Abstract
This study analyzes data from a randomized clinical trial in infants 90 days and younger to develop a pharmacokinetic model to aid physicians in maintaining minimum inhibitory concentrations of vancomycin in young infants with Staphylococcus aureus infection.
Target trough concentrations for vancomycin hydrochloride therapy in infants younger than 90 days are poorly defined. In adults, the pharmacodynamic target for Staphylococcus aureus correlates with the ratio of the area under the concentration-time curve over a 24-hour period (AUC24) to the minimum inhibitory concentration (MIC) of the bacteria exceeding 400 (ie, AUC24/MIC>400).1,2 There are no widely available AUC calculators that we know of for use in infants younger than 90 days to enable clinicians to determine the AUC24 at steady state (approximately 48 hours) based on a trough concentration. We developed a population-based pharmacokinetic model to determine the association between serum trough vancomycin concentrations and AUC24.
Methods
Data were collected from 104 young infants enrolled in a randomized clinical trial of vancomycin between September 1, 2014, and December 31, 2017.4 Data were analyzed using nonlinear mixed-effect modeling (NONMEM version 7; ICON plc) interfaced with PLT tools from September 1, 2018, to December 31, 2018. The first-order conditional estimation method with interaction was used. The covariates of weight, sex, serum creatinine concentration, and albumin level were added in a stepwise manner to the model. Model accuracy was assessed using goodness-of-fit plots and a visual predictive check. The study was approved by the Royal Children’s Hospital Melbourne Human Research Ethics Committee (HREC 34030) and the South Eastern Sydney Local Health District Human Research Ethics Committee (SSA 16/G/335), and parents provided written informed consent to have blood samples taken.
Simulations were performed using Matlab version 9.1.0.441655 (R2016b) (MathWorks). Covariates from a separate database of 1400 infants admitted to the same hospital over an 18-month period aged 0 to 90 days with normal serum creatinine values were used to generate virtual patients. Institutional review board approval was not needed for this cohort because the data were deidentified. Simulations were stratified to generate 1000 concentration-time profiles for each dosage interval based on the British National Formulary for Children guideline.
Results
The final model was a 2-compartment model with a sigmoidal maturation model (MATMOD) for clearance3 and included weight (WT) and serum creatinine (Cr) as covariates, as follows:
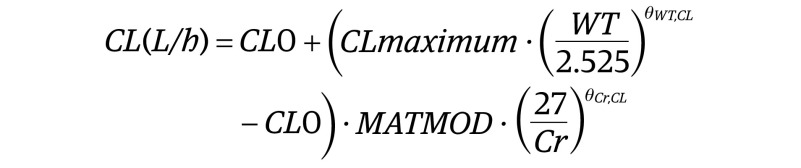 |
 |
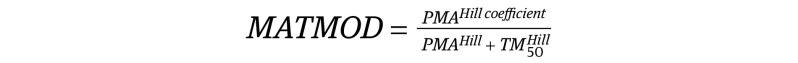 |
where CL0 is the clearance at postmenstrual age (PMA) 0; θWT,CL is the exponent for weight on clearance; θCr,CL is the exponent for creatinine on clearance; and θWT,Vc is the exponent for weight on volume of the central compartment (Vcentral). The θWT,CL was fixed at 0.75 because the model estimate was unstable. Goodness-of-fit plots and visual predictive check showed that the model had good predictive performance.
For S aureus with an MIC of 1 mg/L, the probability of attaining an AUC24 greater than 400 mg.h/L for the various dosage intervals are shown in the Figure. The 48-hour trough concentrations that provided a 90% probability of target attainment were greater than 15 to 20 mg/L for the 6-hour dosage interval and greater than 8 to 15 mg/L for the 12-hour dosage interval. Trough concentrations of approximately 30 mg/L were required to achieve an AUC24 greater than 800 mg.h/L (for an MIC of 2) for a 6-hour dosage interval and would not be achievable without increasing the risk of drug-related toxic effects.
Discussion
To our knowledge, this is the first vancomycin population pharmacokinetic model in young infants developed from prospectively collected data from a randomized clinical trial.4 Studies in adults suggest that trough concentrations of 15 to 20 mg/L are required to achieve an AUC24 greater than 400 mg.h/L, and these targets are often applied to young infants.5 These recommendations from the adult studies are intended for a 12-hour dosage interval. In contrast, in young infants, dosage intervals range between 6 and 24 hours; therefore, the same trough targets cannot be extrapolated. With less-frequent administration of vancomycin, lower trough concentrations can be targeted and the same AUC24 achieved. Our results differ from a previous pharmacokinetic study of young infants who received the drug at 12- or 24-hour intervals. That study reported that with trough concentrations of 7 to 11 mg/L, an AUC24 greater than 400 mg.h/Lwould be achieved in more than 90% of infants.6 This difference may be partially explained by the retrospective data set used in the previous model.
Study limitations include potential sampling or data errors; however, data were prospectively collected after appropriate staff training. Also, because our study population were young infants hospitalized in neonatal units, results can only be generalized to those settings.
The results of this study have important implications for the successful treatment of S aureus infections with vancomycin in young infants. The association between trough concentration and AUC24 for different dosage intervals (Figure) can be used to aid clinicians in the interpretation of trough concentrations in young infants with sepsis.
