Abstract
Cholangiocytes, the epithelial cells lining the intrahepatic and extrahepatic bile ducts, are highly specialized cells residing in a complex anatomic niche where they participate in bile production and homeostasis. Cholangiocytes are damaged in a variety of human diseases termed cholangiopathies, often causing advanced liver failure. The regulation of cholangiocyte transport properties is increasingly understood, as is their anatomical and functional heterogeneity along the biliary tract. Furthermore, cholangiocytes are pivotal in liver regeneration, especially when hepatocyte regeneration is compromised. The role of cholangiocytes in innate and adaptive immune responses, a critical subject relevant to immune-mediated cholangiopathies, is also emerging. Finally, reactive ductular cells are present in many cholestatic and other liver diseases. In chronic disease states, this repair response contributes to liver inflammation, fibrosis and carcinogenesis and is a subject of intense investigation. This Review highlights advances in cholangiocyte research, especially their role in development and liver regeneration, their functional and biochemical heterogeneity, their activation and involvement in inflammation and fibrosis and their engagement with the immune system. We aim to focus further attention on cholangiocyte pathobiology and the search for new disease-modifying therapies targeting the cholangiopathies.
Cholangiocytes line a complex network of interconnecting tubes extending from the Canals of Hering in the liver to the duodenum (FIG. 1). In humans, the total length of this network is estimated to be ~1.25 miles (2 km)1. As with other epithelial cells, cholangiocytes are polarized with distinct apical and basolateral plasma membrane domains and multiple transport functions, many relevant to bile formation. Although cholangiocytes comprise a minority cell population in the liver, they are critical in bile generation, a life-sustaining function of the liver2. Bile is a secretory fluid product of the hepatobiliary system containing a variety of components, including bile acids, electrolytes, lipids, proteins and endobiotic and xenobiotic compounds. These factors contribute to health by aiding digestion, maintaining the enterohepatic circulation and helping to eliminate unwanted compounds from the body. The continuous and extensive network of these cells within and outside the liver results in considerable heterogeneity in cholangiocyte function along the biliary tract. The blood supply to cholangiocytes originates from the hepatic artery and forms a peribiliary plexus (PBP) consisting of a 3D network of blood vessels of homogeneous diameter surrounding bile ducts3. The intimate anatomic association of the PBP with cholangiocytes enables crosstalk that probably both helps regulate normal cholangiocyte function and is associated with cholangiocyte malfunction in disease4. Under healthy circumstances, cholangiocytes have major physiological functions: bile is modified within the ductal lumen via activities at their apical plasma membrane domain; they form a barrier to potentially damaging molecules and microorganisms in bile via their tight junctions and immunoglobulin A (IgA) secretion; and they enable access to the immune and vascular systems via their basolateral plasma membrane domain. These complex processes are regulated by extracellular signals (for example, peptides, nucleotides, hormones and neurotransmitters), biliary constituents (such as bile acids, glucose and vesicles) and physical forces (including flow and pressure) that are reflected in various intracellular pathways, relying mostly on cAMP and Ca2+ signalling as second messengers.
Fig. 1. Ductal bile formation.
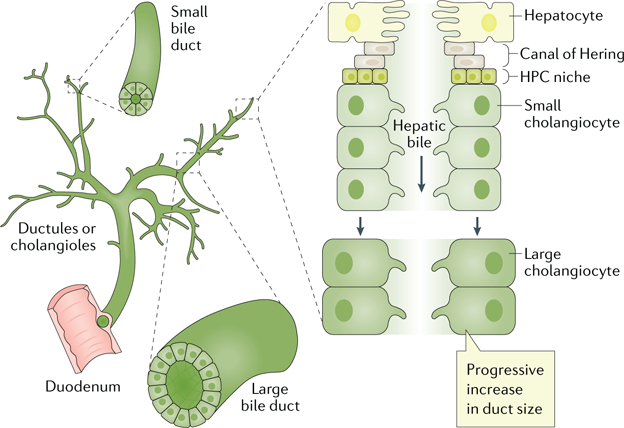
Bile produced by hepatocytes (primary or hepatic bile) is delivered into bile ducts. The Canals of Hering provide the continuum between the hepatocyte canaliculus and the ductules or cholangioles, the small bile ducts and the large bile ducts in which hepatic bile is modified to become ductal bile. A hepatic progenitor cell (HPC) niche is also thought to reside at the interface of the cells lining the Canals of Hering and the hepatocyte plate. Active biliary epithelial transport of electrolytes and solutes occurs in small and large bile ducts and determines the vectorial water movement (that is, absorption or secretion) across cholangiocytes, thus altering ductal bile composition and flow. Adapted with permission from REF.6, Elsevier.
Cholangiocytes are affected during liver injury and participate in the pathobiology of various liver disease states. Although ample evidence demonstrates that hepatocytes, the predominant epithelial cell type in the liver, regenerate, cholangiocytes can also contribute to liver regeneration when hepatocyte regeneration is impaired5. Genetic, infectious, immune-mediated, idiopathic, malignant and vascular diseases can also directly perturb cholangiocyte structure and function, resulting in impaired bile formation (cholestasis), followed by inflammation, fibrosis and liver dysfunction. Although the inflammation and fibrosis might initially be limited to the biliary tract, over time, portal fibrosis worsens and can culminate in hepatic dysfunction, cirrhosis and chronic liver failure. Collectively, these disease syndromes comprise the cholangiopathies, which are unsolved pathophysiological problems and important unmet needs in clinical hepatology6 (BOX 1). Although individually they are uncommon or even rare, as a group the cholangiopathies cause considerable morbidity and mortality, and curative therapies are not available; for example, primary sclerosing cholangitis (PSC) is the indication for 6% of liver transplants in the USA, with an approximate yearly cost of $125 million7.
Box 1 |. Selected cholangiopathies.
Genetic cholangiopathies
Alagille syndrome
ABCB4 deficiency
Caroli syndrome
Cystic fibrosis
Polycystic liver disease (ADPLD, ADPKD and ARPKD)
Infectious cholangiopathies
Cryptosporidium-associated cholangiopathy
Recurrent pyogenic cholangitis
Recurrent cholangitis in patients with a choledochoduodenostomy
Immune-mediated cholangiopathies
Primary biliary cholangitis
Primary sclerosing cholangitis
IgG4-associated cholangitis
Autoimmune cholangitis
Graft versus host disease involving the liver
Eosinophilic or mast cell cholangiopathy
Idiopathic cholangiopathies
Biliary atresia
Sarcoidosis
Malignant cholangiopathies
Cholangiocarcinoma
Vascular cholangiopathies
Hepatic artery thrombosis after liver transplantation
Portal hypertensive biliopathy
ADPLD, autosomal dominant polycystic liver disease;
ADPKD, autosomal dominant polycystic kidney disease;
ARPKD, autosomal recessive polycystic kidney disease;
IgG4, immunoglobulin G4.
Evolving information indicates that cholangiocytes are not necessarily innocent victims in these disease processes but might also initiate and/or actively participate in the cholangiopathies. For example, cholangiocytes participate in inflammation by secreting chemokines and cytokines and can directly modulate the biology of myofibroblasts, the cell type responsible for collagen deposition within the liver6. Thus, in this Review, we address cholangiocytes in development and liver regeneration, their functional and biochemical heterogeneity, their activation and involvement in inflammation and fibrosis and their engagement with the immune system.
Cholangiocyte heterogeneity
The 3D network of ducts inside and outside the liver provides a very large surface area along which cholangiocytes perform fundamental secretory and absorptive processes that regulate bile flow and composition in its transport to the duodenum8–12. Throughout the biliary system, cholangiocytes exhibit morphological, biochemical and functional heterogeneity13. Immature cholangiocytes within the Canals of Hering, as well as from the intrahepatic and extrahepatic peribiliary glands, are poorly differentiated and are considered progenitor cells that participate in epithelium renewal and tissue regeneration. However, cholangiocytes progressively acquire a greater degree of differentiation along the biliary tree (from small to large bile ducts) in terms of cell polarity, expression of receptors and transporters and response to hormones13–17. This differentiation might also in part be due to the differences in vascularization of the cholangiocytes along the biliary tree3. Intrahepatic and extrahepatic bile ducts are surrounded by a complex network of vessels and capillaries derived from the hepatic artery and vein. The oxygen tension and metabolite and substrate composition differ along the biliary tree, promoting the differentiated metabolic, secretory and absorptive features of cholangiocytes. Differentiated cholangiocytes have distinct basolateral and apical (luminal) plasma membranes, the latter containing microvilli that provide a fivefold increase in cell surface area12,18,19 and a single primary cilium able to detect and transmit bile signals and regulate cell function20,21. Although cholangiocytes represent a minor proportion of all liver cells (3–5%), they are responsible for up to 30% of total bile flow in humans, with the other 70% originating from hepatocyte canalicular secretion11; however, their contribution to this process in rodents is probably less22. Bile flow regulation involves the action of multiple ion carriers (such as transporters, exchangers and channels) strategically distributed along the polarized structure of cholangiocytes, as well as various molecules (including hormones, neurotransmitters, peptides and nucleotides) that tightly regulate bile flow and composition through the interaction with intracellular signalling pathways and cascades (such as cAMP and Ca2+) (FIG. 2).
Fig. 2. Molecular mechanisms regulating biliary secretion and absorption.
