Abstract
Introduction: Deep brain stimulation is a safe and effective neurointerventional technique for the treatment of movement disorders. Electrical stimulation of subcortical structures may exert a control on seizure generators initiating epileptic activities. The aim of this review is to present the targets of the deep brain stimulation for the treatment of drug-resistant epilepsy.
Methods: We performed a structured review of the literature from 1980 to 2018 using Medline and PubMed. Articles assessing the impact of deep brain stimulation on seizure frequency in patients with DRE were selected. Meta-analyses, randomized controlled trials, and observational studies were included.
Results: To date, deep brain stimulation of various neural targets has been investigated in animal experiments and humans. This article presents the use of stimulation of the anterior and centromedian nucleus of the thalamus, hippocampus, basal ganglia, cerebellum and hypothalamus. Anterior thalamic stimulation has demonstrated efficacy and there is evidence to recommend it as the target of choice.
Conclusion: Deep brain stimulation for seizures may be an option in patients with drug-resistant epilepsy. Anterior thalamic nucleus stimulation could be recommended over other targets.
Keywords: anterior thalamic nucleus, electrical stimulation, neuromodulation, neurostimulation, refractory epilepsy, target
Introduction
Deep brain stimulation (DBS) is a neurointerventional technique that involves implanting electrodes and a pacemaker-like device to deliver pulses of electricity to specific areas of the brain. Although the mechanism of action remains to be fully elucidated, it is suggested that DBS acts via focal modulation of specific functional circuits within the brain (1, 2). The fact that the same DBS parameters and targets can benefit different neurological disorders suggests that DBS does not act against the pathophysiology of any specific disorder, but rather modulates existing and active pathologic brain circuits and it is well-tolerated (3).
The success of DBS for the treatment of Parkinson's disease (PD), in conjunction with the benefits of being adjustable, reversible, and exhibiting a good safety profile, has prompted investigation into the potential utility of neuromodulation via DBS for other diseases (4). Tens of thousands of patients suffering from different forms of neurological disorders have been treated with DBS worldwide (5), including tremor, dystonia, obsessive–compulsive disorder, depression, Tourette's syndrome, headache, chronic pain, eating disorders, and epilepsy (6). Anti-epileptic drugs (AEDs) can control seizures in most patients with epilepsy. However, at least 30% of adults with epilepsy do not achieve seizure control with AEDs (7) and surgery to remove or disconnect the epileptogenic zone is not always an appropriate option (8). These patients may be candidates for neurostimulation.
The number of potential neural targets in drug-resistant epilepsy (DRE) has increased over the years. The majority of available literature suggests targeting the anterior thalamic nucleus (ATN). In the following sections, we review the clinical outcomes for the most commonly chosen targets for the treatment of epilepsy. We included the ATN, centromedian thalamic nucleus (CMTN), hippocampus, basal ganglia (caudate nucleus, subthalamic nucleus), posterior hypothalamus and cerebellum.
Materials and Methods
We performed a literature search of the Medline®, Embase®, Index Medicus®, Scopus, and Cochrane databases from January 1980 to October 2018 that incorporated Medical Subject Headings and text words for literature related to “deep brain stimulation” and “drug-resistant epilepsy.” We also searched bibliographies of pertinent reviews: original articles reference lists, book chapters and relevant conference proceedings to find additional documents. We included original retrospective and prospective studies assessing the impact of DBS on seizure frequency in patients with DRE regardless of language or country of origin. Children were classified as subjects younger than 18 years. Systematic reviews, meta-analyses, randomized controlled trials, and observational studies were included. We also included studies on experimental, animal or molecular models in search of an integrative review (basic and clinical science). The following outcomes were assessed: seizure reduction; seizure freedom; and time of follow-up. Discrepancies were solved by consensus. Categorical data were expressed as percentages and quantitative data as mean, standard deviation, and range. All statistical analyses were performed with SPSS statistical software package (SPSS for Mac, v.21, SPSS, Inc., Chicago, IL).
Results and Discussion
Of the 429 abstracts identified by the search, 145 were reviewed as full-text articles. Seventy-two articles fulfilled eligibility criteria and described outcomes in 826 patients. The majority of patients were diagnosed with generalized or secondary generalized seizures (75%), while 7.2% included exclusively patients with focal seizures. Age ranged between 5 and 66 years, with a median of 30 years. All patients included in the studies had DRE.
Historical Perspective
The beginnings of DBS date back to the late 19th century. Several authors identified the functional anatomy of the brain using animal models that went against the established beliefs and dogmas of the time (9). Horsley and Clarke (10) were pioneers in the development of stereotactic frameworks for experimental use in animals. Subsequently Spiegel et al. (11) developed the use of X-ray pneumoencephalography in 1947 allowing to visualize the living brain more accurately. As well, this enabled the creation of stereotactic atlases to guide surgeries. In 1950s we saw the introduction of neuro-ablative techniques for the treatment of Parkinsonian tremor, with the study of Albe Fessard et al. (12). They were the first to report the use of high frequency electrical stimulation (~100–200 Hz) targeting the intermediate ventral thalamic nucleus with clinical improvement in tremor severity (12). The emergence of levodopa as a highly effective pharmacological treatment for PD in the 1960s limited the development of DBS, although several authors such as Hosobuchi et al. (13) and other research groups continued their studies in other pathologies, such as chronic pain and disorders with impaired level of consciousness with encouraging results.
