Abstract
Background:
The aim of this study was to compare and evaluate the efficacy of neodymium-doped yttrium aluminum garnet laser with and without herbal and nanohydroxyapatite dentifrices in management of dentinal hypersensitivity (DH).
Materials and Methods:
A total of 180 patients who responded to air-blast test and cold-water test using verbal rating scale (VRS) were included in this study. The patients were randomly assigned to six groups (n = 30): control group (CG), scaling and root planning (SRP) + nanocrystalline hydroxyapatite dentifrices, SRP + nanocrystalline hydroxyapatite dentifrices + laser, SRP + herbal dentifrices, SRP + herbal dentifrices + laser, and SRP + laser. Each group was evaluated at baseline, 1 week, 1 month, and 6 months. In every visit of each patient, their clinical examination was done.
Results:
Among all groups’, repeated ANOVA measures and Kruskal–Wallis test was performed in which laser groups showed maximum reduction in DH in all indices while the CG showed minimum reduction in DH. The VRS values showed maximum reduction in SRP + nanocrystalline hydroxyapatite dentifrices + laser group with mean of 0.3 ± 0.5 and minimum reduction in CG with mean of 3.0 ± 0.5 (P < 0.001).
Conclusion:
Among all the groups, SRP + Nanocrystalline hydroxyapatite + Laser can be an effective treatment modality for DH.
Keywords: Dentinal hypersensitivity, herbal dentifrice, nanocrystalline hydroxyapatite dentifrice, neodymium-doped yttrium aluminum garnet
INTRODUCTION
Ice creams, chocolates, coffee, cold drinks, and many other beverages that are treated to enjoy but sometimes they become triggers of pain in individuals having sensitive teeth. Dentinal hypersensitivity (DH) has become a common problem among populations. It occurs when the dentinal tubules of tooth gets exposed,[1] resulting in an unpleasant sensation while eating or drinking or even by cold air.[2] Technically, the triggers of this pain are the external stimuli which include electrical or mechanical stimulus like pulp testers, osmotic stimulus like sugars, or thermal stimulus like hot and cold.[3,4]
Prevalence of DH varies from place to place and person to person. A study reported by Dhaliwal in 2012[5] reported that 40%–50% of North Indians suffer DH and Rees et al.[6] showed that DH is more prevalent in the adult population, ranging from 4% to 74%. Naidu et al.[7] in South India showed DH prevalence to be 32% in the adult population, while Babu et al.[8] reported that rural population of Southwest India was having DH approximately 62% and males were more affected than females while only 38.1% urban population.
The phenomenon of DH is based on hydrodynamic theory, according to which when dentine comes in contact with an external stimuli, the flow of fluid inside dentinal tubules increases and this movement of fluid causes changes in pressure of fluid and stimulates the pulpal nerve receptors on the dentine which in turn responds in the form of pain.[9] The intensity of pain depends on the intensity of stimuli.[10] DH is caused when the dentinal tubules are exposed. This exposure occurs due to cementum/enamel loss. There are many factors that are responsible of enamel/cementum loss. These include attrition,[11] abrasion,[12] erosion, abfraction,[11] gingival recession,[13] periodontal treatment,[14] bleaching,[14] and root exposure due to age.[15] There are several desensitizing pastes reported in studies that are capable of reducing DH such as potassium nitrate, sodium fluoride, and calcium hydroxide,[16] but permanent cure is still a matter of concern. A new treatment modality to treat DH has been evolved in the form of laser. Treatment of DH using laser was first done in the year 1985,[17] since then many researches have been reported for the treatment of DH using lasers.
The aim of this clinical trial was to evaluate and compare the clinical significance of neodymium-doped yttrium aluminum garnet (Nd:YAG) laser with and without use of nanocrystalline hydroxyapatite and herbal dentifrices after a nonsurgical periodontal therapy.
MATERIALS AND METHODS
The study was a double-blind, randomized, controlled clinical trial. The period of study was 2 years. The protocol of this study was approved by the ethical committee of King George's Medical University (9390/Ethics/R. Cell-16). It was also registered in the Clinical Trial Registry of India (CTRI) (REF/2017/03/013586). A total of 180 participants (sample size calculated using G* Power v.3 software (Faul, Erdfelder, Lang and Buchner, 2007)[18] were selected from the patients referred to the Outpatient Department of Periodontology and Conservative Dentistry and Endodontics, King George's Medical University. The clinical efficacy of every participant was assessed by air-blast test and cold-water test and the response was calculated using Verbal Rating Scale (VRS).[19]
The inclusion criteria included systemically healthy patient having at least two sensitive teeth in each quadrant and willingness to comply with all study requirements and written consent, no treatment received for periodontal disease in the past 3 months, no ongoing treatment for dentine hypersensitivity, and should not be on medication. Patients who smoke or chew tobacco and were alcoholics and had caries were excluded from the study. Pregnant, nursing women, and physical and mental disable patients were also excluded from the study.
