This systematic review and meta-analysis assesses whether HLA-C*06:02 status is associated with a differential response in patients with psoriasis after 6 months of ustekinumab therapy.
Key Points
Question
Is HLA-C*06:02 status associated with a differential response to ustekinumab therapy in patients with psoriasis?
Findings
This systematic review and meta-analysis of 8 studies and 1048 patients showed a risk difference for achievement of 75% improvement in Psoriasis Area and Severity Index (PASI75) after 6 months of ustekinumab therapy in favor of HLA-C*06:02–positive patients. Although HLA-C*06:02–positive patients had a high PASI75 response rate after 6 months, the PASI75 response rate was also high in the HLA-C*06:02–negative group.
Meaning
Based on high PASI75 response rates in both genotype groups, there appears to be no rationale for excluding patients from ustekinumab treatment based on a negative HLA-C*06:02 status.
Abstract
Importance
Previous research showed a differential response to ustekinumab therapy based on HLA-C*06:02 status in patients with psoriasis but consisted mostly of small (and sometimes inconclusive) cohort studies.
Objective
To assess whether HLA-C*06:02 status is associated with a differential response to ustekinumab therapy in patients with psoriasis through a systematic review and a meta-analysis of available data.
Data Sources
A comprehensive search was conducted using MEDLINE, Embase, the Cochrane Library, Web of Science, and gray literature sources. Databases were searched from January 1, 2000, to May 14, 2018. Search strategies included terms and synonyms for psoriasis, HLA-C, and ustekinumab. Languages were restricted to English, French, German, and Dutch.
Study Selection
Studies were included if they reported the association between HLA-C*06:02 status and 75% improvement in Psoriasis Area and Severity Index (PASI75) response to ustekinumab therapy in patients with plaque psoriasis after 6 and/or 3 months of treatment. Randomized clinical trials and observational studies were included. Screening and selection were performed independently by 2 reviewers.
Data Extraction and Synthesis
HLA-C*06:02 genotype status and PASI75 response rates were extracted by 2 reviewers. Data were pooled using random-effects models. Heterogeneity was assessed using the τ2 and I2 statistic. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines were followed.
Main Outcome and Measure
The primary outcome was the risk difference of achieving PASI75 after 6 months of ustekinumab therapy between HLA-C*06:02–positive and HLA-C*06:02–negative patients.
Results
A total of 8 studies were reviewed; 1048 patients were included for meta-analyses, and 937 patients were included for the primary analysis of PASI75 response after 6 months of treatment. Random-effects meta-analysis showed a risk difference of 0.24 (95% CI, 0.14-0.35; P < .001) in favor of HLA-C*06:02–positive patients. The median PASI75 response rate in the HLA-C*06:02–positive group was 92% (pooled, 89%; range, 62%-98%). For HLA-C*06:02–negative patients, the median response rate was 67% (pooled, 62%; range, 40%-84%). Substantial heterogeneity may have been present, with an I2 of 82%.
Conclusions and Relevance
The meta-analysis showed a differential response to ustekinumab therapy based on HLA-C*06:02 status in patients with psoriasis. Although HLA-C*06:02–positive patients had high PASI75 response rates after 6 months, the PASI75 response rate was also high in the HLA-C*06:02–negative group. There appears to be no rationale for excluding patients from ustekinumab treatment based on a negative HLA-C*06:02 status.
Introduction
Psoriasis vulgaris is a chronic, immune-mediated skin disease with a reported prevalence in the Western population varying from 1% to 9%.1,2 For patients with moderate to severe disease, treatment outcomes have vastly improved since the introduction of biologics.3 Nevertheless, treatment failures do occur.3,4 Patients may successfully switch between biologics, indicating that unresponsiveness to 1 agent does not necessarily reflect therapeutic resistance to all biologics.5 In the absence of tools to predict treatment response, finding the right biologic for the individual patient is a process of trial and error. Identifying biomarkers for treatment success or failure could prevent periods of suboptimal therapy and thereby improve quality of life in these patients.
Pharmacogenetics investigates the genetic basis for variability in drug response with respect to both drug efficacy and toxic effects. In a systematic review on pharmacogenetics of biologic treatment in psoriasis, we found that research on this topic has generated divergent and partly conflicting results, especially for tumor necrosis factor inhibitors.6 However, for ustekinumab (an interleukin [IL]–12/IL-23 inhibitor), evidence pointed to HLA-C*06:02 as a possible predictor for treatment response.6 Response rates to ustekinumab were higher in patients with a positive HLA-C*06:02 status in 4 of 5 studies identified in the review. However, the difference was statistically significant in only 2 studies.7,8,9,10,11 A lack of power in these small cohort studies prohibits sound conclusions about the association between this variant and response to ustekinumab.
The objective of this study was to assess whether HLA-C*06:02 status is associated with a differential response to ustekinumab in patients with psoriasis through a meta-analysis of currently available data. Several new studies investigating HLA-C*06:02 in association with ustekinumab treatment response have appeared in the literature during the past year. We extended and updated our previous review to include the latest research about this topic. We hypothesized that HLA-C*06:02 mediates the response to ustekinumab therapy in favor of HLA-C*06:02–positive patients.