As further evidence accumulated through the 1980s in regards to adverse effects of levodopa, such as dyskinesias, and patients who were resistant to treatment, a better understanding of the basal ganglia (14) emerged. Furthermore, the subthalamic nucleus (STN) was identified initially as a central locus of PD (15) and therefore represented an important potential surgical target (16). This was eventually introduced into clinical practice by Pollak et al. (17). Shortly thereafter, they identified the globus pallidus interna (GPi) as another target in 1994 (18).
From these advances, the use of DBS expanded to other pathologies such as epilepsy. The first studies investigating the anti-epileptic effects of DBS in epilepsy were published in the 1970s and 1980s (19–21). Since then, numerous studies have been published evaluating the effectiveness and safety of DBS in epilepsy; however, most studies have limitations as generally report small number of patients with variable results. To date, only one large randomized control clinical trial has been published (22), generating modest evidence of benefit.
Pathophysiology
The basic rationale for DBS as an effective anti-epileptic therapy is similar to what has been postulated for movement disorders: potential cellular inhibition or excitation (neuro-modulation) within the target structure (23). The stimulation will then either help to disrupt seizure propagation or raises the overall seizure threshold. If appropriately coordinated, low frequency stimulation (LFS) has been shown to restore normal neuronal electrical activity, while high frequency stimulation (HFS) is typically more effective at disrupting the propagation of synchronous neuronal activity (24). In hippocampal rat slice models, HFS enhances the inhibitory tone of the network and prevents the synchronization and propagation of epileptiform burst discharges (25).
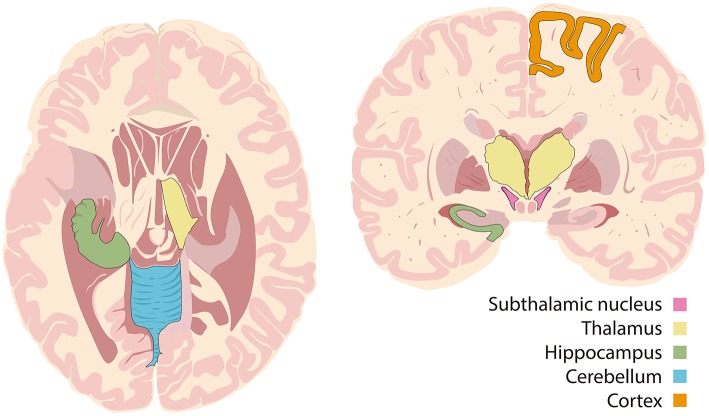
There are three different theories behind the selection of targets: the stimulus can be: (1) directly applied to the suspected seizure onset zone; (2) applied in deep subcortical structures to interrupt epileptic networks or; (3) applied over deeply located fiber bundles, which are connected to different structures within the brain (1, 2). The direct stimulation of the epileptogenic zone alters the tissue excitability and neuronal synchronization and can have an inhibitory effect, without causing functional deficits (1). Direct targets include the hippocampus, amygdala, hypothalamus or specific cortical zones. The indirect stimulation of deep structures may suppress neuronal circuits that favor seizure emergence. These targets include the cerebellum, basal ganglia and thalamus (2). Stimulation of fiber bundles in structures such as fornix or corpus callosum alter the threshold of seizure induction by modulation of neuronal circuits which are located in different but connected brain structures (26) (see Figure 1).
Figure 1.
Targets of deep brain stimulation.
Networks
Papez circuit links hippocampal output via the fornix and mammillary nuclei to the ATN. Projections from the ATN then travel through the cingulum bundle to the parahippocampal cortex and complete the circuit by returning to the hippocampus. Animal studies have supported the role of this circuit as being important in seizure occurrence (27, 28). Alterations in Papez' circuit have been observed in multiple forms of epilepsy. The cerebellum, STN, and CMNT all have projections to the circuit of Papez and have therefore been considered as potentially therapeutic DBS targets for seizure reduction (29).
Another important network is the cortico-thalamo-cortical loop, which has been associated with absence epilepsy (30) and motor seizures (31) in animal models. Lesional studies in non-human primates with focal epilepsy showed almost complete suppression of seizures with thalamotomy (32). More recent studies using optogenetic techniques have shown that both thalamic and cortical neurons can trigger seizures, contrary to previous hypotheses (33–35). It has been proposed that the thalamocortical pathway acts as a “choke point” in the disruption of seizure propagation (30).
Basic Mechanisms
The mechanism of action underlying DBS remains poorly understood. Its action on neuronal circuits is likely multifactorial and complex. The initial DBS study suggested that its effect was inhibitory (36). This inhibition could be explained by either the blockade of depolarization and inactivation of voltage-gated currents, or, alternatively, by activation of GABAergic afferents in the stimulated nucleus (37). It is not completely clear if the therapeutic effects of DBS occur due to the stimulation of neurons, glial cells, passage fibers or afferent inputs to target neurons (38). Some studies have identified that activation thresholds were lowest in myelinated axons, and sequentially increased in unmyelinated axons, dendrites, and cell bodies (39, 40). In addition to orthodromic activation of efferent axons, multiple studies conducted in animal models and humans with PD have shown that DBS excites afferent axons in an antidromic manner (41, 42).
