Key Points
Question
What is the etiology of pneumonia among patients with alcohol use disorder, and is alcohol use disorder associated with poorer outcomes?
Findings
In this cohort study of 137 496 patients with pneumonia, the most common cause of pneumonia among patients with alcohol use disorder was Streptococcus pneumoniae; resistant gram-negative infections were rare. In comorbidity-adjusted models, alcohol use disorder was not significantly associated with inpatient mortality, but patients with alcohol use disorder undergoing alcohol withdrawal more frequently required late mechanical ventilation, vasopressors, and intensive care unit admissions and had increased lengths of stay and hospital costs.
Meaning
This study suggests that alcohol use disorder alone is not an independent risk factor for resistant infection or mortality, but alcohol withdrawal is associated with clinical deterioration and higher use of health care resources.
Abstract
Importance
Patients with alcohol use disorder (AUD) are at elevated risk of developing pneumonia, but few studies have assessed the outcomes of pneumonia in patients with AUD.
Objectives
To compare the causes, treatment, and outcomes of pneumonia in patients with and without AUD and to understand the associations of comorbid illnesses, alcohol withdrawal, and any residual effects due to alcohol itself with patient outcomes.
Design, Setting, and Participants
A retrospective cohort study was conducted of 137 496 patients 18 years or older with pneumonia who were admitted to 177 US hospitals participating in the Premier Healthcare Database from July 1, 2010, to June 30, 2015. Statistical analysis was conducted from October 27, 2017, to August 20, 2018.
Exposure
Alcohol use disorders identified from International Classification of Diseases, Ninth Revision, Clinical Modification codes.
Main Outcomes and Measures
Pneumonia cause, antibiotic treatment, inpatient mortality, clinical deterioration, length of stay, and cost. Associations of AUD with these variables were studied using generalized linear mixed models.
Results
Of 137 496 patients with community-acquired pneumonia (70 358 women and 67 138 men; mean [SD] age, 69.5 [16.2] years), 3.5% had an AUD. Patients with an AUD were younger than those without an AUD (median age, 58.0 vs 73.0 years; P < .001), more often male (77.3% vs 47.8%; P < .001), and more often had principal diagnoses of aspiration pneumonia (10.9% vs 9.8%; P < .001), sepsis (38.6% vs 30.7%; P < .001), or respiratory failure (9.3% vs 5.5%; P < .001). Their cultures more often grew Streptococcus pneumoniae (43.7% vs 25.5%; P < .001) and less frequently grew organisms resistant to guideline-recommended antibiotics (25.0% vs 43.7%; P < .001). Patients with an AUD were treated more often with piperacillin-tazobactam (26.2% vs 22.5%; P < .001) but equally as often with anti–methicillin-resistant Staphylococcus aureus agents (32.9% vs 31.8%; P = .11) compared with patients without AUDs. When adjusted for demographic characteristics and insurance, AUD was associated with higher mortality (odds ratio, 1.40; 95% CI, 1.25-1.56), length of stay (risk-adjusted geometric mean ratio, 1.24; 95% CI, 1.20-1.27), and costs (risk-adjusted geometric mean ratio, 1.33; 95% CI, 1.28-1.38). After additional adjustment for differences in comorbidities and risk factors for resistant organisms, AUD was no longer associated with mortality but remained associated with late mechanical ventilation (odds ratio, 1.28; 95% CI, 1.12-1.46), length of stay (risk-adjusted geometric mean ratio, 1.04; 95% CI, 1.01-1.06), and costs (risk-adjusted geometric mean ratio, 1.06; 95% CI, 1.03-1.09). Models segregating patients undergoing alcohol withdrawal showed that poorer outcomes among patients with AUD were confined to the subgroup undergoing alcohol withdrawal.
Conclusions and Relevance
This study suggests that, compared with hospitalized patients with community-acquired pneumonia but without AUD, those with AUD less often harbor resistant organisms. The higher age-adjusted risk of death among patients with AUD appears to be largely attributable to differences in comorbidities, whereas greater use of health care resources may be attributable to alcohol withdrawal.
This cohort study uses data from the Premier Healthcare Database to compare the causes, treatment, and outcomes in patients hopitalized with community-acquired pneumonia with and without alcohol use disorder.
Introduction
Community-acquired pneumonia (CAP) is the sixth leading cause of death in the United States and the most common cause of infectious disease mortality.1 Underlying conditions including age, immune status, smoking, and comorbidities influence the severity of CAP.2 Alcohol use disorder (AUD) affects 15.1 million US adults3 and approximately 4% of patients hospitalized with pneumonia.4 Compared with patients without AUD, those with AUD tend to have more severe clinical presentations2 and greater use of health care resources, including intensive care.2,4
There are several potential explanations for these poorer outcomes. First, alcohol can affect oropharyngeal flora, promoting colonization with resistant gram-negative organisms.5,6,7 Alcohol consumption also blunts the cough and gag reflexes, predisposing patients to aspirate these organisms.8 Second, alcohol adversely affects immune function and pulmonary clearing mechanisms, impairing the body’s ability to fight infection.9,10 Malnutrition, which is common among patients with AUD, may amplify these effects.11 Third, long-term alcohol use damages organ systems, leading to liver disease, cardiovascular disorders, kidney disease, and cancer.12 Fourth, AUD puts patients at risk for alcohol withdrawal syndrome (AWS), which is itself a cause of increased use of health care resources and mortality.13
Despite the prevalence of AUD, few large studies have evaluated the effects of AUD in pneumonia. None has sought to attribute the poorer outcomes of patients with AUD to these various potential causes. The objective of this study was to better inform management of patients with AUD by identifying the bacterial causes of pneumonia in a large sample of US hospitals, describing antibiotic resistance and treatment patterns, and assessing AUD’s association with outcomes of pneumonia, including the specific contributions of comorbidities, AWS, and any residual differences that are potentially attributable to alcohol’s direct immunosuppressive effects. These questions have important implications for clinical care (eg, choosing initial antibiotic therapy and admission to intensive care) as well as risk adjustment.
