Summary
Impaired locomotion is a frequent and major source of disability in patients with neurological conditions. Different neuroimaging methods have been used to understand the brain substrates of locomotion in various neurological diseases (mainly in Parkinson’s disease) during actual walking, and while resting (using mental imagery of gait, or brain-behavior correlation analyses). These studies, using structural (i.e., MRI) or functional (i.e., functional MRI or functional near infra-red spectroscopy) brain imaging, electrophysiology (i.e., EEG), non-invasive brain stimulation (i.e., transcranial magnetic stimulation, or transcranial direct current stimulation) or molecular imaging methods (i.e., PET, or SPECT) reveal extended brain networks involving both grey and white matters in key cortical (i.e., prefrontal cortex) and subcortical (basal ganglia and cerebellum) regions associated with locomotion. However, the specific roles of the various pathophysiological mechanisms encountered in each neurological condition on the phenotype of gait disorders still remains unclear. After reviewing the results of individual brain imaging techniques across the common neurological conditions, such as Parkinson’s disease, dementia, stroke, or multiple sclerosis, we will discuss how the development of new imaging techniques and computational analyses that integrate multivariate correlations in ‘‘large enough datasets’’ might help to understand how individual pathophysiological mechanisms express clinically as an abnormal gait. Finally, we will explore how these new analytic methods could drive our rehabilitative strategies.
Keywords: Gait disorders, Neuroimaging, Neurological conditions, Methods, Review, Dementia, Parkinson’s disease, Stroke, Multiple Sclerosis
Introduction
Locomotion is often affected in neurological conditions. Gait disorders are found in 60% of patients hospitalized in neurological units [196]. Parkinson’s disease (PD), stroke, multiple sclerosis (MS) and dementia are the leading causes of gait disorders in neurological settings. In PD, the presence of freezing of gait (FOG) — likely the most debilitating gait symptom—is reported in 38.2% in the ‘‘on medication’’ state, and is associated with poor quality of life [157]. After a stroke, 32–47% of stroke patients are not able to walk unsupervised [116,158]; age, severity of the paresis, size of brain lesions and presence of hemianopia are the major factors associated with gait disorders within 30 days post-stroke [35]. In MS, the leading cause of non-traumatic disability in young adults, mobility impairment affects 80% of the patients [149], even in the earliest stages of the disease [195]. Gait impairment in neurological conditions increased with advanced age, especially in older adults with non-Alzheimer’s dementia [196]: gait disorders have been reported in 93% of patients with PD with dementia, 79% with vascular dementia, 75% with dementia with Lewy bodies and 25% with Alzheimer’s disease (AD) [6].
Gait disorders are clinically divided in neurological or non-neurological causes, such as arthritis, or cardiovascular conditions. Among the neurological gait abnormalities, different patterns of gait disorders, such as parkinsonian gait, frontal gait or spastic gait, have been identified [227]. Identifying neurological from non-neurological clinical gait abnormalities is clinically important, because only neurological gait abnormalities have been associated with poor clinical outcomes, such as falls [225] or dementia [227]. Gait has been recently used to define a new pre-dementia syndrome, called the Motoric Cognitive Risk (MCR) syndrome [228]. The MCR syndrome is defined by the presence of slow gait and cognitive complaint; this syndrome represents an early risk factor for developing dementia [226].
The brain correlates of gait have been extensively studied in aging [70] and in individuals with specific neurological diseases [17,47,125] during the last few years using various techniques, such as electroencephalography, magnetic resonance imaging (MRI), nuclear medicine, non-invasive brain stimulation (i.e., transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS)) or functional near infrared spectroscopy (fNIRS). These various techniques give us access to the brain structures and the functional substrates involved in gait control during real walking or during mental imagery of gait. Each neuroimaging technique presents its own advantages and limitations and will be reviewed in detail in this article by including the different common neurological diseases. This review will be articulated around three aspects of the relationship between brain changes and gait disorders in neurological conditions: correlation between brain abnormalities evidenced by the different techniques and clinical measures of gait; online measurements performed with some of these techniques (i.e. fNIRS and EEG) during actual gait; impact on gait modification following stimulation of cortical regions using TMS/tDCS.
We will then discuss the development of future methodological approaches aimed to better understand the pathophysiological mechanisms affecting gait control in neurological diseases. This article is not a systematic review of the literature, but a targeted review that aims to describe the brain-related gait changes in adults with common neurological conditions.
Magnetic resonance imaging
Magnetic Resonance Imaging (MRI) is a non-invasive neuroimaging technique that has been used to identify the structural and functional brain substrates and pathologies associated with human locomotion in a number of neurological conditions — including PD, stroke, Alzheimer’s disease and related dementias (ADRD), and MS (see Table 1). Standard three-dimensional structural images have been used to link locomotion in neurological conditions to brain volume or atrophy in a particular brain region, or a set of regions. The integrity of a particular white matter tract, or a set of white matter tracts, have also been linked to locomotion in neurological conditions using diffusion-weighted imaging (DWI). In addition, fluid attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging (SWI) have been used to link small vessel disease pathologies, including lacunes, white matter hyperintensities (WMH), and microbleeds, to loco-motion in neurological conditions. Finally, functional MRI (fMRI) has been used to linked functional activation of a brain region, or a set of brain regions, during imagined (or motor imagery of) locomotion, as well as to functional connectivity during rest (resting-state fMRI).
Table 1.
Magnetic resonance imaging in neurological diseases.
| Reference | Design, methods, participants | Key findings |
|---|---|---|
| A. Parkinson’s disease | ||
| Maidan, 2016 [123] | Cross-sectional, imagery, fMRI, PD vs. controls | Controls, but not PD participants, had increased activation during virtual navigation relative to virtual walking |
| Maidan, 2017 [124] | RCT, imagery, fMRI, PD. Treadmill training + Virtual imagery (TT+VI) vs. Treadmill training (TT) alone | TT+VI relative to TT had: a) Improved attention and gait speed b) Decreased activation in anterior and inferior frontal gyrus c) Decreased activation in left anterior lobe of cerebellum and mid temporal gyrus |
| Peterson, 2014 [160] | Cross-sectional, imagery, fMRI, PD-FOG+ vs. PD-FOG− | During imagined gait, PD-FOG+ had less activation in right globus pallidus and cerebellar regions than PD-FOG− |
| Peterson, 2014 [161] | Cross-sectional, imagery, fMRI, PD vs. controls | During imagery, PD participants had less activation in globus pallidus and more activity in SMA than controls. Activation of SMA, putamen, globus pallidus and brain stem correlated with gait speed in PD, but not in controls |
| Snijders, 2011 [194] | Cross-sectional, imagery, fMRI, PD-FOG+ vs. PD-FOG− | PD FOG+ participants had increased activation during imagery, and more brain volume atrophy in brainstem than PD-FOG− participants |
| Li, 2018 [111] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG− | Reduced interhemispheric functional connectivity was observed in the inferior parietal lobule of PD FOG+ participants compared to PD FOG− and healthy controls |
| Zhou, 2018 [248] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG− | PD FOG+ participants had reduced regional functional connectivity in SMA and left superior PFC relative to PD FOG− and controls |
| Gilat, 2018 [56] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG− | PD-FOG+ participants had increased functional connectivity between right amygdala and putamen compared to PD-FOG− |
| Mi, 2017 [135] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG− | PD FOG+ participants had increased spontaneous neural activity in anterior cingulate, intraparietal lobule, and decreased activity in superior frontal gyrus, bilateral cerebellum, and thalamus |
| Ma, 2017 [118] | Cross-sectional, resting-state fMRI, PD-TD vs. PD-PIGD, controls | PD-PIGD participants had disrupted functional connectivity in cerebellum relative to PD-TD |
| Shen, 2017 [181] | Cross-sectional, resting-state fMRI, PD vs. controls | Increased functional connectivity between substantia nigra and sensory-motor cortex was observed in PD patients on medication |
| Gallea, 2017 [55] | Cross-sectional, resting-state fMRI, PD vs. controls, with or without impaired postural control and/or sleep disorder | PD patients with sleep disorders displayed decreased functional connectivity between peduncolopontine nucleus and anterior cingulate |
| Wang, 2016 [236] | Cross-sectional, resting-state fMRI and DWI, PD-FOG+ vs. PD-FOG−, controls | PD-PIGD participants had reduced functional connectivity between left putamen and substantia nigra and increased connectivity between substantia nigra and occipital lobe |
| Wang, 2016 [234] | Cross-sectional, resting-state fMRI, PD-TD vs. PD-PIGD, controls | PD FOG + participants had abnormal functional connectivity in pedunculopontine nucleus and visual temporal areas |
| Vervoort, 2016 [230] | Cross-sectional, resting-state fMRI, PD-TD vs. PD-PIGD, PD undetermined, controls | PD-PIGD participants had reduced functional connectivity between caudate and putamen compared to PD TD |
| Lenka, 2016 [109] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG− | PD FOG+ participants had reduced interhemispheric functional connectivity between left parietal opercular cortex and primary somatosensory and auditory regions |
| Vervoort, 2016 [231] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG−, controls | PD-PIGD participants had reduced functional connectivity between the caudate and superior temporal lobe and increased connectivity between the dorsal putamen and precuneus, which correlated with worse dual-task gait performance |
| Jiang, 2016 [82] | Cross-sectional, resting-state fMRI, PD-TD vs. PD-PIGD, controls | PD-PIGD participants had reduced synchronicity of activation in frontal, parietal, occipital, temporal, limbic lobes, basal ganglia and thalamus regions |
| Chen, 2015 [32] | Cross-sectional, resting-state fMRI, PD-TD vs. PD-PIGD, controls | PD-PIGD participants had lower spontaneous neural activity in the putamen and cerebellar posterior lobe, and higher spontaneous neural activation in inferior and superior temporal gyrus, superior frontal gyrus, and parietal gyrus |
| Canu, 2015 [25] | Cross-sectional, resting-state fMRI and DWI, PD-FOG+ vs. PD-FOG− | PD-FOG+ participants had decreased functional connectivity in primary motor and supplementary motor regions of the sensorimotor network, frontoparietal regions of the default mode network, and occipital cortex regions of the visual network - as well as reduced white matter integrity in the pendunculopontine tracts, corpus callosum, corticospinal tract, cingulum, superior longitudinal fasciculus |
| Hou, 2015 [72] | Cross-sectional, resting-state fMRI, PD vs. controls | PD-PIGD participants had increased spontaneous neural activity in right postcentral gyrus, and decreased spontaneous neural activity in putamen, pre and supplementary motor area, frontal lobes, temporal lobes, and insula |
| Fling, 2014[51] | Cross-sectional, resting-state fMRI and DWI, PD vs. controls | PD-FOG+ participants had greater functional connectivity between the supplementary motor and cerebellar regions compared to both PD-FOG− and controls - and was correlated with increased (worse) clinical, self-reported and objective ratings of FOG |
| Tessitore, 2012 [212] | Cross-sectional, resting-state fMRI, PD-FOG+ vs. PD-FOG−, controls | PD-FOG+ participants had reduced connectivity in middle frontal gyrus, angular gyrus and right occipito-temporal gyrus |
| Wen, 2018 [239] | Cross-sectional, DWI, PD-TD+ vs. PD-PIGD, controls | White matter integrity in genu of corpus callosum was more strongly associated with motor severity in PD-FOG+ than PD-TD |
| Hall, 2018 [63] | Cross-sectional, DWI, PD-FOG+ vs. PD-FOG− | PD-FOG+ participants had reduced structural modularity and integration in caudate, thalamus, hippocampus, and superior frontal and parietal cortex regions |
| Pietracupa, 2018 [162] | Cross-sectional, DWI, PD-FOG+ vs. PD-FOG− | PD-FOG+ participants had reduced cortical thickness in cerebellar, superior frontal, paracentral, posterior cingulate, precuneus, pericalcarine and dorsolateral prefrontal cortex regions — as well as reduced white matter integrity in superior longitudinal fasciculus, uncinate fasciculus, cingulate, and inferior longitudinal fasciculus |
| Lenfeld, 2016 [108] | Longitudinal, DWI, PD-FOG+ vs. PD-FOG−, PD-undetermined | PD-PIGD participants had reduced white matter integrity in the prefrontal cortex, external capsule, the and lateral horn of the anterior ventricle relative to PD-TD – the integrity of these regions was also lower at baseline among those that developed PIGD symptoms during follow-up |
| Nagae, 2016 [147] | Cross-sectional, DWI, PD-TD vs. PD-PIGD, controls | PD patients had reduced had reduced white matter integrity in the substantia nigra |
| Youn, 2015 [246] | Cross-sectional, DWI, PD-TD vs. PD-PIGD, controls | PD-FOG+ participants had reduced structural connectivity in regions connected to pedunculopontine regions — including basal ganglia, thalamus and cerebellum |
| Chan, 2014 [29] | Case-control, DWI, PD vs. PIGD, controls | Structural connectivity in the body of the corpus callosum body differentiated PD-PIGD from PD |
| Marumoto, 2012 [128] | Cross-sectional, DWI, INPH vs. PD, controls | INPH participants had reduced white matter integrity in anterior thalamic radiation and forceps minor compared to PD participant. The addition of DWI assisted in the differential diagnosis of INPH from PD beyond what could be deduced from ventricular size alone |
