This randomized clinical trial compares the effect of an intervention (Supporting Asthma Self-Management Behaviors in Older Adults, SAMBA) for older adults with asthma vs usual care.
Key Points
Question
Does a needs-tailored intervention improve outcomes for older adults with asthma?
Findings
In this randomized clinical trial that included 391 adults, intervention patients had significantly better asthma control, quality of life, medication adherence, and inhaler technique than control patients. The proportion of intervention patients with an emergency department visit for asthma was 6% vs 12% in the control group, a significant difference.
Meaning
Older adults with asthma can benefit from tailoring self-management support to the range of psychosocial, health, function, and cognitive barriers they have to asthma control.
Abstract
Importance
Older adults with asthma have worse control and outcomes than younger adults. Interventions to address suboptimal self-management among older adults with asthma are typically not tailored to the specific needs of the patient.
Objective
To test the effect of a comprehensive, patient-tailored asthma self-management support intervention for older adults on clinical and self-management outcomes.
Design, Setting, and Participants
Three-arm randomized clinical trial conducted between February 2014 and December 2017 at primary care practices and personal residences in New York City. Adults 60 years and older with persistent, uncontrolled asthma were identified from electronic medical records at an academic medical center and a federally qualified health center. Of 1349 patients assessed for eligibility, 406 met eligibility criteria, consented to participate, and were randomized to 1 of 3 groups: home-based intervention, clinic-based intervention, or control (usual care). A total of 391 patients received the allocated treatment
Interventions
Screening for psychosocial, physical, cognitive, and environmental barriers to asthma control and self-management with actions to address identified barriers. The intervention was delivered in the home or primary care practices by asthma care coaches.
Main Outcomes and Measures
Primary outcomes were the Asthma Control Test, Mini Asthma Quality of Life Questionnaire, Medication Adherence Rating Scale, metered dose inhaler technique, and emergency department visits for asthma care. Primary analyses compared intervention (home or clinic based) with usual care.
Results
Of the 391 patients who received treatment, 58 (15.1%) were men, and the mean (SD) age was 67.8 (7.4) years. After accounting for baseline scores, scores on the asthma control test were better in the intervention groups vs the control group (difference-in-differences at 3 months, 1.2; 95% CI, 0.2-2.2; P = .02; 6 months, 1.0; 95% CI, 0.0-2.1; P = .049; 12 months, 0.6; 95% CI, −0.5 to 1.8; P = .28; and overall, χ2 = 13.4, with 4 degrees of freedom; P = .01). Emergency department visits were lower at 12 months for the intervention groups vs the control group (16 [6.2%] vs 17 [12.7%]; P = .03; adjusted odds ratio, 0.8; 95% CI, 0.6-0.99; P = .03). Statistically significant improvements were observed for the intervention vs control patients in quality of life (overall effect: χ2 = 10.5, with 4 degrees of freedom; P = .01), medication adherence (overall effect: χ2 = 9.5, with 4 degrees of freedom; P = .049), and inhaler technique (metered-dose inhaler technique, correctly completed steps at 12 months, median [range]: 75% [0%-100%] vs 58% [0%-100%]). No significant differences in outcomes were observed between patients receiving the intervention in home vs practice settings.
Conclusions and Relevance
An intervention directed by patients’ needs and barriers improved asthma outcomes and self-management behaviors among older adults.
Trial Registration
ClinicalTrials.gov identifier: NCT02316223
Introduction
Asthma affects 7% of Americans older than 65 years1 and causes more symptoms and hospitalizations in this age group than in younger patients with asthma.2 While experts have called for interventions specifically targeting this population,3 few relevant studies have been reported.4,5,6
Older adults are prone to poor asthma outcomes owing in part to difficulties with self-management, like daily use of inhaled corticosteroids and proper inhaler technique.7,8,9,10 These difficulties arise because of varied psychosocial, cognitive, physical, and mental health issues.11,12,13 Addressing such a broad range of barriers to self-management would be infeasible for any asthma intervention. Instead, published interventions have typically narrowed their scope, emphasizing generalized asthma education and skills training, with limited tailoring to the specific needs of the individual patient.4,5,14 Yet, a generalized asthma education approach can cognitively overload older patients, distracting them from the information they need most to improve self-care.
We created an asthma self-management support intervention for older adults that screens patients for self-management barriers and targets only the identified problems for support. We compared the effect of the intervention, Supporting Asthma Self-Management Behaviors in Older Adults (SAMBA), with usual care on self-management behaviors and outcomes. We also sought to determine whether delivery of the intervention in the home would have a greater benefit for patients who might have difficulty leaving home.
Methods
Patients and Settings
We conducted a pragmatic randomized clinical trial of SAMBA between February 1, 2014, and December 31, 2017 (Supplement 1). Adults older than 60 years with uncontrolled, moderate or severe persistent asthma were recruited from 3 adult primary care practices of the Mount Sinai Health System and 6 practices of the Institute for Family Health, a federally qualified health center, all in New York City. We excluded patients with other chronic pulmonary diseases or a smoking history greater than 15 pack-years, who required assistance with medication administration, or who did not speak English or Spanish. The study protocol was approved by the institutional review boards of all participating institutions.
Recruitment, Randomization, and Data Collection
Patients were identified by electronic health record query and screened by telephone for eligibility. Verbal informed consent was obtained in person, in English or Spanish, followed by baseline interview then randomization. Analysts and study investigators were blinded to participant assignment.
Patients were randomly assigned to usual care (control), the SAMBA intervention delivered at home, or SAMBA delivered in the primary care clinic. Randomization was in blocks of 6, stratified by site and asthma severity (severe vs moderate). Follow-up interviews were conducted by telephone at 3 and 6 months and in person at 12 months.