Methods
Study Population
We conducted a retrospective cohort study of patients 18 years or older who were admitted between July 1, 2010, and June 30, 2015, to 177 US hospitals participating in the Premier Healthcare Database (Premier Inc),14 an inpatient database developed for measuring quality and use of health care resources. Data were provided by participating hospitals from all regions of the United States and are in most respects representative of US acute care hospitals, although larger hospitals, the southern region, and urban facilities are overrepresented. The Premier Database contains sociodemographic information; hospital and physician information; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes; and date-stamped hospital charge codes for all items charged to the patient or insurer, including medications, laboratory or diagnostic tests, and procedures. Approximately three-quarters of the participating hospitals provide information on actual hospital costs, and the remainder provide cost estimates based on Medicare cost to charge ratios. The microbiology laboratory data, including culture results and antibiotic sensitivity results, were available for hospitals that used SafetySurveillor (Premier Inc), an infection tracking tool. Because the Premier Healthcare Database includes only affirmative charges, missing data on chargeable events are not readily detectable. Missing demographic fields were very rare, and the few such patients with them were omitted. Because all data from the database are deidentified and contain no protected health information, the study protocol was deemed exempt by the institutional review board of The Cleveland Clinic Foundation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients with a primary diagnosis of pneumonia (ICD-9-CM codes: 481, 482.0-482.9, 483.0-483.8, 484.0-484.8, 485, 486, and 507.0) or a primary diagnosis of respiratory failure (ICD-9-CM codes: 518.81, 518.82, 518.84, and 799.1) or sepsis (ICD-9-CM codes: 785.52, 790.7, 995.91, 995.92, and 038.0-038.9) combined with a secondary diagnosis of pneumonia were included in the study (eTable 1 in the Supplement). To increase the specificity of the diagnosis, we also required patients to undergo chest radiography, to have received antibiotics, and to have had blood or respiratory cultures collected by the first hospital day.
Patients with cystic fibrosis, those with potential causes of bacteremia other than pneumonia (cholecystitis, appendicitis, diverticulitis, perforated diverticulum, peritonitis, postoperative anastomotic leaks or abdominal surgical site infections, central line–associated bloodstream infection with positive blood culture results, or endocarditis with Staphylococcus aureus or viridans group Streptococci in blood), and patients receiving long-term mechanical ventilation or who were transferred from another acute care facility were excluded because we were not interested in studying the bacteriology of pneumonia facilitated by these other conditions. Patients with the same organism in blood and urine cultures (representing urinary pathogens), enterococcal infection (which is not a cause of pneumonia), positive Streptococcus or Legionella pneumonia antigen test results in the current admission and within the past 6 months (because antigen positivity may persist for up to 6 months), or the same pneumonia diagnosis in the current admission and a previous admission within 1 year (because previous diagnoses may be carried forward) were also excluded (eFigure in the Supplement).
Baseline Variables
We used ICD-9-CM codes (eTable 2 in the Supplement) to identify AUD (codes 291.0, 291.81-291.89, 291.9, 303.0-303.92, and 305.00-305.02) and AWS (codes 291.81 and 291.0). Patients who had ICD-9-CM codes indicating AUD in remission (codes 303.03, 303.93 and 305.03) were excluded from the study; although they had a high burden of comorbidity, they were presumably not at elevated risk for colonization by resistant gram-negative bacteria, aspiration, immunosuppression due to alcohol, or alcohol withdrawal.
Additional variables included demographic characteristics (age, sex, race, and health insurance status), comorbidities (identified using secondary ICD-9-CM codes and diagnosis related group based on the work of Elixhauser15), risk factors for resistant infections (admission from a skilled nursing facility or intermediate care facility, prior admission within 6 months, dialysis, and immune status),16 hospital characteristics (geographical region, urban vs rural location, bed size, and teaching status), and certain treatments on hospital day 1 (admission to an intensive care unit [ICU], administration of vasopressors, invasive mechanical ventilation [IMV], and type and number of antibiotics administered).
Microbiological Evaluation
We considered all blood and respiratory samples collected by hospital day 1. Positive cultures were used to identify the cause of pneumonia as well as to study antibiotic resistance patterns reported by the hospital laboratories. Organisms were considered resistant to CAP therapy if they demonstrated intermediate or greater resistance to treatment with either a quinolone or a third-generation cephalosporin plus a macrolide.
Outcomes
Outcomes included inpatient mortality, clinical deterioration (as evidenced by late ICU transfer, late IMV, or late vasopressor therapy initiation [ie, after the first hospital day]), length of stay (LOS), and cost. Hospital costs represented the entire cost of hospitalization, including bed charge, laboratory tests, and medications.
Statistical Analysis
Statistical analysis was conducted from October 27, 2017, to August 20, 2018. We summarized and compared baseline characteristics between patients with and without AUD by using frequencies, proportions, and Pearson χ2 tests for categorical variables and medians, quartiles, and Kruskal-Wallis rank analysis of variance tests for continuous variables. We described the frequencies of pneumonia causes in 2 ways: as the fractions of patients from whom the organism was cultured in any blood or respiratory sample both in the subset of patients with any positive culture of either type and among all cultured patients.