Methods
A systematic review and meta-analysis were performed to investigate the association between HLA-C*06:02 and the response to ustekinumab therapy in patients with plaque psoriasis. This study was registered with the PROSPERO international prospective register of systematic reviews before completion. Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines were followed for study methods and reporting.12,13
Search Strategy
Relevant papers published from January 1, 2000, to July 1, 2016, were selected from a systematic review about pharmacogenetics of biologics in psoriasis, previously published by the authors.6 A comprehensive update-search was carried out in MEDLINE, Embase, the Cochrane Library, and Web of Science to cover literature from January 1, 2016, to May 14, 2018. Keywords and MeSH terms for psoriasis, HLA-C, and ustekinumab were used (eAppendix 1 in the Supplement). Search results were restricted to articles written in English, French, German, and Dutch. Reference lists of relevant articles were handsearched for additional studies of interest. In addition, we searched for gray literature using OpenGrey.eu and ClinicalTrials.gov.
Study Selection
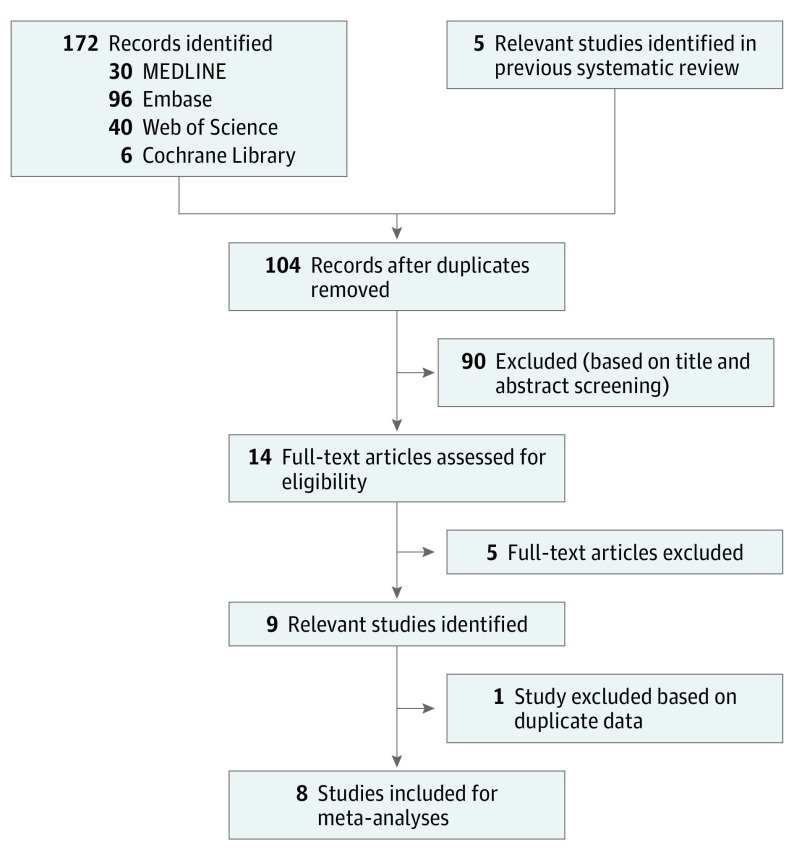
Study screening and selection was performed independently by 2 reviewers (L.J.v.V. and J.M.P.A.v.d.R.). Discrepancies were settled through consensus or resolved by a third reviewer (E.M.G.J.d.J.) when consensus could not be obtained. Studies were included if they reported on HLA-C*06:02 status and 75% improvement in Psoriasis Area and Severity Index (PASI75) response after 6 and/or 3 months in patients with plaque psoriasis treated with ustekinumab (Figure 1).
Figure 1. Study Screening and Selection Flow Diagram.
Both randomized clinical trials and observational studies were included. Case reports, case series (n <10 patients in each case series), poster abstracts, oral communications, and meeting summaries were excluded. For relevant but unpublished studies, research groups were contacted with a request to provide summary data.
Data Extraction
Data extraction was performed by 2 reviewers (L.J.v.V. and J.M.P.A.v.d.R.) independently using a predesigned form. The following data were extracted: reference details, population characteristics, HLA-C*06:02 genotyping data, and PASI75 response data. Authors were contacted if the appropriate data could not be obtained from the published articles or if there was ambiguity regarding duplicate publication of data.
Outcomes of Interest and Definitions
Response was defined as achieving PASI75. The risk difference (RD) between the 2 genotype groups (HLA-C*06:02–positive vs HLA-C*06:02–negative patients) for achievement of PASI75 after 6 months was considered as the primary outcome. A range of 24 to 28 weeks was accepted for this 6-month time point. The secondary outcome was the RD for achievement of PASI75 after 3 months; a range of 12 to 16 weeks was accepted for this time point. When a study reported 2 outcome points within a range (for example, both 12 weeks and 16 weeks), the latest available time point was selected for the analyses. Both primary and secondary outcomes were prespecified as described in the PROSPERO protocol.
The RD is the difference between the observed risks (proportions of individuals with the outcome of interest) in the 2 groups being investigated. In our study, the term risk difference may cause confusion given that occurrence of the outcome of interest (achieving PASI75) is not a negative event but rather a desired outcome. It may be more intuitive to speak of a chance for the outcome (PASI75) to occur rather than risk and, when comparing the 2 genotype groups, to read chance difference instead of RD.