Intervention
The SAMBA intervention has 3 core elements: (1) screening to identify barriers to asthma self-management and control, (2) targeted actions to address the barriers, and (3) reinforcement over time.15 The central feature of SAMBA is comprehensive screening for barriers to asthma control and self-management from among 21 domains of psychosocial, cognitive, physical and mental health, and environmental barriers. These barriers were identified by review of existing research, application of theory,16,17 qualitative research done in preparation for the trial,18 and input from the project’s diverse stakeholder group, including patients.15 Specific actions are then taken to address the identified barriers. During follow-up encounters, the coaches assess asthma control, medication adherence, and inhaler technique, and check on progress with goals. An example of how the intervention works is provided in the Box, and additional examples and all intervention materials are available without charge online at SAMBAforAsthma.com.
Box. Example of the SAMBA Intervention.
Screening
During the initial encounter, the Asthma Care Coach (ACC) administers the screening assessment and identifies the following barriers to asthma control: poor inhaler technique, intermittent use of fluticasone propionate, and evidence of cockroach infestation in the home. Reasons for intermittent medication use identified through screening are patient’s belief that asthma is an intermittent (vs chronic) disease and the cost of the medication.
Targeted Actions
Following screening during the initial and subsequent encounters, the ACC trains the patient on proper inhaler technique, provides brief asthma education focused exclusively on the chronic nature of asthma and the different roles and use of controller (eg, fluticasone) and rescue (albuterol) medications, advises the patient to discuss cost problems with her physician, and makes a referral for the New York City–sponsored pest remediation service available for residents of low-income households. The ACC faxes a summary of identified issues and actions to the patient’s primary care physician.
Reinforcement
During subsequent encounters over 12 months, the ACC observes the patient’s inhaler technique and corrects it if needed, reassesses medication adherence and the underlying contributing factors, and addresses new problems as they arise.
The intervention was delivered by asthma care coaches (ACCs) who were high school– or college-educated employees of the organizations partnering on the study. The ACCs underwent a 2-day, classroom-based training addressing basic asthma pathophysiology and self-management support, motivational interviewing, communication strategies with low-literacy patients, and the SAMBA protocols. Demonstration of competency in delivering the intervention was required before the ACCs were allowed to work independently. All ACCs met monthly to review cases and hear lectures to support new skills and knowledge development.
The SAMBA design embraced the teach-to-goal concept,19 focusing only on topics the patient and ACC agreed required attention and reinforcement. The ACC and patient worked collaboratively, in English or Spanish, to set goals and priorities,20 and ACCs used the teach-back technique to ensure understanding.21 The ACCs reinforced targeted learning by referring patients as needed to a low-literacy asthma educational booklet.22 Intervention duration was 12 months. The ACC recommended a schedule of in-person and telephone encounters but allowed for flexibility.
Primary Outcomes Measures
Asthma control and quality of life were measured with the Asthma Control Test (ACT, range 5-25)23 and the Mini Asthma Quality of Life Questionnaire (mAQLQ, range 1-7).24 Adherence to asthma controller medications (inhaled corticosteroids or leukotriene inhibitors) was measured by self-report using the Medication Adherence Rating Scale (MARS, range 0-5).25 Data on these measures were collected at baseline, and 3, 6, and 12 months. Technique with metered-dose inhalers (MDI) was assessed on placebo devices using a validated checklist at baseline and 12 months.26 Dry-powdered inhaler technique was assessed but not included in outcomes analysis because of low rates of use (n = 108). Hospital and emergency department visit data for the 12 months prior to and following study enrollment were obtained from the New York State Department of Health Statewide Planning and Research Cooperative System.27 Events were attributed to asthma exacerbation if asthma was listed as the first diagnosis.
Secondary outcomes included use of an asthma action plan or peak flow meter, and exposures to or actions taken to avoid common asthma triggers.
Additional Measures
Baseline assessment included identification of cognitive impairment, defined as age and education adjusted score less than 21 on the Montreal Cognitive Assessment (range, 0-30),28 severe physical impairment, defined as 20 points below the standard deviation of normal on the Patient-Reported Outcome Measurement Information System Short Form version 1.0—Physical Function 4a (standardized score range, 22.9-56.9),29,30 and moderate to severe depression, defined as a score of at least 65.8 on the 8-item Patient-Reported Outcomes Measurement Information System short form measure (standardized score range, 38.2-81.3).29,31
Data Analysis
We conducted intention-to-treat analyses of the combined SAMBA intervention (home or clinic based) vs usual care. In subgroup analyses, we compared outcomes between home-based and clinic-based arms.
ACT, mAQLQ, MARS, and inhaler technique scores were examined as continuous repeated measures using random and fixed effects hierarchical linear modeling, with study visit as a longitudinal level 1 factor and group (intervention or control) as a level 2 factor. For all models, preliminary analyses suggested the inclusion of age, education level, and marital status as time-invariant covariates as they were predictive of outcomes. Clinical site was included as a fixed effect. Nested models, with and without inclusion of treatment group effects, were fitted and compared using χ2 analyses; covariate-adjusted means for each group and time point were estimated from the model. We report changes in mean scores between treatment arms as difference-in-differences. The study was not powered for formal assessment of noninferiority.
Because hospitalization rates were low, we only modeled emergency department visits. The outcome of having at least 1 asthma-related emergency department event was analyzed using a generalized linear model with a logit link function, adjusting for age, education level, marital status, and clinical site.