We used mixed logistic regression with random hospital effects to model the dichotomous outcomes of death and signs of clinical deterioration. To understand the relative contributions of comorbidities, AWS, and any residual effects that might be attributed to the direct immunosuppressive effects of alcohol,4 we performed stagewise adjusted analyses. First, we adjusted only for patient demographic characteristics (Table 1). Second, we added to the model the comorbidities and risk factors for resistant infections in Table 1. Third, we stratified results by the presence or absence of AWS. We then measured the remaining effect of AUD, which might be due to the direct immunosuppressive effects of alcohol. Models were prespecified without data-driven variable selection. Analogous sequences of gamma generalized linear mixed models17,18 with log link function were used for LOS and cost. All our models included random hospital intercept effects. In unadjusted and final adjusted analyses, we first expressed AUD as a dichotomous effect and then as a trichotomy, further distinguishing between AUD with and without AWS. Models were fitted using residual subject-specific pseudolikelihood, and Wald statistics and 95% CIs were used for formal inference. In final models, inferences for overall AUD effects were based on sample-size weighted linear combinations of estimated parameters for the subgroups undergoing and not undergoing alcohol withdrawal. Results of logistic models are summarized as odds ratios (ORs) and of gamma models as ratios of geometric means, each with 95% CIs. Analyses were performed using SAS, version 9.4 (SAS Institute Inc). All P values were from 2-sided tests, and results were deemed statistically significant at P < .05.
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%)a | |
|---|---|---|
| No AUD (n = 132 744) | AUD (n = 4752) | |
| Principal diagnosis | ||
| Pneumonia | 71 805 (54.1) | 1958 (41.2) |
| Aspiration pneumonia | 12 946 (9.8) | 519 (10.9) |
| Sepsis | 40 740 (30.7) | 1832 (38.6) |
| Respiratory failure | 7253 (5.5) | 443 (9.3) |
| Demographics | ||
| Age, median (IQR), y | 73.0 (60.0-83.0) | 58.0 (50.0-67.0) |
| Age group, y | ||
| 18-44 | 10 774 (8.1) | 633 (13.3) |
| 45-64 | 32 933 (24.8) | 2669 (56.2) |
| 65-74 | 27 070 (20.4) | 901 (19.0) |
| 75-84 | 32 469 (24.5) | 417 (8.8) |
| ≥85 | 29 498 (22.2) | 132 (2.8) |
| Sex | ||
| Male | 63 466 (47.8) | 3672 (77.3) |
| Female | 69 278 (52.2) | 1080 (22.7) |
| Race | ||
| White | 102 672 (77.3) | 3501 (73.7) |
| Black | 16 351 (12.3) | 814 (17.1) |
| Hispanic | 870 (0.7) | 31 (0.7) |
| Other | 12 851 (9.7) | 406 (8.5) |
| Insurance payer | ||
| Medicare | 96 761 (72.9) | 1973 (41.5) |
| Medicaid | 10 751 (8.1) | 1036 (21.8) |
| Managed care | 14 150 (10.7) | 670 (14.1) |
| Commercial indemnity | 4090 (3.1) | 209 (4.4) |
| Others | 6992 (5.3) | 864 (18.2) |
| HCAP components | ||
| Admitted from SNF or ICF | 10 181 (7.7) | 138 (2.9) |
| Dialysis | 6045 (4.6) | 106 (2.2) |
| Admission within past 6 mo | 13 011 (9.8) | 313 (6.6) |
| Immunosuppressed | 20 644 (15.6) | 673 (14.2) |
| Comorbidities | ||
| Hypertension | 88 151 (66.4) | 2622 (55.2) |
| Fluid and electrolyte disorders | 65 035 (49.0) | 3019 (63.5) |
| Chronic pulmonary disease | 61 501 (46.3) | 2484 (52.3) |
| Diabetes | 43 882 (33.1) | 898 (18.9) |
| Deficiency anemias | 43 005 (32.4) | 1481 (31.2) |
| Congestive heart failure | 37 303 (28.1) | 935 (19.7) |
| Smoker | 22 146 (16.7) | 2905 (61.1) |
| Chronic kidney disease | 24 158 (18.2) | 427 (9.0) |
| Hypothyroidism | 23 046 (17.4) | 347 (7.3) |
| Other neurologic disorders | 22 517 (17.0) | 694 (14.6) |
| Depression | 20 683 (15.6) | 785 (16.5) |
| Obesity | 17 930 (13.5) | 455 (9.6) |
| Weight loss | 16 617 (12.5) | 974 (20.5) |
| Valvular disease | 12 799 (9.6) | 297 (6.3) |
| Coagulopathy | 11 650 (8.8) | 892 (18.8) |
| Peripheral vascular disease | 10 982 (8.3) | 287 (6.0) |
| Pulmonary circulation disease | 10 483 (7.9) | 310 (6.5) |
| Psychoses | 8068 (6.1) | 525 (11.0) |
| Paralysis | 6560 (4.9) | 94 (2.0) |
| Rheumatoid arthritis or collagen vascular disease | 6009 (4.5) | 93 (2.0) |
| Metastatic cancer | 5858 (4.4) | 121 (2.5) |
| Solid tumor without metastasis | 5557 (4.2) | 149 (3.1) |
| Drug abuse | 3623 (2.7) | 845 (17.8) |
| Chronic liver disease | 3294 (2.5) | 970 (20.4) |
| Lymphoma | 2388 (1.8) | 31 (0.7) |
| Chronic blood loss anemia | 1073 (0.8) | 56 (1.2) |
| Peptic ulcer disease with bleeding | 30 (0.02) | 0 |
| AIDS | 72 (0.05) | 8 (0.2) |
Abbreviations: AUD, alcohol use disorder; HCAP, health care–associated pneumonia; ICF, intermediate care facility; IQR, interquartile range; SNF, skilled nursing facility.