Clinical examination
All the selected patients were first subjected to periodontal examination before nonsurgical periodontal treatment, that is, manual scaling and root planning (SRP) done using Hu-Friedy scalers and curettes. After the treatment, they were randomly assigned to different groups. Plaque index (PI) of Silness and Loe (1964)[20] gingival index (GI) of Loe and Silness (1963),[21] calculus index (CI),[22] and periodontal pocket depth (PPD) was examined and recorded in every visit of patient after the respective treatment of each group. It was done to evaluate the relief in pain of dentin. Regular follow-up of each patient was done at baseline, 1 week, 1 month, and 6 months.
Pain scoring
In every visit of each patient for the treatment in respective group, VRS was calculated according to (Clark and Troullos 1990), 0 = No pain; 1 = Mild pain; 2 = Moderate pain; 3 = Severe pain; and 4 = Severe pain that lasts more than 10 s.
Standard protocol for laser treatment
Nd:YAG fiber tip was placed at a distance of 1–2 mm from the target tissue. The power (1W), frequency (10 HZ), and time (60 s/cm2) was adjusted. The procedure was repeated up to 5–20 times at continuous wave mode in sweeping manner. The laser treatment was given to the patients at baseline, 1 week, 1 month, and 6 months, who were randomly assigned to laser groups. (Protocol was followed according to manufacturer's details: Fotona, AT Fidelis, Slovenia).
Treatment groups
Control group (CG); n = 30: The patients of this group were treated with SRP only and were clinically examined in every visit
Test group 1 (SRP + nanocrystalline hydroxyapatite) (TG 1) n = 30: The patients of this group were first treated with SRP and a dentifrice containing nanocrystalline hydroxyapatite (Aclaim toothpaste, Group Pharmaceuticals Ltd., India) was given and was advised to use two times a day and were clinically examined in every visit
Test group 2 (SRP + nanocrystalline hydroxyapatite + laser) (TG 2) n = 30: The patients of this group followed same procedure as TG 1 and treated with laser according to the standard protocol and were clinically examined in every visit
Test group 3 (SRP + herbal) (TG 3) n = 30: The patients of this group were first treated with SRP and a herbal dentifrice (Hiora-K toothpaste, Himalaya Ltd, India) was given and was advised to use two times a day and were clinically examined in every visit
Test group 4 (SRP + herbal + laser) (TG 4) n = 30: The patients of this group followed same procedure as TG 3 and treated with laser according to the protocol and were clinically examined in every visit
Test group 5 (SRP + laser) (TG 5) n = 30: The patients of this group were first treated with SRP and were treated with laser according to the standard protocol. The patients in this group were not given any toothpaste and were clinically examined in every visit.
Statistical analysis
The outcome measures were summarized as mean ± standard deviation (SD). Data were analyzed using Statistical Package for the Social Sciences, 18 (SPSS Inc., Chicago, IL) and MS-Excel. One-way ANOVA and Kruskal–Wallis test were used to evaluate the statistical analysis. Kruskal–Wallis test is used to compare three or more groups.
RESULTS
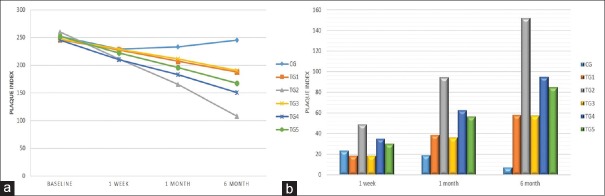
Table 1 and Figure 1a compared the PI values among the six groups. At baseline, minimum mean PI was in TG 1 group (245.9 ± 26.5) while maximum in CG group (252.1 ± 15.9). No significant difference (P = 0.138) was observed at this stage. Post 1 week, a highly significant difference was noted in TG 4 and TG 2 that showed maximal reduction but minimal change was observed in control, TG 3, and TG 1 group. PI persistently reduced maximum in TG 2 group, while no change was evident in CG. Repeated ANOVA measures displayed TG 2 and TG 4 were highly significant among all groups. Table 1 showed alteration in mean PI of all groups from baseline. Post 1 week to 6 months, TG 2 group was significantly reduced and maximally changed [Figure 1b].
Table 1.
Comparison of plaque index among the various groups
| PI | Mean±SD | Between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P* | |
| Baseline | 252.1±15.9 | 245.9±26.5 | 260.1±34.4 | 247.1±18.3 | 245.3±22.0 | 252.0±19.4 | 1.69 | 0.138 |
| 1 week | 229.1±20.9 | 227.5±27.1 | 211.3±35.2 | 228.7±25.9 | 210.3±18.1 | 222.0±19.2 | 3.57 | 0.004 |
| 1 month | 233.3±17.8 | 207.3±26 | 165.4±32.9 | 211.3±31.6 | 182.9±19.4 | 195.5±19.5 | 26.29 | <0.001 |
| 6 months | 245.2±19.3 | 188±27.2 | 108.0±26.5 | 190.2±35.5 | 150.6±22.9 | 167.6±25.0 | 89.21 | <0.001 |
| Within group* | F=33.11, P<0.001 | F=364.48, P<0.001 | F=708.02, P<0.001 | F=111.71, P<0.001 | F=319.90, P<0.001 | F=329.91, P<0.001 | ||
| Comparison of PI change from baseline among the various groups | ||||||||
| PI | Mean±SD | Between groups | ||||||
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| 1 week | 23.0±11.5 | 18.4±7.5 | 48.8±12.1 | 18.4±13.8 | 35.0±14.6 | 30.0±11.2 | 28.77 | <0.001 |
| 1 month | 18.8±11.7 | 38.6±10.0 | 94.7±17.3 | 35.8±20.7 | 62.4±18.8 | 56.5±15.0 | 81.28 | <0.001 |
| 6 months | 6.9±17.0 | 57.9±14.3 | 152.2±24.4 | 56.9±27.4 | 94.7±23.4 | 84.4±21.8 | 146.88 | <0.001 |
*Signifies the comparison between the group was done by using one-way ANOVA. **Signifies the comparison within the groups was done using repeated ANOVA measures. PI: Plaque index, SD: Standard deviation
Figure 1.
