Key Points
Questions
Is intravenous thrombolysis with alteplase efficacious in patients with lacunar stroke?
Findings
In this secondary post hoc analysis of the randomized clinical WAKE-UP trial of magnetic resonance imaging–guided intravenous alteplase administration to patients with stroke of unknown onset, 108 of 503 patients had magnetic resonance imaging–defined lacunar infarcts. Treatment outcomes of alteplase in patients with lacunar infarcts were similar to those in patients with nonlacunar infarcts and resulted in a numerically higher rate of favorable outcome at 90 days (59% vs 46%) and a numerical shift toward better outcomes across all categories of the modified Rankin Scale.
Meaning
Per this analysis, intravenous alteplase appears to be as effective in patients with lacunar stroke as in those with nonlacunar stroke.
This secondary post hoc analysis of data from the WAKE-UP randomized clinial trial compares patients with lacunar and nonlacunar strokes to assess whether intravenous thrombolysis with altaplase is useful in these patients.
Abstract
Importance
The rationale for intravenous thrombolysis in patients with lacunar infarcts is debated, since it is hypothesized that the microvascular occlusion underlying lacunar infarcts might not be susceptible to pharmacological reperfusion treatment.
Objective
To study the efficacy and safety of intravenous thrombolysis among patients with lacunar infarcts.
Design, Setting, and Participants
This exploratory secondary post hoc analysis of the WAKE-UP trial included patients who were screened and enrolled between September 2012 and June 2017 (with final follow-up in September 2017). The WAKE-UP trial was a multicenter, double-blind, placebo-controlled randomized clinical trial to study the efficacy and safety of intravenous thrombolysis with alteplase in patients with an acute stroke of unknown onset time, guided by magnetic resonance imaging. All 503 patients randomized in the WAKE-UP trial were reviewed for lacunar infarcts. Diagnosis of lacunar infarcts was based on magnetic resonance imaging and made by consensus of 2 independent investigators blinded to clinical information.
Main Outcomes and Measures
The primary efficacy variable was favorable outcome defined by a score of 0 to 1 on the modified Rankin Scale at 90 days after stroke, adjusted for age and severity of symptoms.
Results
Of the 503 patients randomized in the WAKE-UP trial, 108 patients (including 74 men [68.5%]) had imaging-defined lacunar infarcts, whereas 395 patients (including 251 men [63.5%]) had nonlacunar infarcts. Patients with lacunar infarcts were younger than patients with nonlacunar infarcts (mean age [SD], 63 [12] years vs 66 [12] years; P = .003). Of patients with lacunar infarcts, 55 (50.9%) were assigned to treatment with alteplase and 53 (49.1%) to receive placebo. Treatment with alteplase was associated with higher odds of favorable outcome, with no heterogeneity of treatment outcome between lacunar and nonlacunar stroke subtypes. In patients with lacunar strokes, a favorable outcome was observed in 31 of 53 patients (59%) in the alteplase group compared with 24 of 52 patients (46%) in the placebo group (adjusted odds ratio [aOR], 1.67 [95% CI, 0.77-3.64]). There was 1 death and 1 symptomatic intracranial hemorrhage according to Safe Implementation of Thrombolysis in Stroke–Monitoring Study criteria in the alteplase group, while no death and no symptomatic intracranial hemorrhage occurred in the placebo group. The distribution of the modified Rankin Scale scores 90 days after stroke also showed a nonsignificant shift toward better outcomes in patients with lacunar infarcts treated with alteplase, with an adjusted common odds ratio of 1.94 (95% CI, 0.95-3.93).
Conclusions and Relevance
While the WAKE-UP trial was not powered to demonstrate the efficacy of treatment in subgroups of patients, the results indicate that the association of intravenous alteplase with functional outcome does not differ in patients with imaging-defined lacunar infarcts compared with those experiencing other stroke subtypes.