Effect sizes were calculated using estimates of variance components from the hierarchical linear modeling. We calculated the absolute risk reduction in poor asthma control and quality of life between intervention and control arms based on the minimal clinically important differences for these constructs (ACT, 3 points; AQLQ, 0.5 points).32,33 We then calculated the number needed to treat to achieve the minimal clinically important differences for one patient
We conducted heterogeneity of treatment effects analyses to test the hypotheses that patients with depression or functional or cognitive impairment would benefit more from the home-based vs clinic-based intervention. Following the modeling strategy for home vs clinic analyses, we included interaction terms of treatment arm by impairment and set significance for the interaction terms at P < .05.
To handle missing data, we employed full information maximum likelihood estimation34 and tested the sensitivity of the maximum likelihood estimation by repeating the main analyses of ACT and mAQLQ scores using multiple imputation (10 imputations per analysis).35 We used the Chained Equation approach36 to Multiple Imputation using Stata, version 15.1, to assemble the imputed data sets. Age, education, and marital status were included in the multiple imputation modeling to generate imputed values. The imputation process revealed very low standard error estimates (0.17 and 0.05) for ACT and mAQLQ, respectively, suggesting stable imputation results.
Study enrollment was planned for 150 individuals per arm (total, n = 450) based on differences between intervention and control of 0.7 for the Asthma Control Questionnaire (ACQ) and 0.5 for the mAQLQ, with 80% power.37,38 We replaced the ACQ with the ACT prior to study implementation. Sample size was adjusted for patient clustering by physicians39 and an all-cause dropout rate of 15%.40 Hierarchical linear modeling was performed using SPSS statistical software release 25.0 (IBM). All other analyses were conducted used SAS, version 9.4 (SAS Institute Inc).
Results
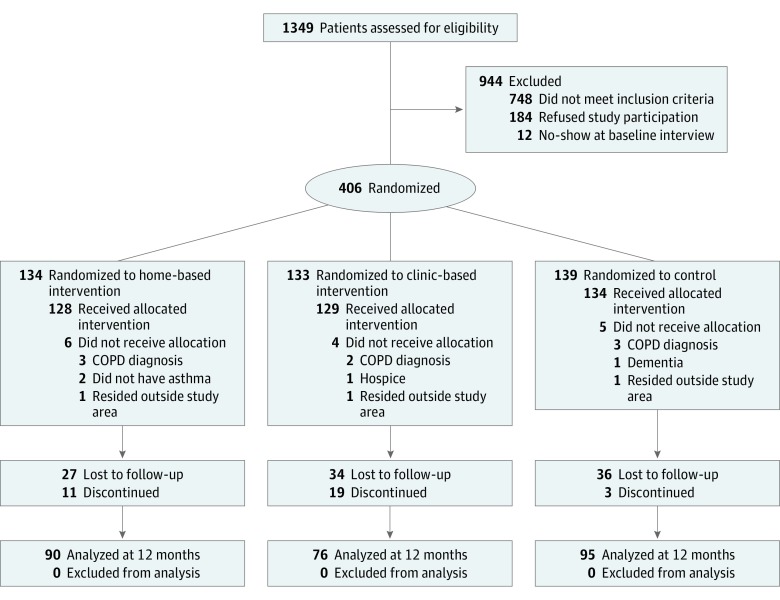
Of 1349 patients assessed for eligibility, 406 met eligibility criteria, consented to participate, and were randomized to intervention or usual care, and 391 received the allocated treatment (Figure). Retention at 12 months was 90 (70.3%), 76 (58.9%), and 95 (70.9%) for the home-based intervention, clinic-based intervention, and usual care arms, respectively (P = .11). Loss to follow-up was similar across study arms, but drop-out varied significantly: home-based arm, 11 (8.6%); clinic-based arm, 19 (14.7%); and usual care, 3 (2.2%) (P = .001). The most common reasons for drop-out were lack of interest (9 [27.3%]), other health concerns (7 [21.2%]), and time commitment (6 [18.1%]).
Figure. Study Enrollment Flowchart.
COPD indicates chronic obstructive pulmonary disease.
There were no significant differences between intervention and usual care patients by demographics, comorbidities (except prevalence of hypertension), and outcomes at baseline (Table 1). The mean (SD) patient age overall was 67.8 (7.4) years; 58 (15.1%) were men; 220 (56.3%) were Hispanic; and 144 (36.8%) had limited English proficiency.
