Key Points
Question
What are the contemporary practice patterns and physician characteristics associated with high arteriovenous graft use for first-time hemodialysis access?
Findings
In this claims-based analysis of more than 85 000 US Medicare patients undergoing first-time permanent hemodialysis access placement, 21% of surgeons had an arteriovenous graft use rate greater than the best-practice threshold of 34%. After accounting for patient characteristics, surgeon factors that were independently associated with high arteriovenous graft use included more than 30 years of clinical experience, practice in a metropolitan setting, and a vascular surgery specialty.
Meaning
Although some of these differences may be explained by patient referral practices, we propose that sharing benchmarked performance data with surgeons could be an actionable step in achieving more high-value care in hemodialysis access surgery.
This study uses Medicare claims data to explore contemporary practice patterns and physician characteristics associated with high rates of use of arteriovenous grafting compared with arteriovenous fistula for hemodialysis access.
Abstract
Importance
Initial hemodialysis access with arteriovenous fistula (AVF) is associated with superior clinical outcomes compared with arteriovenous graft (AVG) and should be the procedure of choice whenever possible. To address the national underuse of AVF in the United States, the Centers for Medicare & Medicaid has established an AVF goal of 66% or greater in 2009.
Objective
To explore contemporary practice patterns and physician characteristics associated with high AVG use compared with AVF use.
Design, Setting, and Participants
This review of 100% Medicare Carrier claims between January 1, 2016, and December 31, 2017, includes both inpatient and outpatient Medicare claims data. All patients undergoing initial permanent hemodialysis access placement with an AVF or AVG were included. All surgeons performing more than 10 hemodialysis access procedures during the study period were analyzed.
Exposures
Placement of an AVF or AVG for initial permanent hemodialysis access.
Main Outcomes and Measures
A surgeon-level AVG (vs AVF) use rate was calculated for all included surgeons. Hierarchical logistic regression modeling was used to identify patient-level and surgeon-level factors associated with AVG use.
Results
A total of 85 320 patients (median age, 70 [range, 18-103] years; 47 370 men [55.5%]) underwent first-time hemodialysis access placement, of whom 66 489 (77.9%) had an AVF and 18 831 (22.1%) had an AVG. Among the 2397 surgeons who performed more than 10 procedures per year, the median surgeon level AVG use rate was 18.2% (range, 0.0%-96.4%). However, 498 surgeons (20.8%) had an AVG use rate greater than 34%. After accounting for patient characteristics, surgeon factors that were independently associated with AVG use included more than 30 years of clinical practice (vs 21-30 years; odds ratio, 0.85 [95% CI, 0.75-0.96]), metropolitan setting (odds ratio, 1.25 [95% CI, 1.02-1.54]), and vascular surgery specialty (vs general surgery; odds ratio, 0.77 [95% CI, 0.69-0.86]). Surgeons in the Northeast region had the lowest rate of AVG use (vs the South; odds ratio, 0.83 [95% CI, 0.73-0.96]). First-time hemodialysis access benchmarking reports for individual surgeons were created for potential distribution.
Conclusions and Relevance
In this study, one-fifth of surgeons had an AVG use rate above the recommended best practices guideline of 34%. Although some of these differences may be explained by patient referral practices, sharing benchmarked performance data with surgeons could be an actionable step in achieving more high-value care in hemodialysis access surgery.
Introduction
End-stage renal disease affects more than 600 000 people in the United States and results in more than $33 billion in Medicare spending annually.1 The type of hemodialysis access chosen for a patient has a direct effect on complication rates and health care costs. Typically, complications and mortality are significantly lower with arteriovenous fistula (AVF) compared with arteriovenous graft (AVG) or central venous catheter use.2,3,4,5,6 In addition, the cost associated with an AVF ($60 000 per person per year) is markedly lower than the cost of an AVG ($72 729 per person per year).7 As a result, the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for hemodialysis access recommend the use of AVF whenever feasible.8
In an effort to improve hemodialysis access outcomes, the Centers for Medicare & Medicaid Services (CMS) and end-stage renal disease networks joined together to create the National Vascular Access Improvement Initiative called the Fistula First Breakthrough Initiative (FFBI).9 The FFBI is a continuous quality improvement project established in 2003 with the primary aim being to increase AVF use to 50%.9 After the initial 50% goal was achieved,10 it was subsequently increased to 66% in 2009 in an effort to match the 60% to 90% AVF rates in Europe and Asia.11
Although nationwide AVF rates are improving, data from the US Renal Data System show that the proportion of patients who achieve first hemodialysis with an AVF ranges from 11% to 22%, depending on their geographic region; as of 2010, no region had achieved the 66% FFBI target for incident AVF use.12 Multiple studies have pointed to limited predialysis nephrology access and late surgeon referral for vascular access creation as reasons for these low rates. As a result, most quality improvement interventions aimed at improving AVF use focus on dialysis center education and predialysis patient care.13,14 However, there are limited data on surgeon hemodialysis access practices to date. The aims of this study were to explore contemporary rates of AVF vs AVG use for first-time permanent hemodialysis access and determine physician characteristics associated with high AVG use.
