Abstract
Importance
Persistent alopecia occurs in a subset of patients undergoing chemotherapy, yet the quality of life (QOL) of these patients and their response to therapy have not been described in a large patient cohort.
Objective
To characterize the clinical presentation of patients with persistent chemotherapy-induced alopecia (pCIA) or endocrine therapy–induced alopecia after chemotherapy (EIAC) and their QOL and treatment outcomes.
Design, Setting, and Participants
A retrospective multicenter cohort of 192 women with cancer treated with cytotoxic agents who received a clinical diagnosis of persistent alopecia (98 with pCIA and 94 with EIAC) between January 1, 2009, and July 31, 2017, was analyzed. All patients were from the dermatology service in 2 comprehensive cancer centers and 1 tertiary-care hospital. Data on demographics, chemotherapy regimens, severity, clinical patterns, and response to hair-growth promoting agents were assessed. Data from the Hairdex questionnaire were used to assess the QOL of patients with alopecia.
Main Outcomes and Measures
The clinical presentation, response to dermatologic therapy, and QOL of patients with pCIA were assessed and compared with those of patients with EIAC.
Results
A total of 98 women with pCIA (median age, 56.5 years [range, 18-83 years]) and 94 women with EIAC (median age, 56 years [range, 29-84 years]) were included. The most common agents associated with pCIA were taxanes for 80 patients (82%); the most common agents associated with EIAC were aromatase inhibitors for 58 patients (62%). Diffuse alopecia was predominant in patients with pCIA compared with patients with EIAC (31 of 75 [41%] vs 23 of 92 [25%]; P = .04), with greater severity (Common Terminology Criteria for Adverse Events, version 4.0, grade 2) among patients with pCIA (29 of 75 [39%] vs 12 of 92 [13%]; P < .001). A negative emotional effect was reported by both groups. After treatment with topical minoxidil or spironolactone, moderate to significant improvement was observed for 36 of 54 patients with pCIA (67%) and for 32 of 42 patients with EIAC (76%).
Conclusions and Relevance
Persistent chemotherapy-induced alopecia is frequently more severe and diffuse when compared with EIAC, and both groups of patients experienced a negative effect. A modest benefit was observed with dermatologic therapy. Additional studies are warranted to develop effective strategies for prevention and effective therapy for pCIA and EIAC.
This cohort study assesses the quality of life and treatment outcomes of patients with persistent chemotherapy-induced alopecia or endocrine therapy–induced alopecia after chemotherapy.
Key Points
Question
What is the quality of life of patients with persistent alopecia after chemotherapy, and what is their response to dermatologic therapy?
Findings
In this cohort study, patients with persistent chemotherapy-induced alopecia showed a predominantly diffuse and more severe alopecia than did patients with endocrine therapy–induced alopecia that developed after completion of chemotherapy, although a negative emotional effect was found in both groups. After treatment with topical minoxidil as a single agent or in combination with oral spironolactone, mild to moderate improvement was observed in both groups.
Meaning
It is important to consider the clinical features and the time to development of alopecia to minimize nonadherence to adjuvant therapies.
Introduction
Incomplete hair regrowth 6 months after completion of chemotherapy has been described as persistent chemotherapy-induced alopecia (pCIA)1 and has been reported for 30% of patients with breast cancer treated with taxane-based chemotherapy2 and for 14% of patients with childhood cancer treated with busulfan and thiotepa-containing regimens.3 Furthermore, up to 25% of patients with breast cancer receiving adjuvant endocrine therapy may develop endocrine therapy–induced alopecia.4
Data on pCIA have been limited, hindering the identification of risk factors and preventive and therapeutic strategies.5,6 This study assesses and compares the quality of life and treatment outcomes of patients with pCIA and patients with endocrine therapy–induced alopecia after chemotherapy (EIAC).