Age in years differs significantly between patients with and without AUD by the Mann-Whitney Wilcoxon rank sum test (P < .001). Other variables also differ significantly (P < .001) between these groups by Pearson uncorrected χ2 test except for immunosuppression (P = .009), deficiency anemia (P = .08), depression (P = .08), chronic blood loss anemia (P = .005), and peptic ulcer with bleeding (P = .30).
Results
Patient characteristics appear in Table 1. Of 137 496 patients hospitalized with pneumonia, the mean (SD) age was 69.5 (16.2) years and 3.5% had an AUD. Compared with patients without AUD, those with AUD were younger (median age, 58.0 vs 73.0 years; P < .001), more often male (77.3% vs 47.8%; P < .001), black (17.1% vs 12.3%; P < .001), and insured by Medicaid (21.8% vs 8.1%; P < .001). Patients with AUD had more comorbid conditions. In particular, they were more likely to smoke (61.1% vs 16.7%; P < .001), have chronic liver disease (20.4% vs 2.5%; P < .001), have weight loss (20.5% vs 12.5%; P < .001), have psychoses (11.0% vs 6.1%; P < .001), and to abuse drugs other than alcohol (17.8% vs 2.7%; P < .001). Patients with AUD also presented with more severe illness: they were more likely to have a principal diagnosis of aspiration pneumonia (10.9% vs 9.8%; P < .001), sepsis (38.6% vs 30.7%; P < .001), or respiratory failure (9.3% vs 5.5%; P < .001) (Table 1); to be admitted to the ICU (39.0% vs 24.3%; P < .001) (Table 2); and to receive vasopressors (11.3% vs 6.2%; P < .001) or IMV (16.4% vs 7.5%; P < .001). Patients with AUD were more likely to have been admitted to larger hospitals (≥401 beds) (42.6% vs 35.7%; P < .001) and teaching hospitals (45.7% vs 40.5%; P < .001), with little variation by geography or urban location (eTable 3 in the Supplement).
Table 2. Initial Treatment.
| Characteristic (Day 0 or 1) | No. (%) | P Valuea | |
|---|---|---|---|
| No AUD (n = 132 744) | AUD (n = 4752) | ||
| Intensive care unit admission | 32 321 (24.3) | 1852 (39.0) | <.001 |
| Vasopressor | 8202 (6.2) | 539 (11.3) | <.001 |
| Invasive mechanical ventilation | 9982 (7.5) | 780 (16.4) | <.001 |
| Antibiotics received, No. | |||
| 1 | 29 454 (22.2) | 969 (20.4) | <.001 |
| 2 | 57 086 (43.0) | 1908 (40.2) | |
| 3 | 28 942 (21.8) | 1129 (23.8) | |
| ≥4 | 17 262 (13.0) | 746 (15.7) | |
| Piperacillin-tazobactam | 29 802 (22.5) | 1246 (26.2) | <.001 |
| Aminoglycosides | 2754 (2.1) | 79 (1.7) | .049 |
| Anti-MRSA agents | 42 212 (31.8) | 1563 (32.9) | .11 |
| Antipseudomonal carbepenem | 4175 (3.1) | 134 (2.8) | .21 |
| Third-generation cephalosporin | 60 004 (45.2) | 2217 (46.7) | .048 |
| Antipseudomonal cephalosporin | 12 465 (9.4) | 344 (7.2) | <.001 |
| Respiratory quinolone | 54 798 (41.3) | 1986 (41.8) | .48 |
| Antipseudomonal quinolone | 50 017 (37.7) | 1793 (37.7) | .94 |
| Macrolide | 53 104 (40.0) | 1968 (41.4) | .05 |
| Guideline antibiotic | |||
| Other antibiotic | 20 380 (15.4) | 763 (16.1) | .50 |
| Fully HCAP | 12 700 (9.6) | 464 (9.8) | |
| Partial HCAP | 22 303 (16.8) | 801 (16.9) | |
| Community-acquired pneumonia | 77 361 (58.3) | 2724 (57.3) | |
Abbreviations: AUD, alcohol use disorder; HCAP, health care–associated pneumonia; MRSA, methicillin-resistant Staphylococcus aureus.
P values are based on Pearson uncorrected χ2 test.
Cause of Pneumonia and Antibiotic Treatment
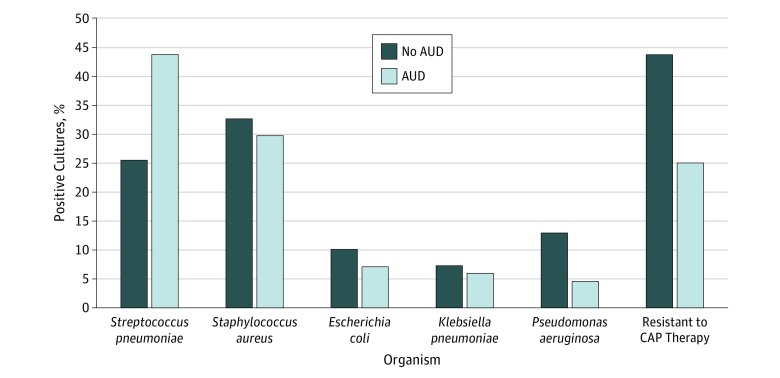
A higher percentage of patients with AUD than patients without AUD yielded positive cultures (13.4% vs 9.1%; P < .001). Among those with positive cultures, patients with AUD more often had Streptococcus pneumoniae (43.7% vs 25.5%; P < .001) and less often Klebsiella pneumoniae (6.0% vs 7.3%; P = .02), Pseudomonas aeruginosa (4.6% vs 12.9%; P < .001), and any organisms resistant to guideline-recommended therapy for CAP (25.0% vs 43.7%; P < .001) than did patients without AUD (Figure 1). Among all patients (including those with negative cultures), the corresponding percentages for those with and without AUD were 5.9% vs 2.3% for S pneumoniae, 0.8% vs 0.7% for K pneumoniae, 0.6% vs 1.2% for P aeruginosa, and 3.3% vs 4.0% for organisms resistant to guideline-recommended therapy for CAP (eTable 4 in the Supplement). Compared with patients without AUD, those with AUD were slightly more likely to receive broad-spectrum antibiotics, including piperacillin-tazobactam (26.2% vs 22.5%; P < .001) but equally as likely to receive anti–methicillin-resistant S aureus agents (32.9% vs 31.8%; P = .11). Two-thirds of patients with AUD who were receiving broad-spectrum antibiotics did not have other risk factors for resistant organisms.