(a) Comparison of plaque index among the various groups and (b) comparison of plaque index change from baseline among the various groups
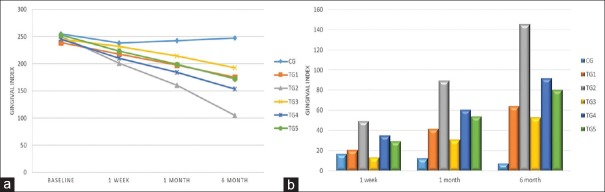
Table 2 and Figure 2a revealed the comparison of mean GI among all the groups. At baseline, the mean GI was insignificant (P = 0.182). Post 1 week to 6 months maximum reduction occurred in TG 2 (200.8 ± 37.7; 160.6 ± 32.5; and 104.7 ± 23.8, respectively) and minimum in CG (238.1 ± 19.3; 242.4 ± 17.9; and 247.4 ± 21.6, respectively). Thus, repeated ANOVA measures showed TG 2 group as highly significant with F value of 555.88. On comparison [Table 2 and Figure 2b] at different time intervals (baseline, 1 week, 1 month, and 6 months) among all the groups observed similar trend of reduction, that is, highest values for TG 2.
Table 2.
Comparison of gingival index among the various groups
| GI | Mean±SD | Between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| Baseline | 254.8±20.1 | 239.0±21.1 | 250.2±38.9 | 245.2±28.6 | 245.0±21.7 | 252.6±18.1 | 1.53 | 0.182 |
| 1 week | 238.1±19.3 | 218.1±26.0 | 200.8±37.7 | 231.8±28.7 | 210.0±21.5 | 223.3±17.8 | 8.39 | <0.001 |
| 1 month | 242.4±17.9 | 197.2±29.6 | 160.6±32.5 | 214.3±30.6 | 184.2±21.6 | 198.6±23.1 | 32.77 | <0.001 |
| 6 months | 247.4±21.6 | 174.9±36.5 | 104.7±23.8 | 192.4±31.1 | 153.1±22.0 | 172.4±30.4 | 83.22 | <0.001 |
| Within group | F=18.48, P<0.001 | F=157.80, P<0.001 | F=555.88, P<0.001 | F=284.71, P<0.001 | F=565.66, P<0.001 | F=189.88, P<0.001 | ||
| Comparison of GI change from baseline among the various groups | ||||||||
| GI | Mean±SD | Between groups | ||||||
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| 1 week | 16.7±7.8 | 20.8±10.8 | 49.4±19.3 | 13.4±7.0 | 35.0±8.7 | 29.3±15.3 | 35.73 | <0.001 |
| 1 month | 12.4±10.8 | 41.7±17.7 | 89.5±22.7 | 30.9±9.5 | 60.8±14.8 | 53.9±21.2 | 74.46 | <0.001 |
| 6 months | 7.4±16.2 | 64.1±26.9 | 145.5±26.6 | 52.8±15.0 | 91.9±17.5 | 80.2±29.2 | 122.33 | <0.001 |
SD: Standard deviation, GI: Gingival index
Figure 2.
(a) Comparison of gingival index among the various groups and (b) comparison of gingival index change from baseline among the various groups
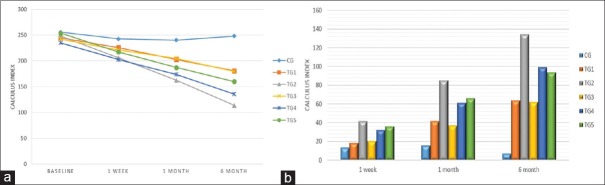
Table 3 and Figure 3a presented comparison of mean CI among six groups. At baseline and post 1 week, maximum reduction was in TG 4 (235.0 ± 21.9; 202.2 ± 20.0), while minimum in CG (255.7 ± 18.9; 242.3 ± 19.5). Post 1 and 6 months, the TG 2 group was maximally reduced. Hence, the repeated ANOVA measures revealed highly significant difference in TG 2 and TG 4 groups. Figure 3b and Table 3 noted highly significant difference (P < 0.001) among all six groups for all follow-up periods and the maximum mean CI change was observed in TG 2 (41.8 ± 15.3, 85.6 ± 22.7, and 134.4 ± 20.0, respectively).