Methods
Study Population
We used 100% Medicare fee-for-service carrier claims from 2016 and 2017 for this study. The study population was all Medicare beneficiaries 18 years and older who had undergone a first-time hemodialysis access procedure (ie, AVF or AVG) for chronic kidney disease or end-stage renal disease between January 1, 2016, and December 31, 2017 (eTables 1 and 2 in the Supplement). We included each patient’s first permanent hemodialysis access procedure during the study period and categorized it as either an AVG (Current Procedural Terminology code 36830) or AVF (codes 36818, 36819, 36820, 36821, and 36825). We excluded patients who had any history of AVG or AVF (n = 23 252); those whose indication for AVG or AVF was not chronic kidney disease or end-stage renal disease (n = 2106); those who had been enrolled in Medicare parts A and B for less than 12 months before their first AVG or AVF (to ensure a minimal washout period of 12 months; n = 12 632); those missing demographic or procedure-setting data (n = 560); and those who had both AVG and AVF billed on the same day as their first hemodialysis access procedure (n = 231). Patients with prior hemodialysis via a central venous catheter were included as long as their AVF or AVG placement was the first recorded permanent hemodialysis access.
The institutional review board of Johns Hopkins School of Medicine approved this study. Informed consent was waived because the data are deidentified and from a publicly available database.
Patient Characteristics
We obtained patients’ demographic information including age, sex, race/ethnicity, and zip code of residence from the Medicare Master Beneficiary Summary File. Zip codes were mapped to Federal Information Processing Standard codes using the sashelp.zipcode file (SAS Inc) and further mapped to core-based statistical area codes using a National Bureau of Economics crosswalk15 to determine the region and type of residence. Based on core-based statistical area codes, the Office of Management and Budget designated counties as metropolitan (with an urban core of ≥50 000 population), or rural (<10 000). We also collected data on use of preoperative vein mapping, which was defined as Current Procedural Terminology codes 93970 and/or 93971 billed within the 90 days before the procedure.
Physician Characteristics
We identified the performing physician for each hemodialysis access procedure using the National Provider Identifier number present on each claim. Physicians who performed 10 or fewer AVF or AVG procedures during the study period were excluded as required by a CMS user agreement. We calculated a physician-level metric for hemodialysis performance, which was defined as the proportion of AVG (vs AVF) performed as the first hemodialysis access (ie, AVG use rate). The denominator for the metric was the total number of first-time hemodialysis procedures performed by a given physician during the study period, and the numerator was the number of first-time AVG procedures performed during the same period. We obtained information on physicians’ characteristics from Medicare Data on Provider Practice and Specialty and Physician Compare National Downloadable File. Physician characteristics of interest included sex, years since graduation from medical school, primary specialty, practice region (eTable 3 in the Supplement), practice location, and the volume of hemodialysis access procedures during the study period, as calculated from the data.
Statistical Analysis
We presented patient characteristics stratified by whether they underwent an AVG or AVF as a first-time hemodialysis access procedure. We presented physician characteristics stratified by whether their AVG use was greater than 34% (defined as high AVG use), in accordance with the defined Fistula First Initiative threshold that initial hemodialysis access should be with an AVF in more than 66% of patients.9
We then used a hierarchical logistic regression model to evaluate patient-level and physician-level characteristics associated with the use of AVG. The outcome modeled was whether a patient received an AVG (vs an AVF) as their first-time hemodialysis access. The first-level covariates in the model consisted of patient characteristics, including age, sex, and race/ethnicity, as well as the procedure setting (hospital vs nonhospital) in which they were treated. The second-level covariates consisted of physician characteristics, including sex, number of years in practice, practice region, practice location, primary specialty, and hemodialysis access procedure volume (based on tertiles). We also included a random intercept for physician to account for patient clustering within a given physician. All covariates were chosen a priori based on clinical suspicion and prior experience on this topic.
Finally, we performed an estimated value analysis of the procedural costs associated with AVF vs AVG, which was defined as all costs billed on the day of the procedure. We calculated the Medicare allowed payment amount for AVF and AVG by summing the professional fees and facility fees that occurred on the day of the hemodialysis access procedure. We limited the included costs to procedures performed in an outpatient setting, because for inpatient procedures, the facility fee was only available for the whole inpatient stay and not by the day. All statistical analyses were performed using SAS Enterprise version 7.1 (SAS Inc) with significance set at P < .05.
Results
Study Cohort
Overall, 85 320 patients underwent a first-time hemodialysis access placement during the study period (Table 1). Of these, 66 489 patients (77.9%) received an AVF, and 18 831 (22.1%) received an AVG. The median age was 70 years for both groups (range, 18-103 years), and 47 370 patients (55.5%) were male. Most patients were white (n = 50 629 [59.3%]) and lived in a metropolitan setting (n = 69 202 [81.1%]). The Southern region had the highest number of patients (n = 36 812 [43.1%]), followed by the Midwest (n = 18 371 [21.5%]) and the West (n = 15 207 [17.8%]). Preoperative vein mapping was performed in 29 280 patients (32.4%).