Introduction
Intravenous thrombolytic therapy with alteplase (recombinant human tissue plasminogen activator) is approved for acute ischemic stroke treatment, independent of stroke subtype.1,2 The vascular pathology underlying lacunar infarction remains incompletely understood, and the efficacy of intravenous thrombolysis in patients with lacunar infarcts is debated, since a thrombotic occlusive process cannot be confirmed.3 As far back as the pioneering work of C. Miller Fisher, MD, half a century ago, lacunar infarcts have been understood to result from the occlusion of small penetrating arteries owing to microatheroma and lipohyalinosis.4 The role of thrombosis in the pathophysiology of lacunar infarctions is uncertain, and consequently, researchers have questioned whether lacunar strokes would benefit from clot-dissolving pharmacological treatment. Concerns about an increased risk of symptomatic intracranial hemorrhage (SICH) in severe leukoaraiosis, which is frequently present in patients with lacunar infarction, and the natural history of a more benign course in a large proportion of lacunar strokes are further arguments against the use of alteplase in these patients.5
Previous randomized clinical trials of stroke thrombolysis have reported results for subgroups based on the causative mechanisms of stroke, including so-called lacunar infarcts, but with diagnoses based on clinical presentation.1,2 This includes studies in which lacunar syndromes may be conflated with minor stroke, defined by neurological severity.6 However, in the short-term clinical setting, the diagnosis of lacunar infarct (ie, an acute subcortical ischemic lesion in the territory of a small penetrating artery) requires magnetic resonance imaging (MRI), which identifies acute lacunar infarction with high sensitivity on diffusion-weighted imaging (DWI).7 This imaging information was not available in previous trials of thrombolysis for acute stroke, which relied on noncontrast computed tomography (CT) for the randomization of patients.8,9
In the WAKE-UP trial (Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke), all patients underwent MRI at screening, and to be randomized, a visible acute ischemic lesion had to be present on DWI.10 In this secondary post hoc analysis of the WAKE-UP trial data, we aimed at studying the efficacy and safety of intravenous thrombolysis among patients with lacunar infarcts diagnosed on prerandomization MRI.
Methods
Study Design
In this exploratory, secondary post hoc analysis, we reviewed imaging and clinical data of all patients randomized in the WAKE-UP trial to identify participants in whom DWI confirmed an acute lacunar infarct. The WAKE-UP trial was a multicenter, double-blind, placebo-controlled randomized clinical trial designed to study the efficacy and safety of intravenous thrombolysis with alteplase, guided by MRI, in patients with an acute stroke of unknown onset time. Inclusion criteria contained the mismatch between an acute ischemic lesion visible on DWI with no corresponding marked parenchymal hyperintensity on fluid-attenuated inversion recovery (FLAIR) as a surrogate marker of lesion age, indicating that the stroke onset is most likely 4.5 hours or less from imaging.11 Patients or their legal representatives provided written informed consent according to national and local regulations. There was an exception from explicit informed consent in emergency circumstances in some countries. For each study site, the competent authorities and the corresponding ethics committee approved the trial. The detailed trial protocol has been published together with its main results (Supplement).10 In this analysis, we examined demographic characteristics, medical history, and clinical and imaging data at baseline and follow-up, including a final follow-up assessment 90 days after stroke.
Definition of Lacunar Infarct
Acute lacunar infarcts were defined according to the neuroimaging standards established in the Standards for Reporting Vascular Changes on Neuroimaging position article as subcortical lesions in the territory of penetrating arteries, with a rounded, ovoid, or tubular shape and a maximum diameter less than 20 mm on the axial plane.7 Acute lacunar infarcts were detected on MRI before randomization, where they presented as a hyperintense signal relative to white or deep gray matter on DWI, with reduced apparent diffusion coefficient in the absence of vessel occlusion in the corresponding brain region. Follow-up MRI performed 22 to 36 hours after treatment served for verification of the correct diagnosis. Diagnosis of an acute lacunar infarct was made by consensus of 2 independent investigators (E.B. and G.T.) who were blinded to clinical information.
Outcome Measures and End Points
Clinical outcome was assessed at 90 days after stroke. Evaluation of efficacy outcomes followed the clinical end points as defined in the WAKE-UP trial. The primary end point was favorable outcome defined as a score of 0 or 1 point on the modified Rankin Scale (mRS). Secondary end points included ordinal analysis of the mRS (so-called shift analysis), treatment response associating outcome on the mRS with stroke severity at baseline (defined as an mRS score of 0 for patients with mild deficit on admission [National Institutes of Health Stroke Scale (NIHSS) score ≤7], an mRS score of 0 or 1 for moderate deficit [NIHSS score, 8-14], and mRS score of 0 to 2 for severe deficit [NIHSS score, >14]); a global outcome score of patients who attained good outcome on 4 scales (mRS score, 0 to 1 point; NIHSS, 0 to 1 point; Barthel Index score, 95-100 points; and Glasgow Outcome Scale, 5 points); and functional health status and quality of life assessed by EuroQol–5 Dimensions at 90 days after stroke. Safety outcomes were mortality and death or dependence defined as a score of 4 to 6 points on the mRS at 90 days after stroke; the incidence of SICH according to the protocols of the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST), European Cooperative Acute Stroke Study II (ECASS II), ECASS III, and National Institute of Neurological Disease and Stroke; and parenchymal hemorrhage type 2 on follow-up imaging 22 to 36 hours after treatment.1,12,13,14,15,16
Statistical Analysis
Baseline characteristics were first compared between patients with lacunar infarcts and nonlacunar infarcts and subsequently between patients with lacunar infarcts assigned to treatment with alteplase vs placebo. Statistical analyses of treatment outcomes were performed in the intention-to-treat population for all patients with available information for clinical end points. To investigate the interaction between stroke subtype (lacunar infarct vs nonlacunar infarct) and treatment outcome on the primary end point, we performed a subgroup analysis on the stratification variable stroke subtype. An unconditional logistic regression model was fitted, associating the log odds of the primary outcome with the covariate of interest, its interaction with the treatment group, and the treatment group itself, adjusted for the baseline stratification parameters age and NIHSS score. The interaction term was tested with the Wald χ2 test, estimating the treatment outcome (odds ratio) and its 95% CI for each category of the categorical variable.
