Abstract
Background
Electronic health records are central to cancer care delivery. Electronic clinical decision support (CDS) systems can potentially improve cancer care quality and safety. However, little is known regarding the use of CDS in clinical oncology and their impact on patient outcomes.
Methods
We performed a systematic review of peer-reviewed studies evaluating outcomes of electronic CDS systems for cancer diagnosis, treatment, and supportive care. Peer-reviewed studies published between 1995 and 2016 were included if they assessed clinical outcomes, patient reported outcomes (PROs), costs, or care delivery process measures.
Results
Electronic database searches yielded 2,439 potentially eligible papers; 24 studies were included after final review. Most studies used an uncontrolled, pre-post intervention design. Twenty-three of 24 studies reported improvement in key study outcomes with use of oncology CDS systems. Twelve studies assessed CDS for computerized chemotherapy order entry, demonstrating reductions in prescribing error rates, medication-related safety events, and workflow interruptions. The remaining studies examined oncology clinical pathways, guideline adherence, systems for collection and communication of PROs, and prescriber alerts.
Conclusions
There is a paucity of data evaluating clinically relevant outcomes of CDS system implementation in oncology care. Currently available data suggests that CDS can have a positive impact on the quality of cancer care delivery. However, there is a critical need to rigorously evaluate CDS systems to better understand how oncology CDS can be implemented to improve patient outcomes.
Keywords: Clinical Decision Support Systems, Critical Pathways, Delivery of Health Care, Medical Order Entry Systems, Neoplasms
Introduction
Clinical decision support (CDS) systems include any electronic system designed to directly aid clinical decision making by utilizing individual patient characteristics to generate patient-specific assessments or recommendations.1,2 These systems require computable biomedical knowledge, person-specific data, and a reasoning or inferencing mechanism that combines knowledge and data to generate and present information to clinicians as care is being delivered.3 Examples of CDS tools include computerized alerts and reminders, clinical guidelines, condition-specific order sets, focused patient data reports, documentation templates, diagnostic support, and contextually relevant reference information. Thus, CDS has the potential to drive evidence-based standardization of cancer care, improving care delivery and patient outcomes. CDS tools have been incorporated across the patient care spectrum, encompassing prevention, diagnosis, and clinical monitoring. Common roles for CDS include computerized physician order entry (CPOE), and electronic health record (EHR) clinical reminder systems.2
CDS improves health care process measures; however, data demonstrating their effectiveness on clinical outcomes and costs are limited.1 Accordingly, real-world uptake of CDS systems has been modest at best.4 Benefits of CDS include improved efficiency and quality of health care delivery and access to medical data; enhanced communication; and potential cost savings.5–14 In 2007, the American Medical Informatics Association sounded a call to action regarding CDS implementation that included three pillars for fully realizing the promise of CDS: 1) Best knowledge available when needed; 2) High adoption and effective use; and 3) Continuous improvement of knowledge and CDS methods.4 Further, the Agency for Healthcare Research and Quality stated the question is not whether CDS systems should be designed and implemented but rather, how to make it easy to do the right thing.15 Nevertheless, effective implementation of a CDS system is a major undertaking, considering the vast amount of clinical data and its variability, availability, and structure across sites.
Clinical oncology is a dynamic, multidimensional health care specialty with complex decision making and care coordination needs, as well as multiple handoffs between primary and specialty care providers.16 In 2013 The Institute of Medicine reported that the cancer care delivery system was in crisis due to a lack of patient-centric care, palliative care, and evidence-based decision-making.17 CDS systems have the potential to significantly improve cancer care delivery, but there are critical gaps in the availability and utilization of effective CDS tools.18 To better understand the current landscape of CDS systems in oncology practice, we conducted a systematic review of the literature describing real world implementation of CDS tools for the diagnosis, treatment, and supportive care of patients with cancer. The objective was to investigate the reported impact of CDS systems on clinically-relevant patient outcomes.
Methods
Study Rationale and Definition
We critically appraised and synthesized the published medical literature to answer the objective question “What evidence supports the use of CDS systems for diagnosis, treatment and supportive care in clinical oncology?” We defined a CDS system as any electronic system in which characteristics of individual patients are used to generate patient-specific assessments or recommendations that are then presented to clinicians to aid in clinical decision making.1
Inclusion and Exclusion Criteria
Inclusion/exclusion criteria were determined a priori. We performed a systematic review of peer-reviewed, English-language studies published between January 1, 1995 and December 31, 2016 that evaluated the implementation of an electronic CDS system in the field of cancer care. Included studies assessed one or more of the following: clinical outcomes (including patient reported outcomes [PROs]); costs; or care delivery process measures (eg, medication prescribing error rates, or compliance with clinical practice guidelines). Studies of decision aids intended primarily for patient use were excluded. Of the 120 studies identified prior to 1995, none met the pre-defined inclusion criteria and were not included in this analysis.
Literature Search Strategy
In a manner consistent with the PRISMA statement, we conducted a comprehensive review of the clinical and scientific literature.19 A trained health sciences librarian (BOB) performed a comprehensive search of the literature to identify studies meeting the inclusion criteria. Databases searched were: PubMed (NLM), EMBASE (Ovid), Academic Search Premier (EBSCO), Web of Science (Thomson Reuters), and Inspec (Elsevier). Relevant narrative reviews, systematic reviews, and meta-analyses were also evaluated for background information, but were not included in this study.