| Kanno, 2011 [85] | Cross-sectional, DWI, INPH vs. AD, PD | Reduced white matter integrity is related to motor and cognitive dysfunction in INPH |
| Skorpil, 2012 [191] | Cross-sectional, DWI, PD vs. controls | PD participants had reduced white matter integrity in substantia nigra relative to controls |
| Karagulle Kendi, 2008 [86] | Cross-sectional, DWI, PD vs. controls | PD participant had reduced structural connectivity in the supplementary motor area, pre-supplementary motor area, and cingulum |
| Surova, 2016 [198] | Cross-sectional, DWI, PD vs. controls | PD participants had white matter integrity changes in the putamen, the thalamus, and the superior longitudinal fasciculus — yet, these changes do not aid in the diagnostic work-up of PD |
| Vervoort, 2016 [232] | Cross-sectional, DWI, PD-TD vs. PD-PIGD, controls | PD-PIGD participants had reduced white matter integrity in superior longitudinal fasciculus and corpus callosum compared to controls — as well as increased grey matter atrophy in the rostro dorsal head of the caudate |
| Vercruysse, 2015 [224] | Cross-sectional, DWI, PD vs. controls | PD participants had reduced white matter integrity in striato-frontal tracts including putamen, caudate, pallidum, subthalamic nucleus, cerebellar peduncle, subthalamic nucleus and pedunculopontine nucleus |
| Gu, 2014 [60] | Cross-sectional, DWI, PD-PIGD+ vs. PD-PIGD−, controls | PD-PIGD is associated with reduced white matter integrity was greater in superior longitudinal fasciculus relative to PD-PIGD |
| Lenfeldt, 2013[107] | Longitudinal, DWI, PD-PIGD, PD-TD | White matter integrity in thalamus was reduced in PD-TD than PD-PIGD |
| Al-Bachari, 2017 [3] | Cross-Sectional, WMH, PD-TD vs., PD-PIGD, controls | PD-PIGD participant had more white matter lesions than PD-TD participants |
| Acharya, 2007 [1] | Cross-Sectional, WMH, old-PD young-PD vs. old-controls, young-control | White matter changes was not different between the groups |
| Sartor, 2017 [176] | Cross-Sectional, WMH, PD | Gait performance was poorer, and reduction in bradykinesia following a single dose of levodopa was reduced, in PD participants with WMH than in those without WMH |
| Arena, 2016 [8] | Cross-Sectional, WMH, PD with acute L-DOPA challenge | PD participant with greater deep WMH burden was less responsive to levodopa on axial motor symptoms |
| Herman, 2013 [68] | Cross-Sectional, WMH, PD | The mean WMHs scores and the percent of subjects with lesions in specific brain regions were similar in the two subtypes |
| Moccia, 2016 [141] | Longitudinal, WMH, PD | Developing PIGD symptoms during follow-up was associated with higher WMH burden |
| Kim, 2015 [95] | Longitudinal, SVD, IPD vs. VP | Cerebral microbleeds were more common in vascular parkinsonism than in idiopathic PD. In IPD, cerebral microbleeds were also associated with white matter hyperintensities and concurrent lacunar infarctions |
| Schneider, 2016 [180] | Longitudinal, SVD, PD vs. PIGD | PD-PIGD had less SWI hypointensity in the putamen and globus pallidus PD patients |
| Rosenberg-Katz, 2016 [171] | Cross-Sectional, brain volume, PD-TD vs. PD-PIGD | PD-PIGD had more atrophy in amygdala and globus pallidus relative to PD-TD. In both PD-PIGD and PD-TD, hippocampal volume was positively associated with dual task gait performance, and putamen volume was negatively associated with FOG score |
| Rubino, 2014 [172] | Cross-Sectional, brain volume, PD-FOG+ vs. PD-FOG− | PD-FOG+ participants had more gray matter atrophy in left posterior parietal gyrus compared with PD-FOG− |
| Tessitore, 2012 [211] | Cross-Sectional, brain volume, PD-FOG+ vs. PD-FOG−, controls | PD-FOG+ had less gray matter volume in cuneus, precuneus, lingual gyrus, and posterior cingulate cortex compared to PD-FOG− and controls |
| B. Stroke | ||
| Carter, 2012 [27] | Cross-Sectional, resting-state fMRI, subacute stroke vs. controls | The extent of corticospinal damage was associated with functional connectivity between the left and right central sulcus |
| Peters, 2018[159] | Cross-Sectional, DWI, post-stroke | Structural connectivity between primary and supplementary motor regions and the cerebral peduncle, thalamus, and red nucleus in the same hemisphere as stroke lesion was associated with upper and lower extremity motor functions post-stroke |
| Jang, 2014 [79] | Cross-Sectional, DWI, chronic stroke | White matter integrity was associated with motor functions in chronic stroke |
| Dubey, 2016 [44] | Cross-Sectional, DWI, chronic stroke, controls | Reduced white matter integrity of corpus callosum was observed in chronic stroke relative to controls and was associated with gait speed |
| Jang, 2013 [78] | Cross-Sectional, DWI, stroke with complete injury of CST | White matter integrity (fiber volume) in unaffected (but not in affected) hemisphere was positively associated with the ability to walk |
| Jayaram, 2012 [81] | Cross-Sectional, DWI and TMS, chronic stroke | Greater relative connectivity between motor cortex and lower limbs in affected and unaffected hemisphere was associated with poorer gait speed |
| Yeo, 2011 [243] | Cross-Sectional, DWI, chronic stroke | White matter integrity in pedunculopontine nucleus was greater in stroke patients with the ability to walk |
| Ahn, 2006 [2] | Cross-Sectional, DWI, ambulatory chronic stroke | Some stroke patients retained the ability to walk despite complete lateral corticospinal tract injury in the affected hemisphere |
| Loos, 2017 [115] | Cross-Sectional, SVD, lacunar vs. non-lacunar stroke | Total cerebral small vessel disease burden was associated with gait impairment in non-lacunar (but not lacunar) stroke patients |
| Callisaya, 2014 [24] | Cross-Sectional, WMH, subcortical infarcts vs. controls | The risk of multiple falls was increased in older adults with three or more subcortical infarcts and highest quartile of white matter hyperintensity volume |
| Choi, 2012 [33] | Cross-Sectional, WMH and microbleeds, subcortical infarcts vs. microbleeds | Subcortical infarcts and microbleeds amplified the negative association of WML volume with gait. Subcortical infarcts, but not microbleeds amplified the negative association of WML volume with postural stability |
| C. Alzheimer’s disease and related dementias | ||
| Olazaran, 2013 [152] | Cross-Sectional, brain volume, DWI, probable AD vs. possible AD, AD-CVD+ | Gait dysfunction was related to brain atrophy in motor cortex, cingulate, insula, caudate (total sample), and cerebellar (total sample and probable AD) regions |
| Verwer, 2017 [233] | Cross-Sectional, SVD, AD vs. MCI, vascular brain injury | High small vessel disease burden, particularly white matter hyperintensities, co-occurred with impairments in physical performance (gait speed, short physical performance battery) |
| Nadkarni, 2009 [146] | Cross-Sectional, subcortical hyperintensities, mild AD vs. controls | Subcortical hyperintensity burden — particularly in frontal and basal ganglia – was associated with stride length and velocity in both mild AD and controls |
| D. Multiple sclerosis | ||
| Sbardella, 2015 [177] | Cross-Sectional, DWI, RRMS vs. controls | Gray matter atrophy and white matter integrity in RRMS was associated with upper-limb motion and cognition, but not 25-foot timed walk |
| Li, 2013 [113] | Cross-Sectional, DWI, RRMS vs. controls | RRMS participants had reduced communicability in frontal and hippocampal/parahippocampal regions. Communicability in superior frontal and superior temporal regions was associated with 25-foot timed walk. Increased communicability was observed in thalamus, putamen, corpus callosum and cingulum |
| Anderson, 2011 [7] | Cross-Sectional, DWI and gray and white matter, RRMS vs. PPMS, controls | White matter integrity in cerebellar peduncle was reduced in PPMS relative to RRMS and controls. In PPMS white matter integrity in cerebellar peduncle was also associated with upper limb function and gait speed |
| Kern, 2011 [90] | Longitudinal, DWI, RRMS vs. controls | White matter integrity associated with fine hand motor control but not 25-foot time walk |
| Freund, 2010 [53] | Longitudinal, DWI, MS vs. controls | White matter integrity of the cortico-spinal tract at baseline was associated with better clinical outcomes |
| Wetter, 2016 [240] | Cross-Sectional, White matter lesions, MS (RRMS,SPMS and PPMS) | White matter lesion volume explained additional variance in six-minute-walk performance after adjusting for age, white matter volume and gray matter volume |
| Sanfilipo, 2005 [175] | Cross-Sectional, DWI, MS vs. controls | MS participants had significantly lower gray matter and parenchymal volume, and a trend towards lower white matter volume, relative to controls. Gray matter atrophy was associated with clinical status, lesion load and central brain atrophy. White matter volume was associated with central brain volume |
| Goncalves, 2018 [57] | Cross-Sectional, corpus callosum index and volume, MS vs. controls | Corpus callosum index was associated with white matter and lesion volume, whole brain volume, some (not all) cognitive measures and functional status, but not with clinical status or 25-foot timed walk |
| Cocozza, 2017 [34] | Cross-Sectional, brain volume, PPMS vs. controls | MS participants had reduced cerebellar volume relative to control. Cerebellar lobules VI, Crus I and VIIIa was associated with cognitive measures. Anterior cerebellum and Lobule I-IV atrophy was associated with the 25-foot timed walk |
| Dupuy, 2016 [45] | Longitudinal, brain volume, RRMS with dimethyl fumurate therapy | RRMS patients on dimethyl fumurate therapy had a reduced rate of whole brain and putamen atrophy |
| Khalid, 2017 [91] | Longitudinal, brain volume, RRMS and PPMS with natalizumab therapy | Natalizumab therapy was not associated with any changes in annual gray matter volume, parenchymal volume, lesion load, clinical status or 25-foot time walk |
| Motl, 2016 [143] | Cross-Sectional, brain volume, MS vs. controls | MS participants had reduced subcortical gray matter lesion load and slower 25-foot timed walk. 25-foot timed walk was associated with subcortical gray matter in both MS and controls. Thalamic volume partially accounted for the difference in 25-foot timed walk between MS and controls |
| Ruggieri, 2015 [173] | Cross-Sectional, brain volume, PPMS vs. controls | PPMS was associated with reduced brain, gray and white matter volume relative to controls. Thalamic atrophy was associated with cortical lesions, particularly in frontal lobes |
| Onu, 2015 [154] | Cross-Sectional, brain volume, RRMS vs. controls | RRMS participants had reduced gray matter volume, particularly in thalamic, putamen, caudate, globus pallidus and nucleus accumbens - and volume in these regions (as well as sensory motor and primary motor regions) was associated with 25-foot timed walk |
| Feys, 2017 [142] | Cross-Sectional, brain volume, MS | The 6-minute walk and 25-foot timed walk is associated with gray matter volume of the pallidum and caudate in MS |
| Maghzi, 2014 [119] | Longitudinal, brain and lesion volume, MS | Baseline volume of the whole, the gray matter and normal appearing white matter was associated with changes in a functional and the 25-foot timed walk in MS |
| Wen, 2015 [238] | Cross-section, gray matter volume, MS vs. controls | Age-adjusted differences between MS and control participants were observed in cortical gray matter, normal appearing white matter — and was associated with cognitive measures, but not clinical status or 25-foot timed walk |
| Tovar-Moll, 2015 [216] | Cross-Sectional, DWI, MS (both RRMS & PPMS) | White matter integrity in corticospinal tract was associated with disability status and 25-foot timed walking speed — but correlation with 25-foot timed walking speed disappeared when correcting for lesion volume |
| Tavazzi, 2018 [209] | RCT, fMRI, MS, neurorehabilitation | Increased functional connectivity in precentral and postcentral gyrus, more restricted functional activation, improved gait and improved balance was observed following 4 weeks of neurorehabilitation |
| Sandroff, 2018 [174] | RCT(secondary analysis), resting-state fMRI, MS | Increased functional connectivity in thalamus, superior frontal gyrus and medial frontal gyrus and improved processing speed was observed following 12-week treadmill walking intervention |
| Sbardella, 2015 [178] | Cross-Sectional, resting-state fMRI and DWI, RRMS vs. controls | Decreased functional connectivity in a number of resting-state functional networks (sensorimotor, visual, cerebellar, basal ganglia, and executive control) was observed in RRMS relative to controls. In RRMS white matter integrity of the corpus callosum was also associated with functional connectivity in cerebellar and auditory networks |
| Klineova, 2016 [100] | Cross-Sectional, DWI, MS with gait impairment | White matter integrity in corticospinal tracts and overall gray and white matter volume was associated with 25-foot timed walk |
| Fritz, 2017 [54] | Cross-Sectional, DWI, RRMS | White matter integrity in corticospinal tract was associated with quantitative gait measures in RRMS |
| Hubbard, 2016 [75] | Cross-Sectional, DWI, MS | Some (median diffusivity, radial diffusivity, axial diffusivity), but not all (fractional anisotropy) measure of structural connectivity was associated with quantitative gait measures |
| Deppe, 2016 [40] | Cross-Sectional, DWI, RRMS vs. controls | RRMS participants in general had reduced white matter integrity and white matter volume relative to controls. Early and mild RRMS had reduced white matter integrity, but not white matter volume relative to controls |
| Feys, 2017 [49] | RCT, DWI and gray and white matter, MS, (‘‘start-to-run’’) running program vs. wait list | Pallidum volume increased following start-to-run program |
fMRI: functional magnetic resonance imaging; PD: Parkinson’s disease; RCT: randomized controlled trial; PD-FOG+: PD with freezing of gait; PD-FOG−: PD without freezing of gait; PD-PGID: PD with postural instability and gait difficulty; PD-TD: PD with tremor dominant phenotype; DWI: diffusion-weighted imaging; CST: corticospinal tract; INPH: idiopathic normal pressure hydrocephalus; TMS: transcranial magnetic stimulation; AD: Alzheimer’s disease; MCI: mild cognitive impairment; WMH: white matter hyperintensities; SVD: small vessel disease; CVD: cardiovascular disease; MS: multiple sclerosis; RRMS: relapsing-remitting MS; PPMS: primary progressive MS; SPMS: secondary progressive MS.