Other studies have shown that DBS stimulates neurons and astrocytes, producing a release of glutamate, D-serine and ATP (43). The activation of astrocytes leads to neuronal modulation through brain flow and neurovascular factors (44, 45). In addition, a “microlesion effect” has been proposed. It is demonstrated by clinical improvement in patients prior to turning the device ON, which favors the astrocytes activation hypothesis (46). Electrotaxis, the mechanism by which progenitor cells migrate through the electric current produced by DBS (47), seems to be based on inducing synthesis of growth factors and gene expression, which enhance neuroplasticity and neurogenesis (48, 49).
Neurostimulation is classified according to the method of stimulation (50). An open-loop neurostimulation system applies chronic, intermittent or continuous stimulation to inhibit epileptiform activity without reference to the patient's clinical symptoms or ongoing electroencephalogram (EEG) activity. Using an implanted seizure detection device, closed-loop stimulation provides more efficient treatment by adjusting the stimulation settings in response to EEG changes, before application of electrical stimuli (2). A burst of stimulation is applied, with the intention of terminating the detected bioelectrical change (51). Recent evidence supports the concept that closed-loop stimulation (feedback-controlled) can be more effective than open-loop therapy (52). The responsive neurostimulator system (RNS, NeuroPace, Inc., Mountain-view, CA, USA) is a prime example of a closed-loop system and has been approved by the FDA for use in epilepsy. This system applies electrical stimulation to a previously defined seizure-onset zone, triggered by detection of electrophysiological signatures of a seizure in real-time (53). RNS has demonstrated efficacy in the reduction of epileptic seizures in long-term studies, increases in quality of life scores and acceptable safety (51, 54).
Technique
The DBS procedure consists of three steps: (1) preoperative planning, (2) surgical implantation, and (3) postoperative assessment. Prior to surgery, stereotactic coordinates for the target region are obtained by merging magnetic resonance imaging (MRI) of the patient's brain with a brain atlas (55). DBS for DRE is carried out with the patient under local or general anesthesia. The surgical procedure typically takes up to seven hours to complete and involves a multidisciplinary team of surgeons, epileptologists, and technical device staff. First, the exact placement and trajectory path for the electrode lead is determined. Next, burr holes are carefully drilled at the planned entry points for the electrodes. Region-specific neuronal activity, as a functional landmark, is used to verify the target structure during surgical procedure. One or more permanent microelectrodes are inserted into the brain using imaging guidance. Intraoperative fluoroscopy and postoperative MRI or computed tomography (CT) scans are acquired to confirm electrode placement. Finally, lead extenders are tunneled subcutaneously down the neck to below the clavicle, in which the pulse generator is implanted.
Old frame-based systems utilize a fixed frame that surrounds the patient's head entirely. New frameless systems are essentially skull-mounted aiming systems. The patient's head is registered to a scan containing the planned trajectory using intraoperative imaging, and a neuro-navigation system is then used to align the surgical trajectory with the plan. Compared to their frame-based counterparts, the new frameless systems provide benefits, such as increased patient comfort and shorter operating times. A recent American meta-analysis found a clinically insignificant loss of accuracy with frameless methods, they can therefore represent a reasonable alternative to frame-based methods (56).
DBS Parameters
Stimulation parameters in epilepsy have been chosen empirically in the last 40 years, based on investigator experience in other pathologies, (primarily movement disorders), and center preferences. In some centers, epilepsy specialists even use electrodes that have been developed specifically for the treatment of PD (57). It is difficult to draw conclusions from existing studies suggesting parameters and predictors of efficacy, as they have been done with a small number of patients.
The evidence is scarce and often contradictory. The SANTE study found no favorable parameters for frequency, voltage, current, or pulse width after a long term follow up (22). There is no clear difference between cycling and continuous stimulation (58), or unilateral and bilateral stimulation (57). To date, usual stimulation parameters are: frequency ≥100 Hz and voltage at 1–10 V for stimulation of the ANT; frequency ≥130 Hz and voltage at 1–5 V for hippocampal and STN stimulation; HFS at voltage 1–10 V for stimulation of the CMNT; and low (10 Hz) or high (200 Hz) stimulation for the cerebellum (59). However, as there are currently no clinical randomized control trials comparing the different stimulation paradigms, the optimal parameters for epilepsy remain unknown.
Most DBS systems use a continuous, high frequency (100–250 Hz) pulse train (55). After surgery, several postoperative outpatient sessions are conducted over the course of 3–6 months, by a clinician who optimizes stimulation parameters based on patient feedback and seizure control (60). Often the clinician is a neurologist or an epilepsy nurse, who determines the optimal parameters including amplitude, frequency and pulse width.
Targets of Stimulation
Thalamus
Anterior Thalamic Nucleus (ATN)
The ATN is the most widely used target for DBS in treatment of DRE (22). It has been preferred because of its size, its distance from vascular structures (24), and its extensive connections. Several studies have indicated that the anterior thalamic region is crucial to the maintenance and propagation of seizures (61). This is explained by its connections to the limbic system through the fornix and mammillothalamic tract with widespread and extended projections to the cingulate, entorhinal cortex, hippocampus, orbitofrontal cortex, and caudate, all of which have been implicated in the pathogenesis of focal epilepsy (62).