For further analyses of treatment outcome of alteplase in patients with lacunar infarcts, we repeated the analysis of primary and secondary end points as in the original trial analysis in this subset of patients. The main efficacy variable was the adjusted odds ratio for favorable outcome (mRS score, 0 or 1 point) using an unconditional logistic regression analysis, fitted to estimate the odds ratio and its 95% CI. The categorical shift in the distribution of mRS scores was analyzed by fitting a proportional-odds logistic regression model. The global outcome score was calculated using a global odds-ratio estimate (Wald-type test) from generalized estimating equations based on a linear logistic regression model. For safety end points (mortality, death or dependence, SICH and parenchymal hemorrhage type 2), we calculated an unconditional logistic regression model to estimate the odds ratio and its 95% CI associated with treatment outcome adjusted for the randomization stratification factors. All analyses were adjusted for the stratification parameters age and NIHSS score. Because all analyses were considered exploratory, all tests were carried out with a 2-sided α level of 5%, without correction for multiple comparisons. We used SAS version 9.4 (SAS Institute) for all analyses.
Results
Patient Characteristics
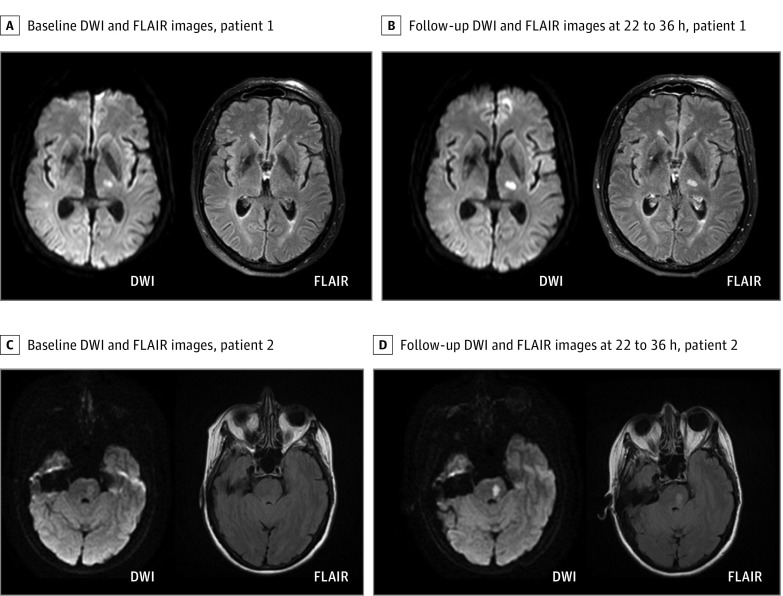
Of 503 randomized patients, 108 (21.5%) presented with a DWI lesion consistent with a lacunar infarct on baseline MRI. In 3 of these, DWI lesions consistent with a lacunar infarct at baseline MRI had completely resolved at follow-up MRI. In all other cases, follow-up MRI confirmed the diagnosis of lacunar infarct from baseline imaging. In 77 patients, lacunar infarcts were located in supratentorial deep white matter or periventricular white matter, while 31 patients presented with lacunar infarcts in the brain stem (examples in Figure 1). In 3 patients with lacunar infarcts, a vessel occlusion was identified on time-of-flight magnetic resonance angiography, but in none of these was the vessel occlusion considered to be associated with the acute lacunar infarct; in 1 patient, a most likely asymptomatic preexisting occlusion of the top of the basilar artery was observed, and in 2, a hypoplastic or previously occluded vertebral artery was observed. Atrial fibrillation was registered in 2 patients (1 patient in the placebo group with a medical history of atrial fibrillation and 1 patient in the alteplase group without previous detection of atrial fibrillation). Imaging data of these patients did not indicate an embolic cause of the detected infarcts.
Figure 1. Magnetic Resonance Imaging in Patients With Lacunar Infarcts.
Example magnetic resonance images of 2 randomized patients with a lacunar infarct in the left internal capsule (A) and brain stem (C). Before randomization for treatment (A and C), both infarcts presented with a hyperintense signal on diffusion-weighted imaging (DWI) without signal alterations in the corresponding area on fluid-attenuated inversion recovery (FLAIR). According to the mandatory DWI-FLAIR mismatch, patients were randomized and treated either with alteplase or placebo. Follow-up magnetic resonance images 22 to 36 hours after treatment (B and D) also show a hyperintense signal on FLAIR corresponding to the lesion area detected on DWI.
