Key Points
Question
Is seizure induction by cortical stimulation during intracranial electroencephalography associated with good surgical outcome in patients with focal drug-resistant epilepsy?
Findings
In this cohort study of 103 patients with focal drug-resistant epilepsy, cortical stimulation induced typical electroclinical seizures in 59 patients (57.3%). Induction of seizures was associated with better surgical outcome; a higher percentage of resected contacts from the seizure-onset zone informed by cortical stimulation, similar to that of spontaneous seizures, was associated with better surgical outcome.
Meaning
Cortical stimulation appears to be reliable in identifying the cortical area responsible for seizure generation and to be associated with surgical outcome.
Abstract
Importance
Cortical stimulation is used during presurgical epilepsy evaluation for functional mapping and for defining the cortical area responsible for seizure generation. Despite wide use of cortical stimulation, the association between cortical stimulation–induced seizures and surgical outcome remains unknown.
Objective
To assess whether removal of the seizure-onset zone resulting from cortical stimulation is associated with a good surgical outcome.
Design, Setting, and Participants
This cohort study used data from 2 tertiary epilepsy centers: Montreal Neurological Institute in Montreal, Quebec, Canada, and Grenoble-Alpes University Hospital in Grenoble, France. Participants included consecutive patients (n = 103) with focal drug-resistant epilepsy who underwent stereoelectroencephalography between January 1, 2007, and January 1, 2017. Participant selection criteria were cortical stimulation during implantation, subsequent open surgical procedure with a follow-up of 1 or more years, and complete neuroimaging data sets for superimposition between intracranial electrodes and the resection.
Main Outcomes and Measures
Cortical stimulation–induced typical electroclinical seizures, the volume of the surgical resection, and the percentage of resected electrode contacts inducing a seizure or encompassing the cortical stimulation–informed and spontaneous seizure-onset zones were identified. These measures were correlated with good (Engel class I) and poor (Engel classes II-IV) surgical outcomes. Electroclinical characteristics associated with cortical stimulation–induced seizures were analyzed.
Results
In total, 103 patients were included, of whom 54 (52.4%) were female, and the mean (SD) age was 31 (11) years. Fifty-nine patients (57.3%) had cortical stimulation–induced seizures. The percentage of patients with cortical stimulation–induced electroclinical seizures was higher in the good outcome group than in the poor outcome group (31 of 44 [70.5%] vs 28 of 59 [47.5%]; P = .02). The percentage of the resected contacts encompassing the cortical stimulation–informed seizure-onset zone correlated with surgical outcome (median [range] percentage in good vs poor outcome: 63.2% [0%-100%] vs 33.3% [0%-84.6%]; Spearman ρ = 0.38; P = .003). A similar result was observed for spontaneous seizures (median [range] percentage in good vs poor outcome: 57.1% [0%-100%] vs 32.7% [0%-100%]; Spearman ρ = 0.32; P = .002). Longer elapsed time since the most recent seizure was associated with a higher likelihood of inducing seizures (>24 hours: 64.7% vs <24 hours: 27.3%; P = .04).
Conclusions and Relevance
Seizure induction by cortical stimulation appears to identify the epileptic generator as reliably as spontaneous seizures do; this finding might lead to a more time-efficient intracranial presurgical investigation of focal epilepsy as the need to record spontaneous seizures is reduced.
This cohort study examines the cortical stimulation of the epileptogenic zone and its association with good or poor outcomes in patients with focal epilepsy.
Introduction
Cortical stimulation is used during intracranial electroencephalography (EEG) in patients with epilepsy for functional mapping and for defining the epileptogenic zone. Despite its wide application, cortical stimulation used in seizure induction was the topic of only 14 original articles published between 1974 and 2015.1 Two of these 14 studies correlated cortical stimulation findings to surgical outcome, but they provided conflicting evidence.2,3 The association of cortical stimulation with surgical outcome remained uncertain.1
A primary aim of this study was to investigate whether the removal of the cortical stimulation–informed seizure-onset zone (SOZ) as a marker for the epileptogenic zone is associated with good surgical outcome. We performed in a large series of patients a detailed neurophysiologic characterization of stereoelectroencephalography (SEEG) data on a single-contact basis, including the superposition of the surgical cavity with the implanted electrodes, and then we correlated these findings with surgical outcome. We focused on cortical stimulation–induced electroclinical seizures that mimic a patient's typical spontaneous seizures. We hypothesized that cortical stimulation–induced seizures mimicked the patient’s spontaneous seizures and therefore provided similar information on the epileptogenic zone as spontaneous seizures do.