In PubMed, the medical subject headings (MeSH) terms defined the concepts of cancer and CDS systems, medical order entry systems, or clinical pathways. For optimal retrieval, all terms were supplemented with relevant title and text words. Full PubMed search parameters are available in the online appendix. Search strategies for EMBASE, Academic Search Premier, Web of Science, and Inspec were adjusted for the syntax appropriate for each database. Published reports in the peer-reviewed literature were identified and all abstracts were reviewed for eligibility. Full-text articles were retrieved and reviewed when additional information was needed. Bibliographies from selected key articles, relevant review articles, and related meta-analyses were reviewed to identify additional publications.
Screening and Full-Text Data Extraction
The study team made every effort to identify all publications meeting the inclusion criteria. Two investigators reviewed each title and abstract for potential inclusion. Titles and abstracts were excluded upon mutual agreement; those not mutually determined to be included or excluded were adjudicated by the third investigator. Each publication that was not excluded after review of the title and abstract was then subjected to a second round of full-text review by 2 members of the study team in the same manner as for titles and abstracts, again with adjudication of discordance by the third investigator.
Data from included studies were abstracted from full-text publications using a data abstraction form that included the study objective and design, intervention, number of subjects, study results and conclusions, location of study by country, number of study sites (single- vs. multi-site), setting (inpatient vs. outpatient), patient diagnosis, subject age range, CDS tool type (stand-alone, EHR-embedded) and name, study or system features, funding source, and conflict of interest declaration.
Results
Electronic database searches yielded 2439 potentially eligible papers (Figure 1). Following review and screening of titles and abstracts, 83 papers were identified for full-text review, and 24 studies were included in the final analysis.
Figure 1.
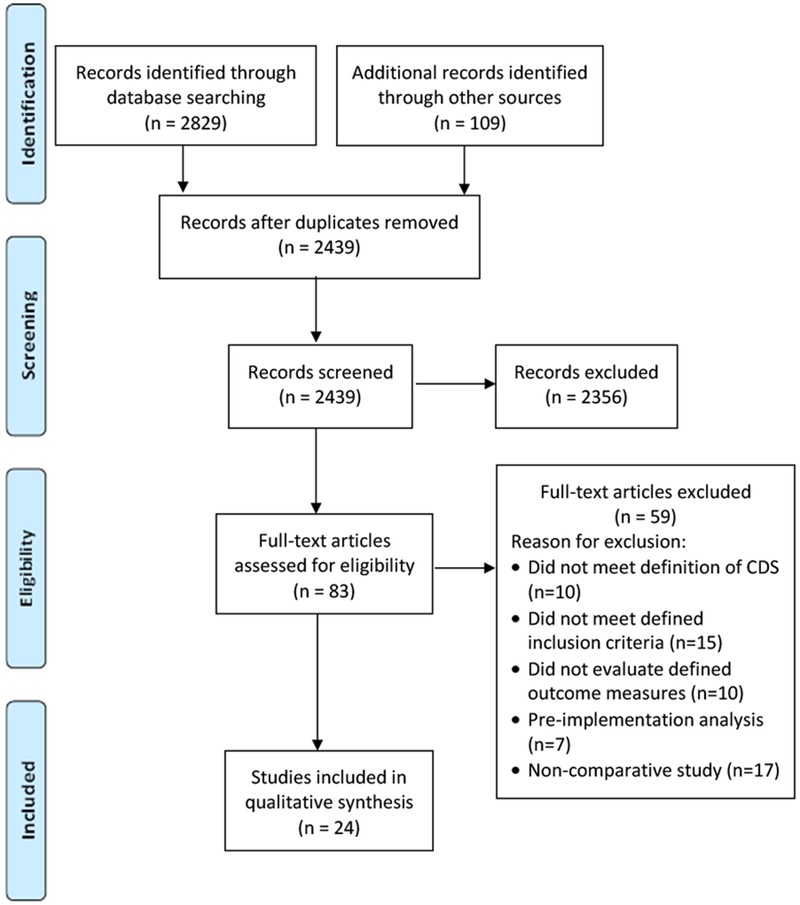
Consort diagram of studies demonstrating evidence supporting the use of electronic clinical decision support systems in cancer care.
Table 1 describes the characteristics of the included studies. Most were conducted in adults (96%) in the United States (US, 38%) or Europe (38%), across any cancer diagnosis (71%), and evaluated a variety of outcomes including patients, clinicians, and clinician hours. The most common study outcomes were chemotherapy orders (n=76619) or chemotherapy order sets (n=9838). Most studies were conducted at a single site (79%), 87% used quasi-experimental designs, and only 3 (13%) studies were randomized controlled trials. Table 2 describes individual study sample sizes, study design, site number, and diagnoses whereas Table 3 summarizes the objectives and outcomes of each included study.
Table 1.
Summary statistics of studies assessing clinical decision support for oncology clinical care.