Table 1. Demographic Characteristics of Patients Undergoing First-time Arteriovenous Fistula or Arteriovenous Graft Placement.
| Patient Characteristics | No. (%) | |
|---|---|---|
| Arteriovenous Fistula | Arteriovenous Graft | |
| No. | 66 489 | 18 831 |
| Age, y | ||
| Median (range) | 70 (18-103) | 70 (20-101) |
| ≤64 | 20 560 (30.9) | 6506 (34.6) |
| 65-74 | 24 864 (37.4) | 5873 (31.2) |
| 75-84 | 17 109 (25.7) | 4786 (25.4) |
| 85-94 | 3904 (5.9) | 1619 (8.6) |
| ≥95 | 52 (0.1) | 47 (0.3) |
| Sex | ||
| Male | 38 950 (58.6) | 8420 (44.7) |
| Female | 27 539 (41.4) | 10 411 (55.3) |
| Race/ethnicity | ||
| White | 41 841 (62.9) | 8788 (46.7) |
| Black | 15 933 (24.0) | 7783 (41.3) |
| Asian | 2160 (3.3) | 654 (3.5) |
| Hispanic | 3251 (4.9) | 815 (4.3) |
| North America native | 1003 (1.5) | 175 (0.9) |
| Other or unknown | 2301 (3.5) | 616 (3.3) |
| Residence | ||
| Metropolitan | 53 364 (80.3) | 15 838 (84.1) |
| Rural | 13 125 (19.7) | 2993 (15.9) |
| Region | ||
| Midwest | 14 350 (21.6) | 4021 (21.4) |
| Northeast | 11 594 (17.4) | 2925 (15.5) |
| South | 28 011 (42.1) | 8801 (46.7) |
| West | 12 244 (18.4) | 2963 (15.7) |
| Other | 290 (0.4) | 121 (0.6) |
| Procedure setting | ||
| Hospital | 63 188 (95.0) | 18 302 (97.2) |
| Nonhospital | 3301 (5.0) | 529 (2.8) |
| Preoperative vein mapping | ||
| Yes | 22 979 (34.6) | 6301 (33.5) |
| No | 43 510 (65.4) | 12 530 (66.5) |
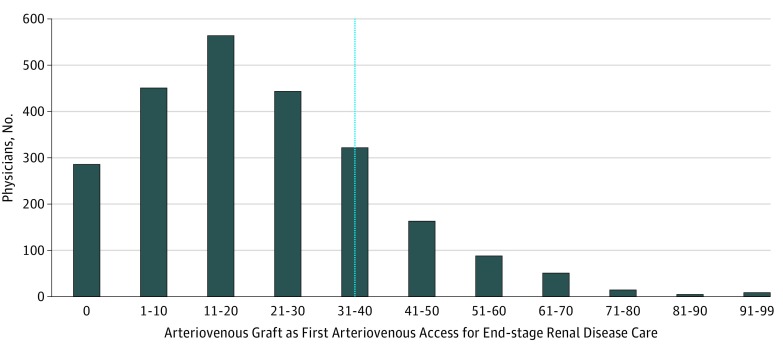
The median physician AVG use rate was 18.2% (range, 0.0%-96.4%). Of the 2397 physicians included in the analysis, 498 (20.8%) had AVG use rates greater than 34% (Table 2; Figure). Most of the physicians were male (n = 2215 [92.4%]), and the median time since medical school graduation was 25 (range, 1-59) years. The most prominent specialty was vascular surgery (n = 1428 [59.6%]), followed by general surgery (n = 755 [31.5%]) and cardiothoracic surgery (n = 175 [7.3%]). Similar to the patient distribution, most physicians practiced in a metropolitan setting (n = 2240 [93.5%]), and the South was the region with the largest proportion of physicians (n = 996 [41.6%]). The median number of first-time hemodialysis access procedures performed per physician during the 2-year study period was 24 (range, 11-300).
Table 2. Characteristics of Physicians, Stratified by Inlier vs Outlier Arteriovenous Graft Use Ratesa.
| Physician Characteristics | Arteriovenous Graft Use Rate, No. (%) | |
|---|---|---|
| Physicians Performing ≤34% Arteriovenous Graft Procedures | Physicians Performing >34% Arteriovenous Graft Procedures | |
| No. | 1899 | 498 |
| Sex | ||
| Male | 1764 (92.9) | 451 (90.6) |
| Female | 135 (7.1) | 47 (9.4) |
| Time since medical school graduation, y | ||
| Median (range) | 25 (2-59) | 25 (1-59) |
| 0-10 | 145 (7.6) | 32 (6.4) |
| 11-20 | 542 (28.5) | 140 (28.1) |
| 21-30 | 554 (29.2) | 134 (26.9) |
| ≥31 | 628 (33.1) | 182 (36.6) |
| Unknown | 30 (1.6) | 10 (2.0) |
| Primary specialty | ||
| Vascular surgery | 1115 (58.7) | 313 (62.9) |
| General surgery | 613 (32.3) | 142 (28.5) |
| Cardiothoracic surgery | 142 (7.5) | 33 (6.6) |
| Other | 29 (1.5) | 10 (2.0) |
| Practice location | ||
| Metropolitan | 1771 (93.3) | 469 (94.2) |
| Rural | 128 (6.8) | 29 (5.8) |
| Practice region | ||
| Midwest | 409 (21.5) | 111 (22.3) |
| Northeast | 368 (19.4) | 76 (15.3) |
| South | 763 (40.2) | 233 (46.8) |
| West | 350 (18.4) | 74 (14.9) |
| Other | 9 (0.5) | 4 (0.8) |
| Volume of first-time hemodialysis access procedures | ||
| Median (range) | 23 (11-300) | 25 (11-247) |
| 11-18 | 645 (34.0) | 171 (34.3) |
| 19-32 | 650 (34.2) | 153 (30.7) |
| ≥33 | 604 (31.8) | 174 (34.9) |
Included physicians who performed more than 10 first-time hemodialysis access procedures during the study period.