The demonstration that cortical stimulation–induced seizures reliably identify the epileptogenic zone could be associated with a substantially reduced duration of the SEEG investigation, a shorter hospitalization, and substantially decreased risks and costs. The probability of having a cortical stimulation–induced seizure has been postulated to be dependent on clinical and a number of anatomo-functional characteristics of the epileptogenic zone.4,5,6 A secondary aim was to identify the patients for whom cortical stimulation could be advantageous.
Methods
Patient Sample
We reviewed all medical records and recordings of patients with drug-resistant focal epilepsy who underwent SEEG during presurgical workup in 2 tertiary epilepsy centers (Montreal Neurological Institute [MNI] in Montreal, Quebec, Canada and Grenoble-Alpes University Hospital [CHUGA] in Grenoble, France) between January 1, 2007, and January 1, 2017. The protocol for this study received approval from the MNI Institutional Review Board. Informed consent was waived by the MNI Director of Professional Services as this research was a retrospective study.
Selection criteria were as follows. First, patients had to have undergone cortical stimulation during the SEEG investigation. The decision to perform cortical stimulation was taken independently of this study by a neurophysiologist (F.D., A.-S.J., L.M., and P.K.). Reasons for exclusion from cortical stimulation included a conflict in scheduling, technical problems with electrodes or recordings, inability of the patient to collaborate during cortical stimulation owing to cognitive impairment, and patient anxiety or acute illness. Second, patients had to have undergone, after SEEG investigation, open epilepsy surgical procedure with a minimum postsurgical follow-up period of 1 year. Third, patients had to have presurgical, peri-implantation, and postsurgical brain imaging (slices ≤2 mm) for exact localization of individual electrode contacts and resection cavity. In case of more than 1 epilepsy surgical procedure, the postsurgical time of follow-up and Engel score7 were counted from the date of the latest operation.
Decision to conduct SEEG was made, and implantation schemes were tailored when phase 1 presurgical evaluation provided insufficient or noncongruent information for direct surgical planning. The SEEG electrodes were implanted stereotactically using an image-guided system.8 The type and extent of the surgical procedure were clinically defined. Use of cortical stimulation to tailor some procedures did not present a bias but allowed us to evaluate whether removal of the cortical stimulation—informed SOZ contacts had prognostic significance.
Analysis of Spontaneous SOZ
We defined spontaneous SOZ according to Spanedda et al.9 In patients with seizures from different locations, all contacts within the separate spontaneous SOZs were classified as SOZ contacts. We analyzed the contacts involved during the first 3 seconds from the onset of the seizures (spontaneous SOZ) in a bipolar montage. The onset was defined by consensus between 2 neurophysiologists (F.D. and C.C.O., A.-S.J. and C.C.O., L.M. and C C.O, or P.K. and C.C.O.). We assessed the different seizure-onset patterns10,11 and indicated whether the onset was diffuse (involvement of ≥1 lobe at onset) or not.11
Cortical stimulation was performed with continuous SEEG video monitoring during 1 or more sessions that lasted 2 to 4 hours while receiving home medication before reduction (at least 24-48 hours after implant to avoid the acute adverse effects of electrode insertion) or after reinstatement of antiepileptic medication. Stimulation while taking medication was done to avoid inducing atypical electroclinical seizures. Atypical electroclinical seizures were rare in our sample, with 7.8% (8 of 102 patients) experiencing atypical seizures at 50-Hz stimulation and 1.5% (1 of 66 patients) experiencing seizures at 1-Hz stimulation. All contacts were stimulated, except if outside the brain; if showing extended periods of artifact; if impedances were not normal; if stimulation was painful; and, for MNI patients, if contacts were unlikely to be part of the epileptogenic zone according to the clinical investigation.5
Biphasic square pulses of currents were applied between 2 adjacent contacts at low frequency (1-Hz pulse width per phase = 1-1.5 milliseconds for MNI patients vs 0.5-1 millisecond for CHUGA patients; train duration = 20-30 seconds for MNI patients vs 40 seconds for CHUGA patients) and at high frequency (50-Hz pulse width per phase = 0.5 millisecond for MNI patients vs 0.5-1 millisecond for CHUGA patients; duration = 5 seconds).
Electrical currents were gradually increased from 0.5 mA to a maximum of 5 mA in mesial temporal structures and to a maximum of 8 mA in the neocortex. Stimulations were repeated every 30 to 60 seconds after baseline activity resumed in the SEEG. Cortical stimulation was ended at a pair of contacts if it induced an electroclinical seizure, a physiological response perceived to be disabling or painful, or if the maximum current was reached. Adverse events during cortical stimulation were negligible when following the described procedure. Cortical stimulation was performed with the aim of inducing seizures. The complications of induced seizures were lower than those occurring in the epilepsy monitoring unit because stimulation was performed with the medical team at bedside and with intravenous medication in place. To our knowledge, no adverse effects outlasted the duration of the stimulation.