| Characteristic | N | % |
|---|---|---|
| Full text articles included in analysis | 24 | 100 |
| Study population | ||
| Adult | 23 | 96 |
| Pediatric | 1 | 4 |
| Cancer Diagnosis | ||
| Any cancer diagnosis | 17 | 71 |
| Multiple cancer diagnoses included | 1 | 4 |
| Breast | 4 | 17 |
| Prostate | 1 | 4 |
| Renal Cell | 1 | 4 |
| Outcome Samples Studied | ||
| Patients | 5730 | - |
| Clinicians | 276 | - |
| Clinician Hours | 228 | - |
| Decisions | 521 | - |
| Chemotherapy Orders | 76619 | - |
| Chemotherapy Order Sets | 9838 | - |
| Medication related events | 212 | - |
| Radiation Therapy Courses | 7904 | - |
| Study Design | ||
| Randomized Controlled Trial | 3 | 13 |
| Non-randomized | 21 | 87 |
| Pre-post | 10 | 48 |
| Cross-sectional | 7 | 33 |
| Other | 4 | 19 |
| Site Number | ||
| Single | 19 | 79 |
| Multi-site | 5 | 21 |
| Site Type | ||
| Inpatient | 7 | 29 |
| Outpatient | 5 | 21 |
| Not Specified | 12 | 50 |
| CDS System Type | ||
| CPOE | 12 | 50 |
| Clinical Pathway | 6 | 25 |
| CPG | 2 | 8 |
| PRO | 3 | 13 |
| Prescriber Alert | 1 | 4 |
| CDS Tool Type | ||
| Integrated into EHR | 13 | 54 |
| Integrated into Other System | 1 | 4 |
| Internet-based | 2 | 8 |
| Standalone | 8 | 33 |
| Country | ||
| United States | 9 | 38 |
| Europe | 9 | 38 |
| Canada | 1 | 4 |
| Korea | 1 | 4 |
| Pakistan | 1 | 4 |
| Study Funding Source | ||
| Not specified | 12 | 50 |
| NIH | 2 | 8 |
| Other National Program | 4 | 16 |
| Foundation | 2 | 8 |
| Industry | 1 | 4 |
| Local/Site | 3 | 13 |
| Taiwan | 3 | 13 |
| Conflict of Interest Disclosure | ||
| Not Reported | 10 | 42 |
| None | 11 | 46 |
| Affiliated with For-profit Entity | 3 | 13 |
Table 2.
Study characteristics for the systematic review of clinical decision support systems used in oncology practice.
| Author (year) | Sample Size | Study Design51 | Number of Sites | Cancer Diagnosis |
|---|---|---|---|---|
| Aziz MT, 201520 | 9,279 chemotherapy orders | Pre-post, no control group | Single | Any |
| Basch, E, 201642 | 766 patients | Randomized controlled trial | Single | Multiple |
| Beer J, 200221 | 836 chemotherapy orders | Non-randomized | Multi- | Any |
| Beriwal S, 201232 | 7,904 radiation therapy courses | Pre-post, no control group | Multi- | Multiple |
| Berry D, 201140 | 765 patients, 262 clinicians | Randomized controlled trial | Multi- | Any |
| Bertsche, T, 200933 | 100 patients | Pre-post, uncontrolled | Single | Any |
| Bouaud J, 200138 | 127 decisions | Cross-sectional with internal control | Single | Breast |
| Bouaud J, 201539 | 394 decisions | Cross-sectional with internal control | Multi- | Breast |
| Chang PL, 200234 | 124 patients | Pre-post, no control group | Single | Renal cell |
| Chen AR, 201122 | 212 medication related events | Pre-post, no control group | Single | Any pediatric |
| Cho E, 201323 | 54,561 chemotherapy orders | Cross-sectional with non-equivalent control | Single | Any |
| Collins CM, 201124 | 538 chemotherapy orders | Pre-post, no control group | Single | Any |
| Elsaid K, 201325 | 1,192 chemotherapy orders | Interrupted time series, no control group | Single | Any |
| Hanauer DA, 201326 | 228 clinician hours | Pre-post, no control group | Single | Any |
| Hsu YC, 200835 | 44 patients | Pre-post, no control group | Single | Prostate |
| Hsu PI, 201543 | 2,512 patients | Three stage study (pre, post 1, post 2), no control group | Single | Any |
| Huertas Fernandez MJ, 200627 | 60 chemotherapy orders | Cross-sectional with non-equivalent control | Single | Any |
| Mattsson TO, 201528 | 5,767 chemotherapy orders | Cross-sectional with non-equivalent control | Multi- | Any |
| Meisenberg BR, 201429 | 9,838 chemotherapy order sets | Quality improvement, no control group | Single | Any |
| Patkar V, 201236 | 1,295 patient cases | Cross-sectional with internal control | Single | Breast |
| Ruland CM, 200341 | 14 clinicians/56 patients | Randomized controlled trial | Single | Any |
| Small MD, 200830 | 1,941 chemotherapy orders | Cross-sectional with non-equivalent control | Single | Any |
| Van Erps J, 201037 | 68 patients | Pre-post, no control group | Single | Any |
| Voeffray M, 200631 | 2,445 chemotherapy orders | Pre-post, no control group | Single | Any |
CPOE=Computerized Physician Order Entry; CPG=Clinical Practice Guideline; NSC=National Science Council
Table 3.
Summary of significant findings for studies assessing clinical decision support for oncology clinical care.
