Abstract
Background
Despite antiretroviral treatment (ART) being an efficacious treatment for HIV, essentially making it a chronic nonterminal illness, two related and frequent concerns for many people living with HIV/AIDS (PLWHA) continue to be HIV-related stigma and life stress. These two variables are frequently associated with depression, substance use, and poorer functional health. Studies to date have not fully examined the degree to which these constructs may be associated within one model, which could reveal a more nuanced understanding of how HIV-related stigma and life stress affect functional health in PLWHA.
Methods
The current study employed hybrid structural equation modeling to examine the interconnectedness and potential indirect relationships of HIV-related stigma and life stress to worse health through substance use and depression, controlling for ART adherence and age. Participants were 240 HIV-infected individuals who completed a biopsychosocial assessment battery upon screening for an RCT on treating depression in those infected with HIV.
Results
Both HIV-related stigma and stressful life events were directly related to depression, and depression was directly related to health. There were significant indirect effects from stigma and stress to health via depression. There were no significant effects involving substance use.
Conclusion
It is important to continue to develop ways to address stigma, stressful life events, and their effects on distress in those living with HIV. Expanding our knowledge of disease progression risk factors beyond ART adherence is important to be able to design adjuvant interventions, particularly because treatment means that people living with HIV have markedly improved life expectancy and that successful treatment means that HIV is not transmittable to others.
Keywords: HIV-related stigma, Stressful life events, HIV, Depression, Substance use
Introduction
With more potent antiretroviral therapy for HIV available, treatment means that people living with HIV/AIDS (PLWHA) have markedly improved life expectancies [1, 2]. Additionally, HIV/AIDS is now in the era of undetectable = untransmittable [3] in that successful treatment means that those living with HIV do not transmit the virus to HIV-negative sexual partners [4-7]. These previously unprecedented developments in HIV prevention and treatment have the potential to greatly mitigate the deleterious effects of HIV-related stigma. However, HIV-related stigma and associated variables, including life stressors, mental health, and substance use, continue to be salient in PLWHA [8]. Such associations are important to examine as they potentially have the power to greatly impact health outcomes among PLWHA. Turan et al.’s [8] conceptual model of HIV-related stigma posits that HIV-related stigma leads to poorer health outcomes via mechanisms such as mental health, psychological resources, interpersonal factors, engagement in care, and stress processes. In addition, and consistent with a trauma-informed model of HIV care, other life stressors (e.g., abuse, loss of financial resources) should also be considered predictors of such mechanisms and health outcomes alongside HIV-related stigma [9, 10].
HIV-related stigma has been associated with both poor physical and mental health [11-13]. Regarding physical health, disease-related stigma is thought to put individuals at risk for other illnesses and worse outcomes for their current illness [14]. For example, in a study of PLWHA, experiencing greater stigma related to their HIV status was associated with greater HIV-related symptoms and lower medication adherence [15]. Regarding psychological outcomes, prospective studies have shown that those who experienced stigma had greater symptoms of psychological distress [16]. For example, experiencing HIV-related stigma has been associated with depression [17-19], a consistent predictor of non-adherence [20]. HIV-related stigma has also been shown to be associated with substance use [13, 19, 21].
Significant life stressors have also been associated with poor physical and mental health in HIV. Once exposed to an adverse life event, an individual is at risk for being exposed to future stressors, creating a life-course of chronic stress that takes a long-range toll on physical health via sustained activation of the stress response which is pathogenic in nature [22, 23]. In the context of HIV, the immune system is compromised and, consequently, stress exacerbates the immunosuppression response leading to poorer health outcomes among PLWHA. Indeed, work with PLWHA has found substantial evidence that stress negatively affects HIV disease progression [24-26]. For example, in a recent 4-year longitudinal study, norepinephrine, a neurohormone related to stress, was associated with greater rate of increase in viral load and decrease in CD4 [27]. Stress also plays an integral role in the pathogenesis of psychological distress, and, in particular, depression [28]. Among a sample of PLWHA, individuals with depression were 3.5 times more likely to have reported experiencing stress [29]. Life stress has also been shown to be a risk factor for drug use [30, 31], including in PLWHA [32].
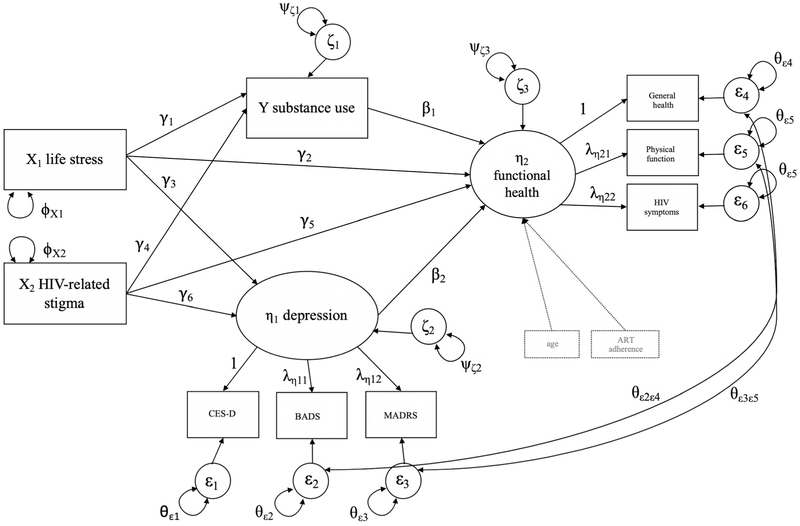
Taken together, research to date has consistently identified pathways of both HIV-related stigma and life stress to important disease-related variables such as substance use, depression, and health outcomes in PLWHA. The associations to depression and substance abuse are also important in and of themselves because both depression [24, 33, 34] and substance use [35-37] have been associated with worse HIV outcomes over and above the effects of antiretroviral use or adherence. Studies to date have not fully examined the degree to which these constructs may be associated within one model, which may reveal a more nuanced understanding of how HIV-related stigma and life stress affect functional health in PLWHA. Further, contextualizing Turan et al.’s model of HIV-related stigma [8] within a trauma-informed HIV care framework may have clinically beneficial implications. For example, scholars have recently posited the need to integrate chronic disease coping and HIV-related stigma management into trauma treatment for PLWHA in order to promote better ART adherence and disease outcomes [38]. Thus, the aim of the current study is to use path analytic procedures to explore the extent that HIV-related stigma and life stress may affect the functional health of PLWHA indirectly through substance use and depression, two potential mechanisms supported by the literature in PLWHA in care, controlling for age and ART adherence. In the current study, depression and substance use are modeled as parallel mechanisms given that prospective studies show reciprocal patterns between the two [39] and the path model accounts for this relationship with the error covariance. Overall, confirming the posited model (see Fig. 1) would have important clinical implications given the continued pervasiveness of stigma and life stress among PLWHA even in the era of such potent HIV treatments. Additionally, confirming the posited model would support preliminary evidence for further investigation of important biological pathways in addition to behavioral ones (e.g., ART adherence).