A typical electroclinical-induced seizure was defined as a typical seizure or aura with clinical semiology resembling spontaneous seizure or aura. All events were required to have electrographic changes with clear spatial and temporal evolution. We analyzed all contacts involved at the onset (time 0) immediately after cessation of the stimulation artifact for 50-Hz stimulation or at the time of seizure onset during 1-Hz stimulation (the first SEEG changes can be visualized owing to fewer artifacts during 1-Hz stimulation); these contacts defined the cortical stimulation–informed SOZ.
Coregistration of Electrode Contacts and Resection Cavities
We coregistered the multisequential and multiplanar magnetic resonance imaging or computed tomography using the MINC toolkit (GNU General Public License). We computed the volume of the surgical cavity by marking it visually using MRIcron (MRIcroGL)12 before extending it by 5 mm using Matlab (MathWorks) to account for sagging, coregistration error, and consequence of partial contact resection. In the case of a surgical procedure prior to implantation, we considered as surgical cavity only the difference between the previous and the new cavity. On the basis of the superposition of the surgical cavity with the SOZ electrodes, we calculated the percentage of resected SOZ contacts for the SOZs (spontaneous or cortical stimulation–informed) and the percentage of resected channels inducing typical electroclinical seizures.
The epileptogenic zone is the smallest region that has to be resected to achieve seizure freedom.13 Hence, in patients with good outcome, the surgical cavity included the epileptogenic zone.12,14 We hypothesized that, if the cortical stimulation–informed SOZ equaled the epileptogenic zone, then the volume of the SOZ was completely resected in the good outcome group and partially resected or not resected in the poor outcome group. We evaluated the performance of the cortical stimulation–informed SOZ for the delineation of the epileptogenic zone by calculating the percentage of the induced SOZ contacts that were resected.
Statistical Analysis
Because many variables were not normally distributed, nonparametric statistics were applied. The differences in the median of 2 groups were assessed with a Wilcoxon rank sum test. The Spearman correlation was computed for numeric variables. A χ2 test was used to determine differences in contingency tables, and a Fisher exact test was used if any of the groups in the contingency table had an expected value lower than 10 under the null hypothesis. A binomial test was performed to determine the difference in rate between groups. Analysis was performed for groups of 5 or more patients only. A 2-sided P ≤ .05 was considered statistically significant.
Results
In total, 103 (35.0%) of 294 patients fulfilled the inclusion criteria (Figure 1). Of the 103 patients, 54 (52.4%) were female and the mean (SD) age was 31 (11) years. Forty-four patients (42.7%) had a good surgical outcome (Engel class I) with a median (range) length of postsurgical follow-up of 42.2 (12-122.1) months. The electroclinical characteristics of the 103 patients are shown in the Table.
Figure 1. Patient Selection Flowchart.
Incomplete data for most patients meant that the preimplantation, implantation, or postimplantation neuroimaging did not fit the inclusion criteria (slices of <2 mm). The rate of good surgical outcome (Engel class I) was 42.7% in patients included in the study and 41.9% in patients excluded (P = .91). CHUGA indicates Grenoble-Alpes University Hospital; MNI, Montreal Neurological Institute; and SEEG, stereoelectroencephalography.
Table. Electroclinical Characteristics of Patients .