We discuss some theoretical values of the RD as examples of how to interpret this metric. The RD theoretically runs from –1.0 to 1.0. In case of an RD of 0, the proportion of patients achieving PASI75 in the HLA-C*06:02–positive and HLA-C*06:02–negative group would be equal (no difference). In case of a positive value for the RD, the proportion of patients achieving PASI75 would be higher in the HLA-C*06:02–positive group compared with the HLA-C*06:02–negative group. A negative value for RD signifies the opposite: the proportion of HLA-C*06:02–negative patients achieving PASI75 is higher compared with the proportion of HLA-C*06:02–positive patients achieving this outcome. For example, an RD of 0.30 signifies that, if 100 HLA-C*06:02–positive and 100 HLA-C*06:02–negative patients were to be treated with ustekinumab, there would be 30 more patients achieving the outcome of interest (PASI75) in the HLA-C*06:02–positive group compared with the HLA-C*06:02–negative group. An RD of –0.30 would signify the opposite, with 30 more responders among HLA-C*06:02–negative compared with HLA-C*06:02–positive patients.
Statistical Analyses
Pooled RDs and 95% CIs were estimated using random-effects models. Between-study variance was quantified using the τ2 statistic, estimated using the Sidik-Jonkman estimator. Heterogeneity was assessed by visual inspection of forest plots, use of the I2 statistic and its connected χ2 test, and 95% prediction intervals (PIs) were calculated to present the expected range of true effects in similar studies.14 A P < .05 was considered statistically significant. Sensitivity analyses were performed to assess the influence of study country of origin, research group, ustekinumab dose, and study design (observational vs randomized clinical trial). Publication bias was assessed only if 10 or more studies were included in the meta-analysis. All statistical analyses were performed with R, version 3.5.1 (R Foundation for Statistical Computing) using the meta package.
Post Hoc Analyses
The clinical importance of an RD may depend on the underlying risk of an event, in this case, the risk of achieving PASI75. For example, an RD of 0.30 could signify a difference between 0% and 30% and between 70% and 100%. To facilitate clinical interpretation of the RDs found for the primary and secondary outcomes, response rates for both genotype groups were plotted per study in the same figure as the RDs (Figure 2 and Figure 3). For the month 6 and month 3 outcomes, PASI75 response rates per genotype group were pooled to give the overall response rate in both HLA-C*06:02–positive and HLA-C*06:02–negative patients. These pooled response rates give an impression of the underlying proportions of PASI75 responders in both genotype groups, on which the RD (primary and secondary outcome) is based. Pooled estimates of proportions with their corresponding 95% CIs were calculated using Freeman-Tukey double arcsine transformation within a random effects model framework. This analysis was performed post hoc and for supporting purposes only (eFigure 1 and eFigure 2 in the Supplement).
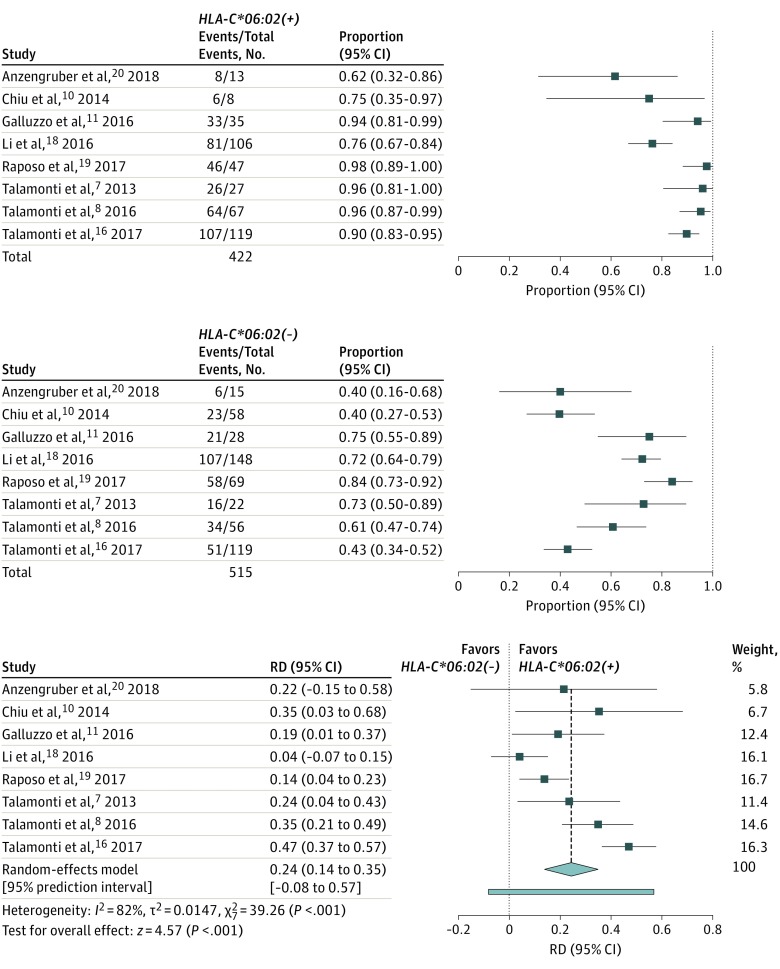
Figure 2. Risk Difference for 75% Improvement in Psoriasis Area and Severity Index (PASI75) Response to Ustekinumab at 6 Months’ Treatment According to HLA-C*06:02 Status.
Event indicates achievement of PASI75; RD, risk difference.