Parkinson’s disease
A large number of MRI studies of locomotion have been completed in PD (see Table 1A). These studies typically contrast the structural and functional brain substrates and pathologies in (a) PD with FOG (PD-FOG+) to PD without FOG (PD-FOG−), or (b) PD with postural instability and gait difficulty (PD-PIGD) to PD with tremor dominant phenotypes (PD-TD). PD-FOG+ relative to PD-FOG− has been associated with more gray matter atrophy [162,172,211], less white matter integrity [25,162,224,234,246], more white matter hyperintensities, altered functional connectivity [46,48,51,111,183,239,248] and altered functional activation patterns during imagined locomotion [160]–in a number of different brain regions, tracts, and functional networks: more specifically, cerebellar, parietal, precuneus, cingulate, and prefrontal cortex regions, white matter tracts that connect these regions (e.g. superior and inferior longitudinal fasciculus, corpus callosum, pedunculopontine nucleus and corticospinal tract) and functional networks that include many of these regions (e.g., fronto-parietal and default mode networks). PD-PIGD relative to PD-TD is also associated with more gray matter atrophy [171], white matter integrity [232] and altered functional connectivity [118,230,236], yet in a more restricted set of brain regions and tracts, including the amygdala, globus pallidus, corpus callosum, superior longitudinal fasciculus, and functional connectivity between the substantia nigra and putamen, and the caudate and putamen.
Stroke
MRI studies of locomotion that have been completed post-stroke are summarized in Table 1B. These studies have linked gait and other motor outcomes post-stroke to white matter integrity [2,44,78,79,81,159,243], small vessel disease pathologies [24,33,115] and functional connectivity [27]. White matter integrity of the corpus callosum and the peduncolopontine nucleus have been linked to gait speed and the ability to walk following a stroke, respectively [44,243]. Increased white matter hyperintensity burden in older adults with three or more subcortical strokes have been shown to increase the risk for multiple falls [24], and subcortical infarcts and microbleeds strengthens the negative association between white matter lesions and gait following a stroke [33]. Finally, a resting-state fMRI study suggests that interhemispheric functional connectivity, particularly between the left and right central sulcus, is associated with the extent of corticospinal damage, which in turn is associated with walking ability [27].
Alzheimer’s disease and related dementias
A more limited number of MRI studies of locomotion have been completed in ADRD (see Table 1C). One study found that gait dysfunction in a sample of older adults with probable AD, possible AD, and AD with cerebrovascular disease, was associated with gray matter atrophy in a number of different brain regions–including the motor cortex, middle cingulate, insula, and the caudate [152]. Another study found that gait impairment in AD was associated with high small vessel disease burden [233]. Finally, a third study found that subcortical white matter hyperintensity burden in frontal and basal ganglia regions was associated with gait speed and stride length in both mild AD and control participants, although the relationship with gait speed was stronger in control participants [146]. Note also that the Motoric Cognitive Risk (MCR) syndrome–a pre-dementia syndrome characterized by slow gait and cognitive complaint [229]–have been linked to gray matter atrophy in supplementary motor, pre-motor, insular and prefrontal cortex regions [10,14], lacunar infarcts in the frontal lobe [235], but not to white matter hyperintensities [134].
Multiple sclerosis
A substantial number of MRI studies of locomotion have been performed in MS (see Table 1D). These studies examined the relationship between neuroimaging measures and locomotion in a) relapsing-remitting MS (RRMS), b) primary progressive MS (PPMS), or c) MS in general. In RRMS, gray matter volume of putamen, caudate, globus pallidus, and nucleus accumbens has been linked to the 25-foot timed walking (25FTW) test [154], while cerebellar volume has been associated with the 25FTW test in PPMS [34]. In MS in general, the 25FTW test and a 6-minute walking test have been linked to gray matter volume of the pallidum and caudate [142], and pallidum volume has been shown to increase following a running intervention [49]. White matter integrity in the corticospinal tract has been linked to quantitative gait parameters in RRMS [54] and the 25FTW test in MS in general [100], while white matter integrity of the cerebellar peduncle has been linked to gait speed in PPMS. Decreased functional connectivity in RRMS relative to controls has also been observed in a number of resting-state functional networks, including sensorimotor, visual, cerebellar, basal ganglia, and executive control networks. Finally, increased functional connectivity in motor and somatosensory cortices has been observed following physical exercise interventions in MS in general [174,209].
Molecular imaging methods
Molecular imaging methods include positron emission tomography (PET) and single photon emission computed tomography (SPECT), which can image the brain during actual and during mental imagery of gait. Some studies have also correlated PET or SPECT imaging results with gait performance. These imaging techniques use radiolabeled ligands that interact in vivo with biological process (i.e., neurotransmitter receptors for the DAT-Scan, amyloid deposition for the Pittsburgh compound B–PiB-PET); measure brain metabolism (i.e., fludeoxyglucose-FDG PET), or the cerebral blood flow (i.e.; SPECT). The molecular imaging studies including patients with neurological conditions have mainly focused on patients with parkinsonism [5,16,18–20,97], or dementia [39].
In PD, the extent of dopaminergic [20,97] and cholinergic [16,18,19] denervation has been largely studied. Dopamine transporter (DAT)-PET has been studied in PD patients: striatal DAT-uptake in de novo PD patients predicted the development of FOG [97]. Interestingly, DAT activity was not associated with gait performance in non-PD population [5] or falls in PD [18]. Cholinergic denervation is a major contributor to poor gait in PD: cholinergic denervation has been associated with falls [18] and slow gait [16]. FOG phenomenon–defined by brief, episodic absence or marked reduction of forward progression of the feet despite intention to walk [150] – frequently observed in PD has been associated with reduced fronto-parietal activation and increased parietal activation while actual walking [205]. Hypoperfusion of bilateral frontal areas has been reported in PD patients with FOG in comparison to those without FOG [76,130], explaining also why FOG patients are presenting poorer executive function performance than non-FOG patients [168,193,206,210].
In the field of dementia, the development of radioligands targeting the pathological process of dementia, such as amyloid- or tau-PET, has greatly improved the in-vivo understanding of the pathophysiological cascade leading to dementia. Amyloid deposition measured by the [18F]florbetapir PET in specific brain regions involving the putamen, the precuneus, the anterior cingulate and the occipital cortex has been associated with gait speed in a cohort of older adults with and without cognitive impairment [39]. An increased β-amyloid deposition measured by the PiB-PET in PD has been associated with an increased severity of postural instability and gait difficulty features [145] that is a phenotype known to be a risk factor for developing PD dementia [166].
Functional near-infrared spectroscopy
Functional near-infrared spectroscopy (fNIRS) is a portable non-invasive optical imaging method. Similar to functional MRI that measures the blood oxygenation level-dependent (BOLD) signal of neural activity [151] – an estimation of variation of deoxy-hemoglobin concentrations – fNIRS uses the light-tissue interaction (within the near-infrared spectrum) to measure the changes in cortical oxygenation [83,197]. The main advantage of fNIRS technique for studying gait control relies on its portable nature to allow a measurement of brain activity during real walking; however, its low spatial resolution (only few centimeters under the skull) represents its main limitation [15]. Several fNIRS studies measuring brain activity while walking have been conducted in aging, showing an increased oxygenation levels in the prefrontal, premotor and supplementary motor area while walking (for a review, see [70]). A systematic review of fNIRS studies focusing on patients with gait disorders has been also recently performed [59]. In neurological conditions, the majority of fNIRS studies while walking have included patients with PD [120–122,148,215] and stroke [4,67,137–140], while few studies involved MS patients [31,69], or patients with early dementia [43]. Although these studies included a limited number of patients with neurological diseases, they demonstrated the feasibility of using fNIRS while walking in neurological conditions. Furthermore, these studies found that prefrontal cortex activations play a key role during walking across this spectrum of various neurological conditions.
Electroencephalography
Electroencephalography (EEG) is another non-invasive brain imaging modality with fNIRS that allows studying cortical activation with a high degree of precision in time (1 ms). One of the strength of EEG is the possibility to assess brain functioning during online walking and to correlate brain functioning with gait measures. Indeed, PET scan or SPECT metabolic changes occur during several minutes following the injection according to the marker used. However, until recently, several concerns limited the use of EEG during real locomotion. First, spatial resolution is low compared to PET, SPECT or MRI. Source localization using scalp electrodes can be used to improve this spatial resolution at a cortical level. The main limit is that signal recorded by scalp electrodes is mostly the consequences of post-synaptic potentials of cortical neurons. Hence, intracerebral recordings of local field potentials (LFPs) with deep brain stimulation electrodes can be used to explore profound source [104,213]. Second, artifacts caused by movement and muscle activity can contaminate EEG activity. Of course, adequate filtering and selection of the epochs not contaminated by obvious artifacts must be performed first. Despite that, two studies [28,99] investigated movement related artifacts in the EEG and found contamination of the EEG data at frequencies from 1 to 150 Hz. As frequencies of EEG investigated during walking include theta (4–7 Hz), alpha (8–12 Hz), beta 1 (13–20 Hz) beta 2 (20–30 Hz) and gamma band (> 30 Hz), these artifacts are taken into account in the analysis of cortical activity during walking and should be removed before considering changes in a power frequency band. The current method to remove these artifacts used Independent Component Analysis (ICA) to separate sources of artifacts from cortical signal [62]. Then, two methods are used, either event related potentials (ERPs) if the EEG signal changes are time-locked to an event; or time frequency analysis to study induced changes in EEG activity in different frequency bands. More recently, some groups used brain connectivity methods to study the links between different cortical areas [64,182] or between cortex and basal ganglia structures [213]. The nonlinear behavior of EEG signals has also been developed using entropy as an index of signal complexity or irregularity [65,201]. The most frequent pattern during normal gait is a modification of alpha- and beta-band power with decrease in beta band power starting during gait initiation over sensorimotor cortex [218]. Changes in alpha and beta power also occur at different phases of gait cycles [61]. The changes of spectral power in the different cortical areas are associated with the level of motor task demands [153].
Electroencephalography in neurological diseases
Recent studies correlated brain changes with gait measures, for example during a FOG in PD (see Table 2a). Some authors also directly assessed online brain functioning while walking in neurological patients; most of these EEG studies in neurological diseases have been conducted in PD patients (Table 2b). They emphasized the role of abnormal alpha and beta oscillations during locomotion and their impact in FOG occurrence. These results are summarized in Table 2b. There are few studies investigating EEG in post-stroke patients, most of them aiming to evaluate EEG-based brain-computer interfaces (BCIs) in rehabilitation, for example detection of movement intention or stopping [131,179]. Other studies aimed to determine the best algorithms to detect FOG for example are excluded from this review.
Table 2a.
Electroencephalography in Neurological Diseases—Studies evaluating the association between brain changes and gait measures.
| Reference | Objective | Methods (task/recordings/signal analysis) | Principal results |
|---|---|---|---|
| Thevathasan, 2012 [213] | Nature of oscillations in the PPN in 7 PD freezers | Gait LFPs of the PPN Time frequency analysis Connectivity (scalp electrodes) |
Peaks of alpha power during gait correlated with gait speed FOG associated with attenuation of alpha activity Alpha oscillations in a network involving the caudal subregion of the PPN and the cerebral cortex |
| Fraix, 2013 [52] | Dynamic changes in PPN area in 7 PD freezers | Step in place/sitting and standing Off and On dopa LFPs Time frequency analysis Connectivity (scalp electrodes) |
ON levodopa increases in alpha power during step in place and decrease in beta and gamma bands. Increased coherence with scalp electrodes. |
| Shine, 2014 [182] | Brain dynamic changes during turning in 24 PD freezers | Turning Scalp EEG Time-Frequency analysis Connectivity |
Increase in theta band power within the central and frontal areas during FOG Increased theta frequency coupling between the central and frontal leads |
| Handojoseno, 2015 [64] | Brain dynamic changes during turning in 4 PD freezers | Turning Scalp EEG Time-Frequency analysis Brain effective connectivity |
Increase power in beta2 band over posterior areas during actual FOG Increased connectivity towards the visual area |
| Handojoseno, 2015 [65] | Brain dynamic changes in 16 PD freezers | Timed up and go Scalp EEG Time frequency analysis Entropy Connectivity |
Posterior alpha power decreases during transition to FOG compared to normal walking decrease of entropy in most frequency bands and electrodes during FOG Connectivity: increase of beta synchronization |
| Syrkin-Nikolau, 2017[201] | STN dynamic changes during gait and turning in 14 PD freezers | Stepping in Place, forward walking, Turning and Barrier Course STN LFP Time-Frequency analysis Entropy |
The FOG STN demonstrated a greater decrease of beta power during stepping and gait than the Non-FOG STN During actual FOG, increased alpha entropy is observed |
Table 2b.