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | No. (%) | P Valuea | ||||
|---|---|---|---|---|---|---|
| Total (n = 391) | Control (n = 134) | Intervention | ||||
| All (n = 257) |
Home Based (n = 128) | Clinic Based (n = 129) | ||||
| Age, mean (SD) | 67.8 (7.4) | 68.4 (8.1) | 67.3 (7.0) | 68.1 (7.4) | 66.5 (6.4) | .18 |
| Age, y | .10 | |||||
| 60-69 | 270 (69.1) | 88 (65.7) | 182 (70.8) | 84 (65.6) | 98 (76.0) | |
| 70-79 | 83 (21.2) | 27 (20.2) | 56 (21.8) | 32 (25.0) | 24 (18.6) | |
| ≥80 | 38 (9.7) | 19 (14.2) | 9 (7.4) | 12 (9.4) | 7 (5.4) | |
| Male sex | 58 (15.1) | 17 (12.7) | 41 (16.0) | 19 (14.8) | 22 (17.1) | .39 |
| Race/ethnicity | .42 | |||||
| White, non-Hispanic | 29 (7.4) | 6 (4.5) | 23 (9.0) | 12 (9.4) | 11 (8.5) | |
| Black, non-Hispanic | 119 (30.4) | 43 (32.1) | 76 (29.6) | 37 (28.9) | 39 (30.2) | |
| Hispanic | 220 (56.3) | 76 (56.7) | 144 (56.0) | 74 (57.8) | 70 (54.3) | |
| Other | 23 (5.9) | 9 (6.7) | 14 (5.5) | 5 (3.9) | 9 (7.0) | |
| Limited English proficiency | 144 (36.8) | 52 (38.8) | 92 (35.8) | 49 (38.3) | 43 (33.3) | .83 |
| Monthly household income <$1350 | 262 (67.0) | 86 (64.2) | 176 (68.5) | 87 (68.0) | 89 (69.0) | .39 |
| Married or partner | 94 (24.0) | 39 (29.1) | 55 (21.4) | 33 (25.8) | 22 (17.1) | .09 |
| Education | .09 | |||||
| <High school | 163 (41.7) | 63 (47.0) | 100 (38.9) | 50 (39.1) | 50 (38.8) | |
| High school graduate | 82 (21.0) | 27 (20.2) | 55 (21.4) | 31 (24.2) | 24 (18.6) | |
| Some college | 84 (21.5) | 31 (23.1) | 53 (20.6) | 25 (19.5) | 28 (21.7) | |
| College graduate | 62 (15.9) | 13 (9.7) | 49 (19.1) | 22 (17.2) | 27 (20.9) | |
| Physical impairment, severe | 58 (14.8) | 22 (16.4) | 36 (14.0) | 16 (12.5) | 20 (15.5) | .52 |
| Cognitive impairment | 204 (52.2) | 75 (56.0) | 129 (50.2) | 63 (49.2) | 66 (51.2) | .28 |
| Moderate-severe depression | 32 (8.4) | 10 (7.8) | 22 (8.7) | 12 (9.6) | 10 (7.9) | .76 |
| Comorbid medical conditions | ||||||
| Diabetes mellitus | 153 (39.1) | 52 (38.8) | 101 (39.3) | 44 (34.4) | 57 (44.2) | .92 |
| High cholesterol | 210 (53.7) | 74 (55.2) | 136 (52.9) | 70 (54.7) | 66 (51.2) | .66 |
| Hypertension | 283 (72.4) | 106 (79.1) | 177 (68.9) | 88 (68.8) | 89 (69.0) | .03 |
| Main outcomes | ||||||
| ACT score, mean (SD) | 14.6 (4.0) | 14.3 (4.0) | 14.8 (3.9) | 15.0 (3.8) | 14.6 (4.0) | .25 |
| mAQLQ score, mean (SD) | 4.4 (1.1) | 4.3 (1.1) | 4.4 (1.2) | 4.4 (1.2) | 4.4 (1.2) | .78 |
| Patients with ≥1 hospitalization prior 12 mo | 6 (1.5) | 3 (2.2) | 4.4 (1.2) | 0 | 3 (2.3) | .42b |
| Patients with ≥1 ED visit prior 12 mo | 40 (10.2) | 13 (9.7) | 27 (10.5) | 17 (13.3) | 10 (7.8) | .86b |
| Medication Adherence Ratings scale score, mean (SD) | 3.7 (0.7) | 3.8 (0.7) | 3.6 (0.7) | 3.6 (0.7) | 3.7 (0.6) | .11 |
| MDI technique,c % correctly completed steps, median (range) | 60 (0-100) | 64 (9-100) | 56 (0-100) | 60 (0-100) | 55 (0-100) | .10 |
Abbreviations: ACT, Asthma Control Test; ED, emergency department; mAQLQ, Mini Asthma Quality of Life Questionnaire; MDI, metered-dose inhaler.
Intervention vs control.
Fisher exact test.
n = 355.
Intervention and usual care patients had low average ACT scores (mean [SD], 14.6 [4.0]). Average mAQLQ scores were also low (mean [SD], 4.4 [1.1]), and 254 (64.9%) had poor quality of life (mAQLQ, <4.7). Hospitalization and emergency department visit rates in the 12 months leading to study enrollment were low (hospitalizations for intervention vs usual care: 3 [2.2%] vs 3 [1.2%], P = .42; emergency department visits: 29 [10.5%] vs 13 [9.7%], P = .86). The mean (SD) MARS score was 3.7 (0.7), and 341 (87.3%) participants had low medication adherence. Poor MDI and dry-powdered inhaler technique were observed for 286 (73.2%) and 238 (60.9%) participants, respectively.
Primary Outcome Analyses
Intervention vs usual care patients had overall significantly greater improvements in asthma control, asthma-related quality of life, and medication adherence (eFigure in Supplement 2). For both ACT and mAQLQ scores, differences were statistically significant at months 3 and 6, but not at 12 months (Table 2). Medication adherence was greater at all 3 time points, and the proportion of correctly performed steps for the MDI technique increased by 15% (absolute increase, 95% CI, 7%-22%; P < .001) at 12 months. Fewer intervention patients had an asthma-related emergency department visit than controls (16 [6.2%] vs 17 [12.7%], P = .03; adjusted odds ratio, 0.8; 95% CI, 0.6-0.99, P = .03). Maximum likelihood estimation and multiple imputation models showed similar results and did not change the significance level of the intervention effect for any outcome.