Fig. 1.
Proposed and initially tested model examining how life stressors and HIV-related stigma affect the health of HIV-infected individuals via substance use and depression
Method
Participants and Procedures
The current study used baseline data from a randomized control trial of cognitive behavioral therapy for adherence and depression in HIV in the USA [40]. All study procedures were approved by the Institutional Review Boards at Massachusetts General Hospital (Boston, MA), Fenway Health (Boston, MA), and the Miriam Hospital (Providence, RI) [41]. Participants were 240 HIV-infected individuals who were 18 years or older, prescribed antiretroviral treatment (ART), and met full inclusion criteria for study participation (e.g., depression). Participants needed to have either a current diagnosis of depression or carry a diagnosis of depression in that they were prescribed an anti-depressant medication for depression and have at least some residual clinically significant depressive symptoms; in other words, participants could be eligible with a wide range of symptomology severity. Recruitment took place from 2009 to 2012 in New England in HIV clinics and also through advertisements in the community. Once screened eligible for the study, participants completed a baseline biopsychosocial assessment battery that included both self-reported and clinician-administered assessments.
Measures
Demographics
Age, sex, race, and ethnicity were collected via self-report.
ART Adherence
ART adherence was a single self-reported item assessing the percentage (0% to 100%) an individual had taken their HIV medications as prescribed in the past 2 weeks with higher scores indicating greater adherence. Similar single-item scales for ART adherence have been shown to be valid and correlated with unannounced pill counts [41].
HIV-Related Stigma
A 5-item self-reported scale, used in previous work [15], assessed the frequency of experienced stigma since being diagnosed with HIV (e.g., “How often do people behave negatively toward you once they learned that you had HIV?”). Responses were rated on a 4-point Likert scale from (1) never to (4) often. A scale score was computed by averaging scores across items (range 1 to 4) with greater scores indicating greater experiences of HIV-related stigma (study α = .94).
Life Stressors
A 12-item self-reported measure, adapted from the Life Experiences Survey [42], assessed exposure to traumatic and stressful life events. Participants responded yes or no to having been exposed to various life stressors in the past 6 months (e.g., “You were physically attacked or sexually abused or assaulted or had your life threatened”; “Loss of your job [fired, laid off, quit, retired]”). Items endorsed were summed to create a total count score (range 0 to 12) with greater scores indicating greater exposure to chronic life stressors.
Substance Use
Participants self-reported on the frequency of past month illicit drug use including marijuana, crack, cocaine, heroin, other opiates, amphetamines, hallucinogens, sedatives, and other substances that were not listed. Marijuana use was excluded from the frequency measure as research has shown marijuana acts as an antiinflammatory and could potentially be beneficial to physical health among PLWHA [43]. We initially ran the analysis with marijuana included; when marijuana was omitted, the pattern of results in terms of statistical significance remained the same. As a result, the non-marijuana model was retained given the empirical evidence of potentially positive effects for physical health. Responses were on a 5-point Likert scale ranging from (0) no use to (4) about every day. The maximum value across substances was retained as a participant’s substance use frequency score (range 0 to 4) with greater scores indicating more frequent substance use.
Depression
Depression was a latent variable made up of three indicators that measure different symptom domains:
Center for Epidemiologic Studies Depression Scale (CES-D) [44]. The CES-D is a self-reported 20-item validated measure of the frequency of depressive symptoms for the past week (e.g., “I thought my life had been a failure”). Responses were rated on a 4-point Likert scale from (0) rarely or none of the time (less than 1 day) to (3) most or all of the time (5–7 days). A total summed score was computed with greater scores indicating greater depressive symptoms (range 0 to 60). A score of 16 suggests a person is at risk for clinical depression (study α = .85).
Behavioral Activation for Depression Scale (BADS) [45]. The BADS is a 25-item self-reported validated measure of the effects of depressive symptoms on behavior for the past week (e.g., “I stayed in bed for too long even though I had things to do”; “I did things to cut myself off from other people”). Responses were on a 7-point Likert scale from (0) not at all to (6) completely. A total summed score was computed with lower scores indicating greater depressive behavior symptoms (range 0 to 150, study α = .87). For the latent depression model, the BADS was reversed scored to have the same directional relationship with depressive symptoms as the other indicators (i.e., higher scores = greater symptoms).