| Variable or Group | No. | Response Rate, % or Median | P Value for Difference in Response Rate | |
|---|---|---|---|---|
| Total Group | Patients With CS-Induced Seizures | |||
| All patients | 103 | 59 | 57.3% | |
| Sex | .24a | |||
| Male | 49 | 31 | 63.3% | |
| Female | 54 | 28 | 51.9% | |
| Age at onset, y | 103 | 59 | Pos = 13 | .37b |
| Neg = 11 | ||||
| Duration, y | 103 | 59 | Pos = 16 | .72b |
| Neg = 14 | ||||
| No. of iEEG electrodes | 103 | 59 | Pos = 12 | .54b |
| Neg = 10.5 | ||||
| History of focal to bilateral tonic clonic seizures | >.99a | |||
| Yes | 60 | 35 | 58.3% | |
| No | 24 | 14 | 58.3% | |
| Neuropathologic disease | .65c | |||
| MCD | 5 | 4 | 80.0% | |
| Gliosis | 63 | 37 | 58.7% | |
| Hippocampal sclerosis | 31 | 15 | 48.4% | |
| Low-grade tumor | 3 | 2 | 66.7% | |
| Vascular malformation | 1 | 1 | 100% | |
| FCD type II | .06a | |||
| Yes | 32 | 13 | 40.6% | |
| No | 64 | 39 | 60.9% | |
| Spontaneous SOZ extent (contacts) | 96 | 55 | Pos = 11 | .96b |
| Neg = 11.5 | ||||
| SOZ location | .47c | |||
| Frontal | 27 | 13 | 48.1% | |
| Parietal | 1 | 1 | 100% | |
| Temporal | 27 | 18 | 66.7% | |
| Occipital | 2 | 1 | 50.0% | |
| Multilobar | 39 | 19 | 48.7% | |
| Seizure-onset pattern | .88c | |||
| LVFA | 40 | 23 | 57.5% | |
| Burst of polyspikes followed by LVFA | 22 | 12 | 54.5% | |
| Sharp activity <13 Hz | 14 | 7 | 50.0% | |
| Decremental response | 6 | 2 | 33.3% | |
| Othere | 14 | 6 | 42.9% | |
| Mesiotemporal involvement | .19a | |||
| Yes | 44 | 27 | 61.4% | |
| No | 52 | 25 | 48.1% | |
| Diffuse seizures | .72a | |||
| Yes | 30 | 18 | 60.0% | |
| No | 73 | 41 | 56.2% | |
| Resection volume, cm3 | 103 | 59 | Pos = 54.5 | .55b |
| Neg = 50.2 | ||||
| Time since most recent seizure | .04c | |||
| <24 h | 11 | 3 | 27.3% | |
| >24 h | 34 | 22 | 64.7% | |
| Type of stimulation | <.001d | |||
| 1 Hz | 66 | 12 | 18.2% | |
| 50 Hz | 102 | 56 | 54.9% | |
Abbreviations: CS, cortical stimulation; FCD, focal cortical dysplasia; iEEG, intracranial electroencephalography; LVFA, low-voltage fast activity; MCD, malformation of cortical development other than FCD type II; Neg, negative response to cortical stimulation (ie, no electroclinical seizure was induced); Pos, positive response to cortical stimulation (ie, an electroclinical seizure was induced); SOZ, seizure-onset zone.
χ2 Test.
Spearman correlation.
Fisher exact test.
Binomial test.
High-amplitude polyspikes: 4 patients; spike and wave: 4 patients; periodic spikes: 3 patients; and delta brush: 3 patients.
Induced Seizures and Surgical Outcome
We induced at least 1 electroclinical seizure in 59 (57.3%) of 103 patients, with a median (range) response rate of 2 (1-13) cortical stimulation–induced seizures per patient. No difference in rates of induced seizures was found between patients in the 2 centers (60.0% CHUGA vs 54.2% MNI; P = .60). The total number of cortical stimulation–induced seizures was 90 (12 at 1-Hz stimulation, and 78 at 50-Hz stimulation).
The percentage of patients with a cortical stimulation–induced electroclinical seizure was higher in the good outcome group compared with the poor outcome group (31 of 44 [70.5%] vs 28 of 59 [47.5%]; P = .02). In addition, the median (range) percentage of resected cortical stimulation–informed SOZ contacts was higher in the good than in the poor outcome group (63.2% [0%-100%] vs 33.3% [0%-84.6%]; Spearman ρ = 0.38; P = .003) (Figures 2 and 3). A median (range) 75.0% (0%-100%) of channels inducing cortical stimulation–induced electroclinical seizures were resected in the good outcome group as opposed to 0% (0%-100%) in the poor outcome group (Spearman ρ = 0.40; P = .002).
Figure 2. Percentages of Resected Volumes in Patients With Good or Poor Surgical Outcome.
Comparable results were found for all 3 measures showing statistically significant differences depending on the outcome. CS indicates cortical stimulation.
aSpearman ρ = 0.32; P = .002.
bSpearman ρ = 0.38; P = .003.
cSpearman ρ = 0.40; P < .002.
Figure 3. Proportion of Resected Contacts in Patients With Good or Poor Surgical Outcome .
A, Examples of Engel class I or good surgical outcome in patients 61, 134, 70, and 81. B, Examples of Engel class IV or poor surgical outcome in patients 260, 255, 180, and 231. For each patient, the coronal, sagittal, and axial planes of the presurgical magnetic resonance imaging are shown. The contacts of the seizure-onset zone informed by cortical stimulation are reconstructed as red dots, and the resection cavity is superimposed in white. The examples illustrate that in patients with good surgical outcome, a higher proportion of the contacts were removed compared with patients with poor outcome. The patient on the second row of panel B had a seizure-onset zone that involved both the left frontal and temporal lobes (the contacts are not visible in the selected planes). The decision to perform a left frontal lobe resection was based on clinical considerations. Its outcome at 1-year of follow-up was an Engel class IV.