Of 108 patients with imaging-defined lacunar infarcts, 74 were men (68.5%). Of 395 patients with nonlacunar infarcts, 251 were men (63.5%). Patients with lacunar infarcts were younger, with a mean age (SD) of 63 (12) years compared with 66 (12) years in patients with nonlacunar stroke (P = .003). Those with lacunar infarcts were less severely affected, with a lower median NIHSS score on admission of 5 points compared with 6 points in those with nonlacunar infarcts (P < .001), and they less frequently had a history of atrial fibrillation (2 [1.9%] vs 57 [14.4%] patients; P < .001). Median DWI lesion volume was smaller for patients with lacunar infarcts (0.7 mL vs 3.8 mL; P < .001). Other baseline characteristics were comparable between patients with lacunar and nonlacunar strokes.
Of 108 patients with a lacunar infarct, 55 patients (50.9%) were assigned to receive treatment with alteplase and 53 patients (49.1%) to receive placebo. Infusion was not given or was not completed in 6 randomized patients (3 patients in the alteplase group and 3 patients in the placebo group). There was a nonsignificant difference in median NIHSS scores (5 [4-7] in the alteplase group vs 4 [3-5] in the placebo group; P = .09). All other baseline parameters did not differ between the groups (Table 1). Three patients were lost to follow-up (2 in the alteplase and 1 in the placebo group).
Table 1. Baseline Characteristics of Patients With Lacunar Infarcts.
| Variable | Patients, No. (%) | P Value | |
|---|---|---|---|
| Alteplase (n = 55) | Placebo (n = 53) | ||
| Age, mean (SD), y | 63 (10) | 62 (13) | >.99 |
| Male | 39 (71) | 35 (66) | .68 |
| Reason for unknown time of symptom onset | |||
| Night sleep | 51 (93) | 49 (93) | .57 |
| Day sleep | 3 (6) | 3 (6) | |
| Aphasia, confusion, or other | 1 (2) | 1 (2) | |
| Time between last-seen-well point and symptom recognition, median (IQR), min | 380 (270-480) | 360 (240-505) | .98 |
| Medical history or risk factors | |||
| Arterial hypertension | 27 (49) | 26 (49) | >.99 |
| Diabetes mellitus | 12 (22) | 6 (11) | .16 |
| Hypercholesterolemia | 18 (33) | 19 (36) | .92 |
| Atrial fibrillation | 1 (2) | 1 (2) | >.99 |
| History of ischemic stroke | 6 (11) | 5 (9) | >.99 |
| National Institute of Health Stroke Scale score, median (IQR) | 5 (4-7) | 4 (3-5) | .09 |
| Diffusion-weighted imaging lesion volume at baseline, median (IQR), mL | 0.8 (0.2-1.2) | 0.7 (0.2-1.1) | .45 |
| Vessel occlusion on time-of-flight magnetic resonance angiography | |||
| Intracranial internal carotid artery occlusion | 0 | 0 | NA |
| Middle cerebral artery occlusion | 0 | 0 | NA |
| Middle cerebral artery branch occlusion | 0 | 0 | NA |
| Othera | 2 (4) | 1 (2) | >.99 |
| Time from symptom recognition to magnetic resonance imaging, median (IQR), min | 165 (127-218) | 174 (138-210) | .80 |
| Time from symptom recognition to treatment initiation, median (IQR), min | 188 (158-250) | 210 (167-245) | .68 |
| Time between last-seen-well point and treatment initiation, median (IQR), min | 598 (434-695) | 590 (471-724) | .83 |
Abbreviations: IQR, interquartile range; NA, not applicable.
For example, anterior or posterior cerebral artery occlusion or vertebral or basilar artery occlusion.
Interaction Between Stroke Subtype Treatment Outcome on the Primary End Point
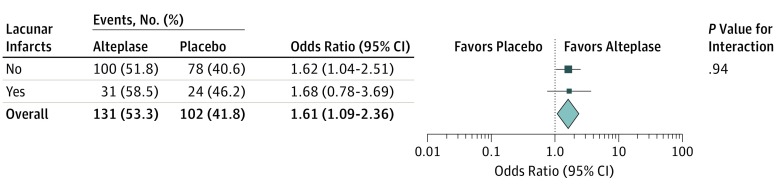
Information on the primary end point was available for 490 patients (105 with lacunar infarct and 385 with nonlacunar infarct). Among all randomized patients, treatment with alteplase was associated with higher odds of favorable outcome with no heterogeneity of treatment outcome for stroke subtypes. The adjusted OR for favorable outcome with alteplase was 1.68 (95% CI, 0.76-3.69) in patients with lacunar infarct, and the adjusted OR was 1.62 (95% CI, 1.04-2.01) in patients with nonlacunar infarct (test for interaction, P = .94; Figure 2).
Figure 2. Association of Alteplase With Favorable Outcome.