Figure. Probability of Attainment of Area Under the Curve (AUC24) Greater Than 400 mg.h/L in Young Infants.
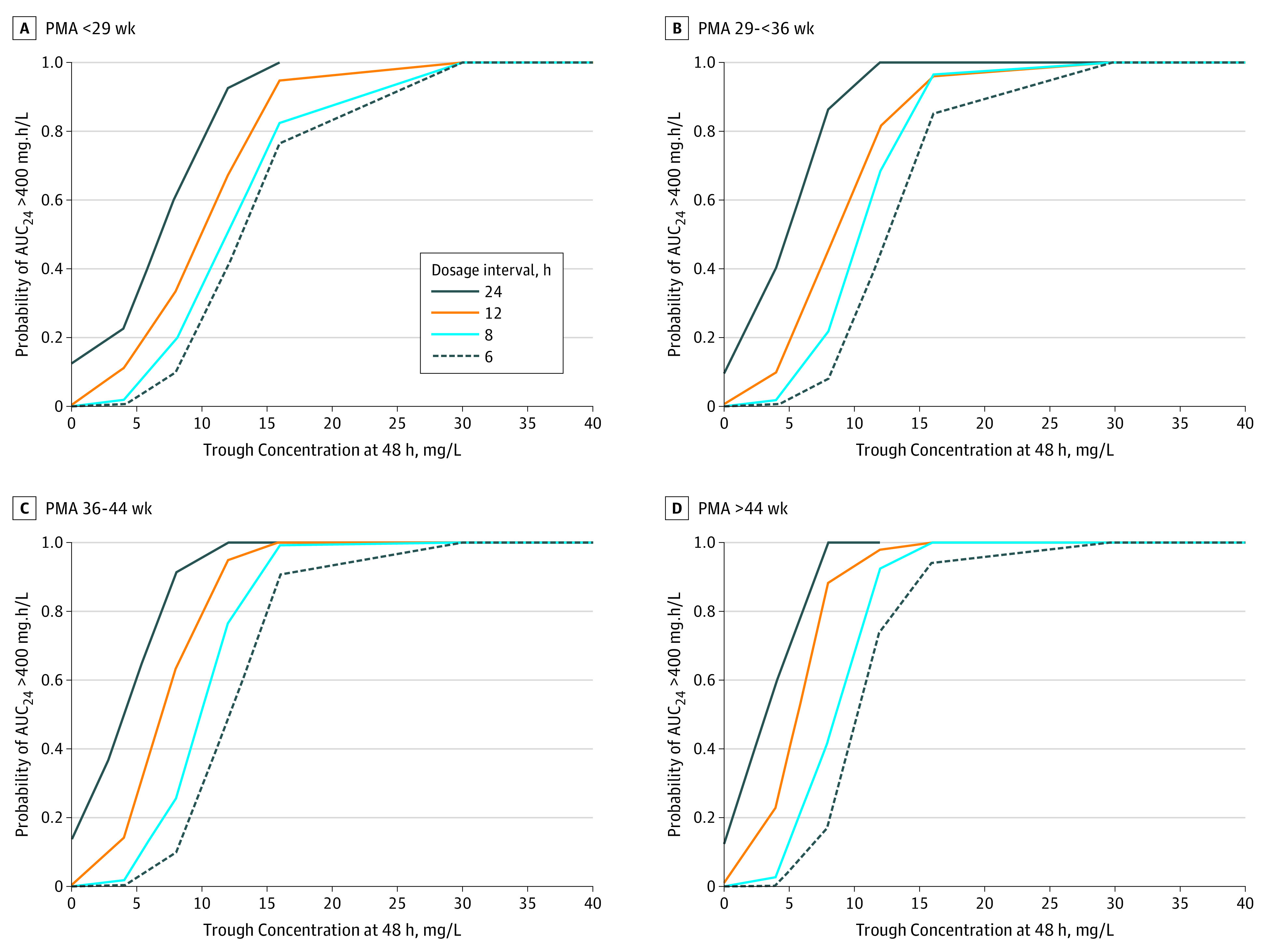
Probabilities are based on 48-hour trough concentrations of vancomycin, administered in 6- , 8-, 12- or 24-hour dosage intervals. Dosage intervals are shown for infant age groups expressed as postmenstrual age (PMA) in weeks.
References
- 1.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi: 10.2165/00003088-200443130-00005 [DOI] [PubMed] [Google Scholar]
- 2.Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654-1663. doi: 10.1128/AAC.01485-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BJ, Holford NH. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child. 2013;98(9):737-744. doi: 10.1136/archdischild-2013-303720 [DOI] [PubMed] [Google Scholar]
- 4.Gwee A, Cranswick N, McMullan B, et al. Continuous versus intermittent vancomycin infusions in infants: a randomized controlled trial. Pediatrics. 2019;143(2):e20182179. doi: 10.1542/peds.2018-2179 [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285-292. doi: 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer A, Hersh AL, El-Komy MH, et al. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob Agents Chemother. 2014;58(11):6454-6461. doi: 10.1128/AAC.03620-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