Figure. National Distribution of US Physicians by Their Arteriovenous Graft Use Rate.
Analysis excludes low-volume surgeons, defined as those performing less than 11 hemodialysis access procedures during the study period. The thin line indicates the 34% threshold used to define physicians who were outliers; all values to the right represent outlying arteriovenous graft use rates.
Independent Factors Associated With First-time AVG
Univariate logistic regression analyses demonstrated patient-level and physician-level characteristics associated with receipt of an AVG for first-time hemodialysis access (Table 3). After accounting for each of these characteristics using a hierarchical logistic regression model, patient-level factors independently associated with AVG use were age 18 to 64 years (adjusted odds ratio [aOR], 1.22 [95% CI, 1.16-1.28]; reference: 65-74 years) and age older than 74 years (aOR: 75-84 years, 1.33 [95% CI, 1.26-1.40]; 85-94 years, 2.18 [95% CI, 2.01-2.36]; 95 years or older, 4.08 [95% CI, 2.54-6.56]; reference: 65-74 years), female sex (aOR, 1.86 [95% CI, 1.79-1.93]), any nonwhite race other than North American native (aOR: black, 2.31 [95% CI, 2.20-2.42]; Asian, 1.31 [95% CI, 1.17-1.47]; Hispanic, 1.14 [95% CI, 1.03-1.26]; other or unknown, 1.29 [95% CI, 1.15-1.44]; reference: white participants), and lack of preoperative vein mapping (aOR, 0.92 [95% CI, 0.87-0.96]; reference: those with preoperative vein mapping; Table 3). Treatment in a nonhospital setting and preoperative vein mapping were protective against AVG use (P ≤ .03; Table 3).
Table 3. Hierarchical Logistic Regression of Patient-Level and Physician-Level Characteristics Associated With Arteriovenous Graft Use.
| Characteristic | Odds Ratio (95% CI)a | |
|---|---|---|
| Unadjusted | Adjusted | |
| Patients | ||
| Age range, y | ||
| 18-64 | 1.34 (1.29-1.40) | 1.22 (1.16-1.28) |
| 65-74 | 1 [Reference] | 1 [Reference] |
| 75-84 | 1.20 (1.14-1.25) | 1.33 (1.26-1.40) |
| 85-94 | 1.77 (1.65-1.89) | 2.18 (2.01-2.36) |
| ≥95 | 3.45 (2.28-5.22) | 4.08 (2.54-6.56) |
| Sex | ||
| Male | 1 [Reference] | 1 [Reference] |
| Female | 1.74 (1.68-1.80) | 1.86 (1.79-1.93) |
| Race/ethnicity | ||
| White | 1 [Reference] | 1 [Reference] |
| Black | 2.29 (2.21-2.38) | 2.31 (2.20-2.42) |
| Asian | 1.43 (1.30-1.57) | 1.31 (1.17-1.47) |
| Hispanic | 1.21 (1.11-1.31) | 1.14 (1.03-1.26) |
| North America native | 0.80 (0.68-0.95) | 0.92 (0.75-1.14) |
| Other or unknown | 1.26 (1.15-1.39) | 1.29 (1.15-1.44) |
| Procedure setting | ||
| Hospital | 1 [Reference] | 1 [Reference] |
| Nonhospital | 0.56 (0.51-0.62) | 0.39 (0.33-0.45) |
| Preoperative vein mapping | ||
| Yes | 0.95 (0.92-0.99) | 0.92 (0.87-0.96) |
| No | 1 [Reference] | 1 [Reference] |
| Physicians | ||
| Sex | ||
| Male | 1 [Reference] | 1 [Reference] |
| Female | 1.08 (1.00-1.16) | 1.06 (0.87-1.28) |
| Time since medical school graduation, y | ||
| 0-10 | 0.91 (0.84-0.98) | 0.83 (0.68-1.03) |
| 11-20 | 0.92 (0.88-0.96) | 0.88 (0.78-1.00)b |
| 21-30 | 0.84 (0.81-0.88) | 0.85 (0.75-0.96) |
| ≥31 | 1 [Reference] | 1 [Reference] |
| Primary specialty | ||
| Vascular surgery | 1 [Reference] | 1 [Reference] |
| General surgery | 0.89 (0.86-0.93) | 0.77 (0.69-0.86) |
| Cardiothoracic surgery | 0.78 (0.73-0.84) | 0.67 (0.55-0.82) |
| Other | 0.93 (0.84-1.03) | 1.07 (0.74-1.57) |
| Practice location | ||
| Metropolitan | 1 [Reference] | 1 [Reference] |
| Rural | 0.89 (0.83-0.96) | 0.80 (0.65-0.98) |
| Practice region | ||
| Midwest | 0.92 (0.88-0.97) | 1.04 (0.91-1.18) |
| Northeast | 0.82 (0.77-0.86) | 0.83 (0.73-0.96) |
| South | 1 [Reference] | 1 [Reference] |
| West | 0.78 (0.75-0.82) | 0.86 (0.75-1.00) |
| Other | 1.28 (0.99-1.66) | 2.54 (1.27-5.06) |
| Volume of first-time hemodialysis access procedures | ||
| 11-18 | 1 [Reference] | 1 [Reference] |
| 19-32 | 1.00 (0.95-1.06) | 0.99 (0.88-1.12) |
| ≥33 | 1.14 (1.09-1.20) | 1.12 (0.99-1.26) |
The outcome being modeled was whether a patient received an arteriovenous graft (vs an arteriovenous fistula) as their first-time hemodialysis access.