| Author (year) | Objective(s) | CDS Tool Type | Study or System Features | Study Target | Key outcomes associated with CDS |
|---|---|---|---|---|---|
| Computerized Physician Order Entry (CPOE) | |||||
| Aziz MT (2015)20 | Compare error rates and severity with paper orders | Integrated in EHR | Oracle-based system with computational intelligence including passive and active alerts | Clinician | Chemotherapy error rate decreased (0.26% vs. 2.4%) |
| Beer J (2002)21 | Compare order review time with paper orders | Integrated in EHR | Electronic order system not described , pharmacist intervention data collected | Clinician | Pharmacist order review time increased 5.15 minutes, with similar intervention rates (7.47% vs 7.14%). |
| Chen AR (2011)22 | Assess medication error rates | Integrated in EHR | Design included features to enhance safety and add order set functionality | Clinician | Medication-related safety events decreased by 39%. |
| Cho E (2013)23 | Compare two CPOE systems for error rates and performance efficiency | Integrated in EHR | Study testing for efficiency outcomes was performed in a controlled space with computers setup for the study. | Clinician | Improved near misses (p < 0.0001); reduced regimen defects, drug omissions and incorrect data input errors more than 70% and efficiency measures (p < 0.0001). |
| Collins CM (2011)24 | Assess process and prescribing errors for oral chemotherapy | Integrated in EHR | Prescriber training was not required due to prior CPOE experience | Clinician | Reduced prescribing error risk 69% [OR =0.31 (95% CI 0.11–0.86)] (P = 0.023) |
| Elsaid K (2013)25 | Assess incidence and types of prescribing errors | Integrated in EHR | Prescriber, nurse and pharmacist education, reminders of form implementation communicated, adherence of form use measured, and a policy prohibiting handwritten orders instituted | Clinician | 30% reduction in prescribing errors |
| Hanauer DA (2013)26 | Quantify CPOE impact on workflow and patient care time | Integrated in EHR | Time spent on each activity and ordered sequence describing and quantify workflow, and patterns of use were measured | Clinician | Workflow fragmentation decreased; average continuous task time increased from 131.2 to 218.3 seconds (P<.01), with an 8-fold decrease in the number of paging interruptions. |
| Huertas Fernandez MJ (2006)27 | Assess errors compared with manual prescribing | Integrated in EHR | Computerized prescriptions validated online by pharmacist; for handwritten orders no pharmacist validation, nursing staff calculates dilutions for treatment preparation. | Clinician | Prescriptions containing at least one error: 100% (manual) vs 13% (CPOE), p < 0.001. Mean error rate = 5 (1-12) for manual and 0 (0-1) for CPOE, p < 0.001 |
| Mattsson TO (2015)28 | Assess incidence, type, severity and related risk factors of prescription dose errors | Integrated in EHR | CPOE system not linked to EHR, comparison was conducted across two institutions. | Clinician | Error rate = 1.60 errors per 100 prescriptions (CPOE) vs 1.84 (paper-based); (OR) = 0.87 [95% CI 0.59-1.29, P = 0.49]. 15 types of errors and 4 risk factors identified. |
| Meisenberg BR (2014)29 | Assess number and type of order errors among hand-written, preprinted and CPOE | Integrated in EHR | Quality improvement initiative, CPOE orders were included after a 2-month run-in time to allow for provider competency. | Clinician | Rate of order sets requiring significant rework reduced from 30.6% (handwritten) to 12.6% (preprinted), P<.001 to 2.2% with CPOE; P <.001). Errors capable of causing harm reduced 4.2% (handwritten) to 1.5% (preprinted), P <.001 to 0.1% with CPOE, P <.001. |
| Small MD (2008)30 | Assess error rates, types and patterns and potential for harm. Compare error rates with order spreadsheets | Integrated in EHR | Comparison of Excel spreadsheet prescriptions with CPOE. Oral chemotherapy not included in CPOE ordering system. | Clinician | CPOE reduced errors 42% (RR 0.58; 95% CI 0.47–0.72). |
| Voeffray M (2006)31 | Assess number of prescribing errors | Integrated in EHR | CPOE system was developed on software without professional programmer support. | Clinician | CPOE decreased error rate from 15% to 5% (95% CI 13%-18%). |
| Clinical Pathways | |||||
| Beriwal S (2012)32 | Assess efficacy of radiation oncology for bone metastases | Standalone | Online system enabled near real-time peer review of treatment choice | Clinician | Treatment with 1-5 fractions in academic vs community sites, 63% vs. 23%; p < 0.0001. Decrease mean number fractions p < 0.0001 |
| Bertsche, T (2009)33 | Asses guideline adherence with pain management tool | Integrated in other system | CDSS was integrated into the drug information system containing current information about formulary drugs | Clinician | Increased CPG concordance (p < 0.001), reduced pain in the intervention group. Physicians accept 85% recommendations |
| Chang PL (2002)34 | Comparison of web- and paper-based pathways | Standalone | Pathway program was on separate computer system in nursing station. | Patient | Similar variance rate, less undetected variances and variance detection time in web-based group, P=0.0193 and 0.0162 |
| Hsu YC (2008)35 | Assess effects of web-based pathway on length of stay and practice variations for radical prostatectomy | Internet-based | Pathway program on separate system in nursing station. | Clinician | Average hospital stay decreased from 11.7 to 9.9 days (P < 0.01). Mean number of practice variations also decreased. |
| Patkar V (2012)36 | Assess breast cancer treatment recommendations and clinical trial eligibility | Standalone | Active evaluation of patient data to offer guideline-based recommendations in real time, | Clinician | CPG concordance for tool recommendations 97% vs. 93% Clinical trial eligibility increased 61% |
| Van Erps J (2010)37 | Assess validity and CPG concordance for anemia management | Standalone | Patient data entered at the point of care with guidance provided to clinicians that cite applicable guidelines. | Clinician | More rapid rate of hemoglobin (Hb) increase (P<0.006) and higher Hb by visit 4 (P=.006) and more rapid rate of Hb increase in post cohort. High concurrent validity. |
| Clinical Practice Guidelines | |||||
| Bouaud J (2001)38 | Assess compliance with tool decisions and transferability to another system | Standalone | CDS system used at point of care for all patients and justification required where not employed and followed a standard procedure | Clinician | Physician compliance with CPGs increased (61.42% to 85.03%; P<.0001 and clinical trial accrual increased 50%. |
