine screening spine imaging was not part of clinical practice during the period of study. Height measurements in boys with DMD, especially nonambulatory boys, may be challenging. In addition, scoliosis and limb contractures may be common in non-ambulatory boys. However, most of the boys in this study were still ambulatory. Detailed prospective studies of linear growth and bone morbidity in boys with DMD are needed, including a larger number of nonambulant participants. Finally, we were unable to evaluate the association of other therapies that may improve growth and skeletal development, such as vitamin D treatment and testosterone therapy.
Conclusions
In summary, in a group of relatively young and mostly ambulant boys with DMD, the overall fracture incidence based on reported fractures was at least 4 times higher, and VF incidence was more than 500 times higher than in healthy boys. We showed for the first time, to our knowledge, that boys receiving daily deflazacort had the greatest likelihood of fracture and confirmed the previously reported increased risk of linear growth impairment. Our analysis failed to identify an association of bisphosphonate with reduction of fracture occurrence in the relatively small number of participants treated prior to first fracture. However, given the high fracture burden and a marked level of linear growth impairment, clinical trials of bone protective therapies and strategies to improve growth in boys with DMD are urgently needed.
eMethods.
eFigure 1. New Fracture Occurrence According to Age Groups
eFigure 2. Multivariate Cox Regression Analysis of Cumulative Hazard According to GC Regimen
eTable. Multivariate Analysis of the Impact of Glucocorticoid Regimen on Changes in Growth Parameters
References
- 1.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;1(5):CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moxley RT III, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25(9):1116-1129. doi: 10.1177/0883073810371004 [DOI] [PubMed] [Google Scholar]
- 3.Passamano L, Taglia A, Palladino A, et al. Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta Myol. 2012;31(2):121-125. [PMC free article] [PubMed] [Google Scholar]
- 4.Bushby K, Finkel R, Birnkrant DJ, et al. ; DMD Care Considerations Working Group . Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77-93. doi: 10.1016/S1474-4422(09)70271-6 [DOI] [PubMed] [Google Scholar]
- 5.Parente L. Deflazacort: therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol Toxicol. 2017;18(1):1. doi: 10.1186/s40360-016-0111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb MM, West NA, Ouyang L, et al. ; Muscular Dystrophy Surveillance, Research, and Tracking Network (MD STARnet) . Corticosteroid treatment and growth patterns in ambulatory males with Duchenne muscular dystrophy. J Pediatr. 2016;173:207-213.e3. doi: 10.1016/j.jpeds.2016.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bello L, Gordish-Dressman H, Morgenroth LP, et al. ; CINRG Investigators . Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85(12):1048-1055. doi: 10.1212/WNL.0000000000001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald DG, Kinali M, Gallagher AC, et al. Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol. 2002;44(10):695-698. doi: 10.1111/j.1469-8749.2002.tb00272.x [DOI] [PubMed] [Google Scholar]
- 9.Bothwell JE, Gordon KE, Dooley JM, MacSween J, Cummings EA, Salisbury S. Vertebral fractures in boys with Duchenne muscular dystrophy. Clin Pediatr (Phila). 2003;42(4):353-356. doi: 10.1177/000992280304200408 [DOI] [PubMed] [Google Scholar]
- 10.Tian C, Wong BL, Hornung L, et al. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26(11):760-767. doi: 10.1016/j.nmd.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 11.Crabtree NJ, Adams JE, Padidela R, et al. Growth, bone health & ambulatory status of boys with DMD treated with daily vs intermittent oral glucocorticoid regimen. Bone. 2018;116:181-186. doi: 10.1016/j.bone.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 12.Ricotti V, Ridout DA, Scott E, et al. ; NorthStar Clinical Network . Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84(6):698-705. doi: 10.1136/jnnp-2012-303902 [DOI] [PubMed] [Google Scholar]
- 13.Ricotti V, Ridout DA, Pane M, et al. ; UK NorthStar Clinical Network . The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatry. 2016;87(2):149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17(4):407-429. doi: [DOI] [PubMed] [Google Scholar]
- 15.Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):17-24. doi: 10.1136/adc.73.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breslow NE, Day NE. The Design and Analysis of Cohort Studies. Vol II. Lyon, France: International Agency for Research on Cancer, World Health Organisation; 1987. [Google Scholar]