Table 2. Primary Outcomes: Intervention vs Control Group.
| Characteristic | Intervention | Control | Intervention Effect (Adjusted) | |||||
|---|---|---|---|---|---|---|---|---|
| Value, Mean (SD) | Δ (95% CI)a | P Valuea | Value, Mean (SD) | Δ (95% CI)a | P Valuea | DiD (95% CI) |
P Value | |
| Asthma Control Test score | ||||||||
| Baseline | 14.8 (3.9) | 14.3 (4.0) | ||||||
| 3 mo | 16.2 (4.4) | 1.4 (0.8 to 2.0) | <.001 | 14.6 (4.8) | 0.4 (−0.5 to 1.2) | .58 | 1.2 (0.2 to 2.2) | .02 |
| 6 mo | 16.2 (4.4) | 1.4 (0.8 to 2.1) | <.001 | 14.6 (4.9) | 0.4 (−0.5 to 1.2) | .37 | 1.0 (0.0 to 2.1) | .049 |
| 12 mo | 17.1 (4.7) | 2.2 (1.5 to 2.9) | <.001 | 16.1 (4.3) | 1.5 (0.5 to 2.6) | <.001 | 0.6 (−0.5 to 1.8) | .28 |
| Overall effectb | χ24 = 13.4 | .01 | ||||||
| Mini Asthma Quality of Life score | ||||||||
| Baseline | 4.3 (1.2) | 4.3 (1.1) | ||||||
| 3 mo | 4.7 (1.2) | 0.4 (0.2 to 0.5) | <.001 | 4.3 (1.3) | 0.0 (−0.2 to 0.2) | .91 | 0.4 (0.1 to 0.6) | .007 |
| 6 mo | 4.7 (1.3) | 0.3 (0.1 to 0.5) | <.001 | 4.3 (1.3) | −0.1 (−0.3 to 0.2) | .62 | 0.4 (0.1 to 0.7) | .02 |
| 12 mo | 4.8 (1.3) | 0.5 (0.3 to 0.7) | <.001 | 4.6 (1.3) | 0.3 (0.0 to 0.5) | .03 | 0.2 (−0.1 to 0.5) | .20 |
| Overall effectb | χ24 = 10.5 | .03 | ||||||
| Medication Adherence Rating scale score | ||||||||
| Baseline | 3.6 (0.7) | 3.8 (0.7) | ||||||
| 3 mo | 3.9 (0.6) | 0.3 (0.2 to 0.4) | <.001 | 3.8 (0.7) | 0.1 (0.0 to 0.3) | .50 | 0.2 (0.1 to 0.4) | .01 |
| 6 mo | 3.9 (0.7) | 0.3 (0.2 to 0.4) | <.001 | 3.8 (0.8) | 0.0 (−0.1 to 0.2) | .69 | 0.2 (0.0 to 0.4) | .02 |
| 12 mo | 3.9 (0.7) | 0.3 (0.1 to 0.4) | <.001 | 3.8 (0.7) | 0.1 (−0.1 to 0.2) | .70 | 0.3 (0.1 to 0.5) | .01 |
| Overall effectb | χ24 = 9.5 | .049 | ||||||
| MDI technique, % correctly completed steps, median (range) | ||||||||
| Baseline | 56 (0 to 100) | 64 (9 to 100) | ||||||
| 12 mo | 75 (0 to 100) | 6 (−14 to 6) | .45 | 58 (0 to 100) | −16 (−1 to −31) | .04 | 15 (7 to 22) | .004 |
| Patients with ≥1 emergency department visit, % | ||||||||
| Baseline | 10.5 | 9.7 | ||||||
| 12 mo | 6.2 | 0.9 (0.7 to 1.0)c | .02 | 12.7 | 1.1 (0.9 to 1.3)c | .33 | 0.8 (0.6 to 0.99)c | .03 |
Abbreviations: DiD, difference-in-differences; MDI, metered-dose inhaler.
Change within study arm relative to baseline.
Effect over all time points.
Odds ratio.
Effect sizes at 12 months were 0.29 (95% CI, 0.07-0.50) for asthma control, 0.20 (95% CI, −0.01 to 0.41) for quality of life, 0.17 (95% CI, −0.04 to 0.38) for medication adherence, and 0.53 (95% CI, 0.31-0.75) for MDI technique (Table 3). The number needed to treat to achieve the minimal clinically important differences in asthma control and quality of life were 9 and 5, respectively, at 6 months, and 30 and 14 at 12 months. The number needed to treat to prevent 1 emergency department visit was 15.
Table 3. Intervention Effect Sizes.
| Characteristic | Effect Size (95% CI)a | ||
|---|---|---|---|
| 3 mo | 6 mo | 12 mo | |
| Control and quality of life | |||
| Asthma Control Test | 0.47 (0.25 to 0.68) | 0.39 (0.17 to 0.60) | 0.29 (0.07 to 0.50) |
| Mini Asthma Quality of Life Questionnaire | 0.34 (0.13 to 0.55) | 0.34 (0.13 to 0.55) | 0.20 (−0.01 to 0.41) |
| Self-management | |||
| Medication Adherence Rating scale | 0.11 (−0.10 to 0.33) | 0.17 (−0.04 to 0.38) | 0.17 (−0.04 to 0.38) |
| Metered-dose inhaler technique | NA | NA | 0.53 (0.31 to 0.75) |
Abbreviation: NA, not applicable.
Cohen d effect size.
Secondary Outcomes Analyses
At 12 months, intervention patients were more likely to report having a peak flow meter than usual care patients (95 [57.2%] vs 34 [35.8%], P < .001). There were no significant differences for having an asthma action plan (56 [33.7%] vs 25 [26.3%], P = .21), using a dehumidifier or air purifier (46 [27.7%] vs 24 [25.3%], P = .67), or reporting triggers in the home (mold: 29 [17.5%] vs 23 [24.2%], P = .19; cockroaches: 54 [32.5%] vs 35 [36.8%], P = .48; pets with fur or feathers: 50 [30.1%] vs 23 [24.2%], P = .31; tobacco smoke: 15 [9.6%] vs 16 [15.8%], P = .14).