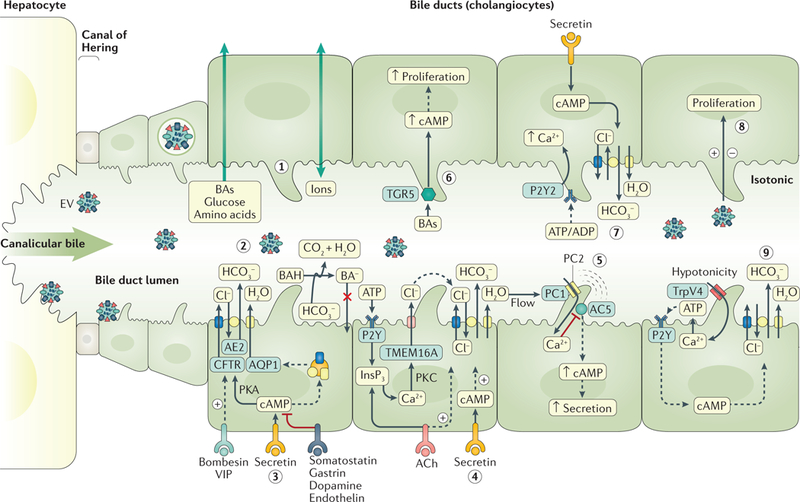
Cholangiocytes regulate the flow, composition and pH of the primary bile generated at the canaliculi of hepatocytes through different mechanisms, including the absorption of bile acids (BAs), glucose and amino acids (step 1) and the secretion of bicarbonate (HCO3 −) and water (step 2). Secretin (step 3) stimulates the apical insertion of intracellular vesicles containing anion exchange protein 2 (AE2), cystic fibrosis transmembrane conductance regulator (CFTR) and aquaporin 1 (AQP1), resulting in chloride secretion through CFTR that is exchanged with bicarbonate via AE2. This bicarbonate generates osmotic force for the movement of water via AQP1. Biliary bicarbonate secretion creates the biliary bicarbonate umbrella that protects cholangiocytes against the damaging effect of toxic protonated BAs (BAHs). Hormones such as bombesin and vasoactive intestinal peptide (VIP) stimulate biliary bicarbonate secretion, whereas somatostatin, gastrin and dopamine inhibit this process. Extracellular nucleotides and nucleosides, via P2Y receptors, and acetylcholine (ACh) also promote baseline and secretin-stimulated bicarbonate secretion, respectively (step 4). The cholangiocyte primary cilium acts as a (step 5) mechanosensor (via polycystin 1 (PC1)), (steps 6–8) chemosensor (via G protein-coupled bile acid receptor 1 (TGR5), P2Y purinoceptor 2 (P2Y2) and extracellular vesicle (EV)) and (step 9) osmosensor (via transient receptor potential channel vanilloid subfamily 4 (TrpV4)), detecting signals in bile and subsequently modifying cell biology and bile flow and composition. AC5, adenylyl cyclase type 5; InsP3, inositol 1,4,5-trisphosphate; PKA, protein kinase A; PKC, protein kinase C; TMEM16A, transmembrane protein 16F.
Bicarbonate secretion.
Biliary bicarbonate (HCO3−) secretion is the central event of bile-salt-independent flow, which is stimulated by the gastrointestinal hormone secretin during the postprandial period11,12,23. Interaction of secretin with its specific G protein-coupled receptor localized to the basolateral membrane of cholangiocytes induces elevated intracellular cAMP levels24 and subsequent protein kinase A activation25. This activation subsequently stimulates the trafficking of intracellular vesicles containing the Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR), the Cl−/HCO3− anion exchange protein 2 (AE2; also known as SLC4A2) and the water channel aquaporin 1 (AQP1) to the apical membrane26. CFTR mediates the apical release of Cl−, which is exchanged with HCO3− by AE2 (REF.23). Biliary bicarbonate secretion drives the movement of water through AQP1, resulting in the alkalization and fluidization of the bile23 (FIG. 2). The biliary secretion of bicarbonate creates an alkaline barrier, the so-called ‘biliary bicarbonate umbrella’, that renders bile acids polar, de-protonated and membrane impermeable27. Biliary bicarbonate neutralizes the acidic pH resulting from meal digestion in the duodenum and favours the absorption of nutrients12. Secretin-stimulated biliary bicarbonate secretion is dependent on the maintenance of the bile acid pool23 and is inhibited by hormones such as somatostatin28,29, gastrin30 and endothelin31. Of note, this choleretic mechanism can be impaired under certain pathological conditions, as in cholestatic disorders such as primary biliary cholangitis (PBC), an immune-mediated disease that results in damage to intrahepatic interlobular bile ducts. PBC is characterized by the downregulation of AE2 expression in cholangiocytes32, resulting in impaired secretin-stimulated biliary bicarbonate secretion33,34. Downregulation of AE2 in PBC cholangiocytes probably has a major aetiopathogenic role, as its experimental downregulation in human cholangiocytes in vitro35,36 or in mice in vivo37–39 results in the development of multiple PBC-like features, such as periportal lymphocytic infiltrates and bile duct damage; increased serum IgM, IgG and hepatic alkaline phosphatase levels; and spontaneous development of specific anti-mitochondrial autoantibodies. The characteristic downregulation of AE2 in PBC cholangiocytes is linked to miR-506 overexpression35,36. Additionally, CFTR mutations occurring in cystic fibrosis might also contribute to cholestasis in these patients40.
The biliary tree is controlled by neurovegetative innervations. Several neurotransmitters regulate the baseline and/or secretin-stimulated biliary bicarbonate secretion. Gastrin-releasing peptide29 and vasoactive intestinal peptide (VIP)41 stimulate baseline biliary bicarbonate secretion, whereas acetylcholine (ACh)11,42 and the α1-adrenergic agonist phenylephrine43 stimulate secretin-dependent biliary bicarbonate secretion. By contrast, dopamine44, α2-adrenergic agonists45 and GABA46 all inhibit secretin-dependent biliary bicarbonate secretion (FIG. 2). Several factors present in bile are also able to influence biliary bicarbonate secretion. Extracellular nucleotides and nucleosides interact with P2Y receptors localized to the apical membrane of cholangiocytes and promote an increase in intracellular Ca2+ levels and subsequent Cl− secretion through apical Ca2+-activated Cl− channels (such as transmembrane protein 16F (TMEM16A; also known as ANO1)) that further promote bicarbonate secretion11,47–49 (FIG. 2).
Cholangiocyte transport.
Cholangiocytes participate in the absorption of different molecules from bile, including bile acids, glucose, amino acids and ions. Bile acids (that is, steroid acids) exist as either a free acid or conjugated to taurine or glycine. Unconjugated bile salts secreted through the canalicular membrane of hepatocytes can be protonated and passively diffuse across the apical membrane of cholangiocytes. They can then be transported by the basolateral membrane into the PBP, from which they can return to hepatocytes via the cholehepatic shunt, an alternative mechanism to the enterohepatic circulation of bile acids50,51. In rat cholangiocytes, conjugated bile acids might be absorbed through the apical sodium-dependent bile salt transporter (ASBT)52 and subsequently via the basolateral truncated ASBT (t-ASBT)53, multidrug resistance protein 3 (MRP3)54,55 and/or the organic solute transporters OSTα and OSTβ12,56–59. Moreover, the vectorial transport of glucose through the apical Na+–glucose cotransporter 1 (SGLT1) and the basolateral glucose transporter 1 (GLUT1) provides an osmotic gradient that favours the reabsorption of water from bile60. Amino acids resulting from the γ-glutamyltranspeptidase (γGT)-dependent degradation of glutathione (including glutamine, cysteine and glycine) promote canalicular bile-salt-independent bile flow. These molecules can then be reabsorbed by sodium-dependent and sodium-independent mechanisms that generate osmotic gradients that favour water absorption and glutathione resynthesis in hepatocytes61,62. Additionally, cholangiocytes possess a variety of transporters able to bidirectionally transport various molecules, including organic and inorganic anions and cations as well as proteins (multidrug resistance proteins perform the majority of these transport functions)12.
Role of the primary cilium.
The primary cilium of cholangiocytes is an antenna-like organelle containing a well-tuned system of receptors and channels able to detect signals in bile that subsequently regulate intracellular signalling mechanisms, ultimately modifying bile flow and/or composition19,63. This non-motile protuberance functions as a mechanosensor (via polycystin 1 and 2 (PC1 and PC2, respectively))64, a chemosensor (via P2Y12 and G protein-coupled bile acid receptor 1 (TGR5; also known as GPBAR1))65,66 and an osmosensor (via transient receptor potential channel vanilloid subfamily 4 (TrpV4))67. Of note, extracellular vesicles (EVs) found in bile can also act as chemosignals that interact with the primary cilium and regulate cell biology68. For example, EVs binding to cilia have been shown to inhibit cholangiocyte proliferation, promoting the quiescent status of the biliary system in normal conditions68.
Cholangiocytes in development and liver repair
The embryological origin of the liver has been studied for many decades; thus, the developmental events that regulate liver organogenesis are becoming well-understood69. However, although the regenerative capacity of hepatocytes has been studied equally as long70, the molecular mechanisms governing biliary regeneration have been more clearly defined in the past decade with the advent of lineage-tracing techniques. Importantly, it seems that many of the key developmental pathways governing liver development become active during the processes of biliary regeneration and/or repair71. This dual role suggests that a better understanding of the developmental biology of the biliary tree might provide insights into therapeutic targeting of these processes during biliary disease. The biliary tree has played a central part in our efforts at understanding liver regeneration as it shares developmental origins with parenchymal hepatocytes, harbours a niche of stem or progenitor-like cells and is activated and expanded in the context of many liver pathologies. Thus, in this section, we briefly outline what is known about human biliary development and how the biliary system is altered during regeneration and repair.
Biliary development.
Around embryonic day (E) 13, bipotent hepatoblasts begin to differentiate towards mature hepatic epithelial cells (hepatocytes or cholangiocytes)69. By E15.5, hepatoblasts nearest the portal mesenchyme become cholangiocyte-like and coalesce to form the ductal plate that then gives rise to primitive ductal structures and ultimately the intrahepatic bile ducts72. The remaining parenchymal hepatoblasts, which lie further from the influence of the portal mesenchyme, differentiate towards hepatocytes. The adoption of the biliary phenotype is orchestrated through spatiotemporal gradients of Notch73, Wnt74, transforming growth factor-β (TGFβ)75 and FGF signalling76, which arise from endothelial cells and/or mesenchymal cells within the portal tract (FIG. 3a). By contrast, differentiation of hepatoblasts towards mature hepatocytes relies on factors such as oncostatin M generated by haematopoietic cells expanding in the fetal liver77, endothelial cell-derived hepatocyte growth factor (HGF)78 and tumour necrosis factor (TNF) generated by Kupffer cells79,80. Jagged 1, a cell surface Notch ligand of principal importance in bile duct development, exerts effects on both differentiation and tubulogenesis72 during cell–cell contact between periportal hepatoblasts and the portal mesenchyme. Indeed, Jagged 1 or Notch 2 mutations lead to the biliary abnormalities seen in patients with Alagille syndrome81, a liver disease associated with ductopenia of intrahepatic interlobular bile ducts.