Figure 1. Cultured Organisms in Patients With Community-Acquired Pneumonia (CAP) by Presence or Absence of Alcohol Use Disorder (AUD).
Heights of the bars are proportional to the fractions of patients among all patients with positive cultures.
Outcomes
In unadjusted analysis, compared with patients without an AUD, those with an AUD were associated with more late ICU admissions (13.4% vs 8.1%; P < .001), need for late IMV (13.7% vs 6.1%; P < .001), late vasopressor use (10.7% vs 5.8%; P < .001), increased median LOS (6 [interquartile range (IQR), 3-10] vs 5 [IQR, 3-8] days; P < .001), and higher median hospitalization cost ($10 425 [IQR, $5705-$21 282] vs $8309 [IQR, $5056-$14 658]; P < .001). Compared with patients with AUD alone, those with AUD and AWS experienced more late ICU admission (26.7% vs 10.6%), late IMV (25.4% vs 10.8%), and vasopressor use (17.0% vs 9.1%); increased median LOS (8.0 [IQR, 5.0-14.0] vs 5.0 [IQR, 3.0-9.0] days); and higher median cost ($16 260.7 [IQR, $8164.9-$32 825.6] vs $9374.8 [IQR, $5289.8-$17 769.7]) (Table 3).
Table 3. Observed (Unadjusted) Outcomes by AUD Category.
| Characteristica | No AUD (n = 132 744) | AUD Without AWS (n = 3747) | AUD With AWS (n = 1005) |
|---|---|---|---|
| In-hospital mortality, No. (%) | 9673 (7.3) | 289 (7.7) | 78 (7.8) |
| Late (≥day 2) ICU admission, No./total No. (%)b | 8134/100 423 (8.1) | 254/2391 (10.6) | 136/509 (26.7) |
| Late (≥day 2) IMV, No./total No. (%)c | 7463/122 762 (6.1) | 343/3185 (10.8) | 200/509 (25.4) |
| Late (≥day 2) vasopressor use, No./total No. (%)d | 7233/124 542 (5.8) | 303/3335 (9.1) | 149/878 (17.0) |
| Length of stay, median (IQR), d | 5.0 (3.0-8.0) | 5.0 (3.0-9.0) | 8.0 (5.0-14.0) |
| Cost, median (IQR), $ | 8308.7 (5056.4-14 657.5) | 9374.8 (5289.8-17 769.7) | 16 260.7 (8164.9-32 825.6) |
Abbreviations: AUD, alcohol use disorder; AWS, alcohol withdrawal syndrome; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range.
Except for in-hospital mortality (P = .52), all variables differed statistically significantly among the 3 groups by Pearson uncorrected χ2 or Kruskal-Wallis rank analysis of variance (length of stay and cost) test.
Patients with ICU admission on day 0 or 1 were excluded.
Patients with IMV on day 0 or 1 were excluded.
Patients with vasopressor use on day 0 or 1 were excluded.
Multivariable Analyses
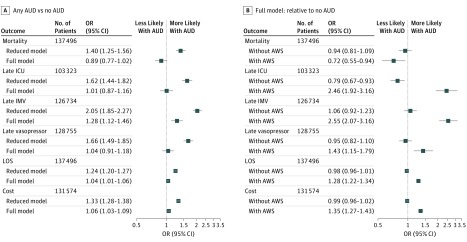
In models that adjusted for age, sex, race, and health insurance, the presence of an AUD was associated with increased mortality (OR, 1.40; 95% CI, 1.25-1.56), late ICU admission (OR, 1.62; 95% CI, 1.44-1.82), late IMV (OR, 2.05; 95% CI, 1.85-2.27), late vasopressor use (OR, 1.66; 95% CI, 1.49-1.85), LOS (risk-adjusted geometric mean ratio, 1.24; 95% CI, 1.20-1.27), and cost (risk-adjusted geometric mean ratio, 1.33; 95% CI, 1.28-1.38). When comorbidities and risk factors for resistance were added, most associations were attenuated (Figure 2A). Alcohol use disorder was no longer associated with mortality (OR, 0.89; 95% CI, 0.77-1.02), late ICU admission (OR, 1.01; 95% CI, 0.87-1.16), or vasopressor use (OR, 1.04; 95% CI, 0.91-1.18). Alcohol use disorder did remain associated with late IMV (OR, 1.28; 95% CI, 1.12-1.46), LOS (risk-adjusted geometric mean ratio, 1.04; 95% CI, 1.01-1.06), and cost (risk-adjusted geometric mean ratio, 1.06; 95% CI, 1.03-1.09).
Figure 2. Associations of Alcohol Use Disorder (AUD) With Outcomes of Hospitalization for Community-Acquired Pneumonia.