Home-Based vs Office-Based Intervention
Outcomes improved within both treatment arms for all measures, but there were no significant differences in outcomes between home-based and clinic-based interventions at 3, 6, or 12 months (eTable 1 in Supplement 2), except greater improvement in quality of life for home-based SAMBA recipients at 6 months. The power to detect a statistically significant difference between the 2 arms at month 12 was 0.13 for the ACT and 0.31 for the mAQLQ.
In heterogeneity of treatment effects analyses, there were no significant interactions of assignment to the home-based arm and physical impairment, cognitive impairment, or depression (eTable 2 in Supplement 2).
Process Measures
Screening for barriers to effective self-management was completed for 240 (93.2%) patients in the intervention (eTable 3 in Supplement 2), with rates ranging from 217 (84.6%) to 257 (100%) across sites (P = .55). The average numbers of in-person and telephone encounters were 3.3 and 4.1, respectively.
Study Completers and Noncompleters
Among those who completed the study, there were no significant differences between intervention and control patients for any baseline measure.
Noncompleters of the study were less likely to be male than completers (9 [7.0%] vs 46 [17.5%], P = .01); have higher rates of cognitive impairment (81 [62.0%] vs 127 [48.8%], P = .02), lower ACT scores (13.8 [3.8] vs 14.9 [4.0], P = .01) and mAQLQ scores (4.0 [1.0] vs 4.4 [1.2], P = .002); and were more likely to have a hospitalization for asthma in the prior 12 months (5 [4.0%] vs 2 [0.7%], P = .02).
Discussion
Compared with older adults receiving usual care, those randomized to the SAMBA asthma self-management support intervention had better asthma control and quality of life, improvements in medication adherence and MDI technique, and fewer asthma-related emergency department visits. These results demonstrate that identification of barriers to asthma self-management with targeted support is an effective method for improving proper asthma self-care, control, and quality of life among older adults.1,41
Additional testing of the SAMBA intervention is needed to firmly establish evidence for its effectiveness given differences in retention between intervention and control arms and diminished treatment effects over time. Nonetheless, this study has a number of strengths that add to its value. This is the largest randomized trial of a self-management support intervention for older adults with asthma yet reported to our knowledge, and it targets a particularly vulnerable older population with very low incomes, low education, and high rates of comorbidity. Another core difference between this and previous studies is the broad scope of barriers to self-management addressed by the intervention and its avoidance of providing information that is not tailored to patients’ needs. Previously reported interventions for older patients with asthma have narrower focus and use nontailored asthma education.5,8 For example, Baptist et al8 randomized 70 patients with asthma (mean age, 73 years) in Ann Arbor, Michigan, to telephone and group education sessions that provided general asthma education combined with patient-driven self-management planning. The intervention improved asthma control and quality of life and reduced some use of urgent services at 12 months.8 Krieger and colleagues42 tested a home-based asthma self-management support intervention in Seattle, Washington, that included adults ages 18 to 65 years (n = 366). Community health workers provided patients with standardized asthma education and assessed their asthma knowledge, challenges controlling asthma, and asthma self-management practices and triggers. The intervention improved mAQLQ scores and reduced the number of days with asthma symptoms but did not reduce need for acute care.
Direct comparison of the SAMBA trial and earlier intervention studies is limited by their different populations, measures, and design (pragmatic vs efficacy trials). Whether the SAMBA intervention’s reliance on an entirely patient-centered approach would result in a marginal benefit over models that mix general asthma education with patient-tailored self-management support must be determined empirically. However, taken together, these studies demonstrate that meaningful improvements in symptom control and quality of life can be achieved with older asthmatics through behavioral interventions, and they provide an evidence-based menu of approaches toward this end.
This study demonstrates the value of patient centeredness and care coaching in supporting older adults with asthma and for ongoing efforts to engage patients in care delivery design and personalization. It also highlights the challenges of engaging vulnerable populations in self-management support, including modest retention rates43 and reduced impact over time despite repeated encounters designed to sustain its effects. To support adoption and sustain impact, participating clinical sites and clinicians were free to deliver the intervention in the fashion that fit their workflow and practice culture and it is unclear how this strategy influenced the intervention’s effects. Future iterations of the intervention will test strategies to improve long-term retention and impact, and may include repeated barrier screening, intentional matching of ACCs with patients, and targeted skills development with ACCs to enhance their ability to engage patients and affect behaviors.
The home-based and clinic-based interventions did not have significantly different outcome effects nor did the home-based intervention favor patients with physical or cognitive impairment or depression as hypothesized. This study was not powered to test noninferiority of the 2 intervention arms. Additionally, retention in the home-based arm was slightly lower than in the clinic-based arm. For these reasons, the question of which environment is better suited for asthma self-management support remains unanswered. The home and office environments each offer unique opportunities to advance the care and self-management of patients with chronic disease and warrant more research to determine when to provide support in each setting and for whom, as well as whether a blend of home-based and office-based self-management support would result in better outcomes.
Limitations
This study has limitations. First, the final sample was 80% of the planned recruitment, reducing the study’s planned statistical power. Second, overall retention in the treatment arms was below 70%. However, analyses that address missing data do not alter any conclusions drawn from the data and there were no significant differences in baseline characteristics between intervention and control patients among those who completed the study. Third, generalizability of findings may be limited to inner city, predominantly older black and Hispanic patients with asthma, similar to those in this study. Additionally, the study included more women than men, which may reflect both higher prevalence of asthma in older women and higher rates of participation of women than men in asthma behavioral interventions.4,5,42,44 Fourth, research assistants conducting study interviews were not blinded to study arm assignment, which raises the possibility of biased documentation of self-reported outcomes. However, the lower emergency department rates among intervention patients, as determined by evaluation of administrative data, is consistent with outcomes of data collected by research assistants, suggesting that lack of blinding is an unlikely contributor to the favorable outcomes observed among intervention patients. Fifth, we observed improvements in some outcomes for patients in the control arm, suggesting that secular effects may have contributed to improvements for intervention patients as well. We are unaware of systematic efforts during the study to improve asthma outcomes in the participating practices.