Electroencephalography in Neurological Diseases—Studies evaluating brain changes while online walking.
| Reference | Objective | Methods (task/recordings/signal analysis) | Principal results |
|---|---|---|---|
| Lau, 2015 [104] | Nature of oscillations in the PPN and STN (14 patients) | Gait LFPs of PPN and STN Time frequency analysis |
The majority of PPN neurons are modulated during gait, contrary to STN neurons |
| Shoushatarian, 2011 [184] | Cortical changes during gait initiation in 20 patients PD (with and without gait initiation difficulties) and controls (young and old) | Gait initiation Scalp EEG ERP |
Differences in MRCP peak amplitude only between young and PD patients (mixing with gait initiation difficulties or not) at Cz |
| Tard, 2016 [208] | Interference of cognitive function and motor preparation in 15 FOG and 15 non FOG PD patients | Gait initiation Scalp EEG ERP Time frequency analysis |
Abnormal increased beta oscillation during motor preparation in FOG group No differences in P3 in the 2 groups |
| Butler, 2017 [23] | Interference of cognitive function and motor preparation in 10 FOG and 10 non FOG PD patients | Gait initiation Scalp EEG ERP |
Motor preparation potential is larger in freezers during gait initiation No differences in P3b in the 2 groups |
PPN: pedunculopontine nucleus; PD: Parkinson’s disease; LFP: local field potential; FOG: freezing of gait; STN: subthalamic nucleus; MRCP: movement related cortical potentials; ERP: event-related potential.
Transcranial magnetic stimulation and transcranial direct current stimulation
Transcranial Magnetic Stimulation involves the delivery of brief magnetic pulses through the skull that can depolarize the underlying neuronal circuits and trigger physiological effects. The main limit of TMS is due to the strength and shape of the induced magnetic field, which mainly depends on the stimulation coil. Focal ones induce accurate but superficial effects and cannot be used to stimulate deep regions (as for example, lower limb motor area in the interhemispheric sulcus). Other coils allow reaching deeper regions but are less focal and induce (sometimes unwanted) effects in widespread territories. Right now, single- and paired-pulses TMS remain an invaluable tool to explore the changes in primary motor cortex excitability that can occur in neurological diseases. It has been extensively used to better understand the pathophysiology of brain networks including lower limb M1 area in gait disorders. TMS may also induce lasting effects on neurons excitability when the stimulus is delivered by trains and can be used to modulate brain activity in disturbed neural circuits most likely via LTP- and LTD-like mechanisms [250]. The net effect of rTMS on brain neurons depends on several parameters such as stimulus intensity, frequency, pattern or train duration. Repetitive TMS may also be influenced by factors that may alter or enhance induction of brain plasticity. For instance, the level of brain excitability [187] or the practice of physical activity before the train [132] are able to change the net effect of rTMS. Another way to induce long lasting changes in cortical networks is to deliver an electrical current through the scalp (tDCS) that shifts the resting membrane potential toward depolarization or hyperpolarization depending on the current flow regarding axonal orientation. Although the mechanism of action of rTMS and tDCS are very different, these two non-invasive brain stimulation (NIBS) techniques share the capacity to decrease or increase brain excitability depending on the paradigm used with effects lasting beyond the duration of the stimulus. In addition to the clinical usefulness to counterbalance the deficits of excitability occurring in neurological disease, the disruptive effect of NIBS on brain circuits is also helpful to understand the role of various cerebral regions in gait and balance processing.
In this chapter, we will firstly focus on TMS studies that aimed at correlating brain excitability changes with clinical aspects of gait alteration. Then, we will consider some of the NIBS studies that allowed assessing the role of cerebral regions in gait control in patients with PD.
Brain excitability changes in neurological diseases with gait disorders
Most TMS studies of neurological diseases have been conducted in PD. In PD patients, only slight changes in lower limb cortical excitability have been shown [222]. An increase in MEP amplitude and reduction in motor threshold was reported in the quadriceps muscle [217], but not in the Tibialis anterior muscle, where only a decrease of intra-cortical facilitation was shown [222]. After stroke, the loss of neurons associated with maladaptive cortical reorganization may lead to functional impairments of gait. As in the upper limbs, TMS studies have revealed a striking unbalance between the affected and the non-affected hemispheres controlling the lower limbs, with a reduction of excitability on the paretic side and an increase on the non-paretic side [155,156,241]. Corticomotor impairment was shown to be more severe in plantarflexor than in dorsiflexor muscles in stroke patients with poor walking recovery [156]. The presence of motor responses in the Tibialis anterior in the post-acute phase of stroke was a good indicator of lower limb motor recovery [163] and the asymmetry between the paretic and non-paretic sides (calculated from MEP motor thresholds or MEP amplitude) directly relates to walking recovery in chronic stroke patients [155,156]. In multiple sclerosis, only one study showed that cortical excitability of the Tibialis anterior was more enhanced than in healthy subjects after a fatiguing exercise [214].
Non-invasive brain stimulation-induced changes in cerebral networks and gait performance in clinical diseases
After first reports showing that rTMS over the vertex could have positive effects on walking performance in PD [126], numerous studies have tried to improve the beneficial influence of rTMS by targeting different cortical areas and looking for the best stimulation paradigms. Although some authors did not find any improvement of gait after rTMS, some studies suggest that high frequency stimulation of the upper limb motor area [106], the lower limb motor area [30,105,129,244] or both of them [93] have positive influence on gait in PD. Similarly, anodal tDCS of M1 area exerts a beneficial effect on gait with a decrease in walking speed [12,245] and a reduction of FOG episodes [36,223]. However, two studies reported no significant effect of tDCS alone over M1, but the same stimulation became effective to improve gait and decrease FOG episodes when coupled with pre-frontal tDCS [96] or motor training [89]. This latter region seems to play an important role in the neural gait network and related motor symptoms in PD, although the results of NIBS on walking performance are controversial with some studies showing gait amelioration after tDCS over the dorsolateral prefrontal cortex [103,127] whereas others did not [200]. One study also reported an improvement of the TUG test after rTMS of the dorsolateral prefrontal cortex [105] and another showed that gait variability and FOG occurrence were decreased after high frequency rTMS over the medial prefrontal cortex [37]. In contrast, neither cerebellum [80], nor premotor cortex stimulation [207] have a positive influence on FOG in PD. Contrasting results have been obtained for the supplementary motor area: whereas a benefit of rTMS for anticipatory postural adjustments duration [77] and FOG [98] were shown; one study did not report significant effects compared with sham stimulation [105]. And tDCS over SMA is not efficient to improve gait initiation [117]. Taken together, these studies confirm the usefulness of non-invasive brain stimulation methods to better understand gait disturbances in PD and suggest a dysfunction of several regions involved in gait control, mainly the prefrontal cortex, SMA, M1 and the cerebellum. In stroke patients, the deficits observed in locomotion are directly the consequence of brain lesions. Right now, NIBS methods have been used to induce plasticity in M1 and improve lower limb motor function but their effectiveness remain difficult to assess (for a review, see [50]).
Advanced methods and perspectives
Differentiating neurological from non-neurological clinical gait abnormalities is nowadays critical in clinics for early onset detection of developing neurodegenerative and mental disorders. Kinetic and kinematic characteristics might be similar between the two disorders, and they are unfit to fully reveal the neural correlates underlying walking deficits further misidentifying the two disorders. A clear distinction of the two entails, therefore, the observation of brain alterations that might be predominant in neurological conditions, and the definition of robust measures of these abnormal neural activity patterns for risk assessment and intervention procedures [242].
The definition of reliable imaging-based biomarkers for clinical decision and therapeutic interventions necessitate the identification of strong associations between brain and objective behavioral measures. The majority of previous works correlated individual signature of gait abnormalities with localized brain features extracted from single imaging technique. However, numerous brain structures, including basal ganglia, cerebellum, frontal and parietal cortices, jointly subserve to generate walking with multifaceted components. Even a priori focal conditions such as stroke have been reconsidered as a distributed network disease [188] leading to a new definition of diaschisis (i.e., connectional diaschisis) that indicates structural and functional changes between brain areas distant to the lesion [26]. Therefore, future studies should investigate multivariate correlations both in terms of brain parcels, acquisition modalities, and gait quantifications [70].
A possible method to identify multiple underlying associations between two sets of variables is canonical correlation analysis (CCA), which seeks significant correlations between combinations of variables in both sets and both at group- and individual-subject level [66]. This method requires large enough datasets for proper validation, but can then be successful in spotting strong relationship between demographic and psychometric measures and functional connections across brain regions within the default mode network [192]. The demand of ‘‘large enough datasets’’ has led to the emergence of several publicly available initiatives to promote data sharing across groups (see Parkinson’s progression markers initiative, Alzheimer’s Disease Neuroimaging initiative, UK biobank for some examples). However, the usage of these datasets requires transfer-learning techniques to match multi-site data acquired from mismatch subjects and with different imaging parameters [112].
In addition, multivariate brain features could be inferred from combinations of anatomo-functional components. Indeed, despite various neurophysiological origins, neuro-logical gait disorders are associated both with structural origin, such as atrophy and/or damage to the grey or white matters in the locomotion networks and reduced integrity (e.g., reduced fractional anisotropy and increased mean diffusivity) between these areas, and with flawed functional networks. However, whether structural and functional abnormalities operate synergistically or in opposition is still unknown. Determining this relationship would lead to a more complete characterization of the pathology and its stage, and, thus, to the definition of more robust and reliable biomarkers. Several approaches have been proposed to map functional connectivity from anatomical features extracted directly from white matter streamlines or enforced from graph-theoretical properties [11,38,169,170]. Brain-graphs can be constructed associating nodes to anatomically defined parcels or recording sensors and edges to structural or functional connections. Measures of information transfer, efficiency, and structure can be extracted from structural and functional-based graph and associated to clinical symptoms [9]. Brain graph measures, for instance, have revealed a decreased efficiency in cortico-basal ganglia motor circuits significantly correlating with Unified Parkinson’s Disease Rating Scale motor scores in PD patients [237]. These results are in line with the pathophysiology (i.e., dopamine depletion of the cortico-basal ganglia) of PD, suggesting that graph-based metrics are suitable biomarkers to track the evolution of the pathology and inform clinical-decision making.
Current approaches applying graph-theoretical analysis extract information from data defined in regular domains such as time or space and do not account for the dynamics of the networks. Moreover, functional connectivity is classically computed by pairwise correlations over the complete resting-state run, disregarding moment-to-moment fluctuations. To overcome these limitations and to allow analyzing spatiotemporal elements, the emerging field of signal processing on graphs has tailored an increasing number of conventional operations to signals expressed at the nodes of the graph [32]. Among others, the main operation deployed to analyze brain activity is the graph Laplacian operator defined from the structural connectome [73]. The eigen decomposition of the Laplacian then defines a spectral graph domain corresponding to the Fourier transform on regular grids [185]. Projections on the eigenvectors of the Laplacian (i.e., Graph Fourier Transform) allows separating low graph frequency components representing signals that are more ‘‘aligned’’ with the structural backbone, and high graph frequency components representing signals that are more ‘‘liberal’’ with respect to brain structure [74]. This separation highlighted that low frequency functional components are associated with greater task switching flexibility [73,133], which is thought to originate in corti-cobasal ganglia-thalamo-cortical loops and is often reduced in patients with neurological syndromes [202]. Additionally, Graph Fourier Transform permits generating surrogate graph signals in which the correlation structure of the signals is imposed by the empirical data allowing stronger statistical assessment, a pivotal feature in clinical applications [73,165].
The dynamics underlying brain networks, however, could also be encoded either through direct voxel- or region-wise signal measures (second-order statistics) or by feeding established brain states into temporal models (first-order methods) [88,167]. In primordial strategies, second-order methods, such as pairwise correlation over temporal sliding-windows, were deployed to compute dynamic functional connectivity. Despite the limiting window length trade-off, this basic sliding-window framework allowed demonstrating that abnormal connectivity in neurodegenerative diseases could be better explained by shortening dwell time in sub-networks configurations, rather than stationary connectivity magnitude, proving the importance of dynamic methods [42,84,94,110]. However, fluctuating brain activity could also be captured with time-resolved analysis where informative timepoints retained by thresholding–either on the original timeseries as in point process analysis [203,204] or on innovation signals [87]–are temporally clustered in spatial co-activation patterns (CAPs) [114]. Spatiotemporal regression of the established repertoire of CAPs allows computing networks-based temporal measures such as occurrence percentage, duration, or state switching frequency, which can be used to related pathological brain dynamics to clinical manifestation. For instance, in a recent study a reduced switching probability among CAPs correlating with motor symptom severity was observed in early stage of PD [249].
Similarly, temporal clustering approaches were applied also to EEG data showing that electrical fluctuations can be discretized in semi-stable potential topographies (a.k.a., microstates), whose temporal properties, identified with analogous temporal measures, change with neurological and psychiatric conditions [92,136]. The EEG microstates demonstrated to be relevant for assessing patient’s clinical status and treatment efficacy [199,247]. For instance, Zappasodi and colleagues [247] showed that preserved microstate duration in post-stroke acute phase correlated with a better effective recovery.