Table 3.
Comparison of calculus index among the various groups
| CI | Mean±SD | Between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| Baseline | 255.7±18.9 | 244.5±23.2 | 247.8±36.0 | 242.0±32.7 | 235.0±21.9 | 253.5±23.0 | 2.35 | 0.055 |
| 1 week | 242.3±19.5 | 225.7±27.0 | 206.0±34.4 | 221.1±32.1 | 202.2±20.0 | 217.0±24.1 | 8.74 | <0.001 |
| 1 month | 240.0±19.6 | 202.6±30.4 | 162.2±32.2 | 204.6±32.3 | 173.7±18.2 | 187.1±25.1 | 31.14 | <0.001 |
| 6 months | 248.2±20.0 | 180.8±34.4 | 113.5±28.3 | 179.8±36.4 | 135.7±18.7 | 159.9±32.5 | 75.95 | <0.001 |
| Within group | F=36.62, P<0.001 | F=154.16, P<0.001 | F=663.78, P<0.001 | F=293.06, P<0.001 | F=551.65, P<0.001 | F=214.62, P<0.001 | ||
| Comparison of CI change from baseline among the various groups | ||||||||
| CI | Mean±SD | Between groups | ||||||
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| 1 week | 13.4±6.5 | 18.8±9.8 | 41.8±15.3 | 20.9±9.5 | 32.8±10.8 | 36.5±15.9 | 27.42 | <0.001 |
| 1 month | 15.7±7.4 | 41.9±17.4 | 85.6±22.7 | 37.4±11.0 | 61.3±14.6 | 66.4±23.6 | 62.42 | <0.001 |
| 6 months | 7.4±9.2 | 63.7±25.9 | 134.4±20.0 | 62.3±16.3 | 99.3±19.4 | 93.7±33.0 | 116.06 | <0.001 |
CI: Calculus index, SD: Standard deviation
Figure 3.
(a) Comparison of calculus index among the various groups and (b) comparison of calculus index change from baseline among the various groups
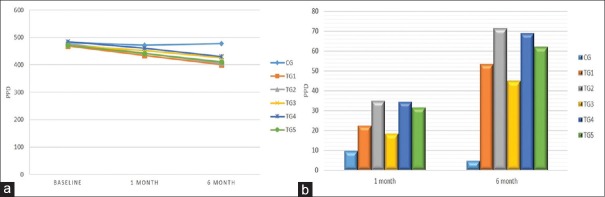
Table 4 and Figure 4a of mean PPD values were reduced nonsignificantly among all groups at the baseline (P = 0.074), but in post 1 and 6 months, significant reduction occurred in TG 1 (434.8 ± 26.5; 400.2 ± 32.7). Repeated ANOVA measures noted that all groups showed a significant difference, but maximum reduction occurred in TG 2 Group. Table 4 and Figure 4b showed that TG 2 group had maximum mean PPD change after 1 month and 6 months with values 34.9 ± 12.2 and 71.3 ± 21.8, respectively, while minimum PPD mean change was in CG.
Table 4.
Comparison of pocket probing depth among the various groups
| PPD | Mean±SD | Between groups | ||||||
|---|---|---|---|---|---|---|---|---|
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| Baseline | 481.5±13.5 | 469.1±28.2 | 476.7±25.1 | 470.1±23.8 | 483.7±20.9 | 472.2±25.6 | 2.05 | 0.074 |
| 1 month | 471.9±12.9 | 434.8±26.5 | 441.8±25.2 | 451.7±26.4 | 461.3±24.3 | 440.8±27.0 | 10.19 | <0.001 |
| 6 months | 477.0±10.7 | 400.2±32.7 | 405.4±23.0 | 425.2±22.5 | 430.5±22.6 | 410.3±27.0 | 41.19 | <0.001 |
| Within group | F=29.00, P<0.001 | F=231.05, P<0.001 | F=273.57, P<0.001 | F=180.98, P<0.001 | F=272.06, P<0.001 | F=250.52, P<0.001 | ||
| Comparison of PPD among the various groups. | ||||||||
| PPD | Mean±SD | Between groups | ||||||
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | F | P | |
| 1 month | 9.5±4.8 | 22.4±9.9 | 34.9±12.2 | 18.4±12.3 | 34.3±13.5 | 31.4±14.6 | 22.72 | <0.001 |
| 6 months | 4.5±7.7 | 53.2±15.7 | 71.3±21.8 | 44.9±15.3 | 68.9±22.9 | 61.9±17.7 | 59.39 | <0.001 |
PPD: Pocket probing depth, SD: Standard deviation
Figure 4.