Conclusions
Older adults receiving a patient-tailored self-management support intervention for asthma, whether in the home or clinic, achieved meaningful improvements in asthma control and quality of life, self-management behaviors, and reductions in emergency department visits compared with patients in usual care. The SAMBA intervention is a promising model of self-management support and disease control for older adults with asthma, and possibly other chronic diseases, though because of lower retention of intervention patients and diminished intervention effects over time, the model should undergo additional refinement and testing before it is widely adopted. This study also demonstrates the challenge of retaining vulnerable patients in behavioral interventions and the need to improve understanding of facilitators and barriers to engagement and retention if interventions directed at social determinants of health and related factors are to succeed.
Trial Protocol.
Supplementary Data Tables and Figures.
eTable 1. Subgroup Analysis of Intervention Patients: Differences in Asthma Outcomes Within and Between Home- and Clinic-based Arms
eTable 2. Subgroup Analysis of Intervention Patients: Heterogeneity of Treatment Effects for Individuals with Cognitive Impairment, Physical Impairment, or Depression
eTable 3. Screening and Follow-up Performance of the Intervention, Overall and by Study Site
eFigure. Asthma Control, Asthma Quality of Life, and Medication Adherence Over Time, Intervention vs. Control
Data Sharing Statement.
Footnotes
SAMBA indicates Supporting Asthma Self-Management Behaviors in Older Adults.
References
- 1.Moorman JE, Zahran H, Truman BI, Molla MT; Centers for Disease Control and Prevention (CDC) . Current asthma prevalence - United States, 2006-2008. MMWR Suppl. 2011;60(1)(suppl):84-86. [PubMed] [Google Scholar]
- 2.Moorman JE, Mannino DM. Increasing U.S. asthma mortality rates: who is really dying? J Asthma. 2001;38(1):65-71. doi: 10.1081/JAS-100000023 [DOI] [PubMed] [Google Scholar]
- 3.Hanania NA, King MJ, Braman SS, et al. ; Asthma in Elderly workshop participants . Asthma in the elderly: Current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128(3)(suppl):S4-S24. doi: 10.1016/j.jaci.2011.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baptist AP, Ross JA, Yang Y, Song PX, Clark NM. A randomized controlled trial of a self-regulation intervention for older adults with asthma. J Am Geriatr Soc. 2013;61(5):747-753. doi: 10.1111/jgs.12218 [DOI] [PubMed] [Google Scholar]
- 5.Goeman D, Jenkins C, Crane M, Paul E, Douglass J. Educational intervention for older people with asthma: a randomised controlled trial. Patient Educ Couns. 2013;93(3):586-595. doi: 10.1016/j.pec.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 6.Martin MA, Catrambone CD, Kee RA, et al. ; Chicago Initiative to Raise Asthma Health Equity Investigative Team . Improving asthma self-efficacy: developing and testing a pilot community-based asthma intervention for African American adults. J Allergy Clin Immunol. 2009;123(1):153-159.e3. doi: 10.1016/j.jaci.2008.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofianou A, Martynenko M, Wolf MS, et al. Asthma beliefs are associated with medication adherence in older asthmatics. J Gen Intern Med. 2013;28(1):67-73. doi: 10.1007/s11606-012-2160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baptist AP, Deol BB, Reddy RC, Nelson B, Clark NM. Age-specific factors influencing asthma management by older adults. Qual Health Res. 2010;20(1):117-124. doi: 10.1177/1049732309355288 [DOI] [PubMed] [Google Scholar]
- 9.Talreja N, Baptist AP. Effect of age on asthma control: results from the National Asthma Survey. Ann Allergy Asthma Immunol. 2011;106(1):24-29. doi: 10.1016/j.anai.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Krauskopf KA, Sofianou A, Goel MS, et al. Depressive symptoms, low adherence, and poor asthma outcomes in the elderly. J Asthma. 2013;50(3):260-266. doi: 10.3109/02770903.2012.757779 [DOI] [PubMed] [Google Scholar]
- 11.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142-151. doi: 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DW, Wolf MS, Feinglass J, Thompson JA. Health literacy, cognitive abilities, and mortality among elderly persons. J Gen Intern Med. 2008;23(6):723-726. doi: 10.1007/s11606-008-0566-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman DP, Zissimopoulos JM. High out-of-pocket health care spending by the elderly. Health Aff (Millwood). 2003;22(3):194-202. doi: 10.1377/hlthaff.22.3.194 [DOI] [PubMed] [Google Scholar]
- 14.Apter AJ, Wang X, Bogen DK, et al. Problem solving to improve adherence and asthma outcomes in urban adults with moderate or severe asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128(3):516-23.e1, 5. doi: 10.1016/j.jaci.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federman AD, Martynenko M, O’Conor R, et al. ; SAMBA Investigators; SAMBA investigators and development team . Rationale and design of a comparative effectiveness trial of home- and clinic-based self-management support coaching for older adults with asthma. Contemp Clin Trials. 2015;44:103-111. doi: 10.1016/j.cct.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 16.Leventhal H, Brissette I, Leventhal EA. The common sense model of self-regulation of health and illness In: Cameron LD, Leventhal H, eds. The self-regulation of health and illness behavior. London, UK: Taylor and Francis Books; 2003:42-65. [Google Scholar]
- 17.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208-220. [PMC free article] [PubMed] [Google Scholar]