Small open-label, uncontrolled, pilot studies have shown clinical benefits. A 49% reduction of seizure frequency was reported in four patients after 44 months of follow-up (58). Similarly, a mean reduction of 75% was observed in four patients with mesial temporal lobe epilepsy (TLE) after treatment with DBS-ATN (63). Lee and colleagues investigated 15 patients with DRE, who underwent placement of bilateral DBS electrodes in the ATN. They showed a significant decrease (70%) in seizure frequency and it was concluded that the short-term outcome of ATN-DBS directly reflects the long-term outcome (64). Additionally, Kerrigan et al. (65) reported four out of five patients with significant reductions in the frequency and severity of seizures after a 36 month follow-up, without any complications. Hodaie et al. (61) reported a mean reduction of 54% in seizure frequency during a 15 month follow-up, also without adverse effects. Finally, Andrade et al. (66) described five of six patients (83%) with at least 50% improvement in seizure frequency over a mean follow-up period of 5 years. Sleep disruption and neuropsychiatric symptoms have been reported as a voltage-dependent adverse effect of DBS in ATN in patients with epilepsy (67).
These studies led to a randomized clinical trial in 2010 called Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) (22). This study was a multicenter, double blind, clinical randomized control trial investigating the use of bilateral DBS of the ATN for the treatment of localization-related epilepsy. One hundred and ten patients with focal or secondarily generalized seizures, intractable to drug treatment, were divided into two groups. Half of the participants received stimulation, and half of the patients received no stimulation over a 3-month blinded phase. Subsequently, all patients received un-blinded stimulation. This trial reported significant improvement in seizure frequency, especially in focal seizures with altered awareness and severe seizures after 25 months of follow-up. Of the 110 patients initially enrolled in the study, 81 (74%) completed the follow-up period. Among these patients, the median decrease in seizure frequency was 56%, ranging widely from a 26% increase in frequency to complete seizure freedom (six patients). Median seizure frequency reduction continued to improve over the 3 years of the trial, with a 41%, 56%, and 68% median seizure frequency reduction at 1 year, 2 years, and 3 years of DBS, respectively, with 29% greater reduction in seizure attacks in the stimulated group compared to the control group, observed during the last month of treatment (22).
No surgery-related symptomatic hemorrhages or deaths were reported, although two participants had transient, stimulation-induced seizures. Other adverse events included paraesthesias at the implant site in 18%, local pain in 11%, and infection in 9% of cases. Depression and memory impairment were more frequent in the stimulated group compared with the controls. Patients with temporal lobe seizures showed a greater benefit during the blinded phase compared with those with extratemporal or multifocal seizure onsets (62). Some studies have suggested that bitemporal mesial epilepsy may be the most responsive to ATN stimulation (22). Direct targeting in the ATN using high-resolution MRI is likely superior to indirect targeting due to extensive individual variation in the location of the ATN and may therefore improve the efficacy of DBS (68). Furthermore, performance of ATN-DBS parameters with simultaneous EEG recording during the ATN-DBS has been suggested to improve the therapeutic efficacy by monitoring of EEG desynchronization (69). As demonstrated in previous studies, DBS had a better effect over time.
Centromedian Nucleus of Thalamus (CMNT)
Dense cluster of axons project from CMNT, a midline thalamic structure, to the dorsolateral part of putamen. The CMNT also projects to the cerebral cortex, principally to the motor and premotor cortices (70). Anatomical patterns of CMNT connections support its role in the pathophysiology of generalized seizures. Animal studies have demonstrated the CMNTs role in the initiation of seizures (71, 72) as well as in improvement of level of postictal consciousness after stimulation of the CMNT (73).
Stimulation of the CMNT in humans for treatment of DRE was first performed by Velasco and colleagues (74). CMNT stimulation appears to be more suitable for the control of absence and generalized seizures, especially in patients with primary or secondary Lennox Gastaut syndrome (LGS) with up to 80% of patients showing a good response. It does not appear to be effective for the treatment of focal seizures with altered awareness (74). Targeting the parvocellular division of the CMNT bilaterally, Velasco et al. (75) observed a reduction in seizure frequency in 13 patients with LGS. However, this was an open-label uncontrolled case series. In the only controlled pilot study of CMNT stimulation, preformed in seven patients, Fisher and colleagues found a 50% reduction in seizure frequency in three patients, treated with 24 h/day continuous stimulator trains (76).
Eleven patients with generalized or frontal lobe DRE epilepsy were recruited at King's College Hospital (London, United Kingdom) and at the University Hospital La Princesa (Madrid, Spain) (77). They underwent bilateral DBS targeting the CMNT. Among the eleven patients, seven (64%) demonstrated improvement. Among the five patients with frontal lobe epilepsy, only one patient (20%) had significant improvement (more than 50% of reduction in seizure frequency) during the blind period; and two (40%) during the long-term extension phase. However, all six patients (100%) with generalized epilepsy had significant improvement in seizure frequency during the blind period; and in the long-term extension phase, five of the six (83%) patients showed more than 50% improvement in the frequency of seizures. Among patients with generalized DRE epilepsy, DBS implantation and stimulation of the CMNT appeared to be effective and safe. One patient (9%) had the device removed 6 months after implantation due to infection and one patient (9%) reported a transient agraphia in the first 4 days following implantation. Improvement in seizure attacks was observed during months 3 to 50 with the DBS device turned OFF (77). Additional large and well-controlled studies for CMNT stimulation are needed to identify the efficacy, mechanism and the target population (Table 1).
Table 1.