(a) Comparison of pocket probing depth among the various groups and (b) comparison of pocket probing depth among the various groups
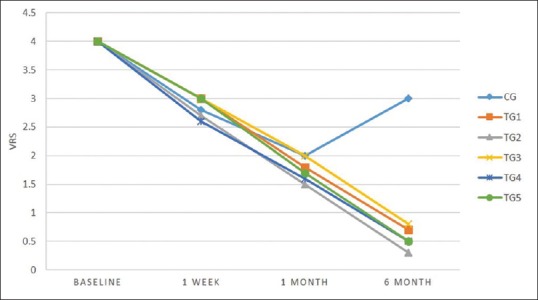
Table 5 and Figure 5 revealed the comparisons of VRS values among six groups. At baseline, mean VRS was 4.0 ± 0.0 and same for all the groups (according to inclusion criteria). After 1 week, reduction in mean VRS was observed in TG 4 group (2.6 ± 0.5), i.e., highly significant (P < 0.001). After 1 and 6 months, significant reduction occurred in TG 2 group (1.5 ± 0.5; 0.3 ± 0.05), while CG showed minimal change.
Table 5.
Comparison of verbal rating scale score among the various groups
| VRS | Mean±SD | Between group* | ||||||
|---|---|---|---|---|---|---|---|---|
| CG | TG 1 | TG 2 | TG 3 | TG 4 | TG 5 | χ2 | P | |
| Baseline | 4.0±0.0 | 4.0±0.0 | 4.0±0.0 | 4.0±0.0 | 4.0±0.0 | 4.0±0.0 | 0.00 | 1.000 |
| 1 week | 2.8±0.4 | 3.0±0.0 | 2.7±0.5 | 3.0±0.0 | 2.6±0.5 | 3.0±0.0 | 36.84 | <0.001 |
| 1 month | 2.0±0.2 | 1.8±0.4 | 1.5±0.5 | 2.0±0.0 | 1.6±0.5 | 1.7±0.4 | 29.65 | <0.001 |
| 6 months | 3.0±0.5 | 0.7±0.5 | 0.3±0.5 | 0.8±0.4 | 0.5±0.5 | 0.5±0.5 | 98.64 | <0.001 |
| Within group# |
χ2=81.90, P<0.001 |
χ2=90.00, P<0.001 |
χ2=89.44, P<0.001 |
χ2=90.00, P<0.001 |
χ2=90.00, P<0.001 |
χ2=90.00, P<0.001 |
||
*Calculated using Kruskal–Wallis Chi-square test, #Calculated using Friedman test. SD: Standard deviation, VRS: Verbal rating scale
Figure 5.
Comparison of verbal rating scale score among the various groups
DISCUSSION
A total of 180 patients were selected for the present study which was accomplished by G * Power v.3 software.[18] The determination of sample size was on patient-based analysis. A minimum of 10 patients per group was required if a difference of 0.8 mm (±0.6 mm SD within each group) and between the two groups was to be detected at a significance level of a = 0.05 with a power of y = 0.80 using a conservative two-tailed testing approach. Hence, the inclusion of at least 30 patients in each group that yielded the adequate statistical power for group comparisons.
The inclusion criteria involved only those patients who had at least two sensitive teeth in each quadrant; similar criteria was also followed by Brahmbhatt et al. 2012[23] to select the patients for DH in their study.
Irving Glickman, a researcher in the field of periodontology, defined epidemiological indices as the attempts to measure the clinical conditions quantitatively on a graduated scale, facilitating difference among populations investigated by the same criteria and methods.[24] In the existing study, all the patients who fulfilled the inclusion criteria were examined for periodontal examination in every visit. It included PI, GI, CI, and PPD indices; with the help of these indices, it was easy to quantitatively calculate the reduction of DH in each patient in entire period of treatment.
PI was introduced by Silness and Loe in 1964 and it is used to measure the plaque thickness on gingiva and tooth surface. In the present study, PI values of each patient before treatment were recorded and the result showed a successive decrease in plaque quantity and DH. From Table 1, it can be clearly observed that there is decrease in PI values of every group as the time interval increases. The maximum reduction was observed in TG 2 group with value F = 708.02, P < 0.001 with a statistically significant difference.
GI was introduced by Loe and Silness in 1963. GI is used to calculate the level of inflammation occurred on the periodontium. In this study, the GI was measured of every patient along with PI. The result of this study showed highly significant difference of GI mean in TG 4 group (F = 565.66, P < 0.001).
CI was introduced by Ennever et al. in 1961; it assesses the presence or absence of sub or supragingival calculus. The present study used CI to measure the reduction in calculus. The result of CI showed a significant reduction in DH as the time interval increased which can be clearly observed in Table 3. The maximum reduction of CI was observed in TG 2 group with high significant difference among other groups with value F = 663.78, P < 0.001.
In this study, PPD mean values showed maximum reduction in TG 2 group (F = 273.57, P < 0.001) that can be observed from Table 4. Overall, the improvement in DH was directly proportional to PI, GI, CI, and PPD mean values which showed a persistent reduction in DH. Out of all the groups’, minimum reduction was observed in CG in which patients were treated with noninvasive periodontal treatment only. Hence, the laser groups along with nanocrystalline hydroxylapatite dentifrice and herbal dentifrice showed a significant reduction in all the indices and reduced DH because these treatment procedures occluded the dentinal tubules and reduced the sensation of pain in sensitive teeth of patients.