Montgomery-Asberg Depression Rating Scale (MADRS) [46]. The MADRS is 10-item clinician-administered validated interview assessing symptoms of depression (e.g., sadness, concentration difficulties, and suicidal thoughts). Responses were on a 6-point severity scale; a total summed score was computed with greater scores indicating greater depressive symptoms (range 0 to 60). A score of 7 or more suggests a person has some level of depression (study α = .72).
Functional Health
Functional health was a latent variable made up of three self-reported indicators, two of which come from the AIDS Clinical Trials Group Quality of Life Health Survey - Short Form (ACTG QOL SF-21), and the other is a measure of HIV symptoms.
ACTG QOL: general health subscale. Lower scores on the general health scale indicate an individual perceives their health as poor and higher scores indicate a perception of better health with the highest score representing a perception of excellent health.
ACTG QOL: physical functioning subscale. Lower scores on the physical functioning scale indicate an individual is limited in performing physical activities due to poor health and higher scores indicate fewer limitations with the highest score representing full functioning with no limitations.
HIV Symptoms. The HIV Symptoms Distress Module [47] is a validated 20-item measure assessing phenomena experienced by those living with HIV (e.g., fevers, dizziness, tingling/hand/foot pain, memory loss), as well as its perceived impact and severity. Responses are on 5-point Likert scale ranging from (0) I do not have this symptom to (4) I have this symptom and it bothers me a lot. For the purposes of the health latent variable, item responses were dichotomized into (0) did not report symptom and (1) reported symptom. A total score was calculated by summing reported symptoms to create a total symptom count (range 0 to 20) with higher scores indicating greater HIV symptomology. For the latent health model, HIV symptom count was reversed scored to have the same directional relationship with health status as the other indicators (i.e., greater scores = less symptoms [better health]).
Data Analysis
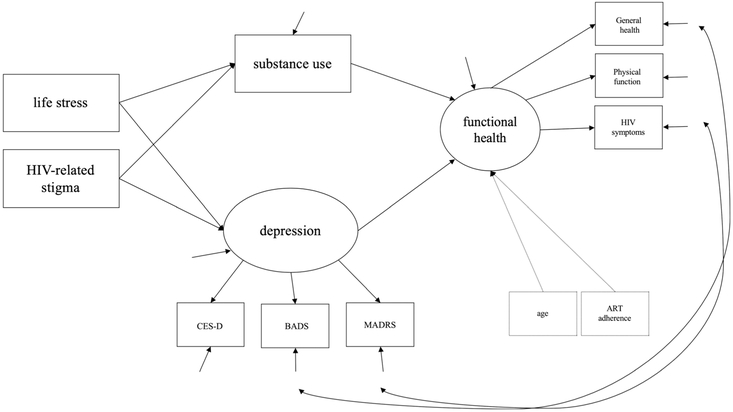
Analyses were conducted in Mplus version 7 [48]. Descriptive statistics were obtained for all variables included in the analyses, including the distribution of scale scores, with appropriate tests for normality which indicated all variables approximated a normal distribution. Hybrid structural equation modeling was used. First, a measurement model was tested using confirmatory factor analysis (CFA) for the two latent variables in the model, depression and functional health. Once fitted, a multivariate structural model was specified and tested using maximum likelihood estimation to examine posited direct and indirect effects. Direct effects included life stressors to functional health, life stressors to substance use, life stressors to depression, HIV-related stigma to functional health, HIV-related stigma to substance use, and HIV-related stigma to depression. Indirect effects included life stressors to functional health via substance use and depression and HIV-related stigma to functional health via substance use and depression. Given that age and ART adherence both can affect functional health, they were included as covariates in the analysis. The proposed and initially tested model is graphically presented in Fig. 1 and the final model is presented graphically in Fig. 2. The chi-square test of f model fit (p > .05), root mean squared error of approximation (RMSEA; < .06), and comparative fit index (CFI; > .95) were used as model fit indices.
Fig. 2.
Final hybrid structural equation model tested examining how life stressors and HIV-related stigma affect the health of HIV-infected individuals via substance use and depression
Results
Participant Characteristics
Table 1 presents the study sample characteristics. The majority of the sample was male (69%), white (61%), middle-aged (M = 47, SD = 8), and, on average, was 85% adherent to ART.
Table 1.