P = .049.
Among physician characteristics, being a vascular surgeon (general surgeon vs vascular surgeon: aOR, 0.77 [95% CI, 0.69-0.86] or cardiothoracic surgeon vs vascular surgeon: aOR, 0.67 [95% CI, 0.55-0.82]) was independently associated with AVG use (Table 3). Physicians with more than 30 years experience since medical school graduation (11-20 years vs ≥31 years: aOR, 0.88 [95% CI, 0.78-1.00] or 21-30 years vs ≥ 31 years: aOR, 0.85 [95% CI, 0.75-0.96]), those practicing in the Northeast region (aOR, 0.83 [95% CI, 0.73-0.96]; reference: the South), and those in rural locations (aOR, 0.80 [95% CI, 0.65-0.98]; reference: metropolitan locations) had lower odds of AVG use (Table 3). There were no significant associations of physician sex or hemodialysis access volume with AVG use after risk adjustment (Table 3).
Description of Top Outlier Physicians
There were 168 physicians with AVG use rates greater than 50%, and 14 physicians with AVG use rates greater than 80% (Table 4). The physician who was the top outlier performed first-time hemodialysis access using an AVG in 96.4% of cases. Among the 14 physicians who were top outliers, the median time from medical school graduation was 35.5 (range, 9-50) years, 100% worked in a metropolitan setting, and all were based primarily in hospital settings. Vascular surgeons composed 50.0% of the group (n = 7), followed by general surgeons (n = 6 [42.9%]). There was also 1 thoracic surgeon (7.1%).
Table 4. Statistics of Top National Outlier Physicians With High Arteriovenous Graft Use (≥80%)a.
| Surgeon | Years Since Graduation | Region | Surgical Specialty | Hemodialysis Access Volume | Arteriovenous Graft Rate, % |
|---|---|---|---|---|---|
| A | 9 | South | Vascular | 28 | 96.4 |
| B | 27 | South | General | 25 | 96.0 |
| C | 39 | South | Vascular | 16 | 93.8 |
| D | 43 | South | Vascular | 15 | 93.3 |
| E | 48 | West | General | 14 | 92.9 |
| F | 16 | West | Vascular | 38 | 92.1 |
| G | 43 | Puerto Rico | General | 11 | 90.9 |
| H | 14 | Midwest | Vascular | 11 | 90.9 |
| I | 46 | West | Vascular | 41 | 90.2 |
| J | 26 | South | General | 17 | 88.2 |
| K | 50 | South | Thoracic | 12 | 83.3 |
| L | 32 | Midwest | Vascular | 35 | 82.9 |
| M | 49 | South | General | 23 | 82.6 |
| N | 13 | South | General | 11 | 81.8 |
Every outlier physician was located in a metropolitan area and based in a hospital.
Estimated Value Analysis
The mean (SD) procedural cost (ie, Medicare-allowed amount) for an AVF was $4526.70 ($2090.10), compared with $5794.00 ($2324.40) for an AVG (P < .001). Had the 498 physicians who were outliers performed their hemodialysis access procedures at the CMS-required AVG rate of 34%, 2228 AVGs could have been performed as AVFs, corresponding to potential savings of $2 823 544.40.
Discussion
Overall, most physicians are achieving the AVF target established by CMS as part of the FFBI.9 However, when considering performance at an individual surgeon level, there appears to be wide variation in quality and clear opportunities for focused improvement. Namely, 21% of surgeons are still using AVG in more than 34% of patients requiring new hemodialysis access. These data suggest that high AVG use is an area of low-value care that can be addressed at the surgeon level.
Most previously published literature reporting trends of hemodialysis access focus on the type of access used at the time of incident hemodialysis3,12 or rates of prevalent dialysis access type.7,11,16 Lynch et al7 reported that the percentage of US facilities achieving the 66% AVF goal increased from 6.4% in 2007 to 19.0% in 2010. Pisoni et al16 subsequently showed that AVF prevalence rose to 68% by 2013 but noted that AVF use at dialysis initiation remained low. An analysis of CMS claims data from 2012 suggested that AVF placement rates for new patients was only 30%.17 In this study, we found a much higher rate of AVF placement for first-time hemodialysis access, which may be attributable to the success of the FFBI.
The FFBI is a quality improvement initiative developed by the end-stage renal disease networks and CMS, along with clinicians, dialysis facilities, and patients, to improve patient-based vascular outcomes. The primary objective of the FFBI is to increase prevalent AVF rates, increase awareness of early referral for vascular access in patients with chronic kidney disease, and educate clinicians about the importance of predialysis hemodialysis access placement. Because the likelihood of starting dialysis with an AVF is significantly improved with earlier (predialysis) access placement,18 much of the current focus on this topic centers around a need for earlier nephrology referral as a means of increasing AVF use.19,20 The argument is that earlier nephrologist referral will lead to earlier surgeon referral, which will allow time for placement and maturation of an AVF before dialysis is initiated.