Clinical studies of Bi-ATN and CMT-DBS for the treatment of Epilepsy.
| Author/year | Mean age (years) | Design | n | Seizure type (s) | Follow up (months) | Average seizure reduction (range) | |
|---|---|---|---|---|---|---|---|
| BI-ATN | |||||||
| Upton et al. (21) | 24 | Open label | 6 | CPS | >36 | 4/6 had “significant clinical control” | |
| Sussman et al. (78) (Abstract) | NR | Open label | 5 | CPS, SGTC | 12–24 | 60% showed “improvement” | |
| Hodaie et al. (61)Andrade et al. (66) | 30 | Single blind | 5 (+1) | GTC, DA, CPS, AA, SGTC, PM | 4–7 years | 55% (24–89%) | |
| Kerrigan et al. (65) | 36 | Open label | 5 | SPS, CPS, SGTC | 20.4 (6–36) | 48% (−57–98%) of “serious seizures” | |
| Lee et al. (79) (& Bi-STN) | 22 | Open label | 3 | TS, DA, HM, AM, SGTC | 6 (2–10) | 75.4% (50–90.6%);3/3 RR | |
| Lim et al. (58) | 27 | Open label | 4 | G, P, STGC | 24 | 49% (35–76%);1/4 RR | |
| Osorio et al. (63) | 31 | Single blind | 4 | (Bi- MTLE)SGTC, CPS, SPS, DA | 36 | 75.6% (53–92%);4/4 RR | |
| Andrade et al. (80) | 29, 45 | Open label | 2 | (DRA)SGTC, MYO, CS, GTC | 120 | 98% of SGTC in one; 66% total in other | |
| Fisher et al. (22) (SANTE) | 36 | Clinical trial (Double blind, randomized, parallel group) | 54 | 55* | CPS, SGTC | 24 | 26% above controls after 2 months; 56% median reduction after 2 years |
| Lee et al. (64) | 31 | Open label | 15 | CPS, GTC, SPS | 39 (24–67) | 70.5% (0–100%);13/15 RR | |
| Oh et al. (81) | 33 | Open label | 9 | CPS, SGTC | 34.6 (22–60) | 57.9% (35.6–90.4%);7/9 RR | |
| Van Gompel et al. (82) (& Bi-HC) | 26, 32 | Open label | 2 | SPS, CPS, SGTC | 3 | 80% in one; 53% in other;2/2 RR | |
| Piacentino et al. (83) | 38 | Open label | 6 | (LGS), CPS, SGTC | >36 | 3/5 RR** | |
| Voges et al. (67) | 37 | Case-Cohort study, Open label | 9 | CPS, SGTC | 28 | 7/9 RR | |
| Lehtimaki et al. (84) | 35 | Open label | 15 | NR | 25.2 (9–52) | 10/15 RR | |
| Salanova et al. (85) (SANTE) | NR | Clinical trial | 83 | SANTE study | 61 | 69% | |
| Krishna et al. (86) | 32 | Open label | 16 | SPS, CPS, SGTC, GTC, MYO, DA | 52 | 65% (−500–99%) at 3 y;11/16 RR | |
| Franco et al. (87) | 51, 48 | Open label | 2 | (SBH)CPS, SGTC | 18, 12 | 61% in one, 75% in other;2/2 RR | |
| Valentin et al. (88) | 15 | Open label | 1 | SPS | 12 | >60% | |
| Nora et al. (89) | 30 | Open label | 1 | GTC | 40 | 87% | |
| Piacentino et al. (90) | 48 | Open label | 1 | CPS, GTC | 60 | 100%*** | |
| Jarvenpaa et al. (91) | 38 | Open label | 16 | NR | 24 | 12/16 RR | |
| CMNT | |||||||
| Velasco et al. (92) | 18 | Open label | 5 | GTC, CPS, MYO, DA | 6–37 | 80–100% GTC;60–80% CPS | |
| Fisher et al. (76) | 28 | Clinical trial (Double blind, cross over) | 6 | GTC | 9 | 30% | |
| Velasco et al. (74) | 19 | Clinical trial (Open label) | 13 | (LGS)AA, GTC, CPS, SGTC | 41.2 (12–4) | 81.6% (53.1–100%) LG;57.3% (13–98.6%) SGTC | |
| Chkhenkeli et al. (93) (& HCN, CDN) | 21–40 range | Single blind | 5 of 54 | SPS, GTC, CPS, SGTC, TS, PM | ≤18 | 4/5 “worthwhile improvement”;1/5 no improvement¥ | |
| Velasco et al. (75) | 13 | Open label | 13 | (LGS) AA, GTC | 46 (23–132) | 80% (30–100%) | |
| Andrade et al. (66) | NR | Open label | 2 | GTC, SPS, CPS, SGTC | ≤7 years | 0/2 RR | |
| Cukiert et al. (94) | 29 | Open label | 4 | DA, AA, MYO, TS, TC, TA | 18 (12–24) | 80% (65–98%) | |
| Valentin et al. (95) | 27 | Open label | 1 | RSE | 6 | 100% | |
| Valentin et al. (77) | 37 | Single blind | 11 | G or FLE | 24 (12–66) | 82% (40–100%) G;49% (0–92%) FLE;6/6 RR in G;1/5 RR in FLE | |
| Son et al. (96) | 29 | Open label | 14 | (LGS) SPS, CPS, GTC, G, DA, MYO, AA | 18.2 | 68% (25–100%);11/14 RR | |
| Valentin et al. (88) | 10, 8 | Open label | 2 | GTC, TS, TA, DA, MYO | 48, 18 | >60% in one; no significant reduction in other | |
Seizure types: G, Generalized; P, Partial; CS, Clonic; SPS, Simple partial seizure; CPS, Complex partial seizure; DA, Drop attack: atonic; DIA, Dialeptic; GTC, Generalized tonic-clonic; AA, Atypical absence; SGTC, Secondarily generalized; PM, Partial motor; TS, Tonic; TC, Tonic-clonic; TA, Typical Absence; HM, Hypermotor; AM, Automotor; MYO, Myoclonic; RSE, Refractory status epilepticus; DRA, Dravet Syndrome; SBH, Subcortical band heterotopia; LGS, Lennox-Gastaut Syndrome.