Participant characteristics and factor loadings for latent variables (N = 240)
| M (SD) or n (%) | ||
|---|---|---|
| Age | 47.4 (8.4) | |
| Male | 165 (68.8%) | |
| Race | ||
| African American or Black | 60 (25.0%) | |
| White | 147 (61.3%) | |
| Native American | 1 (0.4%) | |
| Not listed | 17 (7.1%) | |
| Multi | 15 (6.3%) | |
| Hispanic/Latino | 26 (10.8%) | |
| Sexual minority | 155 (64.6%) | |
| ART adherence (0–100%) | 85.5 (2.1) | |
| Life stressorsa | 2.4 (1.9) | |
| HIV-related stigmab | 2.3 (0.9) | |
| Substance use | ||
| No use | 103 (42.9%) | |
| 1 to 2 times a month | 43 (17.9%) | |
| About once a week | 22 (9.2%) | |
| Several times a week | 25 (10.4%) | |
| About every day | 47 (19.6%) | |
| Standardized factor loadings | ||
| Depression | ||
| CES-Dc | 30.3 (10.0) | .85 |
| BADS (reversed scored)d | 79.6 (22.7) | .61 |
| MADRSc | 25.0 (8.2) | .77 |
| Functional health | ||
| General healthe | 42.6 (20.5) | .87 |
| Physical functioninge | 59.8 (26.5) | .50 |
| HIV symptoms (reversed scored)f | 9.2 (3.6) | .30 |
Range 0–11, higher scores = more stressful events;
Range 1–4, higher scores = more stigma;
Range 0–60, higher scores = greater depressive symptoms;
Range 0–150, higher scores = greater depressive symptoms;
Range 0–100, higher scores = better health/physical functioning;
Range 0–20, higher scores = less symptoms
Measurement Model
The depression and functional health latent factors were specified in a measurement model. Given that how an individual feels physically affects activity level, the error covariance between the general health indicator and the BADS was specified a priori. Results indicated poor model fit, χ2(7) = 27.98, p = .0002; CFI = .94; RMSEA = .11. A covariance between residuals of the depression indicator, MADRS, and the functional health indicator, HIV symptoms, was added as suggested by the data and supported by the conceptual rationale that the MADRS and HIV symptoms assess similar physiological processes. This respecification resulted in a model that fit the data, χ2(6) = 11.14, p = .08; CFI = .99; RMSEA = .06 and thus retained as the final measurement model. Standardized factor loadings for the measurement model are presented in Table 1, and the measurement model, as part of the overall model, is depicted graphically in Fig. 2.
Structural Model
First, the proposed structural model (see Fig. 1) was tested; results demonstrated model fit, χ2(31) = 40.30, p = .12; CFI = .98; RMSEA = .04. Parameter estimates were examined and revealed that the direct effects from life stressors (p = .58) and HIV-related stigma to functional health (p = .30) were not significant. Removing the two nonsignificant direct effects did not worsen the model fit, χ2Δ(2) = 1.56, p = .458, and the respecified model fit the data, χ2(33) = 41.86, p = .139; CFI = .98; RMSEA =.03.
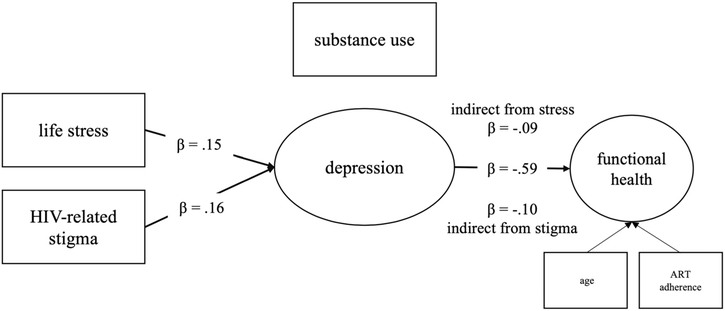
Figure 2 and Table 2 present the final model tested and results from the final model, respectively. Examining direct effects, there was a significant direct effect from life stressors to depression (b = .64, SE = .31, p = .038, 95% CI [.04, 1.24]) and from HIV-related stigma to depression (b = 1.52, SE = .66, p = .022, 95% CI [.22, 2.82]) such that increases in life stressors and HIV-related stigma were associated with increases in depression. Additionally, there was a significant direct effect from depression to functional health (b = − 1.17, SE = .18, p < .001, 95% CI [− 1.52, − .81]) such that increases in depression were associated with decreases in functional health. There were no significant direct effects from life stressors or HIV-related stigma to substance use or from substance use to functional health. Further, life stressors had a significant indirect effect to functional health via depression (b = − .74, SE = .37, p = .045, 95% CI [− 1.47, − .02]) such that increases in life stressors were associated with decreases in functional health through increased depression. HIV-related stigma also had a significant indirect effect to functional health via depression (b = − 1.77, SE = .80, p = .027, 95% CI [− 3.34, − .20]) such that increases in HIV-related stigma were associated with decreases in functional health through increased depression. Table 3 presents the variance/covariance matrix for the data and Fig. 3 depicts the significant direct and indirect pathways.
Table 2.
Final model results (N = 240)
| β | b | SE(b) | b/SE | p | |
|---|---|---|---|---|---|
| Functional health | |||||
| General health | .83 | 1.00 | – | – | – |
| Physical functioning | .51 | .82 | .15 | 5.51 | <.001 |
| HIV symptoms | .33 | .07 | .02 | 3.85 | <.001 |
| Depression | |||||
| CES-D | .86 | 1.00 | – | – | – |
| MADRS | .62 | .60 | .07 | 8.99 | <.001 |
| BADS | .76 | 2.02 | .19 | 10.44 | <.001 |
| Functional health on | |||||
| Depression | − .59 | − 1.17 | .18 | − 6.42 | <.001 |
| Substance use | − .05 | − .49 | .74 | − .67 | .502 |
| Age | − .15 | − .30 | .14 | − 2.17 | .030 |
| ART adherence | .16 | 1.23 | .54 | 2.27 | .023 |
| Depression on | |||||
| Stress | .15 | .64 | .31 | 2.08 | .038 |
| Stigma | .16 | 1.52 | .66 | 2.29 | .022 |
| Substance use on | |||||
| Stress | − .02 | − .01 | .05 | − .26 | .795 |
| Stigma | − .04 | −.07 | .11 | − .62 | .538 |
| Functional health on stress | |||||
| via depression | − .09 | − .74 | .37 | − 2.01 | .045 |
| via substance use | .00 | .01 | .03 | .24 | .808 |
| Total effect | − .09 | − 1.74 | .80 | − 2.16 | .030 |
| Functional health on stigma | |||||
| via depression | − .10 | − 1.77 | .80 | − 2.21 | .027 |
| via substance use | .00 | .03 | .08 | .45 | .650 |
| Total effect | − .09 | − .74 | .37 | − 1.98 | .047 |
| MADRS with HIV symptoms | − .27 | − 5.73 | 1.55 | − 3.69 | <.001 |
| BADS with general health | .26 | 43.77 | 20.54 | 2.12 | .033 |
χ2(33) = 41.86, p = .139; CFI = .98; RMSEA = .034
Table 3.