The analysis of spontaneous seizures (n = 96) revealed similar results. The median (range) percentage of resected contacts of the spontaneous SOZ was 57.1% (0%-100%) in the good outcome group and 32.7% (0%-100%) in the poor outcome group (Spearman ρ = 0.32; P = .002) (Figures 2 and 4). The surgical outcome was associated with the number of resected contacts of the union of both cortical stimulation–informed SOZ and spontaneous SOZ (median good outcome vs poor outcome: 51.0% vs 33.6%; P = .005), but it was not associated with the number of contacts in their intersection (median good outcome vs poor outcome: 30.0% vs 22.0%; P = .18). The median (range) number of electrode contacts involved in the cortical stimulation–informed SOZ was higher compared with that in the spontaneous SOZ (14 [3-78] contacts vs 11 [6-52] contacts; P = .01). The median (range) overlap (calculated as the number of contacts in both informed and spontaneous SOZs divided by the total number of contacts in any of the SOZs) was 54.2% (0%-100%). Figure 4 illustrates 2 patient examples.
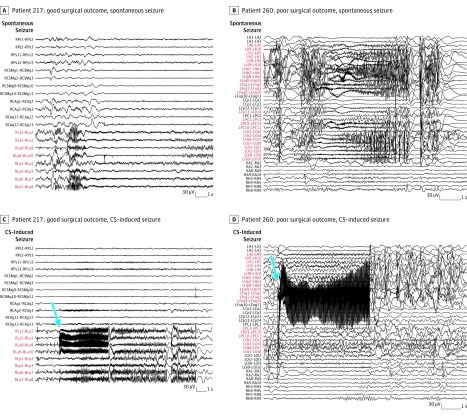
Figure 4. Comparison in Outcomes Between Spontaneous Seizure and Cortical Stimulation–Induced Seizure in 2 Patient Examples .
A and C, Stereoelectroencephalographic (SEEG) recording of patient 217, who had a good surgical outcome. B and D, SEEG recording of patient 260, with poor surgical outcome. Because of functional limitations, only part of the seizure-onset zone was removed in patient 260, as opposed to patient 217. Blue arrows point to the beginning of the cortical stimulation (CS). Contacts RLa 4-6 in patient 217 and contacts LFug 4-6 in patient 260 were stimulated (using 50 Hz). Even though the seizure-onset patterns show differences, the seizure-inducing channels (labeled in red) were similar. LA indicates left amygdala; LCp, left cingulate posterior; LFug, left fusiform gyrus; LH, left hippocampus; LHp, left hippocampus posterior; LOi, left occipital inferior; LOs, left occipital superior; LPC, left precuneus; RA, right amygdala; RCAg, right angular gyrus; RCSMg, right supramarginal gyrus; RH, right hippocampus; RLa, right lesional anterior; RLp, right lesional posterior; RPs, right parietal superior.
Seven patients had only cortical stimulation–induced seizures but no spontaneous seizures. No significant difference in the percentage of good outcome was found between these patients and the whole group (57.1% vs 42.7%; P = .35).
Characteristics of Induced Seizures
The 50-Hz stimulation was more likely to induce electroclinical seizures than the 1-Hz stimulation (56 [54.9%] of 102 patients vs 12 [18.2%] of 66 patients; P < .001); 3 patients had exclusively cortical stimulation responses at 1 Hz. Before the cortical stimulation study, in patients who had a seizure in the past 24 hours, the rate of cortical stimulation–induced seizures was 27.3% (3 of 11), whereas among patients who had more than 24 hours since the most recent seizure, the rate of induced seizures was 64.7% (22 of 34; P = .04). All other electroclinical characteristics were not statistically significant (Table).
When analyzing the results of the 50-Hz stimulation (56 [54.9%] of 102 patients), we found a statistically significant difference in the time elapsed since the most recent spontaneous seizure, with a higher likelihood of a cortical stimulation–induced electroclinical seizure in patients whose most recent seizure was more than 24 hours ago (63.9% for patients with >24 hours vs 25.0% for patients with <24 hours; P = .02). All other electroclinical characteristics were not statistically significant.
When analyzing the results of the 1-Hz stimulation (12 [18.2%] of 66 patients), we found an association with the spontaneous seizure-onset pattern. Low-voltage fast activity (LVFA) was associated with a higher likelihood of a cortical stimulation–induced electroclinical seizure (0 [0%] of 17 patients; P = .05). All other electroclinical characteristics were not statistically significant.