| Bouaud J (2015)39 | Evaluate physician attitudes toward CPG tool advice | Standalone | Evaluated compliance with CPGs and with the CDS system | Clinician | CDS systems and CPG compliance was 75.4% and 86.8%, respectively |
| Patient-Reported Outcomes (PROs) | |||||
| Basch E (2016)42 | Assess web-based PROs with clinician alerts for health-related quality of life (HRQL), survival, ER use, and hospitalization | Internet-based | Automated clinician alerts for severe/worsening symptoms, patient subgroup assignment for computer experience | Patient | Improved HRQL (34% v 18%, P< .001), fewer ER visits (34% v 41%, P=.02), and increased survival at 1 year (75% v 69%, P=.05) in intervention arm compared with usual care. Hospitalization rates were not significantly different. |
| Berry D (2011)40 | Determine tool effect on clinician and patient discussions and duration | Standalone | Electronically collected PROs were provided to clinicians in real time at patient visit | Clinician and Patient | Discussion of symptoms and quality of life issues increased (p = 0.032) with no difference in visit duration |
| Ruland CM (2003)41 | Compare patient-reported symptoms and preferences with those addressed at patient visit. Assess system ease of use, time required, and patient satisfaction. | Standalone | Tablet computers captured symptom reporting prior to consultation and was provided to the patient and clinician for consultation | Clinician and Patient | Symptoms were addressed in 51% intervention vs. 19% control groups. High ease of use, no difference in patient satisfaction between groups. |
| Prescriber Alerts | |||||
| Hsu PI (2015)43 | Evaluate screening and chemoprophylaxis rates for HBV | Integrated in EHR | Testing was recommended for patients who received testing outside of the system. Patients who received treatment outside the system were removed from screening queue. | Clinician | Hard stops improved screening and chemoprophylaxis and reduced severe acute Hepatitis B virus (HBV) exacerbations. Increased screening (99.3% vs. 40.2%, P < 0.001) and HBV prophylaxis rates (95.8% vs. 39.2%, P < 0.001) with therapeutic stage. Lower severe HBV acute exacerbations, 0% vs. 1.2% and 1.2%, respectively; P < 0.01 for both. |
CPOE=Computerized Physician Order Entry; QoL=Quality of Life; CPG=Clinical Practice Guidelines; CDS=Clinical Decision Support; SQLI= Symptoms and quality-of-life issues; HBV=Hepatitis B Virus
Of the 12 studies evaluating computerized physician order entry (CPOE), 20–31 the rate of prescription errors was the primary study outcome in 9 studies. Although errors were defined differently across studies, prescription errors were reduced in each of the 9 studies.20,23–25,27–31 One study evaluated medication-related safety events, demonstrating fewer events with use of CDS.22 Two studies evaluated pharmacy work flow, with one study showing decreased workflow fragmentation and increased continuous task time with CPOE,26 while another study showed increased order review time with CPOE.21
Six studies assessed CDS systems that could be classified as clinical pathways for care delivery processes.32–37 The primary outcome for each of these studies was the association of the clinical pathway CDS system with receipt of guideline-concordant or pathway-recommended care. In general the outcomes associated with use of clinical pathways systems were compared with usual care; however, one study compared the use of an electronic clinical pathway with a paper pathway.34 Most studies were framed as reporting favorable outcomes, including reduced acute care utilization,32,35 increased guideline concordance and reduced symptoms,33 improved identification of eligible subjects for clinical trial participation,36 and improved hemoglobin levels among patients with anemia.37 The comparison between web- and paper-based pathway systems did not find a significant difference in pathway deviations with electronic versus paper systems.34
The remaining 6 studies assessed CDS systems in clinical practice guidelines (2 studies),38,39 PROs (3 studies),40–42 and oncology-specific prescriber alerts (1 study).43 One study evaluated clinical practice guideline concordance with CDS and demonstrated a significant increase in guideline adherence (p<.0001) accompanied by treatment plan modification in 31% of cases, as well as a 50% increase in clinical trial accrual.38 Further, adherence to clinical practice guidelines with CDS system advice was greater than 90% when the CDS tool included clinical practice guideline recommendations.39 CDS tools for obtaining PROs and reporting the results to clinicians demonstrated an increased discussion of symptoms and quality of life issues (p =.03) and symptom monitoring during routine clinical care.40–42 Patients reported a high ease of use and minimal time required; however patient satisfaction was similar between intervention and control groups. One study evaluated prescriber alerts with CDS tools and demonstrated that hard stops for hepatitis B screening prior to chemotherapy treatment were associated with increased screening (99.3% vs. 40.2%, P<.001) and chemoprophylaxis rates (95.8% vs. 39.2%, P<.001) and a reduction in severe exacerbations of liver disease.43
Discussion
We conducted a systematic review to assess the evidence supporting the use of CDS systems in cancer care delivery. Our review included studies assessing cancer diagnosis, treatment, and supportive care. Importantly, among the studies included in this review, most findings are consistent with those of previous non-oncology focused studies in finding that CDS improves care process measures. However, one of the studies we evaluated did not demonstrate favorable findings and some of the findings were not of significant magnitude. These data demonstrate the potential value that CDS systems can bring to oncology clinical practices that have or are about to implement a CDS system.
The largest category of studies included in this review is comprised of studies evaluating the use of CPOE systems in oncology care. These studies demonstrated a positive impact on prescriber errors, safety events and workflow.20–31 CDS tools implementing clinical pathways,32–37 clinical practice guidelines,38,39 PROs,40–42 and prescriber alerts43 were also primarily associated with positive outcomes including reduced length of hospital stays,35 increased guideline utilization and concordance,33,38,39enhanced identification of trial-eligible patients,36 and improvements in symptom management.41 Overall, the included studies suggest that clinical oncology-focused CDS systems appear to be well-accepted and are associated with potentially meaningful improvements in patient care.