Variance/covariance matrix for data
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | General health | 419.88 | ||||||||||
| 2 | Physical functioning | 244.46 | 732.25 | |||||||||
| 3 | HIV symptoms | 19.11 | 21.45 | 12.55 | ||||||||
| 4 | MADRS | − 59.77 | − 60.53 | − 10.11 | 67.95 | |||||||
| 5 | CES-D | − 81.80 | − 47.38 | − 5.67 | 42.90 | 98.34 | ||||||
| 6 | BADS | − 130.04 | − 103.50 | − 16.62 | 85.66 | 146.48 | 502.54 | |||||
| 7 | Substance use | − 1.23 | − 2.48 | − .13 | − .13 | − .81 | 1.43 | 2.42 | ||||
| 8 | HIV-related stigma | − 2.16 | − 1.74 | − .32 | .49 | 1.67 | 2.56 | − .06 | .82 | |||
| 9 | Life stressors | − 4.38 | − 8.37 | − .38 | 2.34 | 3.04 | 3.65 | − .07 | .32 | 3.80 | ||
| 10 | ART adherence | 9.17 | 5.02 | 1.01 | − 1.29 | − 2.62 | − 5.66 | − .36 | − .14 | − .29 | 4.63 | |
| 11 | Age | − 13.01 | − 35.00 | − 2.01 | − 3.10 | − 8.27 | − 16.00 | 1.65 | − .63 | − 3.37 | − 2.77 | 70.52 |
Fig. 3.
Significant direct and indirect pathways from life stress and HIV-related stigma to functional health via depression
Discussion
Studies have consistently supported pathways of both HIV-related stigma and life stress to important disease-related variables such as substance use, depression, and health status in PLWHA, yet the degree to which these associations may be interconnected has not been explicitly articulated. The current study employed a hybrid structural equation model in order to investigate the interplay and potential mechanistic roles such risk factors play. More specifically, analyses examined whether HIV-related stigma and life stressors had indirect effects on functional health via depression and/or substance use. The current study adds to the literature by finding significant indirect effects of both HIV-related stigma and life stressors to functional health via an indirect pathway, through depression. Accordingly, increased levels of stigma and stressors were associated with increased depression and, in turn, increased depression was associated with the decreased latent functional health variable. Additionally, such pathways were supported even when controlling for ART adherence, the primary behavioral variable associated with viral control. In other words, beyond a behavioral path (i.e., via medication adherence), life stress and HIV-related stigma may also affect health through a physiological pathway. Notably, the current study found independent effects of life stress and HIV-related stigma. This finding supports the need to contextualize HIV-related stigma and health conceptual models within trauma-informed HIV care frameworks to consider both stigma and other traumatic life stressors as predictors of poorer health outcomes among PLWHA. Overall, findings suggest that to understand the potential associations of psychosocial variables to health outcomes among PLWHA, a more intricate risk factor framework may be important to consider, rather than siloed direct paths.
HIV-related stigma is an important factor to consider for improving both quality of life and health among PWLHA above and beyond the physiological effects of ART adherence. Indeed, reducing HIV-related stigma and discrimination has been set as an international priority to reach the target goal of 90-90-90 [49] and scholars have called for attention to the long-term negative impacts such stigma can cause [50]. Given that maladaptive emotion regulation and coping strategies have been associated with endorsing stigma [16,51], it would be beneficial to consider targeting such dysfunction to mitigate both the proximal effect of depression and the distal effect of poor health among PWLHA. Further, identifying resiliency factors to promote among PLWHA would be advantageous for intervention design. For example, some work suggests that community support and attachment may buffer the effects of stigma among PWLHA [52, 53]. Although intrapersonal interventions to attenuate the effects of HIV-related stigma among PWLHA are necessary to immediately begin to improve health, the root of the issue does not lie with PWLHA, but rather the stigma itself. Importantly, in addition to the intrapersonal level, it is necessary to consider the other levels of HIV-related stigma (i.e., structural, organizational, community, interpersonal) and design multifaceted interventions. For example, implementing anti-discrimination education and policies, changing community-level attitudes, and implementing strategies to promote interaction with PLWHA have been suggested as methods to decrease HIV-related stigma [54]. Strategies have been proposed for intervening on each level of HIV-related stigma that would be useful to consider in future design and implementation work [55]. Of note, having intersectional stigmatized identities (e.g., HIV-infected, substance user, sexual minority, racial minority, gender minority, sex worker) has been shown to affect physical and mental health among PLWHA [56, 57]. Future research should consider the various potential sources of stigma and explore how they interact to predict poor health outcomes.