Fig. 3. Potential sources of cholangiocytes in development and liver regeneration.
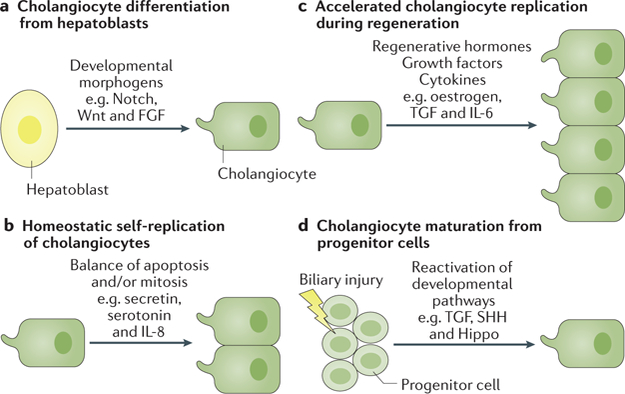
a | Cholangiocytes develop via differentiation from hepatoblasts in response to developmental morphogens. b | Cholangiocyte homeostasis is based on self-replication of pre-existing mature cholangiocytes. c | Cholangiocyte regeneration occurs through an accelerated replication in response to regenerative hormones, growth factors and cytokines. d | Cholangiocyte differentiation from hepatic progenitor cells can occur during biliary injury and repair after reactivation of developmental pathways.
Notably, the extrahepatic bile ducts (consisting of the common bile duct, cystic duct, gallbladder and hepatic ducts) have an embryological origin distinct from that of the intrahepatic bile ducts that develops in concert with the ventral pancreatic ductal system and only later anastomoses with the intrahepatic system. At E8.5, a subset of pancreatic endodermal cells that express both SOX17 and PDX1 represent a pancreatobiliary progenitor compartment that ultimately gives rise to the extrahepatic system. SOX17 seems to be critical in this process and works in concert with several other mediators, including HES1, HNF6 (encoded by ONECUT1), HNF1β and homeobox protein HEX (HHEX)82,83.
Liver regeneration.
In addition to its role in liver development, the biliary system has also been central in discussions related to liver regeneration and repair84, partly owing to the recognition that the biliary tree might harbour hepatic progenitor cells (HPCs) in the terminal ductules and the Canals of Hering85. In general, however, it is thought that maintenance of the normal liver simply requires occasional self-replication of existing adult epithelial cells (hepatocytes and cholangiocytes) through mitosis as opposed to HPC differentiation86. Through this mechanism, a very slow normal rate of liver cell turnover is able to counterbalance occasional apoptotic events to achieve a homeostatic equilibrium (FIG. 3b).
Following partial hepatectomy, a robust liver regeneration programme is activated to accelerate liver cell turnover and restore the lost mass and function. This phenomenon has been recognized for millennia, but the molecular mechanisms have been dissected in more considerable detail only over the past few decades87. Our understanding of liver regeneration has centred primarily on hepatocyte biology, but clearly the biliary system must also be regenerated. The presence of multiple temporal waves of DNA synthesis during the regeneration process suggests that other liver cell types, such as cholangiocytes and non-parenchymal cells, undergo a similar type of accelerated replication after resection to hepatocytes88. Although the bipotent liver stem cell compartment is known to reside in the periportal region, there does not seem to be substantial activation of these cells in the context of normal liver regeneration82,86. This observation suggests that normal liver regeneration is achieved primarily through hypertrophy and proliferation of mature liver cells (FIG. 3c), as opposed to expansion and maturation of the biliary compartment of HPCs. However, HPCs are more prominently expanded in the context of liver injury and repair. The existence of these specialized stem-like cells naturally led to the historical view that HPCs can expand and differentiate into both hepatocytes and cholangiocytes when the normal mechanisms of liver regeneration are overwhelmed89 (FIG. 3d). These small oval-shaped cells (termed oval cells in rodents) have scant cytoplasm and the ability to differentiate into both hepatocytes and cholangiocytes when isolated in vitro. More recently, additional stem cell niches have been described along the larger bile ducts in the peribiliary glands90. Furthermore, most forms of chronic liver disease in humans (especially biliary diseases) and several mouse models of liver disease (especially 3,5-diethoxycarbonyl-1,4-dihidro-collidine (DDC) feeding and the choline-deficient, ethanolamine supplemented diet) are associated with a robust expansion of HPCs91.
Cholangiocyte proliferation is regulated through complex mechanisms involving the effects of various autocrine and paracrine factors. These molecules include, but are not limited to, growth factors (for example, TGF and TNF), cytokines (such as IL-6), neuropeptides (such as ACh) and hormones (for example, testosterone and oestrogen)92. Interestingly, cholangiocytes contain receptors for both male and female sex hormones, which have been shown to promote cholangiocyte proliferation. Oestrogens seem to prevent cholangiocyte apoptosis while also potentiating secretory and proliferative pathways93,94. Likewise, progesterone binds to specific progesterone receptors on cholangiocytes to increase the biliary mass, and anti-progesterone therapy prevents the cholangiocyte growth caused by bile duct ligation95. The role of testosterone was highlighted by work showing that castration or anti-testosterone therapy decreases intrahepatic bile duct mass, reduces secretin-stimulated cAMP levels and blocks ductal secretion in bile-duct-ligated rats96.
Notably, many of the developmental morphogens that regulate liver organogenesis also seem to regulate cell fate decisions in the adult organ (for example, Wnt, SHH and Notch)97. Many genetic lineage-tracing studies have attempted to reconcile the origin and fate of HPCs with mixed results98–108. Currently, the prevailing view is that most parenchymal hepatocyte regeneration is hepatocyte-derived and that HPCs themselves might be derived from de-differentiated hepatocytes109,110, despite their apparent biliary phenotype. However, studies in zebrafish111,112 and in mice102,113 demonstrate that biliary-derived cells can expand and differentiate into parenchymal hepatocytes when mature hepatocytes cannot proliferate or are heavily damaged. Similarly, transdifferentiation of hepatocytes can also form biliary structures in mice with developmental disruption of Notch signalling114. Definitive resolution of any apparent discrepancies between these findings is difficult as none of the animal models precisely recapitulate human disease and because Cre–lox-based lineage tracing, although powerful, has some technical limitations115. Overall, it is clear that the liver is unique in its ability to respond to diverse insults, partially as a result of the profound cellular plasticity that it displays in various contexts116. The remaining challenges will be to reconcile which processes are active in various pathological situations in humans and to devise logical therapeutic interventions on the basis of this underlying pathobiology.
Cholangiocytes in inflammation and fibrosis
Cholangiocytes can be activated by a variety of insults, including infections, cholestasis, ischaemia and xenobiotics117, 118. In many cholangiopathies, PSC and PBC included, the activating insult is unknown. Features that characterize the activated cholangiocyte include increased proliferation and pro-fibrotic and pro-inflammatory secretions119,120. Activated cholangiocytes are also involved in recruitment and crosstalk with immune, vascular and mesenchymal cells, and upon chronic activation the development of biliary fibrosis and cholangiocarcinoma121,122. The broad changes in protein expression and the activated cholangiocyte secretome make the cholangiocyte an active participant in ongoing immunological reactions to biliary injury through pleiotropic autocrine and paracrine mechanisms123. Furthermore, secondary effects of cholestasis on immune cell function through nuclear receptor signalling (for example, via the FXR pathway and/or the vitamin D receptor) further mould this microenvironment124.
Most cholangiopathies share similar pathophysiological mechanisms, including cholestasis, proliferation, apoptosis, inflammation, fibrogenesis and eventually carcinogenesis. At the heart of biliary repair is inflammation. Persistent biliary cell damage and malfunction cause an inflammatory reaction that fuels a pathological reparative reaction, with excessive deposition of scar tissue around the injured ducts and eventually biliary cirrhosis. This complex of inflammatory cells (innate immune cells and T and B cells), mesenchymal cells and activated cholangiocytes is called ductular reaction. Activated cholangiocytes are able to participate in the inflammatory response by secreting chemokines, cytokines and angiogenic growth factors.
The biliary epithelium is exposed to cytokines and chemokines secreted by innate and adaptive immune cells in response to danger-associated molecular patterns (DAMPs), released by damaged liver cells, and/or to pathogen-associated molecular patterns (PAMPs) that originate in the intestine or the bloodstream125. In addition to infection and tissue injury120, epithelial inflammatory reactions might also be stimulated by autonomous cell mechanisms126. In these cases, attempts at restoring normal cell homeostasis sustain a chronic inflammatory response of low-magnitude ‘parainflammation’ (for example, an adaptive response to a persistent cell dysfunction, as shown, for example, for cholangiocytes with defective fibrocystin (congenital hepatic fibrosis)127–129); if normal biliary homeostasis is not restored, the process becomes maladaptive and stimulates the deposition of scar tissue.
Thus, depending on the type (infectious, toxic, autoimmune or inflammatory) and duration (acute or chronic) of the damage, epithelial cells, inflammatory cells and mesenchymal cells are activated and their crosstalk is orchestrated by a variety of autocrine and paracrine signals mediated by chemokines, cytokines and angiogenetic factors. It is important to remember that what is being repaired is an epithelial wound130–132 and that the repair process is driven by signals arising from loss of the homeostatic equilibrium in cholangiocytes. These signals are detected by innate inflammatory cells (such as macrophages and neutrophils) and by cells generating the scaffold (myofibroblasts and portal fibroblasts) and its vasculature (endothelial cells). Together, these cells and their corresponding signals comprise the biliary reparative complex, also called the ductular reaction.