A, Overall association of AUD with outcomes of hospitalization for community-acquired pneumonia. B, Association of AUD with outcomes of hospitalization for community-acquired pneumonia stratified by presence of alcohol withdrawal syndrome (AWS). Late intensive care unit (ICU) admission, late invasive mechanical ventilation (IMV), and late vasopressor use were defined as arising on day 2 or later and were studied only among patients for whom the respective late outcome was not present earlier. Costs were studied conditionally only among patients with positive costs and from hospitals with greater than 50% of all patients also with positive costs. Patients with no cost were excluded. Reduced models were adjusted for age, sex, race, and insurance status. Full models were adjusted for the preceding variables as well as the presence of comorbidities and components of the health care–associated pneumonia definition. LOS indicates length of stay; OR, odds ratio.
When patients with AUD were stratified by the presence of AWS, we did not observe an association between AUD and outcomes for patients without AWS. Those with AWS had significant increases in late ICU admission (OR, 2.46; 95% CI, 1.92-3.16), vasopressor use (OR, 1.43; 95% CI, 1.15-1.79), late IMV (OR, 2.55; 95% CI, 2.07-3.16), LOS (risk-adjusted geometric mean ratio, 1.28; 95% CI, 1.22-1.34), and costs (risk-adjusted geometric mean ratio, 1.35; 95% CI, 1.27-1.43) but lower adjusted mortality (OR, 0.72; 95% CI, 0.55-0.94) (Figure 2B). Although the association of AUD with each outcome was tested in 3 separate models, most P values were less than .01, and Bonferroni-Holm adjustment of P values for these triplicate analyses did not change the statistical significance (α = .05) of any test result. However, after this adjustment, the unexpected lower mortality with AWS is only marginally significant (P = .045) and may be a statistical false-positive.
Discussion
In this large nationwide sample of patients hospitalized with pneumonia, patients with AUD differed from those without AUD in several important ways that might be expected. Patients with AUD were younger, more often male, and more likely to be insured with Medicaid insurance. They also had more comorbidities, especially liver disease, drug abuse, and psychosis; appeared to have more serious pneumonias as measured by admission to the ICU, use of IMV, or use of vasopressors; and experienced longer LOS and higher costs. Age-adjusted differences in mortality appear to have been attributable to alcohol-related comorbidities because they were no longer present after adjustment for comorbidities. Even then, AUD remained associated with poorer clinical outcomes and higher use of health care resources, including the need for mechanical ventilation after admission, longer LOS, and higher costs. These associations appear to be attributable to AWS because they were not present among the subgroup of patients without AWS. We found no evidence that unmeasured factors, such as homelessness, poverty, or direct toxic effects of alcohol on the immune system, contributed to outcomes for patients with AUD. In addition, we found that, despite theoretical reasons to expect gram-negative organisms to predominate, the organism most commonly cultured from patients with AUD was S pneumoniae. Patients with AUD were actually slightly less likely than others to harbor resistant organisms, such as P aeruginosa. Nevertheless, patients with AUD were slightly more likely to receive broad-spectrum antibiotics, primarily because they had a more severe clinical presentation.
The last large study of pneumonia and AUD in the United States was conducted more than 25 years ago. Saitz et al4 examined 23 198 patients admitted to Massachusetts hospitals with a principal diagnosis of pneumonia; similar to our study, they found that 824 patients (3.6%) had an AUD. They also found that, after adjustment for demographics and comorbidities, patients with AUD were more likely to be admitted to the ICU and had higher costs and longer LOS, but mortality was similar to that of patients without AUD. They concluded that alcoholism alone was a factor associated with pneumonia, probably owing to the direct toxic effects of alcohol on the respiratory and immune systems. Although it is true that alcohol decreases mucociliary clearance, impairs alveolar19 and cell-mediated immunity, and decreases the function of alveolar macrophages and neutrophils,20,21,22,23 we found that, after removing patients with AWS—something Saitz et al4 did not do—there was no residual deleterious association of AUD with patient outcomes. Despite the theoretical association of alcohol’s direct toxic effect, AUD by itself was not associated with pneumonia outcomes.
Similarly, there are several reasons to believe that patients with AUD would have infections with gram-negative organisms resistant to recommended empirical therapy for CAP. Alcohol alters the oropharyngeal flora, inviting colonization by gram-negative organisms. It also blunts the cough and gag reflexes,24 predisposing patients to aspiration.25 For these reasons, guidelines from the Infectious Diseases Society of America26 identify alcoholism as a risk factor for gram-negative infections, including Klebsiella and Pseudomonas. However, only 2 small studies support this association: 1 study of 25 patients in the ICU on an island in the Indian Ocean27 and a study of 50 patients, 16 of whom had an AUD, in an emergency department in Barcelona, Spain.28 A third study found that alcoholism is associated with Klebsiella in South Africa and Taiwan but not in the rest of the world.29 In contrast, several much larger prospective and retrospective studies have found that alcoholism is primarily associated with S pneumoniae infection.2,4,30 Our study supports these latter works by presenting more cases than all the other studies combined in a contemporary, multi-institutional sample that is broadly representative of US hospitals. More important, we examined the resistance patterns of the organisms isolated and found that patients with AUD were not more likely to harbor organisms resistant to standard CAP therapy. Given these findings, it may be appropriate to remove alcohol as a risk factor for multidrug-resistant organisms in the next iteration of the guidelines.