Forest plots demonstrate a treatment outcome favoring alteplase in both lacunar and nonlacunar infarcts, with no evidence of a significant interaction between stroke subtype and treatment outcome.
Efficacy Outcomes in Patients With Lacunar Infarct
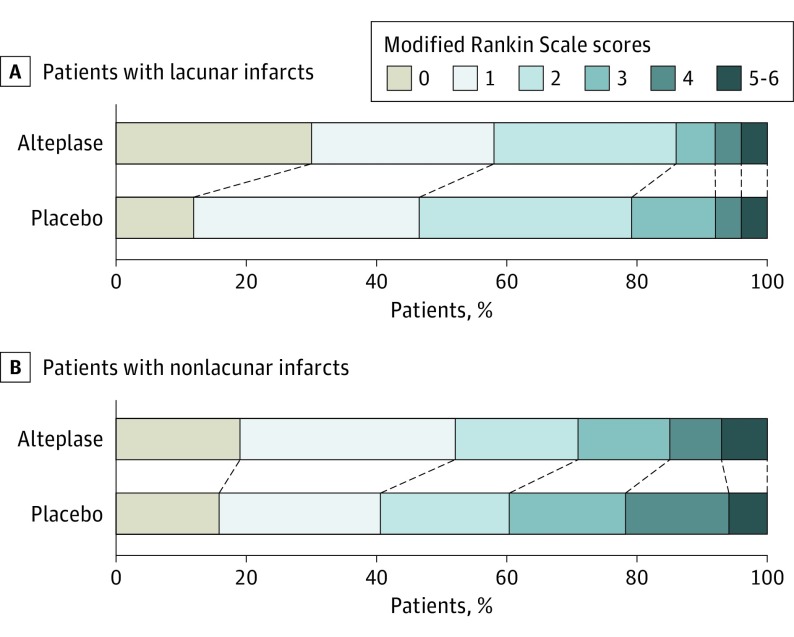
Results of the analysis of efficacy outcomes in 105 patients with lacunar stroke with available information for clinical end points are provided in Table 2. In the subgroup of patients with lacunar stroke, favorable outcome was observed in 31 of 53 patients (59%) in the alteplase group and in 24 of 52 patients (46%) in the placebo group (absolute difference, 12%; adjusted OR, 1.67 [95% CI, 0.77-3.64]; P = .20). The distribution of mRS scores at 90 days after stroke showed a nonsignificant difference in outcomes (adjusted common OR, 1.94 [95% CI, 0.95-3.93]; P = .07; Figure 3). Analysis of treatment response showed a significant benefit of treatment with alteplase with treatment response observed in 19 patients (36%) in the alteplase group compared with 7 patients (14%) in the placebo group (adjusted OR, 3.70 [95% CI, 1.38-9.87]; P = .009). There were further trends in favor of treatment with alteplase for the Global Outcome Score and quality of life measured by the EuroQol–5 Dimensions.
Table 2. Efficacy Outcome Variables.
| Assessment Variable at 90 d | Alteplase (n = 55) | Placebo (n = 53) | Effect Variablea | Adjusted Effect Variable Value (95% CI) | P Value |
|---|---|---|---|---|---|
| Modified Rankin Scale score | |||||
| 0 or 1, No. (%)b | 31 (59) | 24 (46) | Odds ratio | 1.67 (0.77-3.64) | .20 |
| Median (IQR)c | 1 (0-2) | 2 (1-2) | Common odds ratio | 1.94 (0.95-3.93) | .07 |
| Secondary end points | |||||
| Treatment response, No. (%) | 19 (36) | 7 (14) | Odds ratio | 3.70 (1.38-9.87) | .009 |
| Global outcome score | NA | NA | Odds ratio | 1.43 (0.76-2.67) | .27 |
| Beck Depression Inventory, median (IQR) | 5 (1-10) | 5 (2-9) | Odds ratio | 0.08 (−0.34 to 0.49) | .71 |
| EQ-5D sum, median (IQR) | 1 (0-2) | 1 (0-3) | Mean difference (log) | −0.36 (−1.10 to 0.37) | .33 |
| EQ-5D visual analog scale, median (IQR) | 80 (65-90) | 70 (55-88) | Mean difference | 7.04 (−1.07 to 15.14) | .09 |
| Infarct volume at 22 to 36 h, median (IQR), mL | 0.9 (0.4-1.5) | 0.9 (0.4-1.4) | Mean difference (log) | 0.04 (−0.35 to 0.44) | .84 |
Abbreviations: EQ-5D, EuroQol Group–5 dimensions; IQR, interquartile range.
All odds ratios were adjusted for the stratification factors (age and symptom severity).
Modified Rankin Scale scores of 0 or 1 represented favorable outcomes; scores were missing in 3 patients (2 in the alteplase group and 1 in the placebo group).
Categorical shift in the distribution of modified Rankin Scale scores between the 2 treatment groups.