VRS was used to evaluate the status of DH. The maximum scale value was taken as 4 in the present study. Minimum mean value of VRS after 6 months was recorded in TG 2 group (0.3 ± 0.5) and minimum in CG group (3.0 ± 0.5). In agreement with the present study, Dina Al-Tayeb in 2008[25] performed a similar mechanism of clinical examination before periodontal treatment. A total of 52 patients were selected and their PI, GI, and PPD were recorded, and the pain scores were calculated using VRS, which was also similar to the present study. Habashneh et al. in 2017[26] also compared commercially available toothpaste (herbal and nonherbal) and recorded GI, gingival bleeding index (GBI), visual analog scale (VAS) scores, and PI of 50 patients having gingivitis, plaque formation, and DH in every follow-up, similar procedure was followed by the present study.
Plaque is the primary etiological factor for gingival and periodontal diseases that may lead to DH. Thus, the patients among all the groups were treated primarily by manual SRP. It is the gold standard nonsurgical periodontal therapy. CG patients (30) were only treated with SRP. The result after 1 week showed decrease in the sensitivity level but that reoccurred after subsequent follow-up period [Table 5].
The commercial market is swamped with dentifrices that have the capability to treat DH and also reduce the possibilities of gingivitis by reducing dental plaque and improves gingival health. They contain different desensitizing agents which obstruct the dentinal tubules and reduce pain. The reduction in pain can be recorded by different means such as verbal rating scale,[27] VAS,[28] and questionnaires.[29] Nanocrystalline hydroxyapatite dentifrice is a recently developed formula for the betterment of DH. In the existing study, it was used in TG 1 group. Post 6 months, this group noted a reduction in DH (Chi-square = 90.00, P < 0.001). TG 1 group also showed a successive reduction in PPD of DH patients (F = 231.05, P < 0.001), [Table 4]. Vano et al. 2014[30] conducted a study to evaluate the effect of nanohydroxyapatite in reducing DH, which is similar to the present study. The results showed that nanohydroxyapatite dentifrices were much more effective in treating DH after 2 and 4 weeks.
TG 3 group of existing involved the use of herbal dentifrices to treat DH. TG 3 group showed effective results but slowly that can be observed from Tables 1–5. Kumari et al. 2016[31] compared the efficacy of herbal and nonherbal dentifrices against DH on 145 patients having DH and were randomly divided into three groups: placebo, herbal, and nonherbal groups. The result showed herbal dentifrices was more effective than the nonherbal group.
Although in the present study, the protocol of laser treatment was followed according to the manufacturer and mode of action involved occlusion of dentinal tubules, there are mechanisms of lasers reported by several studies that are used to treat DH. Basically, lasers follow different phenomenon to treat DH. Its stimulates the production of ATP, which in turn increases the level of threshold energy of the nerve endings which provides an analgesic effect because production of b-endorphin[32] increases and cyclooxygenase enzyme gets inhibited and pain reduces. Lasers also stimulate odontoblasts to increase the production of secondary dentin.[33,34] It occludes dentinal tubules.[35] The percentage of effective treatment of Nd:YAG laser ranges from 5.2% to 100%. Nd:YAG lasers narrow or occludes the dentinal tubules and blocks direct nerve analgesia due to which the pain is reduced.[36]
The present study compared the efficacy of lasers along with dentifrices and dentifrices alone in treatment of DH. It involved the use of Nd:YAG lasers that followed the manufacturer's protocol to treat DH. Three groups involved lasers: TG 2 group, laser along with nanocrystalline hydroxyapatite; TG 4 group, laser + herbal dentifrices; and TG 5 group, only laser treatment. Out of these three groups, effective results were obtained from TG 2 group that was highly significant among all groups (P < 0.001) that can be clearly observed from Tables 1–5. In agreement with this study, Ali et al.[37] compared a new treatment method for DH. They combined nano-fluorohydroxyapatite (NFH) with Nd:YAG laser. It was an in vitro analysis. They selected 60 freshly extracted human premolar teeth and divided into 6 different groups out of which NFH + Nd:YAG laser was highly significant among other groups. Similarly, Mohammed et al. 2014[38] conducted a study to occlude dentinal tubules by combination of CO2 laser and nanoparticle hydroxylapatite paste. Their result showed a statistically significant difference among all the groups, but better results were obtained with CO2 laser and nanoparticle paste. Similar to our study, they also used Kruskal–Wallis test for statistical analysis.
After TG 2 group, TG 4 group was highly significant compared to other groups [Tables 1–5]. The effect of herbal dentifrices along with Nd:YAG laser on DH was a new treatment modality that has been not reported yet.