Despite the relevance of this extended repertoire of imaging-based biomarkers that exploit network and dynamical organization to better characterize diseases phenotypes and progression, nowadays studies should capitalize on these measures to improve therapeutic strategies. Preliminary applications in this direction utilized fMRI and EEG dynamic brain models to drive neurofeedback showing that network measures-based close-loop instead of single brain region features could target more relevant large-scale interactions leading to potentially more powerful regulation protocols [41,102,190]. Similarly, pretherapeutic grey matter density and network(s) interconnectivity strength could predict clinical effects of stereotactic radio-surgical thalatomy [219–221] and brain stimulation [71], which has been shown to be useful in brain imaging assessment and treatment of gait disturbances. Moreover, these anatomo-functional components and particularly the large-scale impact of regional stimulation detected by dynamical brain networks could optimize the stimulation protocols improving their efficacy and reducing secondary effects [144]. In this framework, the advent of multimodal recordings and stimulation techniques such as TMS-EEG and more recently TMS-fMRI is critical [13,101,186]. Moreover, these applications require the extension of the methods here elucidated and originally designed for analysis of resting-state brain activity to task-based paradigms. Attempts in this direction are, therefore, emerging [21,22,58,164,189] and they would be critical to characterize the neural correlates underlying neurological gait abnormalities.
Conclusion
Complementary neuroimaging techniques have been used in various neurological conditions (mainly in PD) in supine position or during actual walking to study the brain substrates of locomotion. The extent networks involved in the control of locomotion include grey and white matter structures in key regions, such as the prefrontal cortex, the basal ganglia and the cerebellum, explaining the strong relationship between locomotion and cognition (especially executive functions) that are both controlled by these cortical and subcortical regions. However, the role of individual pathophysiological mechanisms encountered in each neurological condition on the phenotype of gait disorders is still not clear. Advanced neuroimaging methods and computational analyses that integrate multivariate correlations, such as information on brain structures, functions, or acquisition modalities, in ‘‘large enough datasets’’, might solve that issue of the phenotypical gait signature due to a unique or combined neurological conditions. These multivariate computational methods will not only ‘‘decode’’ the brain substrate of impaired locomotion, but may also drive the rehabilitative strategy, by using a close loop neurofeedback training (see Fig. 1). The success of these future approaches will depend on the collaborative efforts between neurologists and scientists from mathematics, physics and computer science.
Figure 1.
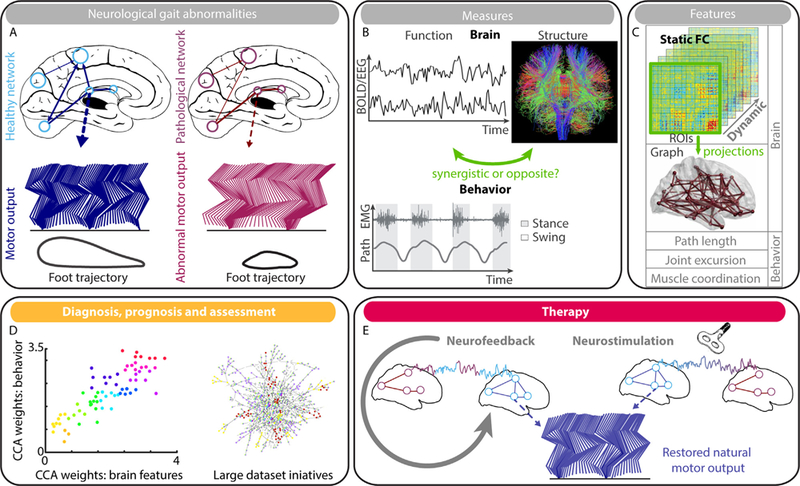
Schematic representation of the contribution of advanced neuroimaging techniques for ‘‘decoding’’ brain substrates of gait disorders and their treatment. A. Disrupted distributed brain networks reflect on neurological gait abnormalities. B. The neural alterations can be captured through both functional and structural modalities; while gait disorders can be characterized by kinetic and kinematic observations. Yet it remains to be elucidated whether anatomo-functional abnormalities operate synergistically or in opposition in the generation of pathological walking. C. Despite this open question, several features can be extracted from neural observations and exploited as biomarkers. However, because correlation between parcels exhibits meaningful temporal variations, brain networks dynamics has to be kept in consideration. The spatiotemporal elements can be captured by graph signal processing techniques, which require projection of the brain activity over the structural backbone, or via first- and second-order statistics, which encode the dynamics directly from voxel- or region-wise signal measures. D. Because of the multifaceted nature of gait and the underlying numerous brain structures, the identified features should be related through multivariate correlation methods, such as canonical correlation analysis (CCA), for a more complete characterization of the pathology and its stage. However, these multivariate techniques require ‘‘large enough datasets’’ for proper validation. E. Finally, the identified brain features and their dynamics should not only inform rehabilitative-decision processes, but also be deployed to directly drive the therapy. For instance, close-loop neurofeedback training can impose neuroregulation of the networks dynamics instead of single brain region; while neurostimulation parameters can be optimized by considering the impact of regional stimulation over dynamical brain networks. The efficacy of these therapies would still require proper assessment via the identified imaging biomarkers.
Acknowledgments
Funding
This study was funded by the Swiss National Science Foundation (320030_173153) and by the Bertarelli Foundation Catalyst Fund (proposal number BC1708).
Footnotes
Disclosure of interest
The authors declare that they have no competing interest.
References
- [1].Acharya HJ, Bouchard TP, Emery DJ, Camicioli RM. Axial signs and magnetic resonance imaging correlates in Parkinson’s disease. Can J Neurol Sci 2007;34:56–61. [DOI] [PubMed] [Google Scholar]
- [2].Ahn YH, Ahn SH, Kim H, Hong JH, Jang SH. Can stroke patients walk after complete lateral corticospinal tract injury of the affected hemisphere? Neuroreport 2006;17:987–90. [DOI] [PubMed] [Google Scholar]
- [3].Al-Bachari S, Vidyasagar R, Emsley HC, Parkes LM. Structural and physiological neurovascular changes in idiopathic Parkinson’s disease and its clinical phenotypes. J Cereb Blood Flow Metab 2017;37:3409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Yahya E, Johansen-Berg H, Kischka U, Zarei M, Cockburn J, Dawes H. Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil Neural Repair 2016;30:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Allali G, Garibotto V, Mainta IC, Armand S, Camicioli R, Ratib O, et al. Dopaminergic denervation is not necessary to induce gait disorders in atypical parkinsonian syndrome. J Neurol Sci 2015;351:127–32. [DOI] [PubMed] [Google Scholar]
- [6].Allan LM, Ballard CG, Burn DJ, Kenny RA. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc 2005;53:1681–7. [DOI] [PubMed] [Google Scholar]
- [7].Anderson VM, Wheeler-Kingshott CA, Abdel-Aziz K, Miller DH, Toosy A, Thompson AJ, et al. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler 2011;17:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arena JE, Cerquetti D, Rossi M, Chaves H, Rollan C, Dossi DE, et al. Influence of white matter MRI hyper-intensities on acute l-dopa response in patients with Parkinson’s disease. Parkinsonism Relat Disord 2016;24:126–8. [DOI] [PubMed] [Google Scholar]
- [9].Bassett DS, Mattar MG. A network neuroscience of human learning: potential to inform quantitative theories of brain and behavior. Trends Cogn Sci 2017;21:250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beauchet O, Allali G, Annweiler C, Verghese J. Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT study. J Gerontol A Biol Sci Med Sci 2016;71:1081–8. [DOI] [PubMed] [Google Scholar]
- [11].Becker CO, Pequito S, Pappas GJ, Miller MB, Grafton ST, Bassett DS, et al. Spectral mapping of brain functional connectivity from diffusion imaging. Sci Rep 2018:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010;81:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bestmann S, Feredoes E. Combined neurostimulation and neuroimaging in cognitive neuroscience: past, present, and future. Ann N Y Acad Sci 2013;1296:11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J. A gray matter volume covariance network associated with the motoric cognitive risk syndrome: a multi-cohort MRI study. J Gerontol A Biol Sci Med Sci 2018. [in press]. [DOI] [PubMed]
- [15].Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 2004;23(Suppl. 1):S275–88. [DOI] [PubMed] [Google Scholar]
- [16].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013;81:1611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bohnen NI, Jahn K. Imaging: What can it tell us about parkinsonian gait? Mov Disord 2013;28:1492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009;73:1670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab 2012;32:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev 2009;46:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bolton TAW, Jochaut D, Giraud AL, Van De Ville D. Brain dynamics in ASD during movie-watching show idiosyncratic functional integration and segregation. Hum Brain Mapp 2018;39:2391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Braun U, Schafer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A 2015;112:11678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Butler JS, Fearon C, Killane I, Waechter SM, Reilly RB, Lynch T. Motor preparation rather than decision-making differentiates Parkinson’s disease patients with and without freezing of gait. Clin Neurophysiol 2017;128:463–71. [DOI] [PubMed] [Google Scholar]
- [24].Callisaya ML, Srikanth VK, Lord SR, Close JC, Brodaty H, Sachdev PS, et al. Sub-cortical infarcts and the risk of falls in older people: combined results of TASCOG and Sydney MAS studies. Int J Stroke 2014;9(Suppl A100):55–60. [DOI] [PubMed] [Google Scholar]
- [25].Canu E, Agosta F, Sarasso E, Volonte MA, Basaia S, Stojkovic T, et al. Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Hum Brain Mapp 2015;36:5064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014;137:2408–22. [DOI] [PubMed] [Google Scholar]
- [27].Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair 2012;26:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Castermans T, Duvinage M, Cheron G, Dutoit T. About the cortical origin of the low-delta and high-gamma rhythms observed in EEG signals during treadmill walking. Neurosci Lett 2014;561:166–70. [DOI] [PubMed] [Google Scholar]
- [29].Chan LL, Ng KM, Rumpel H, Fook-Chong S, Li HH, Tan EK. Transcallosal diffusion tensor abnormalities in predominant gait disorder parkinsonism. Parkinsonism Relat Disord 2014;20:53–9. [DOI] [PubMed] [Google Scholar]
- [30].Chang WH, Kim MS, Cho JW, Youn J, Kim YK, Kim SW, et al. Effect of cumulative repetitive transcranial magnetic stimulation on freezing of gait in patients with atypical Parkinsonism: A pilot study. J Rehabil Med 2016;48:824–8. [DOI] [PubMed] [Google Scholar]
- [31].Chaparro G, Balto JM, Sandroff BM, Holtzer R, Izzetoglu M, Motl RW, et al. Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis. J Neuroeng Rehabil 2017;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen HM, Wang ZJ, Fang JP, Gao LY, Ma LY, Wu T, et al. Different patterns of spontaneous brain activity between tremor-dominant and postural instability/gait difficulty sub-types of Parkinson’s disease: a resting-state fMRI study. CNS Neurosci Ther 2015;21:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, et al. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability population-based study. Stroke 2012;43:1505–10. [DOI] [PubMed] [Google Scholar]
- [34].Cocozza S, Petracca M, Mormina E, Buyukturkoglu K, Podranski K, Heinig MM, et al. Cerebellar lobule atrophy and disability in progressive MS. J Neurol Neurosurg Psychiatry 2017;88:1065–72. [DOI] [PubMed] [Google Scholar]
- [35].Craig LE, Wu O, Bernhardt J, Langhorne P. Predictors of poststroke mobility: systematic review. Int J Stroke 2011;6:321–7. [DOI] [PubMed] [Google Scholar]
- [36].Dagan M, Herman T, Harrison R, Zhou J, Giladi N, Ruffini G, et al. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord 2018;33:642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dagan M, Herman T, Mirelman A, Giladi N, Hausdorff JM. The role of the prefrontal cortex in freezing of gait in Parkinson’s disease: insights from a deep repetitive transcranial magnetic stimulation exploratory study. Exp Brain Res 2017;235:2463–72. [DOI] [PubMed] [Google Scholar]
- [38].Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 2011;12:43–56. [DOI] [PubMed] [Google Scholar]
- [39].Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, et al. Relationship of regional brain beta-amyloid to gait speed. Neurology 2016;86:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Deppe M, Tabelow K, Kramer J, Tenberge JG, Schiffler P, Bittner S, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler 2016;22:73–84. [DOI] [PubMed] [Google Scholar]
- [41].Diaz Hernandez L, Rieger K, Baenninger A, Brandeis D, Koenig T. Towards Using Microstate-Neurofeedback for the Treatment of Psychotic Symptoms in Schizophrenia. A Feasibility Study in Healthy Participants. Brain Topogr 2016;29:308–21. [DOI] [PubMed] [Google Scholar]
- [42].Diez-Cirarda M, Strafella AP, Kim J, Pena J, Ojeda N, Cabrera-Zubizarreta A, et al. Dynamic functional connectivity in Parkinson’s disease patients with mild cognitive impairment and normal cognition. Neuroimage Clin 2018;17:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Doi T, Makizako H, Shimada H, Park H, Tsutsumimoto K, Uemura K, et al. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res 2013;25:539–44. [DOI] [PubMed] [Google Scholar]