Methods
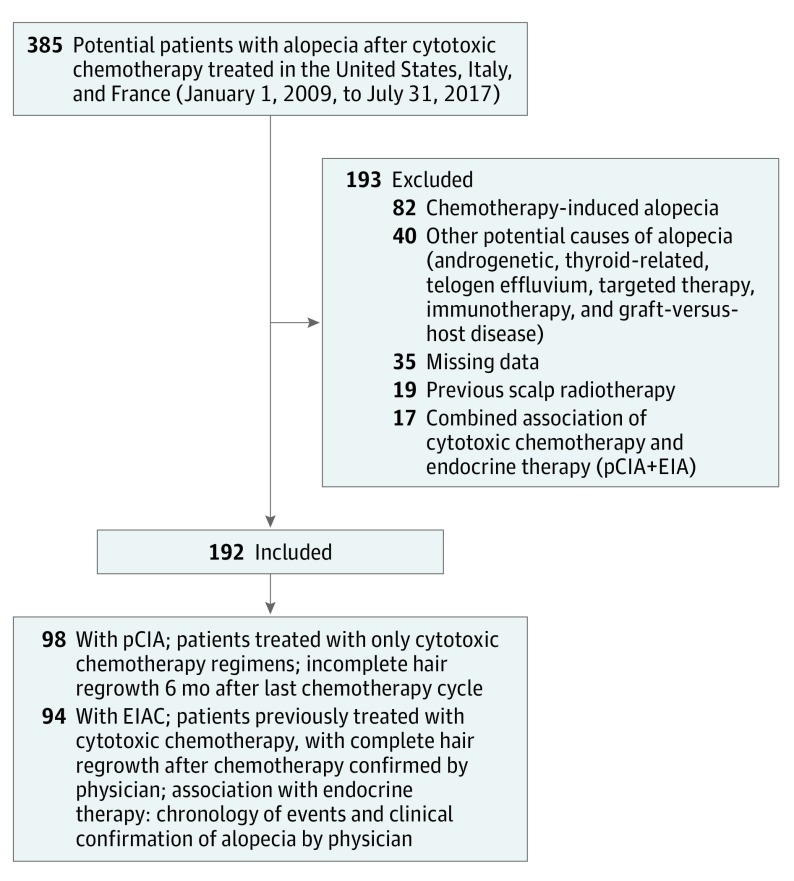
This was an 8-year retrospective, multicenter cohort study conducted between January 1, 2009, and July 31, 2017. Patients were included from 2 comprehensive cancer centers (Memorial Sloan Kettering Cancer Center, New York, New York, and Institut Universitaire du Cancer, Toulouse, France) and the dermatology service of the University Federico II, Naples, Italy. Relevant clinical data were reviewed, including history of alopecia and hair regrowth after chemotherapy. Patients were eligible if they received only systemic cytotoxic chemotherapy with a clinical diagnosis of pCIA or received chemotherapy followed by endocrine therapies but the alopecia was associated with the endocrine therapy (EIAC) (Figure). This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, Institut Universitaire du Cancer, and University Federico II. Written informed consent was obtained from each participant who answered the Hairdex questionnaire.
Figure. Flow Diagram for Patient Inclusion and Case Definition.
EIA indicates endocrine therapy–induced alopecia; EIAC, endocrine therapy–induced alopecia after chemotherapy, and pCIA, persistent chemotherapy-induced alopecia.
Alopecia Grading and Pattern
Standardized photographs of the scalp were reviewed in a blinded fashion by one of us (A.F.-M). The pattern and the severity score of the alopecia were obtained using the basic and specific classification system7 and the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0).8
Trichoscopy
Standardized trichoscopy images were obtained with the Folliscope 2.8 (LeadM Corp) on the midscalp. The total hair diameter and hair density per square centimeter were assessed.
Quality of Life
An alopecia-specific quality of life (QOL) tool (Hairdex questionnaire) was used to assess the QOL of the patients with alopecia, adapted and translated to English from the original German.9 Scores ranged from 0 to 100, with higher scores indicating a lower QOL.