All evidence suggests that inflammation is the primer of the reparative response and biliary fibrosis. Signals from the inflamed ducts activate liver mesenchymal cells and attract them to the bile ducts; in turn, cells in the bile ducts respond to soluble factors or vesicles released by the activated mesenchymal cells. This epithelial– mesenchymal crosstalk requires the complementary expression of an array of agonists and their receptors by epithelial and mesenchymal cells. TGFβ1 and TGFβ2 (REFS133–135), IL-6 and PDGFB134 and CC-motif chemo-kine 2 (CCL2) (REF.135) are some of the most well-studied factors secreted by reactive ductular cells (RDCs) that stimulate myofibroblast activation.
The epithelial component of ductular reaction — RDCs136,137 — displays a biliary phenotype and is organized into irregularly shaped structures. These richly anastomosed structures, usually without a recognizable lumen, are located at the periphery of the portal space, eventually extending into the lobule, following a portal-to-portal pattern. RDCs are a distinct cell population from HPCs and possess reparative rather than regenerative functions, as indicated by several studies showing a strong correlation between the amount of RDCs and portal fibrosis138–140. In addition to the mechanisms described above, RDCs might in part derive from cholangiocytes undergoing senescence, a cell reaction in which the cell is protected from apoptosis and carcinogenesis at the expense of a parainflammatory reaction, or from the ductular metaplasia of periportal hepatocytes, a phenomenon that has been clearly shown for intrahepatic cholangiocarcinomas130,136,137, 141. Thus, RDCs are generated through multiple, highly adaptable mechanisms depending on the nature and intensity of biliary damage. Independently from their histogenesis, RDCs progressively accumulate as an effect of ongoing pathological repair137. More than 20 years after Desmet suggested that RDCs are “the pacemaker of biliary fibrosis”142, several aspects of RDC biology remain elusive. RDCs possess different biological properties from normal cholangiocytes and can acquire a number of morphological and functional features of mesenchymal cells (FIG. 4). De novo expression of epithelial-to-mesenchymal transition (EMT) markers including S100A4, vimentin, Snail and matrix metalloproteinase 2 (MMP2), along with downregulation of the epithelial marker E-cadherin, was observed in RDCs in tissue sections obtained from patients with chronic cholangiopathies131. This ability of RDCs to express EMT markers and increase mobility is necessary to repair the wound. These features led to the concept of ‘partial EMT’ to underline the phenotypic plasticity of RDCs143. Notably, the secretory profile of ductular cells is similar to the senescence-associated secretory response (SASP) of senescent cells. RDCs show heterogeneous expression of senescence markers such as p16 (REF.144). This observation is functionally important as RDC senescence might be a driver of disease processes via the SASP mechanism. This ability to secrete pro-inflammatory cytokines typical of senescent cells might promote progression of the disease by amplifying the inflammatory and fibrotic responses145; therefore, targeting of senescent RCD cells might be a viable therapeutic strategy in cholestatic liver diseases146.
Fig. 4. Ductular reaction and ductular-reactive cells.
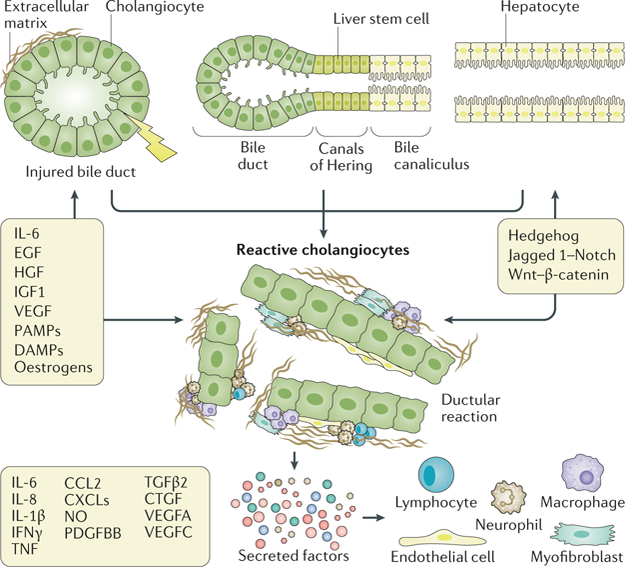
Extensive research has identified many of the morphogenetic mechanisms and molecules involved in the complex signalling and cellular crosstalk network of biliary repair. This crosstalk involves many cytokines, chemokines and signalling molecules. Most of these factors have both paracrine and autocrine effects and can act on multiple cell types. For example, vascular endothelial growth factor (VEGF) can autocrinally stimulate cholangiocyte proliferation, as the VEGF2 receptor is also expressed in cholangiocytes, but VEGF has paracrine effects on the endothelial cells (stimulation of neoangiogenesis) and stimulates mesenchymal cells. At the same time, IL-6 can stimulate cholangiocyte growth and the recruitment of neutrophils. The coexistence of reactive ductular cells and a rich mesenchymal and immune infiltrate constitutes the ductular reaction. The signals between the different infiltrating cell types are integrated into morphogenetic cues enabling cholangiocytes to re-create the biliary architecture owing to the re-expression of Wnt, Hedgehog and Notch signalling. CCL2, CC-chemokine ligand 2; CTGF, connective tissue growth factor; CXCL, CXC-chemokine ligands; DAMPs, damage-associated molecular patterns; EGF, epidermal growth factor; HGF, hepatocyte growth factor; IGF1, insulin-like growth factor 1; nitric oxide (NO); PAMPs, pathogen-associated molecular patterns; PDGFBB, platelet-derived growth factor B homodimer B; TGFβ2, transforming growth factor-β2; TNF, tumour necrosis factor.
An important requisite of biliary repair is the ability of RDCs to re-create the biliary architecture. This mechanism is mediated by morphogenetic pathways that are also involved in biliary development. Among them, Notch and the Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (YAP– TAZ) pathway deserve special consideration because of their known role in maintaining biliary architecture during biliary repair140,147–151.
Notch signalling is heavily involved in biliary repair, specifically in tubulogenesis and biliary transdifferentiation of hepatocytes140. Direct cell–cell interaction between Notch-expressing HPCs and Jagged-1-expressing portal myofibroblasts induces the conversion of HPCs to RDCs. Notch signalling, particularly the Notch 2 receptor, is involved in the generation of branching tubular structures in bile duct repair97,141,148. Although defective Notch signalling negatively affects biliary repair, persistent Notch overactivation might result in liver epithelial cell dysplasia and malignant transformation149. Furthermore, activation of Notch in adult hepatocytes induces their transdifferentiation into biliary phenotype cells that express the biliary markers SOX9 and HNF1β147.
Immunobiology of cholangiocytes
The liver and the bile ducts comprise complex immunological machinery, closely integrated with the mucosal immune system of the gut. Cholangiocytes contribute to homeostasis in this system through the secretion of IgA and various antimicrobial peptides (for example, β-defensin 2, lactoferrin and cathelicidin) into bile152–155. As described earlier, cholangiocytes also participate in the response to injury and repair132. Driven by developments in the understanding of secretory functions of epithelia156,157, research in the past few years has focused on activated cholangiocytes and their immunological functions119,120 (FIG. 5).
Fig. 5. Key aspects of cholangiocyte immunobiology.
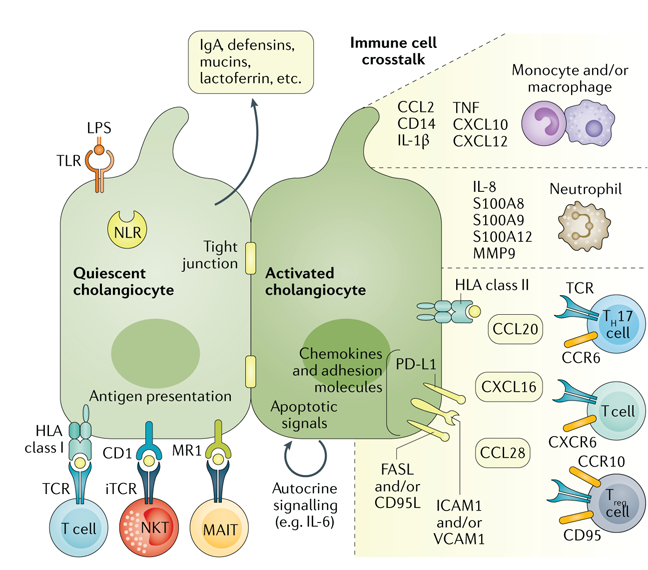
Quiescent cholangiocytes secrete antimicrobial molecules into bile (such as immunoglobulin A (IgA)) and express a range of innate immune receptors (for example, Toll-like receptors (TLRs) and NOD-like receptors (NLRs)) that recognize conserved pathogen-associated molecular patterns. The antigen-presenting capacities of cholangiocytes remain disputed regarding class II and T cell receptor (TCR) interactions, but CD1d and MR1 on cholangiocytes have been shown to effectively present lipid antigens and riboflavin derivatives to natural killer T (NKT) cells and mucosa-associated invariant T (MAIT) cells. Activated cholangiocytes engage in extensive paracrine crosstalk with cells of the immune system, including monocytes and macrophages, neutrophil granulocytes and T cells. Furthermore, autocrine signalling loops (for example, IL-6 signalling) provide further stimulation to augment and modify the activated cholangiocyte phenotype. CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CXCL, CXC-chemokine ligand; CXC-chemokine receptor; ICAM1, intercellular adhesion molecule 1; iTCR, invariant T cell receptor; LPS, lipopolysaccharide; MMP9, matrix metallopeptidase 9; PD-L1, programmed cell death 1 ligand 1; TH17 cell, T helper type 17 cell; TNF, tumour necrosis factor; Treg cell, regulatory T cell; VCAM1, vascular cell adhesion molecule 1.