Targets: ATN, Anterior thalamic nucleus; CMNT, Centromedian nucleus of thalamus; HCN, Head of caudate nucleus; CDN, Cerebellar dentate nucleus.
Seizure onset: Bi, Bilateral; Uni, Unilateral; TLE, Temporal lobe epilepsy; MTLE, medial temporal lobe epilepsy; FLE, Frontal lobe epilepsy.
Outcomes: RR: Responders rate (Seizure reduction ≥50%); Serious Seizures: Potentially injurious seizures (SGTC or CPS associated with falls).
Other: NR, Not report.
SANTE trial control patients (22).
One died, not related to DBS (83).
The patient had long-term significant reduction in seizure frequency even with an absent electric stimulation (90).
Engel Classification (93).
Hippocampus
TLE is the most frequent focal epilepsy syndrome in humans and is frequently associated with hippocampal sclerosis and DRE. Temporal lobe resection is the optimal therapy for patients with refractory mesial TLE (97). Unfortunately, this is contraindicated in a considerable number of patients, including those whose seizures originate in both temporal lobes, those at risk for a postoperative decline in memory, cognitive function and language, and those who have had a previous temporal lobectomy but continue to have seizures originating from the contralateral temporal lobe (98).
Twenty years ago, hippocampal LFS [2-Hz pulses −500 μA base to peak, 1 ms duration biphasic square-wave pulses] was used to trigger seizures in experimental models (99). Recently, studies done in rat models of TLE have demonstrated that LFS applied at a frequency of 1 Hz, significantly reduced the excitability of the neuronal tissue, resulting in decreased seizure frequency (100). Remarkably, hippocampal HFS can protect hippocampal neurons against kainate neurotoxicity in macaques, likely via the inhibition of apoptosis (101).
Continuous electrical stimulation, especially with high frequencies (130 Hz), has been shown to completely inhibit picrotoxin- and high-K+-induced epileptiform activity in animal in vivo models of epilepsy (102, 103). Several clinical investigations support the anti-seizure effect of electrical stimulation of the hippocampus. Direct stimulation over the suspicious epileptogenic zone within the hippocampus is applied in this procedure. Two studies describe a decrease in interictal epileptiform discharges (93, 104). Téllez-Zenteno and his group reported a 15% seizure frequency reduction in four patients after unilateral DBS in the hippocampus (98). Boon et al. (105) studied the effect of DBS in medial temporal lobe in 10 patients with DRE. After mean follow-up of 31 months, one patient was seizure free, one demonstrated a more than 90% reduction in seizure frequency; five of 10 patients had ~50% seizure-frequency reduction; two had a reduction of 30–49%; and one remaining patient was a non-responder, with no change in seizure frequency. No serios clinical adverse effects (except an asymptomatic intracranial hemorrhage in one patient) or alterations in clinical neurological testing have been reported. In another study, McLachlan et al. (106) studied the effect of continuous bilateral electrical stimulation of the hippocampus in two patients with seizures originating from bilateral mesial temporal lobes. Seizure frequency decreased by 33% in both patients during stimulation and remained 25% lower for the 3 months after stimulation was turned off. No consistent changes were seen in objective or subjective measures of memory. No other adverse effects were reported. More surprisingly, Vonck et al. (107) described three patients with neuromodulation of the amygdala-hippocampal junction who exhibited a 50–90% decrease in seizures. The larger prospective, controlled, double-blind study evaluating the effects of Hippocampus-DBS in 16 TLE patients has shown that HFS (130 Hz) is effective in reducing seizure frequency in patients with refractory TLE. Fifty-percent of this cohort became seizure-free (108).
To evaluate anatomical and functional changes in amygdala-hippocampal function after DBS in TLE patients, several studies have been conducted. Velasco et al. (109) found that by extending the period of follow-up from 18 months to seven years, patients could be divided into two groups: patients with normal and abnormal MRI studies. Ninety five percent of patients with non-lesional epilepsy exhibited more than 95% of improvement, while only 50–70% of patients with hippocampal sclerosis identified on MRI showed improvement with DBS. None of these patients exhibited neuropsychological deterioration. Micro-lesions have been reported in a few epileptic patients, following the diagnostic implantation of depth electrodes in the temporal lobe (110, 111). Altogether, DBS appears to be a safe and valuable option for patients who suffer from drug-resistant TLE in whom resective surgery is contraindicated. Hippocampal DBS has also been found to be a relatively safe procedure. No irreversible cognitive or psychiatric deficits were encountered (112). However, even with the potential benefits, hippocampal stimulations cannot be considered as a first line therapy, in lieu of a resective procedure (Table 2).