TG 5 group involved the treatment of DH patients with only Nd:YAG laser. It was also effective in reducing pain in DH patients [Tables 1–5]. There are studies that have used other lasers such as Er, Cr: YSGG, GaAlAs, Er: YAG, and diode lasers to treat DH successfully. Yilmaz et al. 2011[39] compared the effects of two different lasers Er, Cr: YSGG laser and GaAlAs laser to treat DH. The result thus obtained showed that both the lasers were very effective against DH compared to CG and there was no significant difference between the two groups at any follow-up examination. Hashim et al. 2014[40] reported a study to evaluate the efficacy of diode laser (810 nm) for treating DH. The result showed a complete reduction in pain after 1 week in Group 1, while in Group 2, the pain disappeared in 15 min. Yu and Chang 2014[41] evaluated the efficacy of Er: YAG laser in treatment of cervical DH. 20 patients were given laser treatment for 2 min at 60 mJ/pulse with repetition rate 2 Hz. The follow-up time was after 4 weeks. A total of 18 patients showed significant decrease in DH. Mishra et al. 2017[42] reported treatment of DH using diode laser and compared its efficacy with potassium nitrate gel and stannous fluoride gel. All the groups showed a significant decrease in DH, but when statistically compared, diode laser showed highest efficacy against DH. They concluded that 940 nm diode laser is more efficient in treating DH compared to gels.
CONCLUSION
Based on the result obtained from this study, it can be concluded that TG2 group patients showed maximum reduction in DH. While other laser groups, that is, TG4 and TG5 groups were also very effective in reducing pain due to DH. Hence, Lasers are better treatment options for management of DH compared to the dentifrices alone.
Financial support and sponsorship
This study was financially supported by Council of Science and Technology (U.P.).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–6. [PubMed] [Google Scholar]
- 2.Tonguc MO, Ozat Y, Sert T, Sonmez Y, Kirzioglu FY. Tooth sensitivity in fluorotic teeth. Eur J Dent. 2011;5:273–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2013;17(Suppl 1):S63–71. doi: 10.1007/s00784-012-0916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belal MH, Yassin A. A comparative evaluation of CO2 and erbium-doped yttrium aluminium garnet laser therapy in the management of dentin hypersensitivity and assessment of mineral content. J Periodontal Implant Sci. 2014;44:227–34. doi: 10.5051/jpis.2014.44.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhaliwal JS, Palwankar P, Khinda PK, Sodhi SK. Prevalence of dentine hypersensitivity: A cross-sectional study in rural Punjabi Indians. J Indian Soc Periodontol. 2012;16:426–9. doi: 10.4103/0972-124X.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees JS, Jin LJ, Lam S, Kudanowska I, Vowles R. The prevalence of dentine hypersensitivity in a hospital clinic population in Hong Kong. J Dent. 2003;31:453–61. doi: 10.1016/s0300-5712(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 7.Naidu GM, Ram KC, Sirisha NR, Sree YS, Kopuri RK, Satti NR, et al. Prevalence of dentin hypersensitivity and related factors among adult patients visiting a dental school in Andhra Pradesh, Southern India. J Clin Diagn Res. 2014;8:ZC48–51. doi: 10.7860/JCDR/2014/9033.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu B, Hegde MN, Shetty A, Yelapure M. Prevalence of dentinal hypersensitivity in Southwest coastal population of India. Res Rev J Dent Sci. 2016;4:106–9. [Google Scholar]
- 9.Brännström M, Aström A. The hydrodynamics of the dentine; Its possible relationship to dentinal pain. Int Dent J. 1972;22:219–27. [PubMed] [Google Scholar]
- 10.Çolak H. Dentine hypersensitivity: Developing a person centered approach to oral health. Br Dent J. 2015;218:617. [Google Scholar]
- 11.Roberson T, Heymann H, Swift E. 8th ed. Philadelphia, PA: Elsevier; 2006. Art and Science of Operative Dentistry; pp. 268–292. [Google Scholar]
- 12.Gillam D, Chesters R, Attrill D, Brunton P, Slater M, Strand P, et al. Dentine hypersensitivity – Guidelines for the management of a common oral health problem. Dent Update. 2013;40:514. doi: 10.12968/denu.2013.40.7.514. [DOI] [PubMed] [Google Scholar]
- 13.Suge T, Kawasaki A, Ishikawa K, Matsuo T, Ebisu S. Effects of plaque control on the patency of dentinal tubules: An in vivo study in beagle dogs. J Periodontol. 2006;77:454–9. doi: 10.1902/jop.2006.050159. [DOI] [PubMed] [Google Scholar]
- 14.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–32. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 15.Marini MG, Greghi SL, Passanezi E, Sant’ana AC. Gingival recession: Prevalence, extension and severity in adults. J Appl Oral Sci. 2004;12:250–5. doi: 10.1590/s1678-77572004000300017. [DOI] [PubMed] [Google Scholar]