- [44].Dubey P, Lioutas VA, Bhadelia R, Manor B, Novak P, Selim M, et al. Quantitative microstructural deficits in chronic phase of stroke with small volume infarcts: a diffusion tensor 3-D tractographic analysis. Magn Reson Imaging 2016;34:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dupuy SL, Tauhid S, Hurwitz S, Chu R, Yousuf F, Bakshi R. The Effect of dimethyl fumarate on cerebral gray matter atrophy in multiple sclerosis. Neurol Ther 2016;5:215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ehgoetz Martens KA, Hall JM, Georgiades MJ, Gilat M, Walton CC, Matar E, et al. The functional network signature of heterogeneity in freezing of gait. Brain 2018;141:1145–60. [DOI] [PubMed] [Google Scholar]
- [47].Fasano A, Herman T, Tessitore A, Strafella AP, Bohnen NI. Neuroimaging of Freezing of Gait. J Parkinsons Dis 2015;5:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 2017;81:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Feys P, Moumdjian L, Van Halewyck F, Wens I, Eijnde BO, Van Wijmeersch B, et al. Effects of an individual 12-week community-located ‘‘start-to-run’’ program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler 2017. (in press). [DOI] [PubMed]
- [50].Fleming MK, Pavlou M, Newham DJ, Sztriha L, Teo JT. Non-invasive brain stimulation for the lower limb after stroke: what do we know so far and what should we be doing next? Disabil Rehabil 2017;39:714–20. [DOI] [PubMed] [Google Scholar]
- [51].Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One 2014;9:e100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid AL, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PloS one 2013;8:e83919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Freund P, Wheeler-Kingshott C, Jackson J, Miller D, Thompson A, Ciccarelli O. Recovery after spinal cord relapse in multiple sclerosis is predicted by radial diffusivity. Mult Scler 2010;16:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fritz NE, Keller J, Calabresi PA, Zackowski KM. Quantitative measures of walking and strength provide insight into brain corticospinal tract pathology in multiple sclerosis. Neuroimage Clin 2017;14:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gallea C, Ewenczyk C, Degos B, Welter ML, Grabli D, Leu-Semenescu S, et al. Pedunculopontine network dysfunction in Parkinson’s disease with postural control and sleep disorders. Mov Disord 2017;32:693–704. [DOI] [PubMed] [Google Scholar]
- [56].Gilat M, Ehgoetz Martens KA, Miranda-Domínguez O, Arpan I, Shine JM, Mancini M, et al. Dysfunctional limbic circuitry underlying freezing of gait in Parkinson’s disease. Neuro-science 2018;374:119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Goncalves LI, Dos Passos GR, Conzatti LP, Burger JLP, Tomasi GH, Zandona ME, et al. Correlation between the corpus callosum index and brain atrophy, lesion load, and cognitive dysfunction in multiple sclerosis. Mult Scler Relat Disord 2018;20:154–8. [DOI] [PubMed] [Google Scholar]
- [58].Gonzalez-Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci U S A 2012;109:5487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gramigna V, Pellegrino G, Cerasa A, Cutini S, Vasta R, Olivadese G, et al. Near-Infrared Spectroscopy in Gait Disorders: Is It Time to Begin? Neurorehabil Neural Repair 2017;31:402–12. [DOI] [PubMed] [Google Scholar]
- [60].Gu Q, Huang P, Xuan M, Xu X, Li D, Sun J, et al. Greater loss of white matter integrity in postural instability and gait difficulty subtype of Parkinson’s disease. Can J Neurol Sci 2014;41:763–8. [DOI] [PubMed] [Google Scholar]
- [61].Gwin JT, Gramann K, Makeig S, Ferris DP. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011;54:1289–96. [DOI] [PubMed] [Google Scholar]
- [62].Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol 2010;103:3526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hall JM, Shine JM, Ehgoetz Martens KA, Gilat M, Broadhouse KM, Szeto JYY, et al. Alterations in white matter network topology contribute to freezing of gait in Parkinson’s disease. J Neurol 2018;265:1353–64. [DOI] [PubMed] [Google Scholar]
- [64].Handojoseno AM, Gilat M, Ly QT, Chamtie H, Shine JM, Nguyen TN, et al. An EEG study of turning freeze in Parkinson’s disease patients: the alteration of brain dynamic on the motor and visual cortex. Conf Proc IEEE Eng Med Biol Soc 2015;2015:6618–21. [DOI] [PubMed] [Google Scholar]
- [65].Handojoseno AM, Shine JM, Nguyen TN, Tran Y, Lewis SJ, Nguyen HT. Analysis and prediction of the freezing of gait using EEG brain dynamics. IEEE Trans Neural Syst Rehabil Eng 2015;23:887–96. [DOI] [PubMed] [Google Scholar]
- [66].Hardoon DR, Szedmak S, Shawe-Taylor J. Canonical correlation analysis: an overview with application to learning methods. Neural Comput 2004;16:2639–64. [DOI] [PubMed] [Google Scholar]
- [67].Hawkins KA, Fox EJ, Daly JJ, Rose DK, Christou EA, McGuirk TE, et al. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum Mov Sci 2018;59:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Herman T, Rosenberg-Katz K, Jacob Y, Auriel E, Gurevich T, Giladi N, et al. White matter hyperintensities in Parkinson’s disease: do they explain the disparity between the postural instability gait difficulty and tremor dominant subtypes? PLoS One 2013;8:e55193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hernandez ME, Holtzer R, Chaparro G, Jean K, Balto JM, Sandroff BM, et al. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci 2016;370:277–83. [DOI] [PubMed] [Google Scholar]
- [70].Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci 2014;69:1375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol 2017;82:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hou Y, Zhang J, Chen B, Wu T. [Local brain activity in different motor subtypes of Parkinson’s disease with fMRI]. Zhonghua Yi Xue Za Zhi 2015;95:483–8. [PubMed] [Google Scholar]
- [73].Huang WB, Bolton AW, Medaglia JD, Bassett DS, Ribeiro A, Van de Ville D. A graph signal processing perspective on functional brain imaging. Proc IEEE 2018;106:868–85. [Google Scholar]
- [74].Huang WY, Goldsberry L, Wymbs NF, Grafton ST, Bassett DS, Ribeiro A. Graph frequency analysis of brain signals. IEEE J-STSP 2016;10:1189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hubbard EA, Wetter NC, Sutton BP, Pilutti LA, Motl RW. Diffusion tensor imaging of the corticospinal tract and walking performance in multiple sclerosis. J Neurol Sci 2016;363:225–31. [DOI] [PubMed] [Google Scholar]
- [76].Imamura K, Okayasu N, Nagatsu T. Cerebral blood flow and freezing of gait in Parkinson’s disease. Acta Neurol Scand 2012;126:210–8. [DOI] [PubMed] [Google Scholar]
- [77].Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 2009;164:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 2013;44:1099–104. [DOI] [PubMed] [Google Scholar]
- [79].Jang SH, Kim K, Kim SH, Son SM, Jang WH, Kwon HG. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci Lett 2014;572:1–6. [DOI] [PubMed] [Google Scholar]
- [80].Janssen AM, Munneke MAM, Nonnekes J, van der Kraan T, Nieuwboer A, Toni I, et al. Cerebellar theta burst stimulation does not improve freezing of gait in patients with Parkinson’s disease. J Neurol 2017;264:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jayaram G, Stagg CJ, Esser P, Kischka U, Stinear J, Johansen-Berg H. Relationships between functional and structural corticospinal tract integrity and walking post stroke. Clin Neurophysiol 2012;123:2422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jiang S, Wang M, Zhang L, Yuan Y, Tong Q, Ding J, et al. Regional homogeneity alterations differentiate between tremor dominant and postural instability gait difficulty sub-types of Parkinson’s disease. J Neural Transm (Vienna) 2016;123:219–29. [DOI] [PubMed] [Google Scholar]
- [83].Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977;198:1264–7. [DOI] [PubMed] [Google Scholar]
- [84].Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, et al. Non-Stationarity in the ‘‘Resting Brain’s’’ Modular Architecture. PloS one 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kanno S, Abe N, Saito M, Takagi M, Nishio Y, Hayashi A, et al. White matter involvement in idiopathic normal pressure hydrocephalus: a voxel-based diffusion tensor imaging study. J Neurol 2011;258:1949–57. [DOI] [PubMed] [Google Scholar]
- [86].Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P. Altered diffusion in the frontal lobe in Parkinson disease. AJNR Am J Neuroradiol 2008;29:501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Karahanoglu FI, Van De Ville D. Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nat Commun 2015;6:7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Karahanoğlu FI, Van De Ville D. Dynamics of large-scale fMRI networks: Deconstruct brain activity to build better models of brain function. Curr Opin Biomed Eng 2017;3:28–36. [Google Scholar]
- [89].Kaski D, Dominguez RO, Allum JH, Islam AF, Bronstein AM. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clin Rehabil 2014;28:1115–24. [DOI] [PubMed] [Google Scholar]
- [90].Kern KC, Sarcona J, Montag M, Giesser BS, Sicotte NL. Corpus callosal diffusivity predicts motor impairment in relapsing-remitting multiple sclerosis: a TBSS and tractography study. Neuroimage 2011;55:1169–77. [DOI] [PubMed] [Google Scholar]
- [91].Khalid F, Tauhid S, Chua AS, Healy BC, Stankiewicz JM, Weiner HL, et al. A longitudinal uncontrolled study of cerebral gray matter volume in patients receiving natalizumab for multiple sclerosis. Int J Neurosci 2017;127:396–403. [DOI] [PubMed] [Google Scholar]
- [92].Khanna A, Pascual-Leone A, Farzan F. Reliability of resting-state microstate features in electroencephalography. PloS one 2014;9:e114163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord 2006;21:2201–5. [DOI] [PubMed] [Google Scholar]
- [94].Kim J, Criaud M, Cho SS, Diez-Cirarda M, Mihaescu A, Coakeley S, et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain 2017;140:2955–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kim JH, Park J, Kim YH, Ma HI, Kim YJ. Characterization of cerebral microbleeds in idiopathic Parkinson’s disease. Eur J Neurol 2015;22:377–83. [DOI] [PubMed] [Google Scholar]
- [96].Kim MS, Chang WH, Cho JW, Youn J, Kim YK, Kim SW, et al. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson’s disease. Restor Neurol Neurosci 2015;33:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kim R, Lee J, Kim Y, Kim A, Jang M, Kim HJ, et al. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: An analysis of the PPMI cohort. Parkinsonism Relat Disord 2018;51:49–54. [DOI] [PubMed] [Google Scholar]
- [98].Kim SJ, Paeng SH, Kang SY. Stimulation in supplementary motor area versus motor cortex for freezing of Gait in Parkinson’s disease. J Clin Neurol 2018;14:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kline JE, Huang HJ, Snyder KL, Ferris DP. Isolating gait-related movement artifacts in electroencephalography during human walking. J Neural Eng 2015;12:046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Klineova S, Farber R, Saiote C, Farrell C, Delman BN, Tanenbaum LN, et al. Relationship between timed 25-foot walk and diffusion tensor imaging in multiple sclerosis. Mult Scler J Exp Transl Clin 2016;2 [2055217316655365]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Koch PJ, Hummel FC. Toward precision medicine: tailoring interventional strategies based on noninvasive brain stimulation for motor recovery after stroke. Curr Opin Neurol 2017;30:388–97. [DOI] [PubMed] [Google Scholar]
- [102].Koush Y, Meskaldji DE, Pichon S, Rey G, Rieger SW, Linden DEJ, et al. Learning control over emotion networks through connectivity-based neurofeedback. Cereb Cortex 2017;27:1193–202. [DOI] [PubMed] [Google Scholar]
- [103].Lattari E, Costa SS, Campos C, de Oliveira AJ, Machado S, Maranhao Neto GA. Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease? Neurosci Lett 2017;636:165–9. [DOI] [PubMed] [Google Scholar]
- [104].Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E, Grabli D, et al. The integrative role of the pedunculopontine nucleus in human gait. Brain 2015;138:1284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lee SY, Kim MS, Chang WH, Cho JW, Youn JY, Kim YH. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci 2014;32:743–53. [DOI] [PubMed] [Google Scholar]
- [106].Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol 2004;115:2530–41. [DOI] [PubMed] [Google Scholar]
- [107].Lenfeldt N, Hansson W, Larsson A, Nyberg L, Birgander R, Forsgren L. Diffusion tensor imaging and correlations to Parkinson rating scales. J Neurol 2013;260:2823–30. [DOI] [PubMed] [Google Scholar]
- [108].Lenfeldt N, Holmlund H, Larsson A, Birgander R, Forsgren L. Frontal white matter injuries predestine gait difficulties in Parkinson’s disease. Acta Neurol Scand 2016;134:210–8. [DOI] [PubMed] [Google Scholar]
- [109].Lenka A, Naduthota RM, Jha M, Panda R, Prajapati A, Jhunjhunwala K, et al. Freezing of gait in Parkinson’s disease is associated with altered functional brain connectivity. Parkinsonism Relat Disord 2016;24:100–6. [DOI] [PubMed] [Google Scholar]
- [110].Leonardi N, Richiardi J, Gschwind M, Simioni S, Annoni JM, Schluep M, et al. Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest. Neuroimage 2013;83:937–50. [DOI] [PubMed] [Google Scholar]
- [111].Li J, Yuan Y, Wang M, Zhang J, Zhang L, Jiang S, et al. Decreased interhemispheric homotopic connectivity in Parkinson’s disease patients with freezing of gait: A resting state fMRI study. Parkinsonism Relat Disord 2018;52:30–6. [DOI] [PubMed] [Google Scholar]
- [112].Li WZY, Chen X, Xiao Y, Qin Y. Detecting Alzheimer’s disease on small dataset: a knowledge transfer perspective. IEEE J Biomed Health Inform 2018. [in press]. [DOI] [PubMed]