What most prior research and the FFBI fail to discuss are the potential surgeon-based limitations in AVF placement. Although referral for early hemodialysis access placement is important, the need for surgeons to practice responsibly and use AVF for initial access whenever possible is similarly important. Fistulae have been associated with higher nonmaturation rates, a need for more early interventions to achieve function, and a longer time to achieve accessibility than AVG are.21,22,23,24 These technical challenges may drive some surgeons to select AVG placement over AVF placement in certain patients. Based on the distribution of the curve in the Figure, it appears that most surgeons are limiting AVG use to selected patients only. However, there remain a number of physician who were outliers who might benefit from self-evaluation of their practice patterns, some of who are using AVG in more than 80% of cases.
Goodkin et al25 partially addressed the surgeon-level issue when they highlighted the importance of vascular surgery teaching programs supervising adequate numbers of AVF procedures for every trainee to improve access outcomes. This notion is supported by the finding by Saran et al,26 who demonstrated that surgical training is a key component to both AVF placement and maturation rates. However, no targeted initiatives have been implemented to improve AVF rates at the practicing surgeon level. We suggest that an initiative designed to identify and educate surgeons who are performing outside the targeted boundaries of hemodialysis access practice could be an efficient means of augmenting the success of FFBI. This initiative, termed the Improving Wisely campaign,27 could be used to provide peer-to-peer feedback to surgeons who are outliers in an effort to encourage them to think about how they can increase their AVF rates. An Improving Wisely initiative was recently implemented among surgeons providing Mohs procedures,28 and the simple act of receiving a notification about outlier performance was able to significantly reduce the proportion of surgeons performing outside the predefined boundary of appropriate care.9 A similar nonpunitive method of providing surgeons who perform hemodialysis access procedures with a performance report card showing their AVG use rate relative to their peers could be used to improve AVF use among the surgeons we identified as outliers. The Vascular Quality Initiative provides web-based clinical data registries29 that already allows centers to anonymously compare their patients, processes of care, and procedure outcomes with others. This technology could possibly be expanded to provide surgeon-specific feedback as well.
The main surgeon characteristics that were associated with high AVG use rates in this study included more than 30 years in practice and vascular surgery specialty. There have been a number of reports recently demonstrating worse outcomes after surgery performed by older surgeons.30,31 For example, Duclos et al32 found that surgeons aged 35 to 50 years had better outcomes after thyroidectomy than their older colleagues, including a lower probability of recurrent laryngeal nerve palsy and hypoparathyroidism. Walijee et al30 reported lower rates of perioperative mortality after carotid endarterectomy among surgeons aged 41 to 50 years compared with surgeons older than 60 years. Explanations for these observations range from increasing nonclinical workload burdens, challenges with adopting new guidelines and techniques, and physician fatigue in the older age groups.32 Notably, the FFBI was first implemented in 2003,2 which was 15 years ago. Therefore most surgeons who have passed 0 to 20 years since medical school graduation were trained in an era that emphasized the importance of AVF use. Perhaps the older surgeon cohort in this study had been exposed to fewer data supporting AVF over AVG use and/or had less training in AVF creation, which is known to be important for achieving success in this task.26
Alternatively, it is possible that patients with greater complexity are being referred to older surgeons with more experience. This theory would also explain the differences in AVG use among vascular surgeons compared with other specialties. Vascular surgeons learn how to perform hemodialysis access operations as part of their vascular fellowship or residency training, and their board certification requires knowledge about the indications and contraindications for AVF use.33 It seems unlikely that a high number of vascular surgeons would overuse AVG inappropriately, but the hierarchical model in this study suggests this is the case. There were 313 vascular surgeons who performed AVG in more than 34% of cases. Whether these surgeons have developed a specialty practice in complex hemodialysis access care would be informative. The benefit of the unique Medicare database used in this study is that we can actually identify individual outlier surgeons and investigate their practice characteristics. As shown in Table 4, 50% of the 14 physicians who were top outliers for AVG placement are vascular surgeons, most of whom are experienced surgeons with moderate hemodialysis access volume. An interesting extension of this study would be to perform qualitative interviews of the physicians who were top outliers in an effort to better understand what makes their practices so different from their peers. This process has been used to understand factors that differentiate top-performing hospitals from low-performing hospitals in acute myocardial infarction mortality rates,34,35 and it could similarly applied to this cohort.
We also identified significant regional and practice location variation in AVG use rates. Surgeons practicing in the Northeast region have lower AVG rates, as do surgeons practicing in rural settings. Geographic variation in hemodialysis access trends is well described.7,11,12,13 Although the causative mechanisms of these differences are not completely understood, variation in patient populations,7 predialysis nephrology care,12 and/or physician practice patterns13 may play a role. Nguyen et al13 directly addressed some of these concerns in a recent study designed to address significant facility-based variation in the Northwest Renal Network. They demonstrated that by providing targeted education and promoting multidisciplinary teamwork among facilities with less than 40% AVF use, prevalent AVF increased from 31% preintervention to 56% by follow-up 4 years postintervention.36 This is another example of how targeted feedback and constructive educational initiatives can be used to improve patient outcomes.