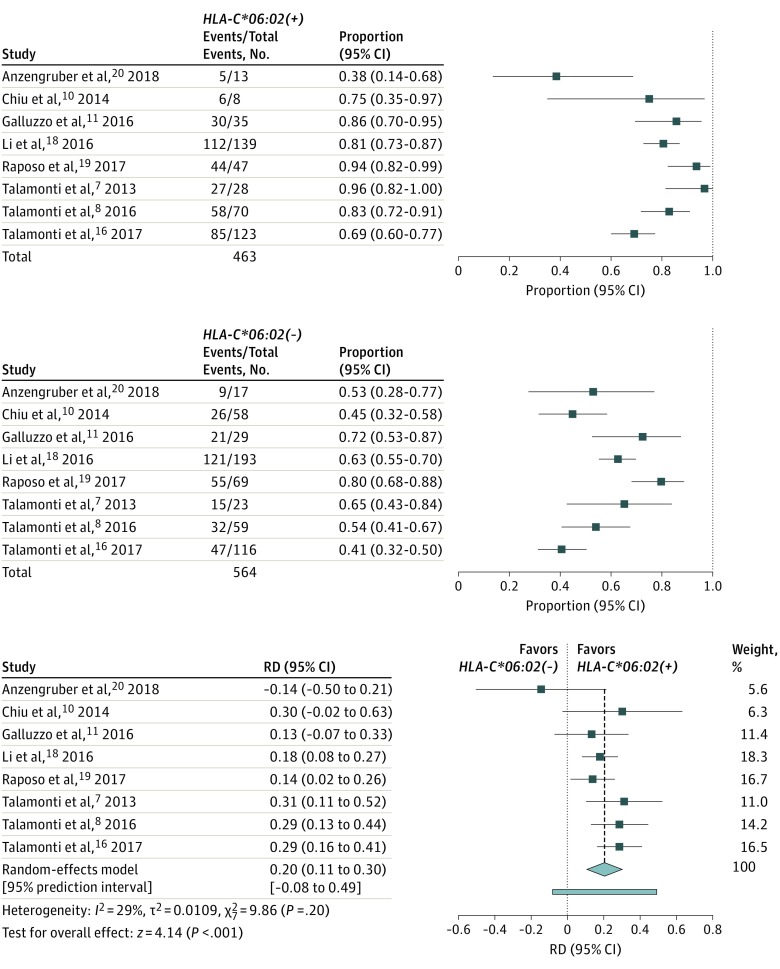
Figure 3. Risk Difference for 75% Improvement in Psoriasis Area and Severity Index (PASI75) Response to Ustekinumab at 3 Months’ Treatment According to HLA-C*06:02 Status.
Event indicates achievement of PASI75.
A post hoc analysis in which treatment response was defined as 90% improvement in PASI (PASI90) instead of PASI75 was performed using available PASI90 data across the included studies (eFigure 3 and eFigure 4 in the Supplement). Pooled RDs for achievement of PASI90 at 6 and 3 months were estimated using random-effects models.
Risk of Bias Assessment
Risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies.15 Risk of bias assessment was performed independently by 2 reviewers (L.J.v.V. and J.M.P.A.v.d.R.). Discrepancies were resolved through discussion, aided by a third reviewer (G.H.) when necessary.
Results
Literature Search and Study Selection
A total of 8 publications were reviewed consisting of 1048 individuals. Five studies about the association between HLA-C*06:02 and ustekinumab treatment were identified from the previously published systematic review about pharmacogenetics in psoriasis.7,8,9,10,11 In 1 of these 5 studies, treatment response was defined as achievement of 50% improvement in PASI rather than PASI75. After failed attempts to contact the authors for retrieval of PASI75 data, that study9 was excluded. Our update-search identified another 99 potentially relevant publications. Title, abstract, and full-text screening led to the selection of 5 additional relevant studies.16,17,18,19,20 There was overlap among patients between 2 studies,16,17 and thus, 1 study17 was excluded. The update-search together with studies identified in the previous systematic review resulted in 8 studies7,8,10,11,16,18,19,20 included in this meta-analysis (Figure 1). Handsearching reference lists of the included articles did not identify any new studies. A list of excluded articles is presented in eAppendix 2 in the Supplement.
Our gray literature search did not retrieve any unpublished studies on this topic (eAppendix 3 and eAppendix 4 in the Supplement). Poster abstracts, oral communications, and meeting summaries were also checked for publication status. We identified 1 relevant but currently unpublished study regarding data from the Psoriasis Stratification to Optimise Relevant Therapy (PSORT) consortium presented at the 47th Annual Meeting of the European Society for Dermatological Research and at Psoriasis Gene to Clinic, 8th International Congress.21,22 However, it was too early for the authors to share the raw data.
Study and Patient Characteristics
Of the 8 total studies, 7 were observational studies (daily practice cohorts)7,8,10,11,16,19,20 and 1 study included patients treated with ustekinumab in phase 3 clinical trials (PHOENIX 1, PHOENIX 2, and ACCEPT).18 The included studies reported 937 patients for the month-6 (primary) analyses and 1027 patients for the month-3 (secondary) analyses. Details about study and patient characteristics are shown in the Table.