Different Currents Applied to Cortical Stimulation
The median (range) current used to induce electroclinical seizures was 2 (0.3-4) mA. A statistically significant difference in the median current was found between stimulating at 1 Hz and stimulating at 50 Hz (n = 12; median [range], 3 [2-3] mA vs n = 78; 2 [0.3-4] mA; P < .001). At 50 Hz, the median current for stimulating mesiotemporal channels was significantly different from the current for other channels (mesiotemporal: n = 30; median [range], 1.8 [0.3-3] mA vs neocortical: n = 48; 2 [0.6-4] mA; P = .05). This difference was not seen for 1 Hz (mesiotemporal: n = 10; median [range], 3 [2-3] mA vs neocortical: n = 2; 3 [3-3] mA; P > .99).
Discussion
During presurgical investigation, cortical stimulation is used to obtain information on the location and extent of the epileptogenic zone. This study demonstrates that seizures induced by cortical stimulation are a good marker of the epileptogenic zone and therefore are associated with surgical outcome.
Typical electroclinical seizures can be induced in 57.3% of patients. We decided a priori to focus on this type of response and not on pure elicited discharges and auras with no accompanying EEG changes, because previous studies have shown that they do not identify surgical outcome.2,15,16 The high variability of elicited discharges can be explained by the background state of neuronal excitability17; pure auras without accompanying EEG changes likely reflect only the symptomatogenic zone.18
Cortical Stimulation and Surgical Outcome
Patients with cortical stimulation–induced typical electroclinical seizures had a higher likelihood of a good surgical outcome compared with patients without such induced seizures. This association is likely explained by the placement and sampling of the SEEG electrodes. In patients in whom no seizures could be induced, the electrodes were probably placed in a cortical area of high neuronal threshold incapable of sufficiently activating the epileptic network to induce seizures. The absence of cortical stimulation–induced seizures might represent a red flag, suggesting the true epileptogenic zone was missed or only partially sampled.
A larger resection of the SOZ as informed by cortical stimulation correlated with a better surgical prognosis. Furthermore, resection of the contacts that induced seizures when stimulated was also important for a good surgical outcome. This latter finding is not consistent with current belief, suggesting that stimulation of any area functionally connected to the epileptogenic zone can induce seizures.17,19,20 One explanation might be that contacts capable of inducing seizures are localized in important epileptic network nodes21 and therefore have to be resected also, provided that these seizures are electroclinical events.
Apart from having the potential to shorten the invasive EEG investigation, cortical stimulation has the additional advantage of providing early bedside assessment of the patient during seizures. Furthermore, it may also help in choosing the contacts for radiofrequency thermocoagulation22 or laser ablation.
It is not surprising that not all contacts of the spontaneous SOZ were removed, given that tailoring of the resection is based not only on the contacts defining the SOZ but also on the tonicity of the ictal discharge23,24 and the interictal EEG findings. Furthermore, the tailoring of the resection takes into account the findings from neuroimaging as well as the presence of eloquent cortex and additional anatomical boundaries.
Characteristics Associated With Induced Seizures
High-frequency stimulation and more-than-24-hour elapsed time since the most recent seizure were associated with a higher likelihood of cortical stimulation–induced seizures. The 50-Hz stimulation was more likely to induce a typical seizure compared with the 1-Hz stimulation, a finding proposed more than 2 decades ago by Munari and colleagues.25 The absence of seizures in the past 24 hours was associated with a higher likelihood of induced seizures. This finding confirmed clinical and experimental experience, suggesting that the more time elapsed since the most recent seizure, the higher the probability of inducing seizures during stimulation.9,26
Electroclinical seizures were believed to be particularly well induced in patients with focal cortical dysplasia type II,27 but we did not find higher rates of cortical stimulation–induced seizures in patients with focal cortical dysplasia type II when compared with different neuropathologies. This study did not investigate how to limit the stimulation sites without losing information, but this is a relevant clinical question that should be examined systematically in the future.
Similarity Between Induced and Spontaneous Seizures
We performed a detailed neurophysiologic characterization of the cortical stimulation–informed SOZ and the spontaneous SOZ on a single-channel basis, allowing the calculation of the percentage of resected SOZ channels. High correlation was demonstrated between the channels involved in the spontaneous SOZ and the cortical stimulation–informed SOZ. This finding extends the results from previous studies suggesting the electroclinical resemblance of cortical stimulation–induced seizures to spontaneous seizures.28,29,30
Seven patients in this series had cortical stimulation–induced electroclinical seizures but no spontaneous seizures during the SEEG investigation. The surgical outcome in this group did not differ from the outcome of the total group, suggesting that cortical stimulation might be extremely valuable to obtain induced electroclinical seizures and might be used as a substitute for the recording of spontaneous seizures.