Response to Therapy
Effectiveness of dermatologic therapy was measured by a single-blinded investigator (A.F.-M), through standardized photographs of the scalp obtained at baseline and at 3 to 6 months of therapy. Response to therapy was assessed using the following 4-point scale: worsening, stabilization, moderate improvement, or significant improvement.10
Statistical Analysis
Descriptive statistics were used for the clinical features and QOL. Univariate regression tests were performed for all variables, and multivariate logistic regression was performed for statistically significant variables, for patients with pCIA or EIAC. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
The demographic and baseline characteristics of 98 female patients with pCIA and 94 female patients with EIAC (Table) revealed that the most common underlying oncologic diagnosis in both groups was breast cancer (79 patients with pCIA [81%]; 89 patients with EIAC [95%]). The most common agent associated with pCIA was taxane-based chemotherapy (80 patients [82%]), whereas aromatase inhibitors were the most common agents associated with EIAC (58 patients [62%]).
Table. Baseline Characteristics of Female Patients With Cancer With Alopecia After Cytotoxic Chemotherapy.
| Baseline Characteristic | pCIA (n = 98) | EIAC (n = 94) | P Valuea |
|---|---|---|---|
| Age, median (range), y | 56.5 (18-83) | 56 (29-84) | .90 |
| Race/ethnicity, No. (%) | |||
| White | 73 (75) | 70 (75) | >.99 |
| Other | 25 (26) | 24 (26) | |
| Primary cancer diagnosis, No. (%) | |||
| Breast | 79 (81) | 89 (95) | .002 |
| Hematologic | 6 (6) | ||
| Ovarian | 2 (2) | 3 (3) | |
| Other (central nervous system, solid, genitourinary, gastrointestinal, or respiratory tract) | 11 (11) | 2 (2) | |
| Therapies, No. (%)b | |||
| Taxane-based chemotherapy | Taxanes, 80 (82) Paclitaxel, 47/80 (59) Docetaxel, 31/80 (39) Combination, 2/80 (2) |
Aromatase inhibitors, 58 (62) Letrozole, 32/58 (55) Anastrozole, 21/58 (36) Exemestane, 5/58 (9) |
NA |
| Cyclophosphamide, methotrexate, and fluorouracil | 13 (13) | SERM, 27 (29) Tamoxifen, 26/27 (96) Toremifene, 1/27 (4) |
NA |
| Other (busulfan, vincristine, carboplatin, lomustine, and doxorubicin) | 5 (5) | Other (leuprolide, fulvestrant), 9 (10) | NA |
| Time with alopecia after chemotherapy completion (pCIA) and after initiation of endocrine therapy (EIAC), median (range), y | 2.0 (0.5-27.3) | 1.0 (0.1-5.3) | |
| Clinical characteristics, No. (%) | |||
| Patients | 75 (77) | 92 (98) | .04 |
| Pattern alopecia (androgenetic alopecia-like pattern) | 44/75 (59) | 69/92 (75) | |
| Diffuse alopecia | 31/75 (41) | 23/92 (25) | |
| Alopecia severity by CTCAE v4.0, No. (%)c | |||
| Grade 1 | 46/75 (61) | 80/92 (87) | <.001 |
| Grade 2 | 29/75 (39) | 12/92 (13) | |
| Eyebrow or eyelashes alopecia, No. (%) | 28/75 (37) | 26/92 (28) | .25 |
| Trichoscopy characteristics | |||
| Patients, No. (%) | 45 (46) | 62 (66) | |
| Hair thickness, mean (SD), μm | 62 (13) | 68 (13) | .02 |
| Hair density, mean (SD), per cm2 | 104 (48) | 116 (35) | .14 |
| Hair shafts per follicular unit, mean (SD), No. | 1.3 (0.3) | 1.5 (0.3) | <.001 |
Abbreviations: CTCAE v4.0, Common Terminology Criteria for Adverse Events, version 4.0; EIAC, endocrine therapy–induced alopecia after chemotherapy; NA, not applicable; pCIA, persistent chemotherapy-induced alopecia; SERM, selective estrogen receptor modulators.
Comparisons of pCIA and EIAC were performed using t test and Fisher exact test.
Patients with EIAC had full hair regrowth after cytotoxic chemotherapy.
Grade 1 is hair loss of less than 50% of normal for that individual and is not obvious from a distance but only on close inspection; it does not require camouflage. Grade 2 is hair loss of 50% or more of normal for that individual and is readily apparent to others; camouflage is necessary if the patient desires, and it is associated with negative psychosocial effects.