Table. Study and Patient Characteristics.
| Source | Country | No. of Patients (Primary) [Secondary]a | Treatment Setting | Outcome Assessment by Week | HLA-C*06:02-Positive Patients, No. (%) | Age, Mean (SD), y | Male, No. (%) | Biological Naivety, No. (%) | Baseline PASI, Mean (SD) | Body Mass Index, Mean (SD)b |
|---|---|---|---|---|---|---|---|---|---|---|
| Anzengruber et al,20 2018 | Switzerland | 30 (28) | Daily practice | 16, 28c | 13 (43) | 50 (12) | 22 (73) | 20 (67) | 10 (5) | NA |
| Chiu et al,10 2014 | Taiwan | 66 | Daily practice | 16, 28 | 8 (12) | 45 (12) | 55 (83) | 28 (42) | 18 (10) | 26 (4) |
| Galluzzo et al,11 2016 | Italy | 64 (63) | Daily practice | 12, 28 | 35 (55) | 46 (12) | 46 (72) | 30 (47) | 17 (8) | NA |
| Li et al,18 2016 | United States | 332 (254) | Randomized clinical triald | 12, 24, 28 | 139 (42) | 47 (11) | 195 (59) | 226 (68) | 19 (7) | 31 (7) |
| Raposo et al,19 2017 | Portugal | 116 | Daily practice | 12, 24 | 47 (41) | 48 (14) | 66 (57) | 93 (80) | 13 (5) | NA |
| Talamonti et al,7 2013 | Italy | 51 (49) | Daily practice | 12, 28 | 28 (55) | 46 (12) | 37 (73) | 10 (20) | 16 (7) | NA |
| Talamonti et al,8 2016 | Italy | 134 (123) [129] | Daily practice | 12, 28 | 73 (54) | 44 (13), 50 (12)e | 83 (62) | 56 (42) | 16 (7), 17 (9)f | 26 (4), 27 (5)g |
| Talamonti et al,16 2017 | Belgium, Italy, and the Netherlands | 255 (238) [239] | Daily practice | 12, 28 | 127 (50) | 51 (12) | 168 (66) | 51 (20) | 17 (7) | 29 (6) |
Abbreviations: NA, not applicable; PASI, Psoriasis Area and Severity Index.
Total number of patients originally included in the study (n = 1048); for the primary outcome of risk difference at 6 months (n = 942); and for the secondary outcome of risk difference at 3 months (n = 1027).
Calculated as weight in kilograms divided by height in meters squared.
Week 28 data provided by the authors.
In the randomized clinical trials used for this study, patients were randomized to ustekinumab vs placebo (PHOENIX-1 and PHOENIX-2) or to ustekinumab vs etanercept (ACCEPT). Patients were not stratified according to genotype before randomization. Of those patients randomized to receive ustekinumab, a subset of patients was later included for pharmacogenetic study of HLA-C*06:02.
HLA-C*06:02–positive patients: 44 (13); HLA-C*06:02–negative patients: 50 (12).
HLA-C*06:02–positive patients: 16 (7); HLA-C*06:02–negative patients: 17 (9).
HLA-C*06:02–positive patients: 26 (4); HLA-C*06:02–negative patients: 27 (5).
Primary Analysis: Risk Difference for PASI75 at 6 Months
All studies provided data about the PASI75 response rates at 6 months (24-28 weeks) of ustekinumab treatment, and response rates were higher among HLA-C*06:02–positive patients in all included studies (Figure 2).7,8,10,11,16,18,19,20 Random-effects meta-analysis revealed a pooled RD of 0.24 (95% CI, 0.14-0.35; P < .001) in favor of HLA-C*06:02–positive patients. This RD signified that if 100 HLA-C*06:02–positive and 100 HLA-C*06:02–negative patients were to be treated with ustekinumab, PASI75 would be achieved in 24 more patients in the HLA-C*06:02–positive group compared with the HLA-C*06:02–negative group.
Response rates and RDs for both the HLA-C*06:02–positive and the HLA-C*06:02–negative groups per study are given in Figure 2. Response rates in the HLA-C*06:02–positive group varied from 62% to 98% (median, 92%). For HLA-C*06:02–negative patients, the response rates were more variable, ranging from 40% to 84% (median, 67%). The pooled response rates (post hoc analysis) were 89% in the HLA-C*06:02–positive group and 62% in the HLA-C*06:02–negative group (eFigure 1 in the Supplement).
Secondary Analysis: Risk Difference for PASI75 at 3 Months
All 8 studies included for the primary analysis (month 6) also reported response rates for month 3 (secondary outcome). In all but 1 study, HLA-C*06:02–positive patients showed a higher response rate at this short-term outcome (Figure 3), with a pooled RD of 0.20 (95% CI, 0.11-0.30; P < .001). Response rates for both genotype groups were plotted alongside the RDs per study (Figure 3). At month 3, response rates in the HLA-C*06:02–positive group varied from 38% to 96% (median, 82%). Among HLA-C*06:02–negative patients, the response rates ranged from 41% to 80% (median, 59%). The pooled response rates (post hoc analysis) were 81% among HLA-C*06:02–positive patients and 59% among HLA-C*06:02–negative patients (eFigure 2 in the Supplement).
Post Hoc PASI90 Analysis
As a post hoc analysis, pooled RDs for achievement of PASI90 after both 6 and 3 months’ treatment were estimated. The PASI90 data were available in 4 of 8 studies for the outcome at 6 months (eFigure 3 in the Supplement) and for 5 of 8 for the outcome at 3 months (eFigure 4 in the Supplement). Random-effects meta-analysis revealed a pooled RD of 0.23 (95% CI, 0.07-0.39; P = .005) in favor of HLA-C*06:02–positive patients after 6 months of treatment and a pooled RD of 0.16 (95% CI, 0.03-0.30; P = .02) in favor of HLA-C*06:02–positive patients after 3 months of treatment.