Figure 3. Distribution of Modified Rankin Scale Scores at 90 Days After Stroke.
Distributions of scores on the modified Rankin Scale show a favoring of the alteplase group compared with the placebo group. Modified Rankin Scale scores range from 0 to 6 (0, no symptoms; 1, no clinically significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death).
Safety Outcomes in Patients With Lacunar Infarct
At 90 days, 1 patient in the alteplase group had died (2%), while no deaths had occurred in the placebo group. Death or dependence occurred in 4 patients (8%) in the alteplase group and 4 patients (8%) in the placebo group (adjusted OR, 1.06 [95% CI, 0.24-4.64]; P = .94). There was 1 case of SICH (2%) meeting criteria of SITS-MOST, ECASS II, ECASS III, and NINDS in the alteplase group, while no cases of SICH were observed in the placebo group. One patient in the alteplase group showed parenchymal hemorrhage type 2 on follow-up imaging; the same patient, in whom intracranial hemorrhage was considered symptomatic, died of SICH 4 days after stroke. No parenchymal hemorrhage type 2 occurred in the placebo group. One severe episode of anaphylaxis was observed in the placebo group.
Discussion
The WAKE-UP trial provided evidence for a clinical benefit of MRI-guided treatment with intravenous alteplase in patients with acute stroke with an unknown time of onset. In this secondary post hoc analysis, we studied the outcome of treatment in patients with lacunar infarcts, motivated by the uncertainty around the role for intravenous thrombolysis in lacunar stroke. As a main result, we found no evidence of heterogeneity of the treatment outcome with regard to stroke subtype (ie, lacunar or nonlacunar infarct). Thus, our data suggest that treatment with alteplase is of similar benefit in patients with lacunar infarcts as in those with other stroke subtypes.
A frequent argument raised against the use of thrombolysis in lacunar stroke is the widespread assumption that lacunar infarcts have a benign natural history with a quick and favorable spontaneous recovery. This assumption has been challenged by reports of a high burden of death and disability in lacunar stroke.17,18 Notably, although most patients with lacunar infarct in this trial presented with only mild to moderate symptom severity, the outcome of lacunar stroke in the trial was far from benign, with less than 50% of patients in the placebo group reaching a favorable outcome after 90 days. At the same time, intravenous alteplase resulted in an absolute increase of 12% of patients with no or minimal nondisabling neurological deficits at 90 days after stroke compared with placebo. The WAKE-UP trial randomized patients with unknown time of stroke onset, who, according to brain imaging findings, were highly likely to be within the time window for effective thrombolysis (ie, within 4.5 hours of symptom onset). There is no reason to assume that these patients differ from patients who are receiving care within 4.5 hours of known symptom onset, with regards to the biological and clinical outcomes of intravenous alteplase usage. The observed treatment benefit in the trial is not only in line with the results of the NINDS trial and the Third International Stroke Trial (IST-3), in which the treatment outcome of intravenous thrombolysis was not modified by stroke subtype judged clinically or with CT,1,2 but also extends the findings from these trials, in that we present what is, to our knowledge, the first analysis of treatment outcome of thrombolysis based on data from a randomized clinical trial with MRI-based diagnosis of stroke subtype.
Previous studies have addressed safety and outcome of intravenous thrombolysis in lacunar stroke and small vessel disease.8,9,19,20,21 Categorization of stroke subtypes was mostly based on clinical presentation of a specific lacunar syndrome and the exclusion of intracranial hemorrhage by noncontrast CT. However, misclassification of lacunar syndromes is common compared with neuroimaging findings.22 Thus, a lacunar syndrome has limited specificity for the final diagnosis of a lacunar infarct, especially in the short-term treatment setting.23
Consensus criteria for diagnosis of lacunar infarct based on MRI findings have been established.7 More recent studies have already used MRI for the diagnosis of lacunar infarcts in the context of thrombolysis.24,25,26 However, available data on thrombolysis in MRI-confirmed lacunar infarction stem from observational studies, which do not allow conclusions regarding the efficacy of thrombolysis caused by the lack of a control group. The WAKE-UP trial relied on MRI for patient enrollment and thus enabled the first (to our knowledge) systematic investigation of the efficacy and safety of thrombolysis among patients with lacunar infarcts in a randomized clinical trial, based on neuroimaging standards for categorization of this stroke subtype.