We believe the results of this study are important because we found that more than one-quarter of patients with pneumonia who had an AUD received an antipseudomonal penicillin, and more than one-third received anti–methicillin-resistant S aureus agents. In most of these patients, AUD was their only risk factor. In addition, the association of S pneumoniae with AUD strongly supports the necessity to promote pneumococcal vaccination of these patients.31,32
On admission to the hospital, abstinence from alcohol can lead to AWS. Monte et al33 assessed factors determining the survival of hospitalized patients with AWS. Cirrhosis, delirium tremens, hallucinations, and seizures increase the risk for adverse outcomes in patients with AWS.33,34,35 Development of delirium tremens is commonly associated with AWS and is a major contributor to AWS-related deaths.36,37 To our knowledge, our study is the first to evaluate the contribution of AWS to outcomes in pneumonia and shows that patients with AWS have increased late transfers to the ICU, need for IMV, and need for vasopressors as well as increased LOS and cost. Prompt management of these patients based on withdrawal severity38 might help reduce the use of health care resources. Paradoxically, the patients with AWS had lower adjusted mortality than patients without AUD. The reasons for this finding are unclear. It is possible that reasons for ICU admission and IMV use among patients with AWS differ from those of other patients with pneumonia and therefore do not carry the same prognostic value. The fact that unadjusted mortality for patients with AWS was slightly higher than that for other patients with AUD supports this hypothesis. Alternatively, it may represent a chance finding.
Limitations
Our study has several limitations. By relying on ICD-9-CM codes, we may have failed to identify some patients with AUD. However, such misclassification seems unlikely to have substantially distorted the effects we observed. Also, we could neither quantify alcohol use nor, with these primarily administrative data, adjust for physiological measures, such as vital signs. This limitation could have resulted in misclassification of pneumonia severity, although we did assess for indirect measures of severity, such as IMV and vasopressor use, and the variables we obtained have excellent prognostic ability for inpatient death.39 We controlled for an extensive list of potential confounders by including them as covariates in mixed logistic regression analyses. An alternative strategy for control of confounding would have been to compare patients with AUD with individually matched sets of patients without AUD treated in the same hospital. We preferred to rely on a more classic modeling approach because our stagewise adjustments would have required different matched sets at each stage; also, close, within-hospital matching using 46 covariates at the final stage would have sacrificed considerable precision by omitting a large fraction of patients without AUD. The use of mixed models, with hospital as the random effects, also accounts for interhospital variability in a manner that supports generalization of results beyond the institutions that contribute information to the Premier Healthcare Database. Our etiologic findings are based on relative frequencies of cultured organisms in culture-positive samples and implicitly assume that these organisms also characterize the unobserved distributions in patients with false-negative samples and among those from whom cultures were not obtained.
Conclusions
In this study, patients with AUD composed 1 in 30 patients hospitalized with pneumonia and had age-adjusted outcomes that were poorer than those of other patients. The reason for this finding appears to be excess comorbidities, such as chronic liver disease, smoking, and malnutrition, among patients with AUD. In addition, patients with AWS were at elevated risk of clinical deterioration and experienced longer LOS and substantially higher cost. Despite theoretical concerns about the effects of alcohol on local flora and defense mechanisms, organisms cultured from patients with pneumonia who had an AUD in this study were not more likely to be resistant to antibiotics for CAP. Treatment should therefore include routine CAP therapy and close monitoring for AWS.
eTable 1. Pneumonia Diagnoses, by ICD-9-CM Code
eTable 2. ICD-9-CM Codes for Alcohol Use Disorders
eTable 3. Hospital Characteristics of Patients, by Absence or Presence of Alcohol Use Disorders
eTable 4. Pneumonia Etiologies, by Absence or Presence of Alcohol Use Disorder and Alcohol Withdrawal Syndrome
eFigure. Inclusion/Exclusion Criteria Considered for Patients Included in the Study
References
- 1.Niederman MS, Mandell LA, Anzueto A, et al. ; American Thoracic Society . Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):-. doi: 10.1164/ajrccm.163.7.at1010 [DOI] [PubMed] [Google Scholar]
- 2.de Roux A, Cavalcanti M, Marcos MA, et al. . Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219-1225. doi: 10.1378/chest.129.5.1219 [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Alcohol Abuse and Alcoholism Alcohol facts and statistics. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics. Updated August 2018. Accessed March 26, 2018.
- 4.Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157(13):1446-1452. doi: 10.1001/archinte.1997.00440340078008 [DOI] [PubMed] [Google Scholar]
- 5.Mackowiak PA, Martin RM, Jones SR, Smith JW. Pharyngeal colonization by gram-negative bacilli in aspiration-prone persons. Arch Intern Med. 1978;138(8):1224-1227. doi: 10.1001/archinte.1978.03630330024009 [DOI] [PubMed] [Google Scholar]
- 6.Dao TT, Liebenthal D, Tran TK, et al. . Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS One. 2014;9(3):e91999. doi: 10.1371/journal.pone.0091999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuxench-López Z, Ramírez-Ronda CH. Pharyngeal flora in ambulatory alcoholic patients: prevalence of gram-negative bacilli. Arch Intern Med. 1978;138(12):1815-1816. doi: 10.1001/archinte.1978.03630370033017 [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Festic E, Park PK, et al. ; United States Critical Illness and Injury Trials Group . Characteristics and outcomes of patients hospitalized following pulmonary aspiration. Chest. 2014;146(4):899-907. doi: 10.1378/chest.13-3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg SS, Zhao X, Hua L, Wang JF, Nelson S, Ouyang J. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide–dependent mechanism, in vivo. Alcohol Clin Exp Res. 1999;23(4):735-744. doi: 10.1111/j.1530-0277.1999.tb04177.x [DOI] [PubMed] [Google Scholar]
- 10.Brown LAS, Harris FL, Ping X-D, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33(3):191-197. doi: 10.1016/j.alcohol.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 11.MacGregor RR. Alcohol and immune defense. JAMA. 1986;256(11):1474-1479. doi: 10.1001/jama.1986.03380110080031 [DOI] [PubMed] [Google Scholar]
- 12.Dguzeh U, Haddad NC, Smith KTS, et al. . Alcoholism: a multi-systemic cellular insult to organs. Int J Environ Res Public Health. 2018;15(6):E1083. doi: 10.3390/ijerph15061083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heavner JJ, Akgün KM, Heavner MS, et al. . Implementation of an ICU-specific alcohol withdrawal syndrome management protocol reduces the need for mechanical ventilation [published online May 25, 2018]. Pharmacotherapy. doi: 10.1002/phar.2127 [DOI] [PubMed] [Google Scholar]
- 14.Premier Premier Healthcare Database: data that informs and performs. https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper. Published July 29, 2018. Accessed May 3, 2019.