- [113].Li Y, Jewells V, Kim M, Chen Y, Moon A, Armao D, et al. Diffusion tensor imaging based network analysis detects alterations of neuroconnectivity in patients with clinically early relapsing-remitting multiple sclerosis. Hum Brain Mapp 2013;34:3376–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu X, Duyn JH. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 2013;110:4392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Loos CM, McHutchison C, Cvoro V, Makin SD, Staals J, Chappell F, et al. The relation between total cerebral small vessel disease burden and gait impairment in patients with minor stroke. Int J Stroke 2017. [1747493017730780]. [DOI] [PubMed]
- [116].Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke 2005;36:1457–61. [DOI] [PubMed] [Google Scholar]
- [117].Lu C, Amundsen Huffmaster SL, Tuite PJ, MacKinnon CD. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: a pilot study. J Neurol 2018;265:2023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ma LY, Chen XD, He Y, Ma HZ, Feng T. Disrupted brain network hubs in subtype-specific Parkinson’s disease. Eur Neurol 2017;78:200–9. [DOI] [PubMed] [Google Scholar]
- [119].Maghzi AH, Revirajan N, Julian LJ, Spain R, Mowry EM, Liu S, et al. Magnetic resonance imaging correlates of clinical outcomes in early multiple sclerosis. Mult Scler Relat Disord 2014;3:720–7. [DOI] [PubMed] [Google Scholar]
- [120].Maidan I, Bernad-Elazari H, Gazit E, Giladi N, Hausdorff JM, Mirelman A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. J Neurol 2015;262:899–908. [DOI] [PubMed] [Google Scholar]
- [121].Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease? Brain Topogr 2017;30:531–8. [DOI] [PubMed] [Google Scholar]
- [122].Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N, et al. The role of the frontal lobe in complex walking among patients with parkinson’s disease and healthy older adults: an fNIRS study. Neurorehabil Neural Repair 2016;30:963–71. [DOI] [PubMed] [Google Scholar]
- [123].Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Deutsch JE, Hausdorff JM, et al. Altered brain activation in complex walking conditions in patients with Parkinson’s disease. Parkinsonism Relat Disord 2016;25:91–6. [DOI] [PubMed] [Google Scholar]
- [124].Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Disparate effects of training on brain activation in Parkinson disease. Neurology 2017;89:1804. [DOI] [PubMed] [Google Scholar]
- [125].Maillet A, Pollak P, Debu B. Imaging gait disorders in parkinsonism: a review. J Neurol Neurosurg Psychiatry 2012;83:986–93. [DOI] [PubMed] [Google Scholar]
- [126].Mally J, Stone TW. Therapeutic and ‘‘dose-dependent’’ effect of repetitive microelectroshock induced by transcranial magnetic stimulation in Parkinson’s disease. J Neurosci Res 1999;57:935–40. [PubMed] [Google Scholar]
- [127].Manenti R, Brambilla M, Rosini S, Orizio I, Ferrari C, Borroni B, et al. Time up and go task performance improves after transcranial direct current stimulation in patient affected by Parkinson’s disease. Neurosci Lett 2014;580:74–7. [DOI] [PubMed] [Google Scholar]
- [128].Marumoto K, Koyama T, Hosomi M, Kodama N, Miyake H, Domen K. Diffusion tensor imaging in elderly patients with idiopathic normal pressure hydrocephalus or Parkinson’s disease: diagnosis of gait abnormalities. Fluids Barriers CNS 2012;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Maruo T, Hosomi K, Shimokawa T, Kishima H, Oshino S, Morris S, et al. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease. Brain Stimul 2013;6:884–91. [DOI] [PubMed] [Google Scholar]
- [130].Matsui H, Udaka F, Miyoshi T, Hara N, Tamaura A, Oda M, et al. Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson’s disease. Mov Disord 2005;20:1272–7. [DOI] [PubMed] [Google Scholar]
- [131].McCrimmon CM, King CE, Wang PT, Cramer SC, Nenadic Z, Do AH. Brain-controlled functional electrical stimulation for lower-limb motor recovery in stroke survivors. Conf Proc IEEE Eng Med Biol Soc 2014:1247–50. [DOI] [PMC free article] [PubMed]
- [132].McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J Appl Physiol 2013;114:1174–82 [1985]. [DOI] [PubMed] [Google Scholar]
- [133].Medaglia JD, Huang WY, Karuza EA, Kelkar A, Thompson-Schill SL, Ribeiro A, et al. Functional alignment with anatomical networks is associated with cognitive flexibility. Nat Hum Behav 2018;2:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mergeche J, Verghese J, Allali G, Wang C, Beauchet O, Kumar V, et al. White Matter Hyperintensities in Older Adults and Motoric Cognitive Risk Syndrome. J Neuroimaging Psychiatry Neurol 2016;1:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Mi TM, Mei SS, Liang PP, Gao LL, Li KC, Wu T, et al. Altered resting-state brain activity in Parkinson’s disease patients with freezing of gait. Sci Rep 2017;7:16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Michel CM, Koenig T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. Neuroimage 2018;180:577–93. [DOI] [PubMed] [Google Scholar]
- [137].Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuroimage 2007;37:1338–45. [DOI] [PubMed] [Google Scholar]
- [138].Miyai I, Suzuki M, Hatakenaka M, Kubota K. Effect of body weight support on cortical activation during gait in patients with stroke. Exp Brain Res 2006;169:85–91. [DOI] [PubMed] [Google Scholar]
- [139].Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke 2003;34:2866–70. [DOI] [PubMed] [Google Scholar]
- [140].Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, et al. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol 2002;52:188–94. [DOI] [PubMed] [Google Scholar]
- [141].Moccia M, Tedeschi E, Ugga L, Erro R, Picillo M, Caranci F, et al. White matter changes and the development of motor phenotypes in de novo Parkinson’s disease. J Neurol Sci 2016;367:215–9. [DOI] [PubMed] [Google Scholar]
- [142].Motl RW, Hubbard EA, Sreekumar N, Wetter NC, Sutton BP, Pilutti LA, et al. Pallidal and caudate volumes correlate with walking function in multiple sclerosis. J Neurol Sci 2015;354:33–6. [DOI] [PubMed] [Google Scholar]
- [143].Motl RW, Zivadinov R, Bergsland N, Benedict RH. Thalamus volume and ambulation in multiple sclerosis: a cross-sectional study. Neurodegener Dis Manag 2016;6:23–9. [DOI] [PubMed] [Google Scholar]
- [144].Muldoon SF, Pasqualetti F, Gu S, Cieslak M, Grafton ST, Vettel JM, et al. Stimulation-based control of dynamic brain networks. Plos Comput Biol 2016;12:e1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Muller ML, Frey KA, Petrou M, Kotagal V, Koeppe RA, Albin RL, et al. Beta-amyloid and postural instability and gait difficulty in Parkinson’s disease at risk for dementia. Mov Disord 2013;28:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer’s disease and aging. Dement Geriatr Cogn Disord 2009;28:295–301. [DOI] [PubMed] [Google Scholar]
- [147].Nagae LM, Honce JM, Tanabe J, Shelton E, Sillau SH, Berman BD. Microstructural Changes within the Basal Ganglia Differ between Parkinson Disease Subtypes. Front Neuroanat 2016;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Nieuwhof F, Reelick MF, Maidan I, Mirelman A, Hausdorff JM, Olde Rikkert MG, et al. Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: feasibility of using a new portable fNIRS device. Pilot Feasibility Stud 2016;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000;343:938–52. [DOI] [PubMed] [Google Scholar]
- [150].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Olazaran J, Hernandez-Tamames JA, Molina E, Garcia-Polo P, Dobato JL, Alvarez-Linera J, et al. Clinical and anatomical correlates of gait dysfunction in Alzheimer’s disease. J Alzheimers Dis 2013;33:495–505. [DOI] [PubMed] [Google Scholar]
- [153].Oliveira AS, Arguissain FG, Andersen OK. Cognitive processing for step precision increases beta and gamma band modulation during overground walking. Brain Topogr 2018;31:661–71. [DOI] [PubMed] [Google Scholar]
- [154].Onu M, Aroceanu A, Ferastraoaru V, Bajenaru O. Gray matter changes in demyelinating disease: correlations with clinical scores. Maedica (Buchar) 2015;10:319–24. [PMC free article] [PubMed] [Google Scholar]
- [155].Palmer JA, Needle AR, Pohlig RT, Binder-Macleod SA. Atypical cortical drive during activation of the paretic and nonparetic tibialis anterior is related to gait deficits in chronic stroke. Clin Neurophysiol 2016;127:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Palmer JA, Zarzycki R, Morton SM, Kesar TM, Binder-Macleod SA. Characterizing differential poststroke corticomotor drive to the dorsi- and plantarflexor muscles during resting and volitional muscle activation. J Neurophysiol 2017;117: 1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol 2014;71:884–90. [DOI] [PubMed] [Google Scholar]
- [158].Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26:982–9. [DOI] [PubMed] [Google Scholar]
- [159].Peters DM, Fridriksson J, Stewart JC, Richardson JD, Rorden C, Bonilha L, et al. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum Brain Mapp 2018;39:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS One 2014;9:e90634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Peterson DS, Pickett KA, Duncan RP, Perlmutter JS, Earhart GM. Brain activity during complex imagined gait tasks in Parkinson disease. Clin Neurophysiol 2014;125:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Pietracupa S, Suppa A, Upadhyay N, Gianni C, Grillea G, Leodori G, et al. Freezing of gait in Parkinson’s disease: gray and white matter abnormalities. J Neurol 2018;265:52–62. [DOI] [PubMed] [Google Scholar]
- [163].Piron L, Piccione F, Tonin P, Dam M. Clinical correlation between motor evoked potentials and gait recovery in post-stroke patients. Arch Phys Med Rehabil 2005;86:1874–8. [DOI] [PubMed] [Google Scholar]
- [164].Pirondini E, Coscia M, Minguillon J, Millan JDR, Van De Ville D, Micera S. EEG topographies provide subject-specific correlates of motor control. Sci Rep 2017;7:13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Pirondini E, Vybornova A, Coscia M, Van de Ville D. A Spectral Method for Generating Surrogate Graph Signals. IEEE Signal Proc Let 2016;23:1275–8. [Google Scholar]
- [166].Post B, Merkus MP, de Haan RJ, Speelman JD, Group CS. Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov Disord 2007;22:1839–51 [quiz 1988]. [DOI] [PubMed] [Google Scholar]
- [167].Preti MG, Bolton TA, Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 2017;160:41–54. [DOI] [PubMed] [Google Scholar]
- [168].Ricciardi L, Bloem BR, Snijders AH, Daniele A, Quaranta D, Bentivoglio AR, et al. Freezing of gait in Parkinson’s disease: the paradoxical interplay between gait and cognition. Parkinsonism Relat Disord 2014;20:824–9. [DOI] [PubMed] [Google Scholar]
- [169].Robinson PA. Determination of effective brain connectivity from functional connectivity using propagator-based interferometry and neural field theory with application to the corticothalamic system. Phys Rev E 2014;90:042712. [DOI] [PubMed] [Google Scholar]
- [170].Robinson PA. Interrelating anatomical, effective, functional brain connectivity using propagators, neural field theory. Phys Rev E 2012;85:011912. [DOI] [PubMed] [Google Scholar]
- [171].Rosenberg-Katz K, Herman T, Jacob Y, Kliper E, Giladi N, Hausdorff JM. Subcortical volumes differ in Parkinson’s disease motor subtypes: new insights into the pathophysiology of disparate symptoms. Front Hum Neurosci 2016;10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Rubino A, Assogna F, Piras F, Di Battista ME, Imperiale F, Chiapponi C, et al. Does a volume reduction of the parietal lobe contribute to freezing of gait in Parkinson’s disease? Parkinsonism Relat Disord 2014;20:1101–3. [DOI] [PubMed] [Google Scholar]
- [173].Ruggieri S, Petracca M, Miller A, Krieger S, Ghassemi R, Bencosme Y, et al. Association of Deep Gray Matter Damage With Cortical and Spinal Cord Degeneration in Primary Progressive Multiple Sclerosis. JAMA Neurol 2015;72:1466–74. [DOI] [PubMed] [Google Scholar]
- [174].Sandroff BM, Wylie GR, Sutton BP, Johnson CL, DeLuca J, Motl RW. Treadmill walking exercise training and brain function in multiple sclerosis: Preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult Scler J Exp Transl Clin 2018;4 [2055217318760641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Sanfilipo MP, Benedict RH, Sharma J, Weinstock-Guttman B, Bakshi R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage 2005;26:1068–77. [DOI] [PubMed] [Google Scholar]
- [176].Sartor J, Bettecken K, Bernhard FP, Hofmann M, Gladow T, Lindig T, et al. White Matter Changes-Related Gait and Executive Function Deficits: Associations with Age and Parkinson’s Disease. Front Aging Neurosci 2017;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Sbardella E, Petsas N, Tona F, Prosperini L, Raz E, Pace G, et al. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PloS one 2013;8:e63250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sbardella E, Tona F, Petsas N, Upadhyay N, Piattella MC, Filippini N, et al. Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Mult Scler 2015;21:1681–92. [DOI] [PubMed] [Google Scholar]