The analysis presented here was a nonprespecified subgroup analysis and has to be considered exploratory. The WAKE-UP trial was not powered to show a significant treatment outcome in subgroups of patients. The subgroup of patients with lacunar stroke made up only 21.5% of all patients randomized in the trial. It is assumed for this reason that CIs for the ORs of treatment outcome cross the null hypothesis, and the observed numerical trend for a benefit of treatment with alteplase is not significant at an α level of .05. Given this limitation, this analysis shows 2 main results: first, the treatment effect of alteplase for patients with lacunar infarction is comparable with the treatment outcome in other stroke subtypes. Second, in patients with lacunar stroke, there is a consistent pattern of better outcome with alteplase no matter which end point is considered. As found in the entire trial population, for patients with lacunar infarct, intravenous alteplase was associated with better scores on the mRS and a shift toward better outcomes across all categories of the mRS compared to placebo. A similar trend favoring treatment with alteplase was observed for the other clinical end points assessed, such as the global outcome score or health-associated quality of life assessed by the EuroQol–5 Dimensions. For treatment response, which defines the target mRS score depending on the initial symptom severity, there was a significant benefit of alteplase treatment, with an absolute increase of 22% of patients showing a treatment response.
In line with previous observations, patients with lacunar infarcts were younger, had smaller lesion volumes, and were less severely affected than patients with nonlacunar infarcts.8,20,21 Except for atrial fibrillation, which was significantly more frequent in patients with nonlacunar infarcts, other vascular risk factors did not differ significantly between groups. This is in accordance with the results of recent studies revealing a largely similar vascular risk factor profile in lacunar infarcts and other ischemic stroke subtypes, except for atrial fibrillation and carotid stenosis, which were much more common in patients with nonlacunar infarcts.17,20,27 The previously assumed excess of hypertension and diabetes among patients with lacunar infarct has already been questioned in a systematic review.28 This excess arose from classification bias and disappeared when only studies using risk factor–free classifications were taken into account.
Mortality and the rate of SICH were low in this study. A single patient in the alteplase group experienced SICH according to the SITS-MOST definition; this case took a fatal course. In this patient, significant hypertension was not adequately treated on admission and during infusion, with repeated increased systolic blood pressure values up to 250 mm Hg. This patient has to be considered a protocol violation and should not have been treated with intravenous alteplase owing to uncontrollable hypertension.10
These results should further inform the debate about thrombolysis in lacunar stroke that was started soon after the results of the NINDS trial were published, owing to a widespread uncertainty about whether patients with stroke without lysable occlusive thromboemboli should be treated with alteplase.3 It was suggested that this would apply to patients with lacunar infarcts, because these infarcts are thought to result from occlusion of penetrating arteries predominantly owing to in situ microatheroma or lipohyalinosis, neither of which may be susceptible to fibrinolysis.4 However, the pathophysiology of lacunar stroke is incompletely understood and, as noted, clinical characteristics alone are a poor guide to underlying pathophysiology. Local thrombi or emboli have been demonstrated in pathological investigations and animal studies of lacunar infarct.29,30 In an exemplary case report,31 a regional perfusion deficit that reversed after intravenous thrombolysis along with clinical improvement has been demonstrated in a patient with lacunar infarct by MRI; this outcome is analogous to MRI patterns of response to thrombolysis in large artery occlusion and supports the potential value of reperfusion treatment in lacunar stroke. The WAKE-UP study protocol did not require the evaluation of stroke causative mechanisms based on a comprehensive diagnostic work-up. Thus, diagnosis of lacunar infarcts was made with no information on the final clinical assessment of stroke causative mechanism but based on imaging findings only, following the Standards for Reporting Vascular Changes on Neuroimaging consensus criteria.7
Conclusions
The WAKE-UP trial was not powered to demonstrate the efficacy of treatment in subgroups of patients. However, these results suggest that intravenous alteplase is safe and improves functional outcome in patients with lacunar infarct with a similar outcome as in patients with other stroke subtypes.
Trial Protocol.
References
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Wardlaw JM, Lindley RI, et al. ; IST-3 collaborative group . The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352-2363. doi: 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan LR, Mohr JP, Kistler JP, Koroshetz W. Should thrombolytic therapy be the first-line treatment for acute ischemic stroke? Thrombolysis—not a panacea for ischemic stroke. N Engl J Med. 1997;337(18):1309-1310. doi: 10.1056/NEJM199710303371812 [DOI] [PubMed] [Google Scholar]