Heterogeneity
We found an I2 of 82% (P < .001) for the RD at 6 months and an I2 of 29% (P = .20) for the RD at 3 months. In addition, PIs were calculated for both outcomes (Figure 2 and Figure 3). The PI presents heterogeneity in the same metric as the original effect size measure, illustrating the range of true effects that can be expected in future settings.14 In case of heterogeneity, PIs will show a wider range of expected treatment effects. For the outcome at 6 months, the pooled RD was 0.24 (95% CI, 0.14 to 0.35; 95% PI, –0.08 to 0.57) (Figure 2). For the outcome at 3 months, the pooled RD was 0.20 (95% CI, 0.11 to 0.30; 95% PI, –0.08 to 0.49) (Figure 3).
Risk of Bias Assessment
Usually the NOS has a maximum of 9 stars. However, 1 of the questions did not attribute to risk of bias assessment for this type of pharmacogenetic study and was excluded from scoring (eTable in the Supplement). The result was a maximum of 8 stars for NOS scoring in this study. We found a 6 of 8 score for all included studies (eTable in the Supplement). Potential for bias was mostly found in comparability of groups. We did not assess funnel plots for publication bias because fewer than 10 studies were included in the meta-analysis.
Sensitivity Analyses
Sensitivity analyses did not show an influence of study country of origin (eFigure 5 in the Supplement), research group (eFigure 6 in the Supplement), ustekinumab dose (eFigure 7 in the Supplement), or study design (eFigure 8 in the Supplement). Pooled RDs for the outcome at 6 months were 0.24 (95% CI, 0.12-0.35) after including only European or North American cohorts, 0.23 (95% CI, 0.07-0.40) after excluding the same research groups, 0.24 (95% CI, 0.14-0.35) after excluding patients who used ustekinumab in a regimen different than prescribed in the drug label, and 0.28 (95% CI, 0.19-0.38) after excluding randomized clinical trials.
Discussion
We performed a systematic review and meta-analysis focusing on HLA-C*06:02 as a possible genetic factor associated with the response to ustekinumab therapy among patients with plaque psoriasis. Our meta-analysis showed a differential response to ustekinumab therapy based on HLA-C*06:02 status after both 6 and 3 months of treatment in favor of HLA-C*06:02–positive patients. Clinical relevance of the RDs found is questionable because underlying response rates were high in both groups.
We assessed the RD for achieving PASI75 in HLA-C*06:02–positive vs HLA-C*06:02–negative patients treated with ustekinumab. For the 6-month outcome, there were higher response rates in HLA-C*06:02–positive patients. For the 3-month outcome, this was the case in all but 1 study. For both outcomes, random-effects meta-analysis revealed a statistically significant gene effect, with a pooled RD of 0.24 (95% CI, 0.14-0.35) at 6 months and 0.20 (95% CI, 0.11-0.30) at 3 months. Although these differences were statistically significant, the clinical significance is debatable. Evaluation of responder proportions per genotype group after 6 months of treatment revealed response rates of approximately 90% among HLA-C*06:02–positive patients and 65% among HLA-C*06:02–negative patients (Figure 2 and eFigure 1 in the Supplement). For the 3-month outcome, response rates were approximately 80% among HLA-C*06:02–positive patients and 60% among HLA-C*06:02–negative patients (Figure 3 and eFigure 2 in the Supplement). These rates showed that, although there was a difference between the genotype groups in terms of RD, the underlying response rates for these RDs were actually high in both genotype groups. Therefore, there appears to be no rationale for excluding patients from ustekinumab therapy based on an HLA-C*06:02–negative status.
Discussion has been raised whether definitions of treatment response in psoriasis should change from PASI75 to PASI90 or even 100% improvement in PASI.23 Although for the newer classes of biologics (anti–IL-17 and anti–IL-23) PASI90 may be considered a realistic treatment goal, at least in a clinical trial setting, the benefits of achieving PASI90 vs PASI75 in terms of increased quality of life have been debated.23,24 We performed a post hoc analysis focusing on the RD of achieving PASI90 after 6 and 3 months based on available PASI90 data across the 8 studies included for the primary analyses. For both time points, a pooled RD in favor of HLA-C*06:02–positive patients was found: 0.23 (95% CI, 0.07-0.39; P = .005) at 6 months and 0.16 (95% CI, 0.03-0.30; P = .02) at 3 months. Of note, these post hoc analyses were based on a limited number of studies (4 for the primary outcome and 5 for the secondary outcome).
With regard to heterogeneity, we found an I2 of 82% for the RD at 6 months and an I2 of 29% for the RD at 3 months. An I2 of 29% might not be important, whereas an I2 of 82% may represent substantial heterogeneity.25 On the basis of these diverging findings, we hypothesized that increased variability at 6 months may occur because, at a later time point, more (nongenetic) factors (eg, environmental factors, stress, or other triggers) could have played a role in response to therapy. In contrast, the chance of other factors to occur is expected to be smaller at an earlier time point. Therefore, the differences between HLA-C*06:02–positive and HLA-C*06:02–negative patients at month 3 may more accurately reflect genetic effects without nongenetic factors creating variability.