Although changing exposure to life stressors is an unlikely treatment target, intervening on biological stress processes and coping strategies is important to consider for better health outcomes among PWLHA. Current findings support a psychoneuroimmunologic model for PLWHA [58]. The model posits that when psychological distress is improved, neuroendocrine functioning is also improved. Such improvement is associated with partial normalization of immune system functions which may regulate HIV replication and/or opportunistic infection risk and lead to minimized viral load and expression of physical clinical symptoms. Multiple clinical psychoneuroimmunology-based intervention methods have shown decreased inflammatory processes that affect health outcomes among PLWHA [59]. For example, in landmark clinical trials examining a cognitive-behavioral stress management (CBSM) intervention among gay and bisexual men, CBSM led to a reduction in the biological stress response and depression which in turn led to an increase in immune function as indicated by increased lymphocytes (e.g., CD4) and decreased viral load among men with a detectable viral load at baseline [60, 61]. In addition to intervening on the stress response, targeting how an individual copes with stressors is an important complement to alleviate the downstream effects of depression and poor health outcomes [62-66]. Future coping work should incorporate biological markers to examine physiological changes parallel to psychological changes. Given that PLWHA pervasively experience life stressors [67, 68], designing and implementing interventions aimed at decreasing pathogenic chronically activated stress responses are crucial in improving the health of PLWHA.
Given the role of both HIV-related stigma and experienced life stressors (i.e., forms of distressing and/or traumatic experiences) in health outcomes for PLWHA, trauma-informed HIV care, to deal with significant life stressors, might be a clinically beneficial focus. If depression does indeed mediate the relationship between traumatic stressors (discrimination, stigma, life events) and health outcomes, then intervening on depression may potentially reduce trauma-specific distress and interrupt the sequelae of trauma to improve HIV treatment outcomes. Indeed, there has been a call for trauma-informed care for PLWHA [9, 10, 69] and extant literature demonstrates the behavioral benefits (e.g., medication adherence) of targeting depression and/or trauma among PLWHA [70-72], but there may be potential physiological benefits as well.
The current analysis did not find any significant direct or indirect paths with substance use. It is possible that it was more difficult to detect effects in the model given that depression was a latent variable and may have overpowered the observed substance use indicator. Recent work has found a mediated relationship between HIV-related discrimination and alcohol use via internalized HIV stigma and stress [73], congruent with the current study’s conceptual model. Future research should not discount the role of substance use given the consistent evidence it is linked to faster disease progression, development of an AIDS-defining illness, and HIV/AIDS-related mortality in PLWHA [35-37].
Although the current study was able to examine multiple established risk factors and their relationships in one model, limitations should be noted. Neither a temporal order nor causality can be determined given the use of cross-sectional data. Moreover, although the path model was based on empirical literature, it is possible an alternative model may also explain the interplay of discussed risk factors for poor health among PLWHA (e.g., bidirectional models where depression and substance use have reciprocal pathways or where health also leads to depression). Given that the entire sample met criteria for participation in a depression-related study, caution should be taken in generalizing to non-depressed samples and may have limited power in the analysis, although there was significant variance in the depression measures (BADS range = 127, IQR = 29; CES-D range = 46, IQR = 15; MADRS range = 43, IQR = 15).
Due to the nature of the secondary analysis, measures were not designed specifically for the study and may not fully reflect the constructs examined. The functional health latent variable was made up of self-reported health and no objective measure of health; thus, findings should be contextualized within this limitation and further research would benefit from examining constructs in relationship to an objective health measure. The HIV-related stigma measure reflected experienced stigma, but other forms of stigma are associated with health outcomes (e.g., internalized, perceived community) and the life stress measure assessed quantity and not quality of stressors; expanded measurement of both constructs should be considered in further research. The depression latent may have had more power to detect effects compared with the substance use indicator and should be considered in interpretation. Additionally, medication adherence was a one-item self-reported measure and may potentially suffer from greater measurement error compared with other measures due to this and caution should be used in interpretations of findings. Gender was collected in a binary form (male/female) and does not account for all gender identities, which is important to do. For example, transgender women have high prevalence of HIV [74] and may have differential effects in the current conceptual model given the intersection of multiple marginalized identities. Further, there was not a reliable measure of socioeconomic status to use as a covariate, which has associations with health-related outcomes among PLWHA [75]. The data were collected from 2009 to 2012 and findings may not reflect the current context for PLWHA, although stigma, stress, depression, and substance use remain as current issues affecting PLWHA [73]. Limitations notwithstanding, the current study was able to provide preliminary evidence to consider broader and more complex biopsychosocial health risk models for PLWHA. Future research should expand on current work.
Despite ART being a powerful treatment for HIV, the current study found that two related and frequent concerns for many PLWHA, HIV-related stigma and life stressors, were associated with functional health, via depression, even when controlling for ART adherence. Although depression and ART adherence have been established as clinical targets for improved health outcomes in PLWHA [76], it is important to continue expanding our knowledge of risk factors to be able to design highly efficacious, multifaceted, multilevel, adjuvant interventions. Current findings support three examples of such clinical targets: life stressors, HIV-related stigma, and depression. Though it may be appropriate to intervene on life stressors at the intrapersonal level, it is vital that HIV-related stigma is targeted at the broader social levels. Now that HIV is essentially a chronic illness that is not transmittable to others when virally suppressed and that treatment means that people living with HIV have markedly improved life expectancy, a focus on psychosocial factors related to general disease management and quality of life among PLWHA is important.
Acknowledgments
Funding Information The project described was supported by R01MH084757 (Safren) from the National Institute of Mental Health. Some of the author time was funded by 9K24DA040489 (Safren) from the National Institute on Drug Abuse.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or any of the other funders.