Two systematic reviews assessing CPOE for inpatients in medicine or intensive care units12 and at the point of care in any clinical setting13 demonstrate a clear benefit to implementing CDS. However, one of the studies in our analysis demonstrated that there was an increase in pharmacist order review time without any impact on intervention rates.21 This underlines the potential for a negative impact of CDS systems on care delivery, and demonstrates the importance of a thorough evaluation of these tools as a part of system implementation. CDS systems incorporated into clinical pathways is associated with increased guideline adherence.32–37 These findings demonstrate the benefit CDS systems can provide to clinicians and are consistent with previous systematic reviews that also demonstrated a positive impact on guideline adherence.10,44 Three studies included in our analysis evaluated the use of CDS systems for PROs, and all demonstrated benefit for one or more outcomes. 40–42 These findings differ somewhat from the findings of a systematic review of 15 studies that assessed the effect of CDS systems on PROs, where a positive effect on symptoms was demonstrated in 3 (20%) studies.45 The impact of a CDS system used with prescriber alerts demonstrated a positive impact and is consistent with that demonstrated in a previous study.43,46
The findings are also consistent with that of a meta-analysis assessing the impact of health information technology (HIT) on cancer care from 2000-July 2014.47 CDS systems were the most common (66%) HIT intervention identified. In this analysis, CDS systems were implemented across several cancer types including breast, colorectal, and prostate for the detection, diagnosis, and treatment but not for survivorship or end of life care. The primary findings demonstrate the beneficial impact differed across the cancer continuum; HIT for diagnosis and treatment was less likely to be associated with benefit compared with prevention. Likewise, those targeting behavioral change were less likely to be beneficial than those targeting improved decision making. The complexity of diagnosis and treatment, the volume of information needed, and the factors associated with behavior change were given as potential reasons for these findings. Within the CDS systems, key factors that appear to contribute to improved outcomes include the use of real-time provider alerts and point of care action on prescription orders and provision of information to clinicians that CDS systems can providr,20,27,32,36,37,40–42 There appear to be resulting factors that create new challenges such as the need to access separate systems and otherwise increase work time of prescribers or other downstream clinicians that may reduce outcomes.21,26,34,35
The current systematic review is reported over 10 years after a call for action by the American Medical Informatics Association regarding the use of CDS.4 The call included directives for achieving desirable levels of patient safety, care quality, patient centeredness, and cost effectiveness. Health systems were called to optimize CDS to improve the quality of health care services and health in the US. However, not all studies evaluating CDS systems have demonstrated clinical practice improvements.48 Thus, it is imperative that systems and tools, both commercially- and locally-developed, are assessed for their effectiveness and impact on patient outcomes. Further, we believe it is essential that these assessments also include outcomes including those associated with clinical care and costs of system implementation, when possible.
Kowamoto, et al, identified features of CDS systems critical for improving clinical practice in any clinical setting.8 These include: 1) automatic provision of decision support as part of clinician workflow; 2) provision of recommendations compared with assessments; 3) provision of decision support at the time and location of decision making; and 4) computer-based decision support. CDS systems with all four features were associated significant improvements in clinical practice.
A major gap in CDS systems use exists across the spectrum of clinical oncology care and further development of tools is warranted. Findings from the limited studies included in this review are largely positive and consistent with most conducted in the general health care population. However, many of the included studies used sub-optimal study designs. Only three studies were randomized controlled trials. Robust quasi-experimental study designs such as interrupted time series designs with a comparator group were rare. Currently, there are fewer than 15 studies assessing the impact of CDS systems currently underway or to be conducted according to clinicaltrials.gov.49 A recent study of CDS in patients with lung cancer demonstrated a reduction in inappropriate G-CSF use without an increase in febrile neutropenia rates illustrates the positive impact these powerful tools can have on clinical oncology care.50 Further analyses of the use of these tools with appropriate study designs and analytic methods are necessary to build the case for wider implementation of CDS systems.
Directions for Future Research
A number of gaps in the current literature were identified during this analysis including a lack of information related to the specific barriers, facilitators, and implementation strategies associated CDS system implementation. The studies did not specify the potential challenges sites may encounter when implementing similar systems or describe identified best practices to support successful implementation or outcomes. In addition, their impact on patient mortality, health care costs, or the costs associated with implementation and CDS system management was also not assessed. Further, outcome assessments for each study did not include the overall impact on patient mortality or the impact of system implementation on health care costs to the institution, payer, or the patient. The costs of system maintenance, upgrades, or enhancements were also not described. These are all crucial elements of CDS systems that must be assessed and understood for optimal implementation to occur.
Study Strengths and Limitations
Strengths of this systematic review include the study team, comprised of a research librarian, two practicing oncologists, and an oncology clinical pharmacist. The review was conducted according to the PRISMA statement to ensure appropriate methods were used. The potential for bias including searching, exclusion criteria, assembling, and publication exist. All efforts to minimize bias were taken where possible. The number of CDS systems that were studied, had no outcome improvement, and were subject to publication bias is unknown.
Conclusions
Currently there are few studies evaluating CDS systems in oncology practice. Comparative studies that report outcomes of care include studies of CDS systems with CPOE, clinical pathways, clinical practice guidelines, PROs and provider alerts. Published studies of CDS systems are largely associated with positive outcomes and are consistent with studies conducted in the non-cancer population. Key features that appear to support positive outcomes include real-time information and point of care action. A small impact on provider behavior with general alerts and increased pharmacist order review times were demonstrated with CPOE. Potential drawbacks may be the need to access separate systems and increased workflow on prescribers or other clinicians. None of the studies assessed the impact of CDS systems on patient survival. There is a critical need for CDS systems development and well-designed studies to demonstrate improvement in patient outcomes—including impacts on survival and efficiency of cancer care delivery.