Table 2.
Clinical studies of HC-DBS for the treatment of Epilepsy.
| Author/year | Mean age (years) | Type of study | n | Seizure type (s) | Follow up (months) | Average seizure reduction (range) |
|---|---|---|---|---|---|---|
| HC | ||||||
| Velasco et al. (113, 114) | 24 | Open label | 10 | (TLE) CPS, SGTC | 2 weeks | 100% after 6 days |
| Vonck, 2002 (115) | 33 | Open label | 3 | (MTLE) CPS, GTC | 5 (3–6) | 77% (50–94%) |
| Vonck et al. (116) | NR | Open label | 7 | (TLE)NR | 14 (5.5–21) | 43% (0–100%) |
| Tellez-Zenteno et al. (98) | 32 | Clinical trial (Double blind, cross over) | 4 | (MTLE)CPS, SGTC | 6 blind | 26% (ON) vs. −49% (OFF) |
| Boon et al. (105) | NR | Open label | 10 | (MTLE)CPS, SPS, SGTC | 31 (15–52) | 50% (< 30–100%) |
| Velasco et al. (109, 117) | 29 | Clinical trial | 9 | (MTLE)CPS, SGTC | 18 (1 blind) | 83% (50–100%);9/9 RR |
| McLachlan et al. (106) | 45, 54 | Clinical trial(Double blind, cross over) | 2 | NR | 3 | 33% (ON) vs. 4% (OFF) |
| Boex et al. (110)Bondallaz et al. (118) | 34 | Open label | 8 | (MTLE) CPS, SGTC | 44 | 67% (0–100%);6/8 RR |
| Tyrand, 2012 (119) | 32 | Open label | 12 | (TLE)NR | 0 | 58.1%* |
| Morrell et al. (53) (RNS trial)Heck et al. (54) (RNS trial) | 34.9 (18–66) | Clinical trial | 95 of 191 | SPS, CPS, SGTC | 3 blind 48 | 38% (ON) vs. 17% (OFF)53%,55% RR |
| Vonck et al. (107) | NR | Open label | 11 | (MTLE)CPS, SPS, SGTC | 96 (67–120) | 70% (0–100%);8/11 RR; |
| Cukiert et al. (57) | 37 | Single blind | 9 | (TLE) CPS, SPS, SGTC | 30.1 | 61% (−50–100%); 7/9 RR |
| Jin et al. (120) | NR | Open label | 3 | CPS, SGTC | 35 | 93% (91–95%) |
| Lim et al. (121) | 35 | Open label | 5 | CPS, SGTC | 38 | 45% (22–72%); 3/5 RR |
| Cukiert et al. (108) | 38.4 | Clinical trial (Double blind, randomized) | 16 | SPS, CPS | 8 (6 blind) | 3/14 RR in CPS, 7/16 RR in SPS |
Seizure types: P, Partial; CS, Clonic; SPS, Simple partial seizure; CPS, Complex partial seizure; DA, Drop attack: atonic; DIA, Dialeptic; GTC, Generalized tonic-clonic; AA, Atypical absence; SGTC, Secondarily generalized; PM, Partial motor; TS, Tonic; HM, Hypermotor; MYO, Myoclonic; PME, Progressive myoclonic epilepsy.
Targets: ATN, Anterior thalamic nucleus; VIM, Ventral intermediate nucleus of thalamus; STN, Subthalamic nucleus; HC, Hippocampus.
Seizure onset: Bi, Bilateral; Uni, Unilateral; TLE, Temporal lobe epilepsy; MTLE, medial temporal lobe epilepsy; FLE, Frontal lobe epilepsy.
Outcomes: RR: Responders rate (Seizure reduction ≥50%).
Other: NR, Not report.
Outcome: Reduction of interictal activity with biphasic stimulation in Hippocampal sclerosis (119).
Basal Ganglia
Caudate Nucleus (CN)
The CN may represent a deep target for the treatment of epilepsy. Low frequency electrical stimulation has been found to be efficacious when targeting the caudate (112). Interestingly, high frequency (30–100 Hz) stimulation of the head of the caudate nucleus (HCN) caused enhancement of epileptiform spikes from the ipsilateral hippocampus and amygdala while, on the contrary, LFS of the caudate reliably produced inhibitory effects bilaterally. The caudate loop is a functional entity comprised of the HCN, thalamus and neocortex. Activation of HCN is associated with hyperpolarization of cortical neurons, suggesting that suppression of seizure activity may be a result of stimulation-induced inhibition of the cortex (122).
Trials studying the effect of caudate stimulation on patients with epilepsy have not been systematically conducted and have yielded only marginal results. Sramka and Chkhenkeli (123) published a study of 74 patients showing reduced interictal epileptiform activity with both caudate and dentate nucleus stimulation. Chkhenkeli et al. (93) stimulated the ventral HCN at low frequency (4–8 Hz) in 38 patients, producing seizure freedom in 21 patients, while improving 35 patients overall (92%). This group was the first to describe benefit with striatal stimulation in cases of drug-resistant TLE. Their study was based on a pathophysiological hypothesis related to the balance of output between pro-convulsant and anti-convulsant structures, however, their work suggests that LFS of the CN is anti-epileptic (124). These results highlight the ability of the basal ganglia to modulate cortical epileptogenicity. Controlled clinical studies are necessary to determine efficacy and safety of this anatomical location. Currently, the CN is not the most frequently targeted structure in the treatment of DRE.