- [179].Sburlea AI, Montesano L, Cano de la Cuerda R, Alguacil Diego IM, Miangolarra-Page JC, Minguez J Detecting intention to walk in stroke patients from pre-movement EEG correlates. J Neuroeng Rehabil 2015;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Schneider E, Ng KM, Yeoh CS, Rumpel H, Fook-Chong S, Li HH, et al. Susceptibility-weighted MRI of extrapyramidal brain structures in Parkinsonian disorders. Medicine (Baltimore) 2016;95:e3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Shen B, Gao Y, Zhang W, Lu L, Zhu J, Pan Y, et al. Resting State fMRI Reveals Increased Subthalamic Nucleus and Sensorimotor Cortex Connectivity in Patients with Parkinson’s Disease under Medication. Front Aging Neurosci 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Shine JM, Handojoseno AM, Nguyen TN, Tran Y, Naismith SL, Nguyen H, et al. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin Neurophysiol 2014;125:569–76. [DOI] [PubMed] [Google Scholar]
- [183].Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 2013;136:3671–81. [DOI] [PubMed] [Google Scholar]
- [184].Shoushtarian M, Murphy A, Iansek R. Examination of central gait control mechanisms in Parkinson’s disease using movement-related potentials. Mov Disord 2011;26:2347–53. [DOI] [PubMed] [Google Scholar]
- [185].Shuman DI, Ricaud B, Vandergheynst P. Vertex-frequency analysis on graphs. Appl Comput Harmon A 2016;40:260–91. [Google Scholar]
- [186].Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul 2009;2:58–80. [DOI] [PubMed] [Google Scholar]
- [187].Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 2004;24:3379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Siegel JS, Ramsey LE, Snyder AZ, Metcalf NV, Chacko RV, Weinberger K, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A 2016;113:E4367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun 2016;7:12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci 2017;18:86–100. [DOI] [PubMed] [Google Scholar]
- [191].Skorpil M, Soderlund V, Sundin A, Svenningsson P. MRI diffusion in Parkinson’s disease: using the technique’s inherent directional information to study the olfactory bulb and substantia nigra. J Parkinsons Dis 2012;2:171–80. [DOI] [PubMed] [Google Scholar]
- [192].Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 2015;18:1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Smulders K, Esselink RA, Bloem BR, Cools R. Freezing of gait in Parkinson’s disease is related to impaired motor switching during stepping. Mov Disord 2015;30:1090–7. [DOI] [PubMed] [Google Scholar]
- [194].Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 2011;134:59–72. [DOI] [PubMed] [Google Scholar]
- [195].Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012;36:154–6. [DOI] [PubMed] [Google Scholar]
- [196].Stolze H, Klebe S, Baecker C, Zechlin C, Friege L, Pohle S, et al. Prevalence of gait disorders in hospitalized neurological patients. Mov Disord 2005;20:89–94. [DOI] [PubMed] [Google Scholar]
- [197].Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry 2002;52:679–93. [DOI] [PubMed] [Google Scholar]
- [198].Surova Y, Lampinen B, Nilsson M, Latt J, Hall S, Widner H, et al. Alterations of diffusion kurtosis and neurite density measures in deep grey matter and white matter in Parkinson’s disease. PLoS One 2016;11:e0157755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Sverak T, Albrechtova L, Lamos M, Rektorova I, Ustohal L. Intensive repetitive transcranial magnetic stimulation changes EEG microstates in schizophrenia: a pilot study. Schizophr Res 2018;193:451–2. [DOI] [PubMed] [Google Scholar]
- [200].Swank C, Mehta J, Criminger C. Transcranial direct current stimulation lessens dual task cost in people with Parkinson’s disease. Neurosci Lett 2016;626:1–5. [DOI] [PubMed] [Google Scholar]
- [201].Syrkin-Nikolau J, Koop MM, Prieto T, Anidi C, Afzal MF, Velisar A, et al. Subthalamic neural entropy is a feature of freezing of gait in freely moving people with Parkinson’s disease. Neurobiol Dis 2017;108:288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron 2014;83:1002–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Frontiers in physiology 2012;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Tagliazucchi E, Balenzuela P, Fraiman D, Montoya P, Chialvo DR. Spontaneous BOLD event triggered averages for estimating functional connectivity at resting state. Neurosci Lett 2011;488:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Tard C, Delval A, Devos D, Lopes R, Lenfant P, Dujardin K, et al. Brain metabolic abnormalities during gait with freezing in Parkinson’s disease. Neuroscience 2015;307:281–301. [DOI] [PubMed] [Google Scholar]
- [206].Tard C, Delval A, Duhamel A, Moreau C, Devos D, Dujardin K. Specific attentional disorders and freezing of Gait in Parkinson’s disease. J Parkinsons Dis 2015;5:379–87. [DOI] [PubMed] [Google Scholar]
- [207].Tard C, Devanne H, Defebvre L, Delval A. Single session intermittent theta-burst stimulation on the left premotor cortex does not alleviate freezing of gait in Parkinson’s disease. Neurosci Lett 2016;628:1–9. [DOI] [PubMed] [Google Scholar]
- [208].Tard C, Dujardin K, Bourriez JL, Molaee-Ardekani B, Derambure P, Defebvre L, et al. Attention modulation during motor preparation in Parkinsonian freezers: a time-frequency EEG study. Clin Neurophysiol 2016;127:3506–15. [DOI] [PubMed] [Google Scholar]
- [209].Tavazzi E, Bergsland N, Cattaneo D, Gervasoni E, Lagana MM, Dipasquale O, et al. Effects of motor rehabilitation on mobility and brain plasticity in multiple sclerosis: a structural and functional MRI study. J Neurol 2018;265:1393–401. [DOI] [PubMed] [Google Scholar]
- [210].Teramoto H, Morita A, Ninomiya S, Shiota H, Kamei S. Relation between freezing of gait and frontal function in Parkinson’s disease. Parkinsonism Relat Disord 2014;20:1046–9. [DOI] [PubMed] [Google Scholar]
- [211].Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, et al. Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol 2012;33:1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord 2012;18:781–7. [DOI] [PubMed] [Google Scholar]
- [213].Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012;135:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Thickbroom GW, Sacco P, Faulkner DL, Kermode AG, Mastaglia FL. Enhanced corticomotor excitability with dynamic fatiguing exercise of the lower limb in multiple sclerosis. J Neurol 2008;255:1001–5. [DOI] [PubMed] [Google Scholar]
- [215].Thumm PC, Maidan I, Brozgol M, Shustak S, Gazit E, Shema Shiratzki S, et al. Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 2018;62:384–7. [DOI] [PubMed] [Google Scholar]
- [216].Tovar-Moll F, Evangelou IE, Chiu AW, Auh S, Chen C, Ehrmantraut M, et al. Diffuse and focal corticospinal tract disease and its impact on patient disability in multiple sclerosis. J Neuroimaging 2015;25:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Tremblay F, Tremblay LE. Cortico-motor excitability of the lower limb motor representation: a comparative study in Parkinson’s disease and healthy controls. Clin Neurophysiol 2002;113:2006–12. [DOI] [PubMed] [Google Scholar]
- [218].Trieu Phat L, Brantley JA, Fangshi Z, Contreras-Vidal JL. Electrocortical amplitude modulations of human level-ground, slope, and stair walking. Conf Proc IEEE Eng Med Biol Soc 2017:1913–6. [DOI] [PubMed] [Google Scholar]
- [219].Tuleasca C, Najdenovska E, Regis J, Witjas T, Girard N, Champoudry J, et al. Pretherapeutic functional neuroimaging predicts tremor arrest after thalamotomy. Acta Neurol Scand 2018;137:500–8. [DOI] [PubMed] [Google Scholar]
- [220].Tuleasca C, Regis J, Najdenovska E, Witjas T, Girard N, Thiran JP, et al. Visually-sensitive networks in essential tremor: evidence from structural and functional imaging. Brain 2018;141:e47. [DOI] [PubMed] [Google Scholar]
- [221].Tuleasca C, Witjas T, Najdenovska E, Verger A, Girard N, Champoudry J, et al. Assessing the clinical outcome of Vim radiosurgery with voxel-based morphometry: visual areas are linked with tremor arrest! Acta Neurochir (Wien) 2017;159:2139–44. [DOI] [PubMed] [Google Scholar]
- [222].Vacherot F, Attarian S, Vaugoyeau M, Azulay JP. A motor cortex excitability and gait analysis on Parkinsonian patients. Mov Disord 2010;25:2747–55. [DOI] [PubMed] [Google Scholar]
- [223].Valentino F, Cosentino G, Brighina F, Pozzi NG, Sandrini G, Fierro B, et al. Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord 2014;29:1064–9. [DOI] [PubMed] [Google Scholar]
- [224].Vercruysse S, Leunissen I, Vervoort G, Vandenberghe W, Swinnen S, Nieuwboer A. Microstructural changes in white matter associated with freezing of gait in Parkinson’s disease. Mov Disord 2015;30:567–76. [DOI] [PubMed] [Google Scholar]
- [225].Verghese J, Ambrose AF, Lipton RB, Wang C. Neurological gait abnormalities and risk of falls in older adults. J Neurol 2010;257:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 2014;83:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 2002;347:1761–8. [DOI] [PubMed] [Google Scholar]
- [228].Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2013;68:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 2012;68:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Vervoort G, Alaerts K, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, et al. Functional connectivity alterations in the motor and fronto-parietal network relate to behavioral heterogeneity in Parkinson’s disease. Parkinsonism Relat Disord 2016;24:48–55. [DOI] [PubMed] [Google Scholar]
- [231].Vervoort G, Heremans E, Bengevoord A, Strouwen C, Nackaerts E, Vandenberghe W, et al. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience 2016;317:36–46. [DOI] [PubMed] [Google Scholar]
- [232].Vervoort G, Leunissen I, Firbank M, Heremans E, Nackaerts E, Vandenberghe W, et al. Structural brain alterations in motor subtypes of Parkinson’s disease: evidence from probabilistic tractography and shape analysis. PLoS One 2016;11:e0157743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Verwer JH, Biessels GJ, Heinen R, Exalto LG, Emmelot-Vonk MH, Koek HL. Occurrence of Impaired Physical Performance in Memory Clinic Patients With Cerebral Small Vessel Disease. Alzheimer Dis Assoc Disord 2018;32:214–9. [DOI] [PubMed] [Google Scholar]
- [234].Wang M, Jiang S, Yuan Y, Zhang L, Ding J, Wang J, et al. Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. J Neurol 2016;263: 1583–92. [DOI] [PubMed] [Google Scholar]
- [235].Wang N, Allali G, Kesavadas C, Noone ML, Pradeep VG, Blumen HM, et al. Cerebral small vessel disease and motoric cognitive risk syndrome: results from the Kerala-Einstein study. J Alzheimers Dis 2016:1–9. [DOI] [PMC free article] [PubMed]
- [236].Wang Z, Chen H, Ma H, Ma L, Wu T, Feng T. Resting-state functional connectivity of subthalamic nucleus in different Parkinson’s disease phenotypes. J Neurol Sci 2016;371:137–47. [DOI] [PubMed] [Google Scholar]
- [237].Wei L, Zhang J, Long Z, Wu GR, Hu X, Zhang Y, et al. Reduced topological efficiency in cortical-basal Ganglia motor network of Parkinson’s disease: a resting state fMRI study. PloS one 2014;9:e108124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Wen J, Yablonskiy DA, Luo J, Lancia S, Hildebolt C, Cross AH. Detection and quantification of regional cortical gray matter damage in multiple sclerosis utilizing gradient echo MRI. Neuroimage Clin 2015;9:164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Wen MC, Heng HSE, Lu Z, Xu Z, Chan LL, Tan EK, et al. Differential white matter regional alterations in motor subtypes of early drug-naive Parkinson’s disease patients. Neurorehabil Neural Repair 2018;32:129–41. [DOI] [PubMed] [Google Scholar]
- [240].Wetter NC, Hubbard EA, Motl RW, Sutton BP. Fully automated open-source lesion mapping of T2-FLAIR images with FSL correlates with clinical disability in MS. Brain Behav 2016;6:e00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Wheaton LA, Villagra F, Hanley DF, Macko RF, Forrester LW. Reliability of TMS motor evoked potentials in quadriceps of subjects with chronic hemiparesis after stroke. J Neurol Sci 2009;276:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Woo CW, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 2017;20:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Yeo SS, Ahn SH, Choi BY, Chang CH, Lee J, Jang SH. Contribution of the pedunculopontine nucleus on walking in stroke patients. Eur Neurol 2011;65:332–7. [DOI] [PubMed] [Google Scholar]
- [244].Yip CW, Cheong PW, Green A, Prakash PK, Fook-Cheong SK, Tan EK, et al. A prospective pilot study of repetitive transcranial magnetic stimulation for gait dysfunction in vascular parkinsonism. Clin Neurol Neurosurg 2013;115:887–91. [DOI] [PubMed] [Google Scholar]
- [245].Yotnuengnit P, Bhidayasiri R, Donkhan R, Chaluaysrimuang J, Piravej K. Effects of Transcranial Direct Current Stimulation Plus Physical Therapy on Gait in Patients With Parkinson Disease: A Randomized Controlled Trial. Am J Phys Med Rehabil 2018;97:7–15. [DOI] [PubMed] [Google Scholar]
- [246].Youn J, Lee JM, Kwon H, Kim JS, Son TO, Cho JW. Alterations of mean diffusivity of pedunculopontine nucleus pathway in Parkinson’s disease patients with freezing of gait. Parkinsonism Relat Disord 2015;21:12–7. [DOI] [PubMed] [Google Scholar]
- [247].Zappasodi F, Croce P, Giordani A, Assenza G, Giannantoni NM, Profice P, et al. Prognostic Value of EEG Microstates in Acute Stroke. Brain Topogr 2017;30:698–710. [DOI] [PubMed] [Google Scholar]
- [248].Zhou C, Zhong X, Yang Y, Yang W, Wang L, Zhang Y, et al. Alterations of regional homogeneity in freezing of gait in Parkinson’s disease. J Neurol Sci 2018;387:54–9. [DOI] [PubMed] [Google Scholar]
- [249].Zhuang X, Walsh RR, Sreenivasan K, Yang Z, Mishra V, Cordes D. Incorporating spatial constraint in co-activation pattern analysis to explore the dynamics of resting-state networks: An application to Parkinson’s disease. Neuroimage 2018;172:64–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Ziemann U TMS induced plasticity in human cortex. Rev Neurosci 2004;15:253–66. [DOI] [PubMed] [Google Scholar]