- 15.Manitoba Centre for Health Policy. Concept: Elixhauser Comorbidity Index. http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?printer=Y&conceptID=1436. Updated January 17, 2019. Accessed April 25, 2018.
- 16.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care–associated pneumonia. Arch Intern Med. 2008;168(20):2205-2210. doi: 10.1001/archinte.168.20.2205 [DOI] [PubMed] [Google Scholar]
- 17.Mcculloch CE, Neuhaus JM. Generalized linear mixed models based in part on the article “Generalized linear mixed models” by Charles E. McCulloch, which appeared in the Encyclopedia of Environmetrics In: Encyclopedia of Environmetrics. Wiley Online Library; 2013. doi: 10.1002/9780470057339.vag009.pub2 [DOI] [Google Scholar]
- 18.Stroup WW. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications Boca Raton, FL: CRC Press; 2012. https://www.crcpress.com/Generalized-Linear-Mixed-Models-Modern-Concepts-Methods-and-Applications/Stroup/p/book/9781439815120. Accessed October 4, 2018.
- 19.Kershaw CD, Guidot DM. Alcoholic lung disease. Alcohol Res Health. 2008;31(1):66-75. [PMC free article] [PubMed] [Google Scholar]
- 20.Guarneri JJ, Laurenzi GA. Effect of alcohol on the mobilization of alveolar macrophages. J Lab Clin Med. 1968;72(1):40-51. [PubMed] [Google Scholar]
- 21.Nair MP, Kronfol ZA, Schwartz SA. Effects of alcohol and nicotine on cytotoxic functions of human lymphocytes. Clin Immunol Immunopathol. 1990;54(3):395-409. doi: 10.1016/0090-1229(90)90053-S [DOI] [PubMed] [Google Scholar]
- 22.Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52. [PMC free article] [PubMed] [Google Scholar]
- 23.Glassman AB, Bennett CE, Randall CL. Effects of ethyl alcohol on human peripheral lymphocytes. Arch Pathol Lab Med. 1985;109(6):540-542. [PubMed] [Google Scholar]
- 24.Berkowitz H, Reichel J, Shim C. The effect of ethanol on the cough reflex. Clin Sci Mol Med. 1973;45(4):527-531. [DOI] [PubMed] [Google Scholar]
- 25.Krumpe PE, Cummiskey JM, Lillington GA. Alcohol and the respiratory tract. Med Clin North Am. 1984;68(1):201-219. doi: 10.1016/S0025-7125(16)31250-0 [DOI] [PubMed] [Google Scholar]
- 26.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paganin F, Lilienthal F, Bourdin A, et al. . Severe community-acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J. 2004;24(5):779-785. doi: 10.1183/09031936.04.00119503 [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Solá J, Junqué A, Estruch R, Monforte R, Torres A, Urbano-Márquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649-1654. doi: 10.1001/archinte.1995.00430150137014 [DOI] [PubMed] [Google Scholar]
- 29.Ko W-C, Paterson DL, Sagnimeni AJ, et al. . Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8(2):160-166. doi: 10.3201/eid0802.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz M, Ewig S, Torres A, et al. . Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160(3):923-929. doi: 10.1164/ajrccm.160.3.9901107 [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane JT, Finch RG, Ward MJ, Macrae AD. Hospital study of adult community-acquired pneumonia. Lancet. 1982;2(8292):255-258. doi: 10.1016/S0140-6736(82)90334-8 [DOI] [PubMed] [Google Scholar]
- 32.Mufson MA, Kruss DM, Wasil RE, Metzger WI. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974;134(3):505-510. doi: 10.1001/archinte.1974.00320210115016 [DOI] [PubMed] [Google Scholar]
- 33.Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol. 2010;45(2):151-158. doi: 10.1093/alcalc/agp087 [DOI] [PubMed] [Google Scholar]
- 34.McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862. doi: 10.1136/jnnp.2007.128322 [DOI] [PubMed] [Google Scholar]
- 35.Mirijello A, D’Angelo C, Ferrulli A, et al. . Identification and management of alcohol withdrawal syndrome. Drugs. 2015;75(4):353-365. doi: 10.1007/s40265-015-0358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson JA, Suelzer CJ, Eckert GJ, Zhou XH, Dittus RS. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11(7):410-414. doi: 10.1007/BF02600188 [DOI] [PubMed] [Google Scholar]
- 37.Rahman A, Paul M. Delirium tremens (DT) In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. http://www.ncbi.nlm.nih.gov/books/NBK482134/. Accessed June 28, 2018. [Google Scholar]
- 38.Mayo-Smith MF; American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal . Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA. 1997;278(2):144-151. doi: 10.1001/jama.1997.03550020076042 [DOI] [PubMed] [Google Scholar]
- 39.Rothberg MB, Pekow PS, Priya A, et al. . Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382. doi: 10.1371/journal.pone.0087382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Pneumonia Diagnoses, by ICD-9-CM Code
eTable 2. ICD-9-CM Codes for Alcohol Use Disorders
eTable 3. Hospital Characteristics of Patients, by Absence or Presence of Alcohol Use Disorders
eTable 4. Pneumonia Etiologies, by Absence or Presence of Alcohol Use Disorder and Alcohol Withdrawal Syndrome
eFigure. Inclusion/Exclusion Criteria Considered for Patients Included in the Study