- 16.Al-Saud LM, Al-Nahedh HN. Occluding effect of Nd:YAG laser and different dentin desensitizing agents on human dentinal tubules in vitro: A scanning electron microscopy investigation. Oper Dent. 2012;37:340–55. doi: 10.2341/10-188-L. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K, Funai H, Shirasuka T, Wakabayashi H. Effects of Nd:YAG-laser in treatment of cervical hypersensitive dentine. Jpn J Conserv Dent. 1985;28:760–5. [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 19.Clark GE, Troullos ES. Designing hypersensitivity clinical studies. Dent Clin North Am. 1990;34:531–44. [PubMed] [Google Scholar]
- 20.Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta odontol scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 21.Löe H, Silness J. Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol Scand. 1963;21:533–1. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 22.Ennever J, Sturzenberger CP, Radike AW. Calculas surface index for scoring clinical calculus studies. J Periodontol. 1961;32:54–7. [Google Scholar]
- 23.Brahmbhatt N, Bhavsar N, Sahayata V, Acharya A, Kshatriya P. A double blind controlled trial comparing three treatment modalities for dentin hypersensitivity. Med Oral Patol Oral Cir Bucal. 2012;17:e483–90. doi: 10.4317/medoral.17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glickman I, Carranza FA. Philadelphia, Pa: Saunders; 1990. Glickman's Clinical Periodontology. [Google Scholar]
- 25.Tayeb-Al D. Management of root-dentine hypersensitivity following non-surgical periodontal therapy: Clinical and scanning electron microscopic study. Egypt Dent J. 2008;54:1–12. [Google Scholar]
- 26.Al Habashneh R, Farasin R, Khader Y. The effect of a triclosan/copolymer/fluoride toothpaste on plaque formation, gingivitis, and dentin hypersensitivity: A single-blinded randomized clinical study. Quintessence Int. 2017;48:123–30. doi: 10.3290/j.qi.a37384. [DOI] [PubMed] [Google Scholar]
- 27.Hansen EK. Dentin hypersensitivity treated with a fluoride-containing varnish or a light-cured glass-ionomer liner. Scand J Dent Res. 1992;100:305–9. doi: 10.1111/j.1600-0722.1992.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 28.Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–13. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagata T, Ishida H, Shinohara H, Nishikawa S, Kasahara S, Wakano Y, et al. Clinical evaluation of a potassium nitrate dentifrice for the treatment of dentinal hypersensitivity. J Clin Periodontol. 1994;21:217–21. doi: 10.1111/j.1600-051x.1994.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 30.Vano M, Derchi G, Barone A, Covani U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014;45:703–11. doi: 10.3290/j.qi.a32240. [DOI] [PubMed] [Google Scholar]
- 31.Kumari M, Naik SB, Martande SS, Pradeep AR, Singh P. Comparative efficacy of a herbal and a non-herbal dentifrice on dentinal hypersensitivity: A randomized, controlled clinical trial. J Invest Clin Dent. 2016;7:46–52. doi: 10.1111/jicd.12133. [DOI] [PubMed] [Google Scholar]
- 32.Benedicenti A. Manuale di laser terapia del cavo orale. Castello: Maggioli; 1982. p. 159. [Google Scholar]
- 33.Furuoka M, Yokoi T, Fukuda S, Usuki M, Matsuo S, Taniguchi K, et al. Effects of gaAlAs laser diode in treatment of hypersensitive dentine. Fukuoka Shika Daigaku Gakkai Zasshi. 1988;15:42–8. [PubMed] [Google Scholar]
- 34.Yamaguchi M, Ito M, Miwata T. Clinical study on the treatment of hypersensitive dentin by GaAlAs laser diode using double blind test. Aichi Gakuin Daigaku Shigakkai Shi. 1990;28:703–7. [PubMed] [Google Scholar]
- 35.Midda M, Renton-Harper P. Lasers in dentistry. Br Dent J. 1991;170:343–6. doi: 10.1038/sj.bdj.4807548. [DOI] [PubMed] [Google Scholar]
- 36.Asnaashari M, Moeini M. Effectiveness of lasers in the treatment of dentin hypersensitivity. J Lasers Med Sci. 2013;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 37.Ali S, Safi-El K, Dayem R. Treatment of dentin hypersensitivity with a combination of nanofluor hydroxyapatite and Nd:YAG laser. Int Arab J Dent. 2013;4:83–8. [Google Scholar]
- 38.Mohammed AA, Mahmood AS, Al-karadaghi TS, Kurzmann C, Laky M, Franz A, et al. The Effects of CO2 laser with or without nanohydroxyapatite paste in the occlusion of dentinal tubules. Sci World J. 2014;2014:1–8. doi: 10.1155/2014/798732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y. Clinical evaluation of Er, Cr: YSGG and gaAlAs laser therapy for treating dentine hypersensitivity: A randomized controlled clinical trial. J Dent. 2011;39:249–54. doi: 10.1016/j.jdent.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Hashim NT, Gasmalla BG, Sabahelkheir AH, Awooda AM. Effect of the clinical application of the diode laser (810 nm) in the treatment of dentine hypersensitivity. BMC Res Notes. 2014;7:31. doi: 10.1186/1756-0500-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu CH, Chang YC. Clinical efficacy of the Er:YAG laser treatment on hypersensitive dentin. J Formos Med Assoc. 2014;113:388–91. doi: 10.1016/j.jfma.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Mishra N, Agarwal S, Mishra P, Sharma KS, Sharma C. A comparative evaluation of diode laser, stannous fluoride gel and potassium nitrate gel in the treatment of dentinal hypersensitivity-a clinical study. IP Ann Prosthodont Restor Dent. 2017;3:118–22. [Google Scholar]