Immunobiology of quiescent cholangiocytes.
Luminal cholangiocyte secretion comprises IgA and a broad range of other proteins with potential antimicrobial and immunological functions158. In rodents, hepatocytes effectively transport secretory IgA into bile157, whereas in humans hepatocytes do not express the secretory component and biliary IgA secretion is performed by cholangiocytes159. Immunoglobulins are the second-most abundant protein fraction in human bile after albumin (which also harbours immune function properties)160. The biliary immunoglobulins, secretory IgA in particular, contribute to the local antimicrobial defence systems in the bile ducts and upper intestine and might be involved in the clearance of systemic antigens161,162. Alterations in the hepatobiliary IgA system are observed in chronic liver diseases, in particular chronic alcoholic liver disease163. Other luminal secretions from cholangiocytes (such as defensins, mucins, lactoferrin and cathelicidin) contribute to the basic antimicrobial defence systems of bile152,153,158 and are typically upregulated during infections.
Cholangiocytes constitutively express Toll-like receptors (TLRs) that respond to conserved PAMPs (for example, lipopolysaccharide (LPS) binds TLR4)164,165. In quiescent cholangiocytes, TLR expression is most pronounced at the luminal membrane164, and biliary infections lead to upregulation of relevant TLRs164,166–168. TLR signalling pathways (such as NF-κB signalling) have important roles in cholangiocyte activation under these conditions117. Activation of TLRs has also been implicated in a variety of other biliary disease states, from cystic fibrosis and biliary atresia169,170 (a paediatric liver disease of unknown aetiology characterized by loss of intrahepatic and extrahepatic bile ducts) to PSC and PBC171–173. In PSC, a predominant hypothesis for disease development has been gut leakage of LPS and other bacterial products into the portal circulation due to concomitant IBD174,175. Although there is some evidence of TLR activation occurring in PSC173,176,177, this might also occur as a secondary phenomenon such as bacterial colonization following endoscopic retrograde cholangiography. Cholangiocytes also express cytoplasmic pattern recognition receptors (for example, NOD-, LRR- and pyrin domain-containing 3 (NLRP3)), and the NLRP3 inflammasome has been suggested to have a similar role in cholestatic liver disease as the TLRs173,178,179.
A potential role of the cholangiocyte as a professional antigen-presenting cell has been much debated. As with other nucleated cells, cholangiocytes constitutively express HLA class I molecules180. Upon activation, cholangiocytes also express HLA class II molecules181,182 but lack the B7 family members B7.1 (CD80) and B7.2 (CD86) co-stimulatory molecules that enable interactions with CD28 and CTLA4 on T cells. This finding probably means that the HLA class II expression on activated cholangiocytes is an epiphenomenon, but alternative co-stimulatory or inefficient class II antigen presentations remain theoretical possibilities. More recently, however, it has been shown that cholangiocytes are capable of presenting antigens to unconventional T cells such as mucosa-associated invariant T (MAIT) cells and natural killer T (NKT) cells. These T cells are enriched in compartments proximal to the gut microflora, the intestine and the liver in particular183, and their invariable T cell receptors react to antigens presented by pre-set HLA class I-like molecules. The target antigens for MAIT cells are bacterial B vitamins (riboflavin derivatives) presented on the MR1 molecule, whereas NKT cells react to lipids presented by the CD1d molecule. After exposure to bacteria, cholangiocytes activate MAIT cells, causing pro-inflammatory cytokine release184. Similarly, cholangiocytes have been shown to activate NKT cells upon exposure to relevant lipids185. Although both NKT and MAIT cells have been suggested to be involved in autoimmune liver diseases186–189, it is not clear whether this involvement is driven by antigens presented by cholangiocytes.
Immunobiology of the activated cholangiocyte.
Cholangiocytes can be activated by a variety of insults, including infections, cholestasis, ischaemia and xenobiotics117,118, although in most human cholangiopathies the insult is unclear. Activated cholangiocytes are characterized by increased proliferation and pro-fibrotic and pro-inflammatory secretions119,120. In this context, cholangiocytes are an active participant in the ongoing immunological processes123. Furthermore, secondary effects of cholestasis on immune cell function also occur124.
Crosstalk between activated cholangiocytes and T cells has been explored primarily in the context of autoimmune liver diseases. The interaction involves cholangiocyte expression of relevant adhesion molecules (such as intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1))181,190 and other contact-dependent mechanisms (for example, programmed cell death 1 ligand 1 (PD-L1))191, as well as bidirectional cytokine and chemokine communication (for example, activation of CXC-chemokine receptor 6 (CXCR6)-expressing T cells by cholangiocyte-derived CXC-chemokine ligand 16 (CXCL16))190. The crosstalk recruits T cells to the sites of biliary injury (for example, by CCL20 released by cholangiocytes positioning CC-chemokine receptor 6 (CCR6)-expressing T cells)192 and modulates relevant T cell activity — for example, by inducing persistence of effector T cells at sites of injury193. In PSC and PBC, in which T cell-mediated cholangiocyte destruction has been proposed to be involved in pathogenesis194,195, the crosstalk between cholangiocytes and T cells might hold clues for therapeutic interventions. Crosstalk between activated cholangiocytes and macrophages is involved in the chemoattraction of monocytes (via signalling molecules such as CCL2, IL-1 and a broad variety of chemokines including CXCL10 and CXCL12)119,196,197 and the regulation of macrophage effector functions. These functions include cytokine secretions (for example, TNF)181, amplification of apoptotic signalling to cholangiocytes198 and the resulting signalling cascades that generate a pro-fibrotic peribiliary microenvironment that also involves hepatic stellate cells and portal myofibroblasts135,199. The presence of proteins including CD14 in bile and immunohistochemistry support the involvement of macrophages in the biliary microenvironment in disease states such as PSC200,201. IL-8 (REFS202,203), probably derived largely from activated cholangiocytes, is another protein consistently found upregulated in the bile of patients with cholestatic liver disease204–206. Although it serves as a potent chemoattractant for monocytes, IL-8 secretion could also be involved in shaping the strong neutrophil signature observed in biliary disease states such as PSC200,207. Numerous proteins found in bile probably reflect neutrophil activation in the biliary microenvironment (for example, S100A8, S100A9, S100A12 and MMP9), albeit more so in PSC than other biliary diseases200,207. Additional cytokines secreted by cholangiocytes (such as IL-6) are probably involved in autocrine signalling208, reinforcing the cholangiocyte–immune system crosstalk, promoting cholangiocyte proliferation and, over time, facilitating cholangiocarcinoma development during chronic biliary inflammation209,210.
Future directions
Much remains to be understood regarding the mechanisms of cholangiocyte pathobiology. Future advances will need to both exploit new technical advances and address fundamental gaps in our knowledge. The field of cholangiocyte biology has been held back by the absence of a cholangiocyte-specific promoter to develop and examine the phenotypes of cholangiocyte gene modifications using Cre recombinase technology. One strategy to gain cell specificity for cholangiocyte Cre expression could use liver-specific promoters in transgenic animals to drive expression of a flippase (FLP) recombinase in hepatoblasts during embryonic development. The FLP recombinase would be expressed in the descendants of the hepatoblasts, including both hepatocytes and cholangiocytes. A second construct expressing a Cre recombinase driven by cytokeratin 19 (which is expressed only in cholangiocytes within the liver) and held in check by a FLP recognition target (FRT)-flanked stop codon would then be introduced into the animals. The FLP recombinase would excise the stop codon, permitting expression of Cre only in cholangiocytes. Although conceptually attractive, we are not aware of anyone establishing this mouse to date, and other approaches would be welcome and are encouraged.
Two validated and now established technical advances include the development of patient-derived induced pluripotent stem cells (iPSCs), which can be differentiated into cholangiocytes, and the generation of 3D organoids. Several groups have now reported on iPSCs differentiated into cholangiocytes and cholangiocyte organoids with considerable success and insight211–223. For example, organoids have been used to identify a clonogenic subpopulation of mouse cholangiocytes and unique surface markers for this proliferative cell population213. In addition, iPSC-derived cholangiocytes have been used to regenerate the extrahepatic bile duct in the mouse224. These new tools will allow further mechanistic studies to improve our understanding of the mechanisms of human cholangiocyte biology and generate more functional in vitro models.
A myriad of pertinent questions persist regarding cholangiocyte pathobiology (BOX 2). This list is certainly not exclusive but rather highlights many relevant knowledge gaps that can be addressed with current methodologies. We hope these questions will help guide future research priorities and emphasize to funding agencies the importance of these questions to human health. We look forward to the future answers to these and other questions.
Box 2 |. Key questions.
How is cholangiocyte regeneration regulated?
What cholangiocyte cell death processes occur and predominate?
Does elimination of reactive ductular cells reverse liver injury?
How do cholangiocytes interact with innate and adaptive immune cells?
How do cholangiocytes interact with the intestinal and biliary microbiome?
How do cholangiocytes modify and engage the biomatrix of the biliary system?
What is the effect of changes in cholangiocyte-mediated alterations of bile duct permeability?
What is the role of cholangiocytes in drug and xenobiotic metabolism and transport?
Conclusions
Despite comprising only ~5% of the cells in the liver, cholangiocytes are essential for health. Considerable information now exists regarding their highly complex and regulated transport functions and contributions to bile composition and flow. The role of cholangiocytes in liver regeneration has also become more sharply defined. Current scientific investigation is focused on their role in liver immunobiology, inflammation and fibrosis, a line of enquiry that is important in unravelling the pathogenic mechanisms causing the cholangiopathies. We encourage more work on these processes, which will hopefully result in better therapy for these diseases.
Key points.
Cholangiocytes are epithelial cells lining the intrahepatic and extrahepatic bile ducts; they are heterogeneous in size and function and contribute to bile composition and flow by solute transport processes.