Supplementary Material
Acknowledgement
The guidance of Terry Field and Diana Buist, members of the Health Care Systems (HCS) Cancer Research Network (CRN) and CRN Scholar Program Mentors, and members of the who contributed to the development of this project. The overall goal of the CRN is to conduct collaborative research to determine the effectiveness of preventive, curative and supportive interventions for major cancers that span the natural history of those cancers among diverse populations and health systems. HealthPartners Institute is an Affiliate Member of the HCS Research Network. We would also like to acknowledge Mandy Fraser, Amanda Lacy, and Lauryn Davin who provided technical assistance during the literature review process and with manuscript preparation and submission.
Research Support
This work was supported by a grant from the National Cancer Institute, U24 CA171524; PI L. Kushi, with no additional funding provided specifically for this work.
Footnotes
Author Disclosures of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Lobach D, Sanders GD, Bright TJ, et al. Enabling health care decisionmaking through clinical decision support and knowledge management. Evid Rep Technol Assess (Full Rep) 2012:1–784. [PMC free article] [PubMed] [Google Scholar]
- 2.Castaneda C, Nalley K, Mannion C, et al. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J Clin Bioinforma 2015;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Decision Support (CDS). Health IT Policymaking, Regulation, & Strategy. (Accessed March 13, 2018, at https://www.healthit.gov/policy-researchers-implementers/clinical-decision-support-cds.)
- 4.Osheroff JA, Teich JM, Middleton B, Steen EB, Wright A, Detmer DE. A roadmap for national action on clinical decision support. J Am Med Inform Assoc 2007;14:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ash JS, McCormack JL, Sittig DF, Wright A, McMullen C, Bates DW. Standard practices for computerized clinical decision support in community hospitals: a national survey. J Am Med Inform Assoc 2012;19:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lusignan S, Chan T. The development of primary care information technology in the United kingdom. J Ambul Care Manage 2008;31:201–10. [DOI] [PubMed] [Google Scholar]
- 7.Roshanov PS, Fernandes N, Wilczynski JM, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013;346:f657. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–38. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742–52. [DOI] [PubMed] [Google Scholar]
- 11.Bonnabry P, Despont-Gros C, Grauser D, et al. A risk analysis method to evaluate the impact of a computerized provider order entry system on patient safety. J Am Med Inform Assoc 2008;15:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 2003;163:1409–16. [DOI] [PubMed] [Google Scholar]
- 13.Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med 2012;157:29–43. [DOI] [PubMed] [Google Scholar]
- 14.Swedish Pres of EU e Health for a Healthier Europe! Opportunities for a better use of healthcare resources. 2009. (Accessed March 13, 2018, at https://joinup.ec.europa.eu/sites/default/files/document/2014-12/eHealth%20for%20a%20Healthier%20Europe%20-%20Opportunities%20for%20a%20better%20use%20of%20healthcare%20resources.pdf.)
- 15.Berner ES. Clinical decision support systems: State of the Art. AHRQ Publication No 09-0069-EF;Rockville, Maryland: Agency for Healthcare Research and Quality; June 2009. [Google Scholar]
- 16.Clauser SB, Wagner EH, Aiello Bowles EJ, Tuzzio L, Greene SM. Improving modern cancer care through information technology. American journal of preventive medicine 2011;40:S198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delivering High-quality Cancer Care: Charting a new course for a system in crisis, 2013. (Accessed March 13 2018, at http://nationalacademies.org/hmd/reports/2013/delivering-high-quality-cancer-care-charting-a-new-course-for-a-system-in-crisis.aspx.) [PubMed]
- 18.Seidman AD. Computer-Assisted Decision Support in Medical Oncology: We Need It Now. The ASCO Post 2016 April 10, 2016 [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz MT, Ur-Rehman T, Qureshi S, Bukhari NI. Reduction in chemotherapy order errors with computerised physician order entry and clinical decision support systems. HIM J 2015;44:13–22. [DOI] [PubMed] [Google Scholar]
- 21.Beer JD, Dobish R, Chambers C. Physician order entry: a mixed blessing to pharmacy? J Oncol Pharm Practice 2002;8:119–26. [Google Scholar]
- 22.Chen AR, Lehmann CU. Computerized provider order entry in pediatric oncology: design, implementation, and outcomes. Journal of oncology practice / American Society of Clinical Oncology 2011;7:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho E, Kim HJ, Kim GM, et al. Assessment of efficiency and safety of the comprehensive Chemotherapy Assistance Program for ordering oncology medications. Int J Med Inform 2013;82:504–13. [DOI] [PubMed] [Google Scholar]
- 24.Collins CM, Elsaid KA. Using an enhanced oral chemotherapy computerized provider order entry system to reduce prescribing errors and improve safety. International journal for quality in health care : journal of the International Society for Quality in Health Care / ISQua 2011;23:36–43. [DOI] [PubMed] [Google Scholar]
- 25.Elsaid K, Truong T, Monckeberg M, McCarthy H, Butera J, Collins C. Impact of electronic chemotherapy order forms on prescribing errors at an urban medical center: results from an interrupted time-series analysis. International journal for quality in health care : journal of the International Society for Quality in Health Care / ISQua 2013;25:656–63. [DOI] [PubMed] [Google Scholar]