Limitations
The limitations of this study deserve consideration. First, both arterial and venous diameters have been shown to correlate with AVF maturation success37,38; in patients with inadequate vasculature characteristics, an AVG may be preferred to AVF because of the high likelihood that the AVF will fail.39 The Medicare claims database does not include anatomic data, and thus these characteristics could not be accounted for. A longitudinal investigation assessing patient-based secondary AVF rates among those patients who initially received an AVG may be useful in differentiating preferential practice from anatomical restrictions in favor of AVG. Second, we were unable to account for patient obesity in this analysis, because this diagnosis has been shown previously to be inaccurately captured by International Classification of Diseases, Ninth Revision codes.40,41 Third, we only evaluated permanent hemodialysis access types in this study in an effort to focus on AVG use as a surgeon-specific metric. More than 80% of patients still initiate dialysis with a central venous catheter in the United States,16 which is a quality improvement metric being actively addressed by CMS.14 Finally, we designed our study to assess first-time AVF vs AVG use rates by individual surgeons. In an attempt to limit the analysis to first-time hemodialysis access attempts, we required that patients have a minimum of 1 year of Medicare enrollment in which no prior documented hemodialysis access procedures were performed. It is possible, however, that patients had earlier hemodialysis access attempts prior to enrolling in Medicare that we were unable to capture.
Conclusions
Most surgeons achieve the CMS goal of more than 66% AVF for first-time hemodialysis access placement, but one-fifth of surgeons have AVG use rates higher than the recommended best practices guideline of 34%. We identified a number of surgeon characteristics associated with outlying, including years since medical school graduation, surgical specialty, and region of practice, and location of practice, which may possibly represent variation in hemodialysis access experience and/or patient referral practices. We propose that that sharing benchmarked performance data with surgeons could be an actionable step in achieving more high-value care in hemodialysis access surgery.
eTable 1. Current Procedural Terminology codes used to identify hemodialysis access procedures
eTable 2. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes for chronic kidney disease and end-stage renal disease
eTable 3. Region definitions by state.
References
- 1.US Renal Data System Chapter 11: costs of ESRD. https://www.usrds.org/2012/view/v2_11.aspx. Updated 2012. Accessed October 29, 2018.
- 2.Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol. 2007;2(5):1043-1053. doi: 10.2215/CJN.01080307 [DOI] [PubMed] [Google Scholar]
- 3.Praehauser C, Breidthardt T, Moser-Bucher CN, et al. The outcome of the primary vascular access and its translation into prevalent access use rates in chronic haemodialysis patients. Clin Kidney J. 2012;5(4):339-346. doi: 10.1093/ckj/sfs055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravani P, Gillespie BW, Quinn RR, et al. Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol. 2013;24(10):1668-1677. doi: 10.1681/ASN.2012121234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manns B, Tonelli M, Yilmaz S, et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. J Am Soc Nephrol. 2005;16(1):201-209. doi: 10.1681/ASN.2004050355 [DOI] [PubMed] [Google Scholar]
- 6.Domenick Sridharan N, Fish L, Yu L, et al. The associations of hemodialysis access type and access satisfaction with health-related quality of life. J Vasc Surg. 2018;67(1):229-235. doi: 10.1016/j.jvs.2017.05.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch JR, Wasse H, Armistead NC, McClellan WM. Achieving the goal of the Fistula First breakthrough initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis. 2011;57(1):78-89. doi: 10.1053/j.ajkd.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vascular Access 2006 Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(suppl 1):S176-S247. doi: 10.1053/j.ajkd.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 9.CGS Fistula First Breakthrough Initiative provides roadmap to reach goal of 66%. https://cgsmedicare.com/jc/pubs/news/2009/1009/cope10862.html. Published April 16, 2009. Accessed May 6, 2019.
- 10.Spergel LM. Has the Fistula First Breakthrough Initiative caused an increase in catheter prevalence? Semin Dial. 2008;21(6):550-552. doi: 10.1111/j.1525-139X.2008.00501.x [DOI] [PubMed] [Google Scholar]
- 11.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219-3226. doi: 10.1093/ndt/gfn261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarkowsky DS, Hicks CW, Arhuidese I, et al. Quality improvement targets for regional variation in surgical end-stage renal disease care. JAMA Surg. 2015;150(8):764-770. doi: 10.1001/jamasurg.2015.1126 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen VD, Lawson L, Ledeen M, et al. Successful multidisciplinary interventions for arterio-venous fistula creation by the Pacific Northwest Renal Network 16 vascular access quality improvement program. J Vasc Access. 2007;8(1):3-11. doi: 10.1177/112972980700800102 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services CMS ESRD measures manual for the 2018 performance period/2020 payment year. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/ESRD-Manual-v30.pdf. Published 2018. Accessed October 29, 2018.
- 15.National Bureau of Economic Research CBSA to FIPS county crosswalk. http://www.nber.org/data/cbsa-fips-county-crosswalk.html. Published 2018. Accessed November 20, 2018.
- 16.Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis. 2015;65(6):905-915. doi: 10.1053/j.ajkd.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services FY 2012 online performance index. https://www.cms.gov/About-CMS/Agency-Information/PerformanceBudget/downloads/CMSOPAFY2012.pdf. Published 2012. Accessed October 9, 2018.