Low-Frequency Stimulation
Low-frequency stimulation showed higher rates of cortical stimulation–induced seizures in LVFA than non-LVFA seizure-onset patterns. The LVFA is the prototype of a seizure-onset pattern, which is associated with high epileptogenicity,23 which might be particularly relevant for inducing seizures at low frequencies. Furthermore, the current study suggests that low-frequency stimulation is more likely to trigger seizures in structures with low epileptogenic threshold.6,27 No seizure was induced at 1 Hz in patients with a frontal generator. One advantage of low-frequency stimulation is its lack of a major electrical artifact and its visualization of the spatial evolution of the induced discharge.31 Furthermore, the study of corticocortical evoked potentials, possible with low-frequency stimulation, is of interest in understanding the effective connectivity of pathological and physiologic networks.31,32
Limitations
This study has limitations. Because of the study design requiring high-resolution neuroimaging with implanted electrodes and adequate postsurgical imaging and follow-up, we excluded a substantial number of patients. Most of these patients were studied before 2010, and thus often had no adequate peri-implantation imaging, or were referred from other hospitals for SEEG and subsequent surgical procedure, and hence lacked adequate postsurgical imaging. The study’s strict neuroimaging criteria, which required precise coregistration of the neuroimaging, were key to the main outcome of this study. Generalizability of the data was supported by similar rates of postsurgically seizure-free patients included and excluded from this study.
Conclusions
Seizures induced by cortical stimulation appear to identify the epileptic generator in a similar way as spontaneous seizures do. This finding might lead to a more time-efficient intracranial presurgical investigation of focal epilepsy by reducing the need to record spontaneous seizures.
References
- 1.Kovac S, Kahane P, Diehl B. Seizures induced by direct electrical cortical stimulation–mechanisms and clinical considerations. Clin Neurophysiol. 2016;127(1):31-39. doi: 10.1016/j.clinph.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 2.Bernier GP, Richer F, Giard N, et al. Electrical stimulation of the human brain in epilepsy. Epilepsia. 1990;31(5):513-520. doi: 10.1111/j.1528-1157.1990.tb06099.x [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Lüders HO, Tuxhorn I, et al. Localization of epileptic auras induced on stimulation by subdural electrodes. Epilepsia. 1997;38(12):1321-1329. doi: 10.1111/j.1528-1157.1997.tb00070.x [DOI] [PubMed] [Google Scholar]
- 4.Chauvel P, Landré E, Trottier S, et al. Electrical stimulation with intracerebral electrodes to evoke seizures. Adv Neurol. 1993;63:115-121. [PubMed] [Google Scholar]
- 5.Jacobs J, Zijlmans M, Zelmann R, et al. Value of electrical stimulation and high frequency oscillations (80-500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2010;51(4):573-582. doi: 10.1111/j.1528-1167.2009.02389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trébuchon A, Chauvel P. Electrical stimulation for seizure induction and functional mapping in stereoelectroencephalography. J Clin Neurophysiol. 2016;33(6):511-521. doi: 10.1097/WNP.0000000000000313 [DOI] [PubMed] [Google Scholar]
- 7.Engel J. Surgical Treatment of the Epilepsies. 2nd ed New York, NY: Raven Press; 1993. [Google Scholar]
- 8.Frauscher B, von Ellenrieder N, Zelmann R, et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain. 2018;141(4):1130-1144. doi: 10.1093/brain/awy035 [DOI] [PubMed] [Google Scholar]
- 9.Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia. 1997;38(12):1300-1314. doi: 10.1111/j.1528-1157.1997.tb00068.x [DOI] [PubMed] [Google Scholar]
- 10.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain. 2014;137(Pt 1):183-196. doi: 10.1093/brain/awt299 [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Jiménez D, Nekkare R, Flores L, et al. Prognostic value of intracranial seizure onset patterns for surgical outcome of the treatment of epilepsy. Clin Neurophysiol. 2015;126(2):257-267. doi: 10.1016/j.clinph.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 12.Rummel C, Abela E, Andrzejak RG, et al. Resected brain tissue, seizure onset zone and quantitative EEG measures: towards prediction of post-surgical seizure control. PLoS One. 2015;10(10):e0141023. doi: 10.1371/journal.pone.0141023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124(Pt 9):1683-1700. doi: 10.1093/brain/124.9.1683 [DOI] [PubMed] [Google Scholar]