- 26.Hanauer DA, Zheng K, Commiskey EL, Duck MG, Choi SW, Blayney DW. Computerized prescriber order entry implementation in a physician assistant-managed hematology and oncology inpatient service: effects on workflow and task switching. Journal of oncology practice / American Society of Clinical Oncology 2013;9:e103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huertas Fernandez MJ, Baena-Canada JM, Martinez Bautista MJ, Arriola Arellano E, Garcia Palacios MV. Impact of computerised chemotherapy prescriptions on the prevention of medication errors. Clin Transl Oncol 2006;8:821–5. [DOI] [PubMed] [Google Scholar]
- 28.Mattsson TO, Holm B, Michelsen H, Knudsen JL, Brixen K, Herrstedt J. Non-intercepted dose errors in prescribing anti-neoplastic treatment: a prospective, comparative cohort study. Ann Oncol 2015;26:981–6. [DOI] [PubMed] [Google Scholar]
- 29.Meisenberg BR, Wright RR, Brady-Copertino CJ. Reduction in chemotherapy order errors with computerized physician order entry. Journal of oncology practice / American Society of Clinical Oncology 2014;10:e5–9. [DOI] [PubMed] [Google Scholar]
- 30.Small MD, Barrett A, Price GM. The impact of computerized prescribing on error rate in a department of Oncology/Hematology. J Oncol Pharm Pract 2008;14:181–7. [DOI] [PubMed] [Google Scholar]
- 31.Voeffray M, Pannatier A, Stupp R, Fucina N, Leyvraz S, Wasserfallen JB. Effect of computerisation on the quality and safety of chemotherapy prescription. Qual Saf Health Care 2006;15:418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beriwal S, Rajagopalan MS, Flickinger JC, Rakfal SM, Rodgers E, Heron DE. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated National Cancer Institute-Designated Comprehensive Cancer Center Network. Int J Radiat Oncol Biol Phys 2012;83:1246–51. [DOI] [PubMed] [Google Scholar]
- 33.Bertsche T, Askoxylakis V, Habl G, et al. Multidisciplinary pain management based on a computerized clinical decision support system in cancer pain patients. Pain 2009;147:20–8. [DOI] [PubMed] [Google Scholar]
- 34.Chang PL, Li YC, Lee SH. The differences in health outcomes between Web-based and paper-based implementation of a clinical pathway for radical nephrectomy. BJU Int 2002;90:522–8. [DOI] [PubMed] [Google Scholar]
- 35.Hsu YC, Tsui KH, Chen CL, Lee SH, Wu YS, Chang PL. Web-based clinical pathway for reducing practice variations in radical prostatectomy. Chang Gung Med J 2008;31:567–75. [PubMed] [Google Scholar]
- 36.Patkar V, Acosta D, Davidson T, Jones A, Fox J, Keshtgar M. Using computerised decision support to improve compliance of cancer multidisciplinary meetings with evidence-based guidance. BMJ Open 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Erps J, Aapro M, MacDonald K, et al. Promoting evidence-based management of anemia in cancer patients: concurrent and discriminant validity of RESPOND, a web-based clinical guidance system based on the EORTC guidelines for supportive care in cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2010;18:847–58. [DOI] [PubMed] [Google Scholar]
- 38.Bouaud J, Seroussi B, Antoine EC, Zelek L, Spielmann M. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: impact upon physician prescribing behaviour. Stud Health Technol Inform 2001;84:420–4. [PubMed] [Google Scholar]
- 39.Bouaud J, Spano JP, Lefranc JP, et al. Physicians’ Attitudes Towards the Advice of a Guideline-Based Decision Support System: A Case Study With OncoDoc2 in the Management of Breast Cancer Patients. Stud Health Technol Inform 2015;216:264–9. [PubMed] [Google Scholar]
- 40.Berry DL, Blumenstein BA, Halpenny B, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol 2011;29:1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruland CM, White T, Stevens M, Fanciullo G, Khilani SM. Effects of a computerized system to support shared decision making in symptom management of cancer patients: preliminary results. J Am Med Inform Assoc 2003;10:573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016;34:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu PI, Lai KH, Cheng JS, et al. Prevention of acute exacerbation of chronic hepatitis B infection in cancer patients receiving chemotherapy in a hepatitis B virus endemic area. Hepatology 2015;62:387–96. [DOI] [PubMed] [Google Scholar]
- 44.Damiani G, Pinnarelli L, Colosimo SC, et al. The effectiveness of computerized clinical guidelines in the process of care: a systematic review. BMC health services research 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum D, Raj SX, Oberholzer R, et al. Computer-Based Clinical Decision Support Systems and Patient-Reported Outcomes: A Systematic Review. Patient 2015;8:397–409. [DOI] [PubMed] [Google Scholar]
- 46.Scott GP, Shah P, Wyatt JC, Makubate B, Cross FW. Making electronic prescribing alerts more effective: scenario-based experimental study in junior doctors. J Am Med Inform Assoc 2011;18:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarver WL, Menachemi N. The impact of health information technology on cancer care across the continuum: a systematic review and meta-analysis. J Am Med Inform Assoc 2016;23:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA 1998;280:1339–46. [DOI] [PubMed] [Google Scholar]
- 49.ClinicalTrials.gov. U.S. National Library of Medicine. (Accessed September 13, 2018, at https://clinicaltrials.gov/ct2/home.)
- 50.Adeboyeje G, Agiro A, Malin J, Fisch MJ, DeVries A. Reducing Overuse of Colony-Stimulating Factors in Patients With Lung Cancer Receiving Chemotherapy: Evidence From a Decision Support-Enabled Program. Journal of oncology practice / American Society of Clinical Oncology 2017;13:e337–e45. [DOI] [PubMed] [Google Scholar]
- 51.Harris AD, McGregor JC, Perencevich EN. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inform Assoc 2006;13:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