Diffuse alopecia was more frequent among patients with pCIA than among patients with EIAC (31 of 75 [41%] vs 23 of 92 [25%]; P = .04), with greater severity (CTCAE v4.0 grade 2) among patients with pCIA than among patients with EIAC (29 of 75 [39%] vs 12 of 92 [13%]; P < .001). Trichoscopy revealed that patients with pCIA had fewer hair shafts per follicular unit than those with EIAC (mean [SD] number, 1.3 [0.3] vs 1.5 [0.3]; P < .001), thinner hairs when compared with those with EIAC (mean [SD], 62 [13 μm] vs 68 [13 μm]; P = .02), and a lower hair density per centimeters squared than 22 healthy female patients with androgenetic alopecia (104 vs 127; P = .04).11
Data on QOL were available for 41 of 98 patients with pCIA (42%) and for 58 of 92 patients with EIAC (63%). Multivariate analysis showed a negative emotional effect in both groups (Hairdex score: patients with pCIA, 48; and patients with EIAC, 45; P = .008), and for patients with pCIA with greater severity of alopecia (57 with grade 2 vs 40 with grade 1; P < .001) (eFigure in the Supplement).
A total of 54 patients with pCIA (55%) were treated with topical minoxidil, 5% (45 patients received only minoxidil and 9 patients received minoxidil and oral spironolactone). In the pCIA group, 36 patients (67%) showed a moderate to significant improvement, and for 18 patients (33%), the alopecia was stable or progressed. A total of 42 patients with EIAC (45%) were treated with topical minoxidil, 5% (37 patients received only minoxidil and 5 patients received minoxidil and oral spironolactone), with an overall moderate to significant improvement for 32 patients (76%). No statistically significant differences were found between the pCIA and EIAC groups for dermatologic therapy outcomes (P = .50). No differences in outcomes were found between treatment with minoxidil and treatment with spironolactone in the pCIA group or the EIAC group.
Discussion
Cytotoxic chemotherapies may induce persistent damage or even depletion of the epithelial hair follicle stem cells required for regeneration of the hair follicle.12 This damage may explain the significantly predominantly diffuse alopecia observed among patients with pCIA (31 of 75 [41%]), with a greater severity (CTCAE G2) (29 of 75 [39%]), fewer mean (SD) hair shafts per follicular unit (1.3 [0.3]), and thinner hair (65 [13] μm) compared with patients with EIAC. This study suggests that both pCIA and EIAC are clinically different. Hence, it is important to consider the clinical features and the chronology of events to minimize nonadherence to adjuvant therapies. In addition to the scalp, eyebrow and eyelash alopecia were also present among 37% of patients with pCIA, which may also have contributed to a negative psychosocial effect, as demonstrated in QOL analyses.13
Scalp cooling has been shown to prevent severe alopecia among 51% of patients with breast cancer undergoing adjuvant chemotherapy regimens14; however, robust data on prevention of pCIA have not been reported, to our knowledge. We found an overall moderate to significant clinical improvement in 36 of 54 patients with pCIA (67%) who were treated with topical minoxidil, 5%, and/or oral spironolactone. This outcome is lower than that seen among patients with EIAC who were treated with topical minoxidil, 5%, and/or oral spironolactone (76%) and lower than the improvement seen in chemotherapy-naive patients with breast cancer with endocrine therapy–induced alopecia who were treated with topical minoxidil, 5% (80%).4 Although the combination of minoxidil and spironolactone seems to be a promising therapy, further research is needed. However, spironolactone should be used with caution because of the hypothetical risk of hormonal stimulation of endocrine receptor–positive tumors, a concern that has not been substantiated.15
Multivariate analysis showed a negative emotional effect in both groups compared with other domains such as symptoms and functioning. Our results are consistent with a previous report of endocrine therapy–induced alopecia, in which patients were also more emotionally affected (42 of 100).4 The emotional distress found in this cohort study suggests that even patients with a clinically low severity grade of alopecia may experience negative emotional effects owing to their persistent alopecia.