Subthalamic Nucleus (STN)
STN DBS has been explored as an option to treat motor seizures through the disruption of pathological cortical synchronization. Inhibition of the STN may potentially release the inhibitory effect of the substantia nigra pars reticulata on the dorsal midbrain anticonvulsant zone, thus raising the seizure threshold. This mechanistic rationale has arisen from observations in animal models (125, 126). The compact and distinct anatomical structure of the STN makes it a superior surgical target for electrode stimulation, which has been previously demonstrated in DBS-STN for treatment of PD.
Benabid et al. (127) reported a series of three patients with DRE who were implanted with STN-DBS. All patients were reported to exhibit important reductions in seizure frequency with stimulation; the first two patients exhibited reductions of 83 and 50%. Afterwards, the group reported a case describing a 5-year-old girl with DRE caused by focal centroparietal dysplasia, followed for 30 months. She had a 81% improvement in the number of seizures. This was most substantially reflected in reduction in cluster seizures (89%) and diurnal seizures (88%) (128).
Small studies have been conducted in severely impaired patients with severe DRE. Chabardes et al. (129) demonstrated a mean of 60% seizure frequency reduction in 80% of patients (4/5). They included patients aged from 5 to 38 years (17.6 ± 12.7). The stimulation was well-tolerated and appeared to demonstrate efficacy, however there were some complications related to the procedure in 40% (2/5) of patients; namely, a device infection in one patient, and a post-implantation subdural hematoma in a second patient, requiring re-operation.
Forty percent (2/5) of patients who underwent STN-DBS at the Cleveland Clinic Foundation reported a seizure rate reduction of 60% at 10 months of follow-up and 80% at 16 months (130). STN/substantia nigra DBS resulted in 30–100% reduction in seizure frequency of five patients with drug-resistant myoclonic epilepsy (131). STN-DBS may be a favorable target in certain epilepsy syndromes, but controlled, blinded trials are required to demonstrate efficacy and safety.
Cerebellum
The cerebellar nuclei have been some of the oldest targets in DBS, with initial uncontrolled trials in the 1970s (20) demonstrating potential efficacy in the treatment of epilepsy. Nuclear activation of inhibitory Purkinje cells, likely results in the suppression of excitatory cerebellar output to the thalamus and therefore, decreased excitatory thalamo-cortical projections, resulting in overall decreased cortical excitability (132). Disrupting thalamo-cortical activity has proven to be a useful approach to stop generalized spike-and-wave discharges in mice. It has been demonstrated that cerebellar nuclei are modulators of pathological oscillations during absence seizures (133).
Seventy six percent of epileptic patients (87/115) treated with cerebellar nuclei modulation demonstrated benefit with a reduction in seizure frequency. Overall, 27% reported seizure freedom and 49% reported reduction in seizure frequency and severity. Those patients with generalized tonic-clonic seizures benefited the most (134).
Velasco et al. (135) conducted a double blind, randomized control pilot study with five DRE patients with motor seizures. They implanted stimulating electrodes on the supero-medial cerebellar cortex and evaluated the efficacy and safety. After 6 months of stimulation, all patients reported a seizure rate reduction, on average 41% (14–75%) compared with the control group. At the end of 24 months, the three (60%) patients who completed follow up reported a further seizure reduction of 24% (11–38%) (135). In regards to the safety profile of the intervention, 60% of patients required another procedure owing to electrode migration, and 20% (one patient) suffered a severe local infection that finally resulted in long-term antibiotic therapy and removal of the entire stimulation system (136). After these preliminary studies, cerebellar stimulations have not been used in recent studies.
Hypothalamus
Due to the presence of epileptiform activity during depth electrode recordings of the mammillary bodies, the posterior hypothalamus has been suggested as a DBS target (137). The mammillo-thalamic tract stimulation has been used to treat gelastic seizures secondary to hypothalamic hamartomas, and has shown an improvement in seizure frequency and severity (138). A report of two patients by Franzini et al. (139) showed a reduction up to 80% in seizure frequency from baseline after 9 months of follow-up. This target may be unfavorable due to consequences of potential hemorrhage in this region during electrode implantation, as well as possible alterations in sleep-wake cycle (140). A recent study targeting the posteromedial hypothalamus reported nine patients with DRE, associated with intractable aggressive behavior, achieved a significant decrease in the frequency of epileptic seizures after up to 5 years of follow-up, achieving an average seizure reduction of 89.6% (141). Current experience with hypothalamic stimulation is too limited to draw firm conclusions. Other targets such as the caudal zona incerta, nucleus accumbens and fornix have been also explored (see Table 3).
Table 3.
Clinical studies of STN, Cerebellum (CB), HNC, CZI, pHT, NA, and Fornix -DBS for the treatment of Epilepsy.
| Author/year | Mean age (years) | Type of study | n | Seizure type (s) | Follow up (months) | Average seizure reduction (range) |
|---|---|---|---|---|---|---|
| STN | ||||||
| Benabid et al. (128) | 5 | Open label | 1 | SPS | 30 | 80.7% |
| Chabardes et al. (129) | 18 | Open label | 5 | TS, CS, GTC, HM | 18 (8–30) | 51.4% (0–80.7%) |
| Shon et al. (142) | 23, 22 | Open label | 2