Cholangiocytes contribute to liver regeneration, especially when hepatocyte regeneration is compromised, as is often the case in human chronic liver diseases.
Cholangiocytes can become activated and participate in inflammation by secreting chemokines and cytokines and can also directly modulate the biology of myofibroblasts, the cell type responsible for collagen deposition within the liver.
Cholangiocytes can become senescent and participate in the senescence-associated secretory phenotype, a cell fate also characterized by cytokine generation and release.
Cholangiocytes participate in hepatic immunobiology, particularly by expressing Toll-like receptors (TLRs), contributing to immunoglobulin A (IgA) biology, and by cellular crosstalk with the innate and adaptive immune system.
Cholangiocytes are damaged in a variety of human liver diseases termed the cholangiopathies, which are in need of optimized therapies and represent a current unmet need in clinical medicine.
Acknowledgements
J.M.B. has received grant support from the Spanish Ministries of Economy and Competitiveness (FIS PI15/01132, FIS PI18/01075 and Miguel Servet Programme CON14/ 00129) co-financed by ‘Fondo Europeo de Desarrollo Regional’ (FEDER); ISCIII (CIBERehd), Spain; BIOEF (Basque Foundation for Innovation and Health Research); EiTB Maratoia BIO15/CA/016/BD; the Department of Health of the Basque Country (2017111010); and the Scientific Foundation of the Spanish Association Against Cancer (AECC). The authors also acknowledge the US National Institutes of Health grants DK63947 (G.J.G.); DK057993, DK084567 and DK24031 (N.F.L.); and DK100575 and DK113339 (R.C.H.). G.J.G., N.F.L. and R.C.H. further acknowledge support from the Carlos Family Foundation and the Mayo Clinic. M.S. has recieved support from the US National Institutes of Health Grants DK079005, DK096096 and DK34989, the Silvio O. Conte Digestive Diseases Research Core Centers and PSC Partners Seeking a Cure.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Masyuk AI, M. T. & LaRusso NF in Physiology of the Gastrointestinal Tract (ed. Ghishan FK) 1003–1023 (Elsevier Inc., 2018). [Google Scholar]
- 2.Boyer JL Bile formation and secretion. Compr. Physiol 3, 1035–1078 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudio E et al. Cholangiocytes and blood supply. World J. Gastroenterol 12, 3546–3552 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morell CM, Fabris L & Strazzabosco M Vascular biology of the biliary epithelium. J. Gastroenterol. Hepatol 28 (Suppl. 1), 26–32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvaro D et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132, 415–431 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Lazaridis KN, Strazzabosco M & Larusso NF The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127, 1565–1577 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Lazaridis KN & LaRusso NF Primary sclerosing cholangitis. N. Engl. J. Med 375, 1161–1170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaigre FP Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 137, 62–79 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Raynaud P, Carpentier R, Antoniou A & Lemaigre FP Biliary differentiation and bile duct morphogenesis in development and disease. Int. J. Biochem. Cell Biol 43, 245–256 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Strazzabosco M & Fabris L Functional anatomy of normal bile ducts. Anat. Rec 291, 653–660 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banales JM, Prieto J & Medina JF Cholangiocyte anion exchange and biliary bicarbonate excretion. World J. Gastroenterol 12, 3496–3511 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP & LaRusso NF Physiology of cholangiocytes. Compr. Physiol 3, 541–565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y et al. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp. Biol. Med 238, 549–565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpini G et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am. J. Physiol 272, G1064–G1074 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Alpini G et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110, 1636–1643 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Ishii M, Vroman B & LaRusso NF Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 97, 1236–1247 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Kanno N, LeSage G, Glaser S, Alvaro D & Alpini G Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology 31, 555–561 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Ludwig J, Ritman EL, LaRusso NF, Sheedy PF & Zumpe G Anatomy of the human biliary system studied by quantitative computer-aided threedimensional imaging techniques. Hepatology 27, 893–899 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Vroman B & LaRusso NF Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab. Invest 74, 303–313 (1996). [PubMed] [Google Scholar]
- 20.Huang BQ et al. Isolation and characterization of cholangiocyte primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol 291, G500–G509 (2006). [DOI] [PubMed] [Google Scholar]
- 21.De La Iglesia FA & Porta EA Ciliated biliary epithelial cells in the livers of non-human primates. Experientia 23, 49–51 (1967). [DOI] [PubMed] [Google Scholar]
- 22.Boyer JL in Physiology of Membrane Disorders (eds Andreoli TE, Hoffman JF, Fanestil DD & Schultz S) 609–636 (Springer US, 1986). [Google Scholar]
- 23.Banales JM et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 43, 266–275 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Lenzen R, Alpini G & Tavoloni N Secretin stimulates bile ductular secretory activity through the cAMP system. Am. J. Physiol 263, G527–G532 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Alvaro D, Mennone A & Boyer JL Role of kinases and phosphatases in the regulation of fluid secretion and Cl−/HCO3- exchange in cholangiocytes. Am. J. Physiol 273, G303–G313 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Tietz PS et al. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J. Biol. Chem 278, 20413–20419 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Hohenester S et al. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 55, 173–183 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Tietz PS, Alpini G, Pham LD & Larusso NF Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am. J. Physiol 269, G110–G118 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Kaminski DL & Deshpande YG Effect of somatostatin and bombesin on secretin-stimulated ductular bile flow in dogs. Gastroenterology 85, 1239–1247 (1983). [PubMed] [Google Scholar]
- 30.Glaser SS et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am. J. Physiol 273, G1061–G1070 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Caligiuri A et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am. J. Physiol 275, G835–G846 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Medina JF, Martinez A, Vazquez JJ & Prieto J Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology 25, 12–17 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Melero S et al. Defective regulation of cholangiocyte Cl−/HCO3(−) and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology 35, 1513–1521 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Prieto J et al. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology 117, 167–172 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Banales JM et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 56, 687–697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erice O et al. MicroRNA-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology 67, 1420–1440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salas JT et al. Ae2a, b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology 134, 1482–1493 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Concepcion AR et al. Anion exchanger 2 is critical for CD8(+) T cells to maintain pHi homeostasis and modulate immune responses. Eur. J. Immunol 44, 1341–1351 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Concepcion AR et al. CD8+T cells undergo activation and programmed death-1 repression in the liver of aged Ae2a, b−/− mice favoring autoimmune cholangitis. Oncotarget 6, 28588–28606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo C, Battezzati PM, Strazzabosco M & Podda M Liver and biliary problems in cystic fibrosis. Semin. Liver Dis 18, 227–235 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Nyberg B, Einarsson K & Sonnenfeld T Evidence that vasoactive intestinal peptide induces ductular secretion of bile in humans. Gastroenterology 96, 920–924 (1989). [PubMed] [Google Scholar]
- 42.Hirata K & Nathanson MH Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology 121, 396–406 (2001). [DOI] [PubMed] [Google Scholar]
- 43.LeSage GD et al. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology 40, 1116–1127 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Glaser S et al. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am. J. Physiol. Gastrointest. Liver Physiol 284, G683–G694 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Kanno N et al. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology 35, 1329–1340 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Francis H et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am. J. Physiol. Cell Physiol 293, C1252–C1262 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Dutta AK et al. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl− channel activated by extracellular nucleotides, in biliary epithelium. J. Biol. Chem 286, 766–776 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman RM, Feranchak AP, Salter KD, Wang Y & Fitz JG Endogenous ATP release regulates Cl− secretion in cultured human and rat biliary epithelial cells. Am. J. Physiol 276, G1391–G1400 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Concepcion AR, Lopez M, Ardura-Fabregat A & Medina JF Role of AE2 for pHi regulation in biliary epithelial cells. Front. Physiol 4, 413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann AF The enterohepatic circulation of bile acids in mammals: form and functions. Front. Biosci 14, 2584–2598 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Lamri Y, Erlinger S, Dumont M, Roda A & Feldmann G Immunoperoxidase localization of ursodeoxycholic acid in rat biliary epithelial cells. Evidence for a cholehepatic circulation. Liver 12, 351–354 (1992). [DOI] [PubMed] [Google Scholar]
- 52.Lazaridis KN et al. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodiumdependent bile acid transporter. J. Clin. Invest 100, 2714–2721 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazaridis KN et al. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc. Natl Acad. Sci. USA 97, 11092–11097 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kool M et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc. Natl Acad. Sci. USA 96, 6914–6919 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soroka CJ, Lee JM, Azzaroli F & Boyer JL Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33, 783–791 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Ballatori N et al. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42, 1270–1279 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Ballatori N et al. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front. Biosci 14, 2829–2844 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benedetti A et al. Carrier-mediated transport of conjugated bile acids across the basolateral membrane of biliary epithelial cells. Am. J. Physiol 272, G1416–G1424 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Hirohashi T, Suzuki H, Takikawa H & Sugiyama Y ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J. Biol. Chem 275, 2905–2910 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Lazaridis KN, Pham L, Vroman B, de Groen PC & LaRusso NF Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am. J. Physiol 272, G1168–G1174 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Ballatori N, Jacob R & Boyer JL Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J. Biol. Chem 261, 7860–7865 (1986). [PubMed] [Google Scholar]
- 62.Ballatori N, Jacob R, Barrett C & Boyer JL Biliary catabolism of glutathione and differential reabsorption of its amino acid constituents. Am. J. Physiol 254, G1–G7 (1988). [DOI] [PubMed] [Google Scholar]