References
- 1.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teeraananchai S, Kerr S, Amin J, Ruxrungtham K, Law M. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–66. [DOI] [PubMed] [Google Scholar]
- 3.Goodenow MM. Director’s update: why is U=U a game changer?: Office of AIDS Research National Institutes of Health; 2018. [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–47. 10.1016/s2352-3018(18)30132-2. [DOI] [PubMed] [Google Scholar]
- 6.Rodger A, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in MSM couples with suppressive ART: the PARTNER2 study extended results in gay men. 22nd International AIDS Conference; July 23-27; Amsterdam, Netherlands; 2018. [Google Scholar]
- 7.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 8.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. Am J Public Health. 2017;107(6):863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Resources and Services Administration (HRSA). Executive summary: trauma and HIV. Rockville, MD: HIV/AIDS Bureau Division of Policy and Data Consultation Overview; 2015. [Google Scholar]
- 10.Nightingale VR, Sher TG, Mattson M, Thilges S, Hansen NBJA. Behavior. The effects of traumatic stressors and HIV-related trauma symptoms on health and health related quality of life. AIDS Behav. 2011;15(8):1870–8. 10.1007/s10461-011-9980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logie C, Gadalla T. Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care. 2009;21(6):742–53. [DOI] [PubMed] [Google Scholar]
- 12.Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7):e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, Holtgrave DR. The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless and unstably housed persons living with HIV. AIDS Behav. 2009;13(6):1222–32. [DOI] [PubMed] [Google Scholar]
- 14.Link BG, Phelan JC. Stigma and its public health implications. Lancet. 2006;367(9509):528–9. [DOI] [PubMed] [Google Scholar]
- 15.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How does stigma “get under the skin”? The mediating role of emotion regulation. Psychol Sci. 2009;20(10):1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charles B, Jeyaseelan L, Pandian AK, Sam AE, Thenmozhi M, Jayaseelan V. Association between stigma, depression and quality of life of people living with HIV/AIDS (PLHA) in South India–a community based cross sectional study. BMC Public Health. 2012;12(1):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RS, Kochman A, Sikkema KJ. Internalized stigma among people living with HIV-AIDS. AIDS Behav. 2002;6(4):309–19. [Google Scholar]
- 19.Levi-Minzi MA, Surratt HL. HIV stigma among substance abusing people living with HIV/AIDS: implications for HIV treatment. AIDS Patient Care STDs. 2014;28(8):442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelman EJ, Cole CA, Richardson W, Boshnack N, Jenkins H, Rosenthal MS. Stigma, substance use and sexual risk behaviors among HIV-infected men who have sex with men: a qualitative study. Prev Med Rep. 2016;3:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell AL, Tasker JG, Lucion AB, Fiedler J, Munhoz CD, Wu TYJ, et al. Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. J Neuroendocrinol. 2018;30: e12641 10.1111/jne.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46(2):205–19. 10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- 24.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70(5):539–45. [DOI] [PubMed] [Google Scholar]
- 25.Mugavero MJ, Raper JL, Reif S, Whetten K, Leserman J, Thielman NM, et al. Overload: the impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multi-site HIV cohort study. Psychosom Med. 2009;71(9):920–6. 10.1097/PSY.0b013e3181bfe8d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kołodziej J Effects of stress on HIV infection progression. HIV AIDS Rev. 2016;15(1):13–6. 10.1016/j.hivar.2015.07.003. [DOI] [Google Scholar]
- 27.Ironson G, O’Cleirigh C, Kumar M, Kaplan L, Balbin E, Kelsch CB, et al. Psychosocial and neurohormonal predictors of HIV disease progression (CD4 cells and viral load): a 4 year prospective study. J AIDS Behav. 2015;19(8):1388–97. 10.1007/s10461-014-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–7. [DOI] [PubMed] [Google Scholar]
- 29.Slot M, Sodemann M, Gabel C, Holmskov J, Laursen T, Rodkjaer L. Factors associated with risk of depression and relevant predictors of screening for depression in clinical practice: a cross-sectional study among HIV-infected individuals in Denmark. HIV Med. 2015;16(7):393–402. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141(1):105–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reif S, Mugavero M, Raper J, Thielman N, Leserman J, Whetten K, et al. Highly stressed: stressful and traumatic experiences among individuals with HIV/AIDS in the Deep South. AIDS Care. 2011;23(2):152–62. 10.1080/09540121.2010.498872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–71. [DOI] [PubMed] [Google Scholar]
- 34.Ironson G, Fitch C, Stuetzle R. Depression and survival in a 17-year longitudinal study of people with HIV: moderating effects of race and education. Psychosom Med. 2017;79(7):749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multi-center cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–9. [DOI] [PubMed] [Google Scholar]
- 37.Carrico AW. Substance use and HIV disease progression in the HAART era: implications for the primary prevention of HIV. Life Sci. 2011;88(21–22):940–7. [DOI] [PubMed] [Google Scholar]
- 38.López CM, Hahn CK, Gilmore AK, Danielson CK. Tailoring cognitive behavioral therapy for trauma-exposed persons living with HIV. Cogn Behav Pract. 2019. 10.1016/j.cbpra.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry. 2004;56(10):730–7. 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 40.Safren SA, Bedoya CA, O’Cleirigh C, et al. Treating depression and adherence (CBT-AD) in patients with HIV in care: A three-arm randomized controlled trial. Lancet. 2016;3(11):e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, et al. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care. 2009;8(6):367–74. 10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psychol. 1978;46(5):932–46. [DOI] [PubMed] [Google Scholar]