- 14.Cuello-Oderiz C, von Ellenrieder N, Sankhe R, et al. Value of ictal and interictal epileptiform discharges and high frequency oscillations for delineating the epileptogenic zone in patients with focal cortical dysplasia. Clin Neurophysiol. 2018;129(6):1311-1319. doi: 10.1016/j.clinph.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Sperling MR, O’Connor MJ. Auras and subclinical seizures: characteristics and prognostic significance. Ann Neurol. 1990;28(3):320-328. doi: 10.1002/ana.410280304 [DOI] [PubMed] [Google Scholar]
- 16.Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol. 2004;115(4):982-989. doi: 10.1016/j.clinph.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 17.Lesser RP, Lee HW, Webber WR, Prince B, Crone NE, Miglioretti DL. Short-term variations in response distribution to cortical stimulation. Brain. 2008;131(Pt 6):1528-1539. doi: 10.1093/brain/awn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bancaud J, Talairach J, Geier S, Scarabin JM. Electro-Encéphalographie et Stereo-électro-encéphalographie Dans les Tumeurs Cérébrales et L’épilepsie. Paris, France: Edifor; 1973. [Google Scholar]
- 19.Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 1999;89(2):335-346. doi: 10.1016/S0306-4522(98)00330-3 [DOI] [PubMed] [Google Scholar]
- 20.David O, Bastin J, Chabardès S, Minotti L, Kahane P. Studying network mechanisms using intracranial stimulation in epileptic patients. Front Syst Neurosci. 2010;4:148. doi: 10.3389/fnsys.2010.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K, Lina JM, Gotman J, Grova C. SPARK: Sparsity-based analysis of reliable k-hubness and overlapping network structure in brain functional connectivity. Neuroimage. 2016;134:434-449. doi: 10.1016/j.neuroimage.2016.03.049 [DOI] [PubMed] [Google Scholar]
- 22.Dimova P, de Palma L, Job-Chapron AS, Minotti L, Hoffmann D, Kahane P. Radiofrequency thermocoagulation of the seizure-onset zone during stereoelectroencephalography. Epilepsia. 2017;58(3):381-392. doi: 10.1111/epi.13663 [DOI] [PubMed] [Google Scholar]
- 23.Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131(Pt 7):1818-1830. doi: 10.1093/brain/awn111 [DOI] [PubMed] [Google Scholar]
- 24.David O, Blauwblomme T, Job AS, et al. Imaging the seizure onset zone with stereo-electroencephalography. Brain. 2011;134(Pt 10):2898-2911. doi: 10.1093/brain/awr238 [DOI] [PubMed] [Google Scholar]
- 25.Munari C, Kahane P, Tassi L, et al. Intracerebral low frequency electrical stimulation: a new tool for the definition of the “epileptogenic area”? Acta Neurochir Suppl (Wien). 1993;58:181-185. [DOI] [PubMed] [Google Scholar]
- 26.Mucha RF, Pinel PJ. Postseizure inhibition of kindled seizures. Exp Neurol. 1977;54(2):266-282. doi: 10.1016/0014-4886(77)90269-2 [DOI] [PubMed] [Google Scholar]
- 27.Chassoux F, Devaux B, Landré E, et al. Stereoelectroencephalography in focal cortical dysplasia: a 3D approach to delineating the dysplastic cortex. Brain. 2000;123(Pt 8):1733-1751. doi: 10.1093/brain/123.8.1733 [DOI] [PubMed] [Google Scholar]
- 28.Cherlow DG, Dymond AM, Crandall PH, Walter RD, Serafetinides EA. Evoked response and after-discharge thresholds to electrical stimulation in temporal lobe epileptics. Arch Neurol. 1977;34(9):527-531. doi: 10.1001/archneur.1977.00500210029003 [DOI] [PubMed] [Google Scholar]
- 29.Wieser HG. Electro-Clinical Features of the Psychomotor Seizure. New York, NY: Butterworth; 1983. [Google Scholar]
- 30.Tardy N. Electro-clinical correlation between seizures induced by direct cortical stimulation and spontaneous seizures: relevance to define the epileptogenic zone [MD thesis]. Grenoble, France: Grenoble-Alpes University; 2017. https://dumas.ccsd.cnrs.fr/dumas-01826169. Accessed Month X, 2019.
- 31.David O, Job AS, De Palma L, Hoffmann D, Minotti L, Kahane P. Probabilistic functional tractography of the human cortex. Neuroimage. 2013;80:307-317. doi: 10.1016/j.neuroimage.2013.05.075 [DOI] [PubMed] [Google Scholar]
- 32.Keller CJ, Honey CJ, Mégevand P, Entz L, Ulbert I, Mehta AD. Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci. 2014;369(1653):1653. doi: 10.1098/rstb.2013.0528 [DOI] [PMC free article] [PubMed] [Google Scholar]