- 63.Masyuk AI, Masyuk TV & LaRusso NF Cholangiocyte primary cilia in liver health and disease. Dev. Dyn 237, 2007–2012 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masyuk AI et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masyuk AI et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am. J. Physiol. Gastrointest. Liver Physiol 295, G725–G734 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masyuk AI et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol 304, G1013–G1024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gradilone SA et al. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl Acad. Sci. USA 104, 19138–19143 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masyuk AI et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol 299, G990–G999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ober EA & Lemaigre FP Development of the liver: Insights into organ and tissue morphogenesis. J. Hepatol 68, 1049–1062 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Michalopoulos GK Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 65, 1384–1392 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Strazzabosco M & Fabris L Development of the bile ducts: essentials for the clinical hepatologist. J. Hepatol 56, 1159–1170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemaigre FP Molecular mechanisms of biliary development. Prog. Mol. Biol. Transl Sci 97, 103–126 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Tchorz JS et al. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 50, 871–879 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Decaens T et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology 47, 247–258 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Clotman F et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev 19, 1849–1854 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanai M et al. FGF signaling segregates biliary cell-lineage from chick hepatoblasts cooperatively with BMP4 and ECM components in vitro. Dev. Dyn 237, 1268–1283 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Kamiya A & Gonzalez FJ TNF-alpha regulates mouse fetal hepatic maturation induced by oncostatin M and extracellular matrices. Hepatology 40, 527–536 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Schmidt C et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373, 699–702 (1995). [DOI] [PubMed] [Google Scholar]
- 79.Gordillo M, Evans T & Gouon-Evans V Orchestrating liver development. Development 142, 2094–2108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin D & Monga SP Cellular and molecular basis of liver development. Compr. Physiol 3, 799–815 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet 16, 243–251 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Zong Y & Stanger BZ Molecular mechanisms of liver and bile duct development. Wiley Interdiscip. Rev. Dev. Biol 1, 643–655 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Merino-Azpitarte M et al. SOX17 regulates cholangiocyte differentiation and acts as a tumor suppressor in cholangiocarcinoma. J. Hepatol 67, 72–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Itoh T Stem/progenitor cells in liver regeneration. Hepatology 64, 663–668 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Duncan AW, Dorrell C & Grompe M Stem cells and liver regeneration. Gastroenterology 137, 466–481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stanger BZ Cellular homeostasis and repair in the mammalian liver. Annu. Rev. Physiol 77, 179–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Preziosi ME & Monga SP Update on the mechanisms of liver regeneration. Semin. Liver Dis 37, 141–151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michalopoulos GK & DeFrances MC Liver regeneration. Science 276, 60–66 (1997). [DOI] [PubMed] [Google Scholar]
- 89.Itoh T & Miyajima A Liver regeneration by stem/ progenitor cells. Hepatology 59, 1617–1626 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Lanzoni G, Cardinale V & Carpino G The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: a new reference frame for disease and regeneration. Hepatology 64, 277–286 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Sato K et al. Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology 69, 420–430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alvaro D, Gigliozzi A & Attili AF Regulation and deregulation of cholangiocyte proliferation. J. Hepatol 33, 333–340 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Svegliati-Baroni G et al. Estrogens maintain bile duct mass and reduce apoptosis after biliodigestive anastomosis in bile duct ligated rats. J. Hepatol 44, 1158–1166 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Alvaro D et al. Estrogens and the pathophysiology of the biliary tree. World J. Gastroenterol 12, 3537–3545 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glaser S et al. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol 295, G124–G136 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Yang F et al. Castration inhibits biliary proliferation induced by bile duct obstruction: novel role for the autocrine trophic effect of testosterone. Am. J. Physiol. Gastrointest. Liver Physiol 301, G981–G991 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boulter L et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med 18, 572–579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Espanol-Suner R et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143, 1564–1575 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Furuyama K et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet 43, 34–41 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Huch M et al. In vitro expansion of single Lgr5+liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamimoto K et al. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. eLife 5, e15034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu WY et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol 17, 971–983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malato Y et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Invest 121, 4850–4860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodrigo-Torres D et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology 60, 1367–1377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sackett SD et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 49, 920–929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schaub JR, Malato Y, Gormond C & Willenbring H Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep 8, 933–939 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarlow BD, Finegold MJ & Grompe M Clonal tracing of Sox9+liver progenitors in mouse oval cell injury. Hepatology 60, 278–289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yanger K et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 15, 340–349 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Overi D et al. Contribution of resident stem cells to liver and biliary tree regeneration in human diseases. Int. J. Mol. Sci 19, 2917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Michalopoulos GK & Khan Z Liver stem cells: experimental findings and implications for human liver disease. Gastroenterology 149, 876–882 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choi TY, Ninov N, Stainier DY & Shin D Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He J, Lu H, Zou Q & Luo L Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Raven A et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 547, 350–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schaub JR et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lemaigre FP Determining the fate of hepatic cells by lineage tracing: facts and pitfalls. Hepatology 61, 2100–2103 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Kopp JL, Grompe M & Sander M Stem cells versus plasticity in liver and pancreas regeneration. Nat. Cell Biol 18, 238–245 (2016). [DOI] [PubMed] [Google Scholar]
- 117.O’Hara SP, Karlsen TH & LaRusso NF Cholangiocytes and the environment in primary sclerosing cholangitis: where is the link? Gut 66, 1873–1877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O’Hara SP, Tabibian JH, Splinter PL & LaRusso NF The dynamic biliary epithelia: molecules, pathways, and disease. J. Hepatol 58, 575–582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinto C, Giordano DM, Maroni L & Marzioni M Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim. Biophys. Acta 1864, 1270–1278 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Strazzabosco M et al. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim. Biophys. Acta 1864, 1374–1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mederacke I et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun 4, 2823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blechacz B & Gores GJ Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 48, 308–321 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adams DH Biliary epithelial cells: innocent victims or active participants in immune-mediated liver disease? J. Lab. Clin. Med 128, 528–530 (1996). [DOI] [PubMed] [Google Scholar]
- 124.Zhu C, Fuchs CD, Halilbasic E & Trauner M Bile acids in regulation of inflammation and immunity: friend or foe? Clin. Exp. Rheumatol 34, 25–31 (2016). [PubMed] [Google Scholar]
- 125.Fabris L, Spirli C, Cadamuro M, Fiorotto R & Strazzabosco M Emerging concepts in biliary repair and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol 313, G102–G116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaffe E et al. beta-Catenin and interleukin-1beta-dependent chemokine (C-X-C motif) ligand 10 production drives progression of disease in a mouse model of congenital hepatic fibrosis. Hepatology 67, 1903–1919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chovatiya R & Medzhitov R Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Medzhitov R Origin and physiological roles of inflammation. Nature 454, 428–435 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Kotas ME & Medzhitov R Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fabris L et al. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am. J. Pathol 156, 1599–1612 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fabris L & Strazzabosco M Epithelial-mesenchymal interactions in biliary diseases. Semin. Liver Dis 31, 11–32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fabris L, Brivio S, Cadamuro M & Strazzabosco M Revisiting epithelial-to-mesenchymal transition in liver fibrosis: clues for a better understanding of the “reactive” biliary epithelial phenotype. Stem Cells Int 2016, 2953727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Milani S, Herbst H, Schuppan D, Stein H & Surrenti C Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am. J. Pathol 139, 1221–1229 (1991). [PMC free article] [PubMed] [Google Scholar]
- 134.Kinnman N et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab. Invest 83, 163–173 (2003). [DOI] [PubMed] [Google Scholar]
- 135.Kruglov EA, Nathanson RA, Nguyen T & Dranoff JA Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol 290, G765–G771 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Roskams TA, Libbrecht L & Desmet VJ Progenitor cells in diseased human liver. Semin. Liver Dis 23, 385–396 (2003). [DOI] [PubMed] [Google Scholar]
- 137.Roskams TA et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39, 1739–1745 (2004). [DOI] [PubMed] [Google Scholar]
- 138.Franchitto A et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann. Transl Med 1, 27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Francis H et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ ERK½ pathway. J. Hepatol 41, 528–537 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Fabris L et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am. J. Pathol 171, 641–653 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Demetris AJ, Seaberg EC, Wennerberg A, Ionellie J & Michalopoulos G Ductular reaction after submassive necrosis in humans. Special emphasis on analysis of ductular hepatocytes. Am. J. Pathol 149, 439–448 (1996). [PMC free article] [PubMed] [Google Scholar]
- 142.Desmet VJ Histopathology of cholestasis. Verh. Dtsch. Ges. Pathol 79, 233–240 (1995). [PubMed] [Google Scholar]
- 143.Brivio S, Cadamuro M, Fabris L & Strazzabosco M Epithelial-to-mesenchymal transition and cancer invasiveness: what can we learn from cholangiocarcinoma? J. Clin. Med 4, 2028–2041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sasaki M et al. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am. J. Clin. Pathol 133, 212–223 (2010). [DOI] [PubMed] [Google Scholar]