In addition, heterogeneity may be explained by either clinical or methodological diversity between included studies. For example, differences in race/ethnicity and biologic naivety (Table) could be factors. We were unable to perform a formal sensitivity analysis on race/ethnicity of the study population because it was reported in only 3 of 8 included studies. However, sensitivity analysis for study country of origin (as a substitute for race/ethnicity) did not alter the findings for month 6. Presence of heterogeneity is also reflected by the wide PI at both 6 and 3 months. The PI represents the expected range of RDs to be found in future (study) settings. Considering the primary outcome at 6 months (Figure 2), the 95% PI ranged from –0.08 to 0.57. Although most of this interval was above zero, indicating an RD in favor of HLA-C*06:02–positive patients in most settings, the interval overlapped zero, indicating that in some settings, there may have been no difference in response between the genotype groups or even a small beneficial difference in favor of HLA-C*06:02–negative patients. For the secondary outcome at 3 months, the 95% PI also overlapped zero (Figure 3).
HLA-C*06:02 is located in the major psoriasis susceptibility locus, termed PSORS1, which lies within the major histocompatibility complex on chromosome 6.26 Major histocompatibility complex alleles have been associated with numerous autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and type I diabetes.27 Although suggestive of an autoimmune mechanism for psoriasis development, autoantigens have not been identified, and the precise role of HLA-C*06:02 in disease pathogenesis remains ill understood.28 Likewise, the suspected pharmacogenetic mechanism for involvement of HLA-C*06:02 in the response to ustekinumab is unknown.
Limitations
Our study has several limitations. Potential studies of interest could have been missed because of language restrictions. Exclusion of case reports and series, meeting summaries, and abstracts could have led to selection bias, although among the excluded abstracts, only one was identified as relevant and attempts were made to retrieve the unpublished data for this particular abstract. In any meta-analysis, there is the possibility of nonsignificant studies being left unpublished entirely, and with the small number of studies included, a funnel plot could not be used to reliably evaluate publication bias. In addition, there was a lack of details about population demographics for the included studies, barring insight into possible causes of heterogeneity. Most important, race/ethnicity of the included patients was not explicitly mentioned in most studies.
Conclusions
This meta-analysis showed a differential response to ustekinumab therapy based on HLA-C*06:02 status in patients with psoriasis. Although HLA-C*06:02–positive patients had high response rates at 6 months, response rates were also high in the HLA-C*06:02–negative group. Therefore, the findings suggest that patients should not be excluded from ustekinumab treatment based on a negative HLA-C*06:02 status. Furthermore, heterogeneity complicated generalizability and thus implementation of our findings, which may prohibit the current use of HLA-C*06:02 as a single predictor for ustekinumab treatment response in patients with psoriasis in clinical practice. Realization of personalized treatment in psoriasis care may be dependent on finding a set of biomarkers, rather than a single marker, that in combination are strongly predictive of therapeutic response.
eAppendix 1. Search Strategy
eAppendix 2. List of Excluded Articles
eAppendix 3. Gray Literature Search OpenGrey.eu
eAppendix 4. Gray Literature Search ClinicalTrials.gov
eFigure 1. Pooled Proportions for Response Rates at 6 Months for HLA-C*06:02–positive (A) and HLA-C*06:02–negative (B) Patients Treated With Ustekinumab.
eFigure 2. Pooled Proportions for Response Rates at 3 Months for HLA-C*06:02 positive (A) and HLA-C*06:02–negative (B) Patients Treated With Ustekinumab
eFigure 3. Risk Difference for Response to Ustekinumab (PASI90) at 6 Months’ Treatment According to HLA-C*06:02 Status
eFigure 4. Risk Difference for Response to Ustekinumab (PASI90) at 3 Months’ Treatment According to HLA-C*06:02 Status
eFigure 5. Sensitivity Analysis Based on Exclusion of Non-European/Non-North American Cohorts
eFigure 6. Sensitivity Analysis Based on Exclusion of Same Research Groups
eFigure 7. Sensitivity Analysis Based on Exclusion of Non-according-to-label Dosing
eFigure 8. Sensitivity Analysis Based on Exclusion of Randomized Clinical Trials
eTable. Risk of Bias Assessment
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385. doi: 10.1038/jid.2012.339 [DOI] [PubMed] [Google Scholar]
- 2.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227-255. doi: 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170(2):274-303. doi: 10.1111/bjd.12663 [DOI] [PubMed] [Google Scholar]
- 4.Zweegers J, Otero ME, van den Reek JM, et al. Effectiveness of biologic and conventional systemic therapies in adults with chronic plaque psoriasis in daily practice: a systematic review. Acta Derm Venereol. 2016;96(4):453-458. doi: 10.2340/00015555-2276 [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Chen Z, Gong Y, Shi Y. A review of switching biologic agents in the treatment of moderate-to-severe plaque psoriasis. Clin Drug Investig. 2018;38(3):191-199. doi: 10.1007/s40261-017-0603-3 [DOI] [PubMed] [Google Scholar]
- 6.van Vugt LJ, van den Reek JMPA, Coenen MJH, de Jong EMGJ. A systematic review of pharmacogenetic studies on the response to biologics in patients with psoriasis. Br J Dermatol. 2018;178 (1):86-94. doi: 10.1111/bjd.15753 [DOI] [PubMed] [Google Scholar]
- 7.Talamonti M, Botti E, Galluzzo M, et al. Pharmacogenetics of psoriasis: HLA-Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br J Dermatol. 2013;169(2):458-463. doi: 10.1111/bjd.12331 [DOI] [PubMed] [Google Scholar]
- 8.Talamonti M, Galluzzo M, Chimenti S, Costanzo A. HLA-C*06 and response to ustekinumab in Caucasian patients with psoriasis: outcome and long-term follow-up. J Am Acad Dermatol. 2016;74(2):374-375. doi: 10.1016/j.jaad.2015.08.055 [DOI] [PubMed] [Google Scholar]
- 9.Chiu HY, Huang PY, Jee SH, et al. HLA polymorphism among Chinese patients with chronic plaque psoriasis: subgroup analysis. Br J Dermatol. 2012;166(2):288-297. doi: 10.1111/j.1365-2133.2011.10688.x [DOI] [PubMed] [Google Scholar]
- 10.Chiu HY, Wang TS, Chan CC, Cheng YP, Lin SJ, Tsai TF. Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. Br J Dermatol. 2014;171(5):1181-1188. doi: 10.1111/bjd.13056 [DOI] [PubMed] [Google Scholar]
- 11.Galluzzo M, Boca AN, Botti E, et al. IL12B (p40) gene polymorphisms contribute to ustekinumab response prediction in psoriasis. Dermatology. 2016;232(2):230-236. doi: 10.1159/000441719 [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 19, 2018.