- 4.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1965;15:774-784. doi: 10.1212/WNL.15.8.774 [DOI] [PubMed] [Google Scholar]
- 5.Álvarez-Sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013;12(7):689-705. doi: 10.1016/S1474-4422(13)70055-3 [DOI] [PubMed] [Google Scholar]
- 6.Khatri P, Kleindorfer DO, Devlin T, et al. ; PRISMS Investigators . Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320(2):156-166. doi: 10.1001/jama.2018.8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Smith EE, Biessels GJ, et al. ; Standards for Reporting Vascular Changes on Neuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluri F, Hatz F, Rutgers MP, et al. Intravenous thrombolysis in patients with stroke attributable to small artery occlusion. Eur J Neurol. 2010;17(8):1054-1060. doi: 10.1111/j.1468-1331.2010.02961.x [DOI] [PubMed] [Google Scholar]
- 9.Shobha N, Fang J, Hill MD. Do lacunar strokes benefit from thrombolysis? evidence from the Registry of the Canadian Stroke Network. Int J Stroke. 2013;8(Suppl A100):45-49. doi: 10.1111/j.1747-4949.2012.00932.x [DOI] [PubMed] [Google Scholar]
- 10.Thomalla G, Simonsen CZ, Boutitie F, et al. ; WAKE-UP Investigators . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 11.Thomalla G, Cheng B, Ebinger M, et al. ; STIR and VISTA Imaging Investigators . DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10(11):978-986. doi: 10.1016/S1474-4422(11)70192-2 [DOI] [PubMed] [Google Scholar]
- 12.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 13.Larrue V, von Kummer R R, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001;32(2):438-441. doi: 10.1161/01.STR.32.2.438 [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, et al. ; The European Cooperative Acute Stroke Study (ECASS) . Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995;274(13):1017-1025. doi: 10.1001/jama.1995.03530130023023 [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Fieschi C, et al. ; Second European-Australasian Acute Stroke Study Investigators . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998;352(9136):1245-1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 17.Norrving B. Lacunar infarcts: no black holes in the brain are benign. Pract Neurol. 2008;8(4):222-228. doi: 10.1136/jnnp.2008.153601 [DOI] [PubMed] [Google Scholar]
- 18.Arboix A, Blanco-Rojas L, Martí-Vilalta JL. Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother. 2014;14(3):261-276. doi: 10.1586/14737175.2014.884926 [DOI] [PubMed] [Google Scholar]
- 19.Hsia AW, Sachdev HS, Tomlinson J, Hamilton SA, Tong DC. Efficacy of IV tissue plasminogen activator in acute stroke: does stroke subtype really matter? Neurology. 2003;61(1):71-75. doi: 10.1212/01.WNL.0000071228.56362.36 [DOI] [PubMed] [Google Scholar]
- 20.Mustanoja S, Meretoja A, Putaala J, et al. ; Helsinki Stroke Thrombolysis Registry Group . Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke. 2011;42(1):102-106. doi: 10.1161/STROKEAHA.110.597534 [DOI] [PubMed] [Google Scholar]
- 21.Fuentes B, Martínez-Sánchez P, Alonso de Leciñana M, et al. ; Madrid Stroke Network . Efficacy of intravenous thrombolysis according to stroke subtypes: the Madrid Stroke Network data. Eur J Neurol. 2012;19(12):1568-1574. doi: 10.1111/j.1468-1331.2012.03790.x [DOI] [PubMed] [Google Scholar]
- 22.Potter G, Doubal F, Jackson C, Sudlow C, Dennis M, Wardlaw J. Associations of clinical stroke misclassification (‘clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis. 2010;29(4):395-402. doi: 10.1159/000286342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toni D, Iweins F, von Kummer R, et al. Identification of lacunar infarcts before thrombolysis in the ECASS I study. Neurology. 2000;54(3):684-688. doi: 10.1212/WNL.54.3.684 [DOI] [PubMed] [Google Scholar]
- 24.Griebe M, Fischer E, Kablau M, et al. Thrombolysis in patients with lacunar stroke is safe: an observational study. J Neurol. 2014;261(2):405-411. doi: 10.1007/s00415-013-7212-8 [DOI] [PubMed] [Google Scholar]
- 25.Lahoti S, Gokhale S, Caplan L, et al. Thrombolysis in ischemic stroke without arterial occlusion at presentation. Stroke. 2014;45(9):2722-2727. doi: 10.1161/STROKEAHA.114.005757 [DOI] [PubMed] [Google Scholar]
- 26.Eggers CCJ, Bocksrucker C, Seyfang L; Austrian Stroke Unit Registry Collaborators . The efficacy of thrombolysis in lacunar stroke—evidence from the Austrian Stroke Unit Registry. Eur J Neurol. 2017;24(6):780-787. doi: 10.1111/ene.13288 [DOI] [PubMed] [Google Scholar]
- 27.Arboix A. Cardiovascular risk factors for acute stroke: risk profiles in the different subtypes of ischemic stroke. World J Clin Cases. 2015;3(5):418-429. doi: 10.12998/wjcc.v3.i5.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson C, Sudlow C. Are lacunar strokes really different? a systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005;36(4):891-901. doi: 10.1161/01.STR.0000157949.34986.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36(2):65-73. doi: 10.1001/archneur.1979.00500380035003 [DOI] [PubMed] [Google Scholar]
- 30.Futrell N. Lacunar infarction: embolism is the key. Stroke. 2004;35(7):1778-1779. doi: 10.1161/01.STR.0000131930.41057.48 [DOI] [PubMed] [Google Scholar]
- 31.Chalela JA, Ezzeddine M, Latour L, Warach S. Reversal of perfusion and diffusion abnormalities after intravenous thrombolysis for a lacunar infarction. J Neuroimaging. 2003;13(2):152-154. doi: 10.1111/j.1552-6569.2003.tb00172.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.