- 145.He S & Sharpless NE Senescence in health and disease. Cell 169, 1000–1011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moncsek A et al. Targeting senescent cholangiocytes and activated fibroblasts with B cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−)) mice. Hepatology 67, 247–259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morell CM et al. Notch signaling and progenitor/ ductular reaction in steatohepatitis. PLOS ONE 12, e0187384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fiorotto R et al. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J. Hepatol 59, 124–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geisler F & Strazzabosco M Emerging roles of Notch signaling in liver disease. Hepatology 61, 382–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zanconato F et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat. Med 24, 1599–1610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Panciera T, Azzolin L, Cordenonsi M & Piccolo S Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol 18, 758–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harada K et al. Peptide antibiotic human beta-defensin-1 and −2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology 40, 925–932 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Saito K & Nakanuma Y Lactoferrin and lysozyme in the intrahepatic bile duct of normal livers and hepatolithiasis. An immunohistochemical study. J. Hepatol 15, 147–153 (1992). [DOI] [PubMed] [Google Scholar]
- 154.Nagura H, Smith PD, Nakane PK & Brown WR IGA in human bile and liver. J. Immunol 126, 587–595 (1981). [PubMed] [Google Scholar]
- 155.D’Aldebert E et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology 136, 1435–1443 (2009). [DOI] [PubMed] [Google Scholar]
- 156.Sternlieb I Special article: functional implications of human portal and bile ductular ultrastructure. Gastroenterology 63, 321–327 (1972). [PubMed] [Google Scholar]
- 157.Brown WR & Kloppel TM The liver and IgA: immunological, cell biological and clinical implications. Hepatology 9, 763–784 (1989). [DOI] [PubMed] [Google Scholar]
- 158.Farina A et al. A step further in the analysis of human bile proteome. J. Proteome Res 10, 2047–2063 (2011). [DOI] [PubMed] [Google Scholar]
- 159.Brandtzaeg P et al. Production and secretion of immunoglobulins in the gastrointestinal tract. Ann. Allergy 59, 21–39 (1987). [PubMed] [Google Scholar]
- 160.Naldi M, Baldassarre M, Domenicali M, Bartolini M & Caraceni P Structural and functional integrity of human serum albumin: Analytical approaches and clinical relevance in patients with liver cirrhosis. J. Pharm. Biomed. Anal 144, 138–153 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Aagaard BD, Heyworth MF, Oesterle AL, Jones AL & Way LW Intestinal immunisation with Escherichia coli protects rats against Escherichia coli induced cholangitis. Gut 39, 136–140 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harmatz PR, Kleinman RE, Bunnell BW, Bloch KJ & Walker WA Hepatobiliary clearance of IgA immune complexes formed in the circulation. Hepatology 2, 328–333 (1982). [DOI] [PubMed] [Google Scholar]
- 163.van de Wiel A, Delacroix DL, van Hattum J, Schuurman HJ & Kater L Characteristics of serum IgA and liver IgA deposits in alcoholic liver disease. Hepatology 7, 95–99 (1987). [DOI] [PubMed] [Google Scholar]
- 164.Chen XM et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol 175, 7447–7456 (2005). [DOI] [PubMed] [Google Scholar]
- 165.Harada K et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab. Invest 83, 1657–1667 (2003). [DOI] [PubMed] [Google Scholar]
- 166.Ninlawan K et al. Opisthorchis viverrini excretory/ secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol. Int 59, 616–621 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ikeda H et al. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab. Invest 87, 559–571 (2007). [DOI] [PubMed] [Google Scholar]
- 168.Oya S et al. Inhibition of Toll-like receptor 4 suppresses liver injury induced by biliary obstruction and subsequent intraportal lipopolysaccharide injection. Am. J. Physiol. Gastrointest. Liver Physiol 306, G244–G252 (2014). [DOI] [PubMed] [Google Scholar]
- 169.Fiorotto R et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF- kappaB-mediated inflammatory response in mice. Gastroenterology 141, 1498–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harada K et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology 46, 1146–1154 (2007). [DOI] [PubMed] [Google Scholar]
- 171.Wang AP et al. Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis. J. Autoimmun 25, 85–91 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Moritoki Y et al. AMA production in primary biliary cirrhosis is promoted by the TLR9 ligand CpG and suppressed by potassium channel blockers. Hepatology 45, 314–322 (2007). [DOI] [PubMed] [Google Scholar]
- 173.Matsushita H et al. TLR4, TLR9, and NLRP3 in biliary epithelial cells of primary sclerosing cholangitis: relationship with clinical characteristics. J. Gastroenterol. Hepatol 30, 600–608 (2015). [DOI] [PubMed] [Google Scholar]
- 174.Karlsen TH, Folseraas T, Thorburn D & Vesterhus M Primary sclerosing cholangitis — a comprehensive review. J. Hepatol 67, 1298–1323 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Hov JR & Karlsen TH The microbiome in primary sclerosing cholangitis: current evidence and potential concepts. Semin. Liver Dis 37, 314–331 (2017). [DOI] [PubMed] [Google Scholar]
- 176.Karrar A et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology 132, 1504–1514 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Mueller T et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int 31, 1574–1588 (2011). [DOI] [PubMed] [Google Scholar]
- 178.Maroni L et al. Nlrp3 activation induces Il-18 synthesis and affects the epithelial barrier function in reactive cholangiocytes. Am. J. Pathol 187, 366–376 (2017). [DOI] [PubMed] [Google Scholar]
- 179.Tian J et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J 30, 4202–4213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Auth MK et al. Establishment and immunological characterization of cultured human gallbladder epithelial cells. Hepatology 18, 546–555 (1993). [PubMed] [Google Scholar]
- 181.Ayres RC, Neuberger JM, Shaw J, Joplin R & Adams DH Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut 34, 1245–1249 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Broome U, Scheynius A & Hultcrantz R Induced expression of heat-shock protein on biliary epithelium in patients with primary sclerosing cholangitis and primary biliary cirrhosis. Hepatology 18, 298–303 (1993). [DOI] [PubMed] [Google Scholar]
- 183.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J & Moody DB The burgeoning family of unconventional T cells. Nat. Immunol 16, 1114–1123 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Jeffery HC et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol 64, 1118–1127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schrumpf E et al. The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology 62, 1249–1259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bottcher K et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote pro-fibrogenic hepatic stellate cell activation. Hepatology 68, 172–186 (2018). [DOI] [PubMed] [Google Scholar]
- 187.Jiang X et al. The immunobiology of mucosal-associated invariant T cell (MAIT) function in primary biliary cholangitis: Regulation by cholic acid-induced Interleukin-7. J. Autoimmun 90, 64–75 (2018). [DOI] [PubMed] [Google Scholar]
- 188.Kita H et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology 123, 1031–1043 (2002). [DOI] [PubMed] [Google Scholar]
- 189.Schrumpf E et al. The role of natural killer T cells in a mouse model with spontaneous bile duct inflammation. Physiol. Rep 5, e13117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Heydtmann M et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol 174, 1055–1062 (2005). [DOI] [PubMed] [Google Scholar]
- 191.Kamihira T et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology 41, 151–159 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Oo YH et al. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J. Hepatol 57, 1044–1051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Afford SC et al. Vascular cell adhesion molecule 1 expression by biliary epithelium promotes persistence of inflammation by inhibiting effector T cell apoptosis. Hepatology 59, 1932–1943 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Grant AJ, Lalor PF, Salmi M, Jalkanen S & Adams DH Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 359, 150–157 (2002). [DOI] [PubMed] [Google Scholar]
- 195.Borchers AT, Shimoda S, Bowlus C, Keen CL & Gershwin ME Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin. Immunopathol 31, 309–322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Adams DH & Afford SC The role of cholangiocytes in the development of chronic inflammatory liver disease. Front. Biosci 7, e276–e285 (2002). [DOI] [PubMed] [Google Scholar]
- 197.Locatelli L et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 63, 965–982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Alabraba EB et al. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology 47, 552–562 (2008). [DOI] [PubMed] [Google Scholar]
- 199.Marra F et al. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am. J. Pathol 152, 423–430 (1998). [PMC free article] [PubMed] [Google Scholar]
- 200.Vesterhus M et al. Novel serum and bile protein markers predict primary sclerosing cholangitis disease severity and prognosis. J. Hepatol 66, 1214–1222 (2017). [DOI] [PubMed] [Google Scholar]
- 201.Cameron RG, Blendis LM & Neuman MG Accumulation of macrophages in primary sclerosing cholangitis. Clin. Biochem 34, 195–201 (2001). [DOI] [PubMed] [Google Scholar]
- 202.Zweers SJ et al. Elevated interleukin-8 in bile of patients with primary sclerosing cholangitis. Liver Int 36, 1370–1377 (2016). [DOI] [PubMed] [Google Scholar]
- 203.Dong R & Zheng S Interleukin-8: a critical chemokine in biliary atresia. J. Gastroenterol. Hepatol 30, 970–976 (2015). [DOI] [PubMed] [Google Scholar]
- 204.Isse K, Harada K & Nakanuma Y IL-8 expression by biliary epithelial cells is associated with neutrophilic infiltration and reactive bile ductules. Liver Int 27, 672–680 (2007). [DOI] [PubMed] [Google Scholar]
- 205.Morland CM, Fear J, Joplin R & Adams DH Inflammatory cytokines stimulate human biliary epithelial cells to express interleukin-8 and monocyte chemotactic protein-1. Biochem. Soc. Trans 25, 232S (1997). [DOI] [PubMed] [Google Scholar]
- 206.Tabibian JH et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab. Invest 94, 1126–1133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Reinhard L et al. S100A9 is a biliary protein marker of disease activity in primary sclerosing cholangitis. PLOS ONE 7, e29821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Johnson C et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest. Cancer 1, 58–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Isomoto H et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology 42, 1329–1338 (2005). [DOI] [PubMed] [Google Scholar]
- 210.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH & Gores GJ Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology 128, 2054–2065 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Andersson ER et al. Mouse model of Alagille syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology 154, 1080–1095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vyas D et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology 67, 750–761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li B et al. Adult mouse liver contains two distinct populations of cholangiocytes. Stem Cell Rep 9, 478–489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sampaziotis F et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med 23, 954–963 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Sampaziotis F et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat. Protoc 12, 814–827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Loarca L et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Lab. Invest 97, 1385–1396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cervantes-Alvarez E et al. Current strategies to generate mature human induced pluripotent stem cells derived cholangiocytes and future applications. Organogenesis 13, 1–15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.De Assuncao TM, Jalan-Sakrikar N & Huebert RC Regenerative medicine and the biliary tree. Semin. Liver Dis 37, 17–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dianat N et al. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology 60, 700–714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.De Assuncao TM et al. Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes. Lab. Invest 95, 684–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ogawa M et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol 33, 853–861 (2015). [DOI] [PubMed] [Google Scholar]
- 222.Takayama K et al. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochem. Biophys. Res. Commun 474, 91–96 (2016). [DOI] [PubMed] [Google Scholar]
- 223.Johanson CE Benzodiazepine self-administration in rhesus monkeys: estazolam, flurazepam and lorazepam. Pharmacol. Biochem. Behav 26, 521–526 (1987). [DOI] [PubMed] [Google Scholar]
- 224.Alberts R et al. Genetic association analysis identifies variants associated with disease progression in primary sclerosing cholangitis. Gut 67, 1517–1524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