- 18.O’Conor R, Martynenko M, Gagnon M, et al. ; Supporting Asthma Self-Management Behaviors Among Aging Adults (SAMBA) investigators . A qualitative investigation of the impact of asthma and self-management strategies among older adults. J Asthma. 2017;54(1):39-45. doi: 10.1080/02770903.2016.1193602 [DOI] [PubMed] [Google Scholar]
- 19.Baker DW, DeWalt DA, Schillinger D, et al. “Teach to goal”: theory and design principles of an intervention to improve heart failure self-management skills of patients with low health literacy. J Health Commun. 2011;16(suppl 3):73-88. doi: 10.1080/10810730.2011.604379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36. [Google Scholar]
- 21.Paasche-Orlow M. Caring for patients with limited health literacy: a 76-year-old man with multiple medical problems. JAMA. 2011;306(10):1122-1129. doi: 10.1001/jama.2011.1203 [DOI] [PubMed] [Google Scholar]
- 22.Sobel RM, Paasche-Orlow MK, Waite KR, Rittner SS, Wilson EA, Wolf MS. Asthma 1-2-3: a low literacy multimedia tool to educate African American adults about asthma. J Community Health. 2009;34(4):321-327. doi: 10.1007/s10900-009-9153-9 [DOI] [PubMed] [Google Scholar]
- 23.Jia CE, Zhang HP, Lv Y, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131(3):695-703. doi: 10.1016/j.jaci.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 24.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14(1):32-38. doi: 10.1034/j.1399-3003.1999.14a08.x [DOI] [PubMed] [Google Scholar]
- 25.Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy Asthma Immunol. 2009;103(4):325-331. doi: 10.1016/S1081-1206(10)60532-7 [DOI] [PubMed] [Google Scholar]
- 26.van Beerendonk I, Mesters I, Mudde AN, Tan TD. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. 1998;35(3):273-279. doi: 10.3109/02770909809068218 [DOI] [PubMed] [Google Scholar]
- 27.New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS). https://www.health.ny.gov/statistics/sparcs/. Accessed July 24, 2018.
- 28.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77(13):1272-1275. doi: 10.1212/WNL.0b013e318230208a [DOI] [PubMed] [Google Scholar]
- 29.Cella D, Riley W, Stone A, et al. ; PROMIS Cooperative Group . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.System P-ROMI PROMIS Physical Function Scoring Manual. 2018; http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Physical_Function_Scoring_Manual.pdf. Accessed August 14, 2018.
- 31.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. 2014;26(2):513-527. doi: 10.1037/a0035768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719-23.e1. doi: 10.1016/j.jaci.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 33.Juniper EF, Price DB, Stampone PA, Creemers JP, Mol SJ, Fireman P. Clinically important improvements in asthma-specific quality of life, but no difference in conventional clinical indexes in patients changed from conventional beclomethasone dipropionate to approximately half the dose of extrafine beclomethasone dipropionate. Chest. 2002;121(6):1824-1832. doi: 10.1378/chest.121.6.1824 [DOI] [PubMed] [Google Scholar]
- 34.Enders CK. A primer on the use of modern missing-data methods in psychosomatic medicine research. Psychosom Med. 2006;68(3):427-436. doi: 10.1097/01.psy.0000221275.75056.d8 [DOI] [PubMed] [Google Scholar]
- 35.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 36.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40-49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902-907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 38.Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee . Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616-621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 39.Friedman LM, DeMets DL, eds. Fundamentals of clinical trials. 3rd ed St. Louis, Missouri: Mosby; 1996. [Google Scholar]
- 40.Federman AD, Wolf MS, Sofianou A, et al. Asthma outcomes are poor among older adults with low health literacy. J Asthma. 2014;51(2):162-167. doi: 10.3109/02770903.2013.852202 [DOI] [PubMed] [Google Scholar]
- 41.New York State Department of Health New York State Asthma Surveillance Summary Report. https://www.health.ny.gov/statistics/ny_asthma/pdf/2013_asthma_surveillance_summary_report.pdf. Published October 2013. Accessed May 9, 2019.
- 42.Krieger J, Song L, Philby M. Community health worker home visits for adults with uncontrolled asthma: the HomeBASE Trial randomized clinical trial. JAMA Intern Med. 2015;175(1):109-117. doi: 10.1001/jamainternmed.2014.6353 [DOI] [PubMed] [Google Scholar]
- 43.Kramer CB, LeRoy L, Donahue S, et al. Enrolling African-American and Latino patients with asthma in comparative effectiveness research: Lessons learned from 8 patient-centered studies. J Allergy Clin Immunol. 2016;138(6):1600-1607. doi: 10.1016/j.jaci.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 44.Oraka E, Kim HJ, King ME, Callahan DB. Asthma prevalence among US elderly by age groups: age still matters. J Asthma. 2012;49(6):593-599. doi: 10.3109/02770903.2012.684252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Supplementary Data Tables and Figures.
eTable 1. Subgroup Analysis of Intervention Patients: Differences in Asthma Outcomes Within and Between Home- and Clinic-based Arms
eTable 2. Subgroup Analysis of Intervention Patients: Heterogeneity of Treatment Effects for Individuals with Cognitive Impairment, Physical Impairment, or Depression
eTable 3. Screening and Follow-up Performance of the Intervention, Overall and by Study Site
eFigure. Asthma Control, Asthma Quality of Life, and Medication Adherence Over Time, Intervention vs. Control
Data Sharing Statement.