- 17.Armitage P, Berry G. Statistical Methods in Medical Research. 3rd ed. Oxford, England: Blackwell; 1994. [Google Scholar]
- 18.Bonifati MD, Ruzza G, Bonometto P, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344-1347. doi: [DOI] [PubMed] [Google Scholar]
- 19.Griggs RC, Miller JP, Greenberg CR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87(20):2123-2131. doi: 10.1212/WNL.0000000000003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon RJ, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C. Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone. 2016;85:9-14. doi: 10.1016/j.bone.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva TD, Massetti T, Monteiro CB, et al. Pain characterization in Duchenne muscular dystrophy. Arq Neuropsiquiatr. 2016;74(9):767-774. doi: 10.1590/0004-282X20160107 [DOI] [PubMed] [Google Scholar]
- 22.Halton J, Gaboury I, Grant R, et al. ; Canadian STOPP Consortium . Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24(7):1326-1334. doi: 10.1359/jbmr.090202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LoCascio V, Ballanti P, Milani S, et al. A histomorphometric long-term longitudinal study of trabecular bone loss in glucocorticoid-treated patients: prednisone versus deflazacort. Calcif Tissue Int. 1998;62(3):199-204. doi: 10.1007/s002239900417 [DOI] [PubMed] [Google Scholar]
- 24.Guenther HL, Felix R, Fleisch H. Comparative study of deflazacort, a new synthetic corticosteroid, and dexamethasone on the synthesis of collagen in different rat bone cell populations and rabbit articular chondrocytes. Calcif Tissue Int. 1984;36(2):145-152. [DOI] [PubMed] [Google Scholar]
- 25.Canalis E, Avioli L. Effects of deflazacort on aspects of bone formation in cultures of intact calvariae and osteoblast-enriched cells. J Bone Miner Res. 1992;7(9):1085-1092. [DOI] [PubMed] [Google Scholar]
- 26.Chung H, Kang YS, Hwang CS, et al. Deflazacort increases osteoclast formation in mouse bone marrow culture and the ratio of RANKL/OPG mRNA expression in marrow stromal cells. J Korean Med Sci. 2001;16(6):769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loftus J, Allen R, Hesp R, et al. Randomized, double-blind trial of deflazacort versus prednisone in juvenile chronic (or rheumatoid) arthritis: a relatively bone-sparing effect of deflazacort. Pediatrics. 1991;88(3):428-436. [PubMed] [Google Scholar]
- 28.Aicardi G, Benso L, Vignolo M, et al. Dose-dependent effects of deflazacort and prednisone on growth and skeletal maturation. Br J Rheumatol. 1993;32(suppl 2):39-43. doi: 10.1093/rheumatology/32.suppl_2.39 [DOI] [PubMed] [Google Scholar]
- 29.Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P. A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatr Nephrol. 1997;11(4):418-422. doi: 10.1007/s004670050308 [DOI] [PubMed] [Google Scholar]
- 30.Shieh PB, Mcintosh J, Jin F, et al. ; AND THE ACT DMD STUDY GROUP . Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: a post HOC analysis from the ACT DMD trial. Muscle Nerve. 2018;58(5):639-645. doi: 10.1002/mus.26191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace AM, Tucker P, Williams DM, Hughes IA, Ahmed SF. Short-term effects of prednisolone and dexamethasone on circulating concentrations of leptin and sex hormone-binding globulin in children being treated for acute lymphoblastic leukaemia. Clin Endocrinol (Oxf). 2003;58(6):770-776. doi: 10.1046/j.1365-2265.2003.01790.x [DOI] [PubMed] [Google Scholar]
- 32.Joseph S, McCarrison S, Wong SC. Skeletal fragility in children with chronic disease. Horm Res Paediatr. 2016;86(2):71-82. doi: 10.1159/000447583 [DOI] [PubMed] [Google Scholar]
- 33.Bell JM, Shields MD, Watters J, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2017;1:CD010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Söderpalm AC, Magnusson P, Ahlander AC, et al. Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord. 2007;17(11-12):919-928. doi: 10.1016/j.nmd.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 35.Wong SC, Dobie R, Altowati MA, Werther GA, Farquharson C, Ahmed SF. Growth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr Rev. 2016;37(1):62-110. doi: 10.1210/er.2015-1026 [DOI] [PubMed] [Google Scholar]
- 36.Wood CL, Ahmed SF. Bone protective agents in children. Arch Dis Child. 2018;103(5):503-508. doi: 10.1136/archdischild-2016-311820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. New Fracture Occurrence According to Age Groups
eFigure 2. Multivariate Cox Regression Analysis of Cumulative Hazard According to GC Regimen
eTable. Multivariate Analysis of the Impact of Glucocorticoid Regimen on Changes in Growth Parameters