- 16.Talamonti M, Galluzzo M, van den Reek JM, et al. Role of the HLA-C*06 allele in clinical response to ustekinumab: evidence from real life in a large cohort of European patients. Br J Dermatol. 2017;177(2):489-496. doi: 10.1111/bjd.15387 [DOI] [PubMed] [Google Scholar]
- 17.van den Reek JMPA, Coenen MJH, van de L'Isle Arias M, et al. Polymorphisms in CD84, IL12B and TNFAIP3 are associated with response to biologics in patients with psoriasis. Br J Dermatol. 2017;176(5):1288-1296. doi: 10.1111/bjd.15005 [DOI] [PubMed] [Google Scholar]
- 18.Li K, Huang CC, Randazzo B, et al. HLA-C*06:02 allele and response to IL-12/23 inhibition: results from the ustekinumab phase 3 psoriasis program. J Invest Dermatol. 2016;136(12):2364-2371. doi: 10.1016/j.jid.2016.06.631 [DOI] [PubMed] [Google Scholar]
- 19.Raposo I, Carvalho C, Bettencourt A, et al. Psoriasis pharmacogenetics: HLA-Cw*0602 as a marker of therapeutic response to ustekinumab. Eur J Dermatol. 2017;27(5):528-530. [DOI] [PubMed] [Google Scholar]
- 20.Anzengruber F, Ghosh A, Maul JT, Drach M, Navarini AA. Limited clinical utility of HLA-Cw6 genotyping for outcome prediction in psoriasis patients under ustekinumab therapy: a monocentric, retrospective analysis. Psoriasis (Auckl). 2018;8:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dand N. Genetic variation contributes to response to biologics: initial findings of the Psoriasis Stratification to Optimise Relevant Therapy (PSORT) consortium. Br J Dermatol. 2017;177(5):e237. [Google Scholar]
- 22.Dand N; PSORT Consortium. Psoriasis Stratification to Optimise Relevant Therapy (PSORT): genome-wide study reveals genetic drivers of response to biologic therapy in psoriasis. J Invest Dermatol. 2017;137(10)(suppl 2):S225. doi: 10.1016/j.jid.2017.07.185 [DOI] [Google Scholar]
- 23.Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645-648. doi: 10.1111/jdv.12817 [DOI] [PubMed] [Google Scholar]
- 24.Abrouk M, Nakamura M, Zhu TH, Farahnik B, Koo J, Bhutani T. The impact of PASI 75 and PASI 90 on quality of life in moderate to severe psoriasis patients. J Dermatolog Treat. 2017;28(6):488-491. doi: 10.1080/09546634.2016.1278198 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. http://www.handbook-5-1.cochrane.org. Accessed on December 12, 2016.
- 26.Capon F. The genetic basis of psoriasis. Int J Mol Sci. 2017;18(12):E2526. doi: 10.3390/ijms18122526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122. doi: 10.1146/annurev.genom.6.080604.162324 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178 (4):854-862. doi: 10.1111/bjd.16083 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eAppendix 2. List of Excluded Articles
eAppendix 3. Gray Literature Search OpenGrey.eu
eAppendix 4. Gray Literature Search ClinicalTrials.gov
eFigure 1. Pooled Proportions for Response Rates at 6 Months for HLA-C*06:02–positive (A) and HLA-C*06:02–negative (B) Patients Treated With Ustekinumab.
eFigure 2. Pooled Proportions for Response Rates at 3 Months for HLA-C*06:02 positive (A) and HLA-C*06:02–negative (B) Patients Treated With Ustekinumab
eFigure 3. Risk Difference for Response to Ustekinumab (PASI90) at 6 Months’ Treatment According to HLA-C*06:02 Status
eFigure 4. Risk Difference for Response to Ustekinumab (PASI90) at 3 Months’ Treatment According to HLA-C*06:02 Status
eFigure 5. Sensitivity Analysis Based on Exclusion of Non-European/Non-North American Cohorts
eFigure 6. Sensitivity Analysis Based on Exclusion of Same Research Groups
eFigure 7. Sensitivity Analysis Based on Exclusion of Non-according-to-label Dosing
eFigure 8. Sensitivity Analysis Based on Exclusion of Randomized Clinical Trials
eTable. Risk of Bias Assessment