- 18.Hod T, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS. Arteriovenous fistula placement in the elderly: when is the optimal time? J Am Soc Nephrol. 2015;26(2):448-456. doi: 10.1681/ASN.2013070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazmi WH, Obrador GT, Khan SS, Pereira BJ, Kausz AT. Late nephrology referral and mortality among patients with end-stage renal disease: a propensity score analysis. Nephrol Dial Transplant. 2004;19(7):1808-1814. doi: 10.1093/ndt/gfg573 [DOI] [PubMed] [Google Scholar]
- 20.Kessler M, Frimat L, Panescu V, Briançon S. Impact of nephrology referral on early and midterm outcomes in ESRD: epidémiologie de l’insuffisance renale chronique terminale en Lorraine (EPIREL): results of a 2-year, prospective, community-based study. Am J Kidney Dis. 2003;42(3):474-485. doi: 10.1016/S0272-6386(03)00805-9 [DOI] [PubMed] [Google Scholar]
- 21.Disbrow DE, Cull DL, Carsten CG III, Yang SK, Johnson BL, Keahey GP. Comparison of arteriovenous fistulas and arteriovenous grafts in patients with favorable vascular anatomy and equivalent access to health care: is a reappraisal of the Fistula First Initiative indicated? J Am Coll Surg. 2013;216(4):679-685. [DOI] [PubMed] [Google Scholar]
- 22.Asif A, Roy-Chaudhury P, Beathard GA. Early arteriovenous fistula failure: a logical proposal for when and how to intervene. Clin J Am Soc Nephrol. 2006;1(2):332-339. doi: 10.2215/CJN.00850805 [DOI] [PubMed] [Google Scholar]
- 23.Bylsma LC, Gage SM, Reichert H, Dahl SLM, Lawson JH. Arteriovenous fistulae for haemodialysis: a systematic review and meta-analysis of efficacy and safety outcomes. Eur J Vasc Endovasc Surg. 2017;54(4):513-522. doi: 10.1016/j.ejvs.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 24.Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol. 2013;8(5):810-818. doi: 10.2215/CJN.00730112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodkin DA, Pisoni RL, Locatelli F, Port FK, Saran R. Hemodialysis vascular access training and practices are key to improved access outcomes. Am J Kidney Dis. 2010;56(6):1032-1042. doi: 10.1053/j.ajkd.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 26.Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247(5):885-891. doi: 10.1097/SLA.0b013e31816c4044 [DOI] [PubMed] [Google Scholar]
- 27.Makary MA, Mehta A, Xu T. Improving wisely using physician metrics. Am J Med Qual. 2018;33(1):103-105. doi: 10.1177/1062860617704504 [DOI] [PubMed] [Google Scholar]
- 28.Krishnan A, Xu T, Hutfless S, et al. ; the American College of Mohs Surgery Improving Wisely Study Group . Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153(6):565-570. doi: 10.1001/jamadermatol.2017.1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Society for Vascular Surgery Vascular Quality Initiative. http://www.vqi.org/. Updated 2019. Accessed February 17, 2019.
- 30.Waljee JF, Greenfield LJ, Dimick JB, Birkmeyer JD. Surgeon age and operative mortality in the United States. Ann Surg. 2006;244(3):353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsugawa Y, Newhouse JP, Zaslavsky AM, Blumenthal DM, Jena AB. Physician age and outcomes in elderly patients in hospital in the US: observational study. BMJ. 2017;357:j1797. doi: 10.1136/bmj.j1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duclos A, Peix JL, Colin C, et al. ; CATHY Study Group . Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012;344:d8041. doi: 10.1136/bmj.d8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Board of Surgery Vascular surgery: content outline for the in-training (VSITE), qualifying (VSQE) and recertification examinations. http://www.absurgery.org/xfer/VS-ITE-QE-RECERT.pdf. Published 2015. Accessed October 15, 2018.
- 34.Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? a qualitative study. Ann Intern Med. 2011;154(6):384-390. doi: 10.7326/0003-4819-154-6-201103150-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherlin EJ, Curry LA, Thompson JW, et al. Features of high quality discharge planning for patients following acute myocardial infarction. J Gen Intern Med. 2013;28(3):436-443. doi: 10.1007/s11606-012-2234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen VD, Griffith CN, Reus J, et al. Successful AV fistula creation does not lead to higher catheter use: the experience by the Northwest Renal Network 16 Vascular Access Quality Improvement Program, four years follow-up. J Vasc Access. 2008;9(4):260-268. doi: 10.1177/112972980800900407 [DOI] [PubMed] [Google Scholar]
- 37.Silva MB Jr, Hobson RW II, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302-307. [DOI] [PubMed] [Google Scholar]
- 38.Lauvao LS, Ihnat DM, Goshima KR, Chavez L, Gruessner AC, Mills JL Sr. Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49(6):1499-1504. doi: 10.1016/j.jvs.2009.02.018 [DOI] [PubMed] [Google Scholar]
- 39.Woo K, Ulloa J, Allon M, et al. Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg. 2017;65(4):1089-1103.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ammann EM, Kalsekar I, Yoo A, Johnston SS. Validation of body mass index (BMI)-related ICD-9-CM and ICD-10-CM administrative diagnosis codes recorded in US claims data. Pharmacoepidemiol Drug Saf. 2018;27(10):1092-1100. doi: 10.1002/pds.4617 [DOI] [PubMed] [Google Scholar]
- 41.Lloyd JT, Blackwell SA, Wei II, Howell BL, Shrank WH. Validity of a claims-based diagnosis of obesity among medicare beneficiaries. Eval Health Prof. 2015;38(4):508-517. doi: 10.1177/0163278714553661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Current Procedural Terminology codes used to identify hemodialysis access procedures
eTable 2. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes for chronic kidney disease and end-stage renal disease
eTable 3. Region definitions by state.