- 43.Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, et al. Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy–treated human immunodeficiency virus–infected individuals. Clin Infect Dis. 2018;66(12):1872–82. 10.1093/cid/cix1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 45.Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The behavioral activation for depression scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess. 2007;29(3):191–202. [Google Scholar]
- 46.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–9. [DOI] [PubMed] [Google Scholar]
- 47.Justice A, Holmes W, Gifford A, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001. ;54(12):S77–90. [DOI] [PubMed] [Google Scholar]
- 48.Muthén L, Muthén B. Mplus Version 7. Los Angeles, CA; 1998. [Google Scholar]
- 49.UNAIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 50.Kalichman SC. The harms of internalized AIDS stigma: acomment on Tsai et al. Ann Behav Med. 2013;46(3):256–7. [DOI] [PubMed] [Google Scholar]
- 51.Varni SE, Miller CT, McCuin T, Solomon S. Disengagement and engagement coping with HIV/AIDS stigma and psychological well-being of people with HIV/AIDS. J Soc Clin Psychol. 2012;31(2):123–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brener L, Callander D, Slavin S, de Wit J. Experiences of HIV stigma: the role of visible symptoms, HIV centrality and community attachment for people living with HIV. AIDS Care. 2013;25(9):1166–73. [DOI] [PubMed] [Google Scholar]
- 53.Earnshaw VA, Lang SM, Lippitt M, Jin H, Chaudoir SR. HIV stigma and physical health symptoms: do social support, adaptive coping, and/or identity centrality act as resilience resources? AIDS Behav. 2015;19(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? JIAS. 2013;16(3S2):18734 10.7448/IAS.163.18734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahajan AP, Sayles JN, Patel VA, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Logie CH, James L, Tharao W, Loutfy MR. HIV, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS Med. 2011;8(11):e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Earnshaw VA, Kalichman SC. Stigma experienced by people living with HIV/AIDS In: Liamputtong P, editor. Stigma, discrimination and living with HIV/AIDS: a cross-cultural perspective. New York: Springer; 2013. p. 23–38. [Google Scholar]
- 58.Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173–88. [DOI] [PubMed] [Google Scholar]
- 59.Moraes LJ, Miranda MB, Loures LF, Mainieri AG, Mármora CHC. A systematic review of psychoneuroimmunology-based interventions AU - Moraes, Lucam. J Psychol Health Med. 2018;23(6): 635–52. 10.1080/13548506.2017.1417607. [DOI] [PubMed] [Google Scholar]
- 60.Antoni MH, Carrico AW, Durán RE, Spitzer S, Penedo F, Ironson G, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68(1):143–51. [DOI] [PubMed] [Google Scholar]
- 61.Antoni MH, Cruess DG, Klimas N, Maher K, Cruess S, Kumar M, et al. Stress management and immune system reconstitution in symptomatic HIV-infected gay men over time: effects on transitional naive T cells (CD4+CD45RA+CD29+). Am J Psychiatry. 2002;159(1):143–5. 10.1176/appi.ajp.159.1.143. [DOI] [PubMed] [Google Scholar]
- 62.Remien RH, Exner T, Kertzner RM, Ehrhardt AA, Rotheram-Borus MJ, Johnson MO, et al. Depressive symptomatology among HIV-positive women in the era of HAART: a stress and coping model. Am J Community Psychol. 2006;38(3–4):275–85. [DOI] [PubMed] [Google Scholar]
- 63.Carrico AW, Antoni MH, Weaver KE, Lechner SC, Schneiderman N. Cognitive—behavioural stress management with HIV-positive homosexual men: mechanisms of sustained reductions in depressive symptoms. Chronic Illn. 2005;1(3):207–15. [DOI] [PubMed] [Google Scholar]
- 64.Carrico AW, Antoni MH, Durán RE, Ironson G, Penedo F, Fletcher MA, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-positive gay men treated with HAART. Ann Behav Med. 2006;31(2):155–64. [DOI] [PubMed] [Google Scholar]
- 65.Rodkjaer LO, Laursen T, Seeberg K, Drouin M, Johansen H, Dyrehave C, et al. The effect of a mind–body intervention on mental health and coping self-efficacy in HIV-infected individuals: a feasibility study. J Altern Complement Med. 2017;23(5):326–30. [DOI] [PubMed] [Google Scholar]
- 66.O’Cleirigh C, Ironson G, Smits JAJ. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behav Ther. 2007;38(3):314–23. 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, et al. Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J Public Health. 2006;96(6):1028–30. 10.2105/ajph.2005.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22(9):1286–91. 10.1007/s11606-007-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sales JM, Swartzendruber A, Phillips AL. Trauma-informed HIV prevention and treatment. Curr HIV/AIDS Rep. 2016;13(6):374–82. 10.1007/s11904-016-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. 2014;47(3):259–69. 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dale SK, Safren SA. Striving towards empowerment and medication adherence (STEP-AD): a tailored cognitive behavioral treatment approach for black women living with HIV. Cogn Behav Pract. 2018;25(3):361–76. 10.1016/j.cbpra.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sikkema KJ, Mulawa MI, Robertson C, Watt MH, Ciya N, Stein DJ, et al. Improving AIDS care after trauma (ImpACT): pilot outcomes of a coping intervention among HIV-infected women with sexual trauma in South Africa. AIDS Behav. 2018;22(3):1039–52. 10.1007/s10461-017-2013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crockett KB, Kalichman SC, Kalichman MO, Cruess DG, Katner HP. Experiences of HIV-related discrimination and consequences for internalised stigma, depression and alcohol use. Psychol Health. 2019:1–15. 10.1080/08870446.2019.1572143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention [CDC]. HIV Among Transgender People. 2018. https://www.cdc.gov/hiv/group/gender/transgender/. Accessed on 3/22/19.
- 75.Burch LS, Smith CJ, Phillips AN, Johnson MA, Lampe FC. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. AIDS. 2016;30(8):1147–62. 10.1097/qad.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 76.Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression–a systematic review of interventions. Psychol Health Med. 2011;16(5):493–527. [DOI] [PubMed] [Google Scholar]