Limitations
Larger prospective studies are needed to identify the incidence, risk factors, and severity of pCIA and EIAC compared with baseline, all of which are part of an ongoing study (NCT02530177). Referrals were predominantly for women likely because of their greater awareness of persistent postchemotherapy alopecia. However, sex was not an exclusion criterion. Controlled studies are also needed to identify more effective therapies for pCIA and EIAC.
Conclusions
The predominantly diffuse alopecia found among patients with pCIA was associated with a lower QOL. In addition, although patients with pCIA and EIAC may benefit from therapies for androgenetic alopecia, a better understanding of the pathobiology of pCIA and EIAC is critical to the development of rational therapies. These findings may contribute to effective pretherapy counseling and the development of studies to identify preventive and therapeutic methods for persistent alopecia after chemotherapy and in the adjuvant setting.
eFigure. Normalized Hairdex Score Means (n = 99)
References
- 1.Freites-Martinez A, Shapiro J, van den Hurk C, et al. . CME part 2: hair disorders in cancer survivors: persistent chemotherapy-induced alopecia, persistent radiotherapy-induced alopecia, and hair growth disorders related to endocrine therapy or cancer surgery [published online April 13, 2018]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2018.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang D, Kim I-R, Lee D-Y, et al. . Incidence of permanent chemotherapy-induced alopecia among breast cancer patients: a five-year prospective cohort study. Ann Oncol. 2017;28(suppl_10):mdx655.022. doi: 10.1093/annonc/mdx655.022 [DOI] [Google Scholar]
- 3.Kinahan KE, Sharp LK, Seidel K, et al. . Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2012;30(20):2466-2474. doi: 10.1200/JCO.2011.39.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freites-Martinez A, Shapiro J, Chan D, et al. . Endocrine therapy–induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154(6):670-675. doi: 10.1001/jamadermatol.2018.0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonia A, Cota C, Setterfield JF, Goldberg LJ, Fenton DA, Stefanato CM. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76(5):948-957. doi: 10.1016/j.jaad.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 6.Tosti A, Palamaras I, Miteva M, Misciali C. Docetaxel and permanent alopecia. J Am Acad Dermatol. 2013;68(5):e151. doi: 10.1016/j.jaad.2010.06.064 [DOI] [PubMed] [Google Scholar]
- 7.Lee WS, Ro BI, Hong SP, et al. . A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57(1):37-46. doi: 10.1016/j.jaad.2006.12.029 [DOI] [PubMed] [Google Scholar]
- 8.Chen AP, Setser A, Anadkat MJ, et al. . Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67(5):1025-1039. doi: 10.1016/j.jaad.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Fischer TW, Schmidt S, Strauss B, Elsner P. Hairdex: a tool for evaluation of disease-specific quality of life in patients with hair diseases [in German]. Hautarzt. 2001;52(3):219-227. doi: 10.1007/s001050051293 [DOI] [PubMed] [Google Scholar]
- 10.Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5(1):6-11. doi: 10.4103/0974-7753.114700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BS, Chan JY, Monselise A, McElwee K, Shapiro J. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66(1):166-167. doi: 10.1016/j.jaad.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 12.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14(2):e50-e59. doi: 10.1016/S1470-2045(12)70553-3 [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Winstanley J, Boyle F, O’Reilly A, White M, Antill YC. Madarosis: a qualitative study to assess perceptions and experience of Australian patients with early breast cancer treated with taxane-based chemotherapy. Support Care Cancer. 2018;26(2):483-489. doi: 10.1007/s00520-017-3852-z [DOI] [PubMed] [Google Scholar]
- 14.Nangia J, Wang T, Osborne C, et al. . Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317(6):596-605. doi: 10.1001/jama.2016.20939 [DOI] [PubMed] [Google Scholar]
- 15.Rozner RN, Freites-Martinez A, Shapiro J, Geer EB, Goldfarb S, Lacouture ME. Safety of 5α-reductase inhibitors and spironolactone in breast cancer patients receiving endocrine therapies [published online November 22, 2018]. Breast Cancer Res Treat. doi: 10.1007/s10549-018-4996-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Normalized Hairdex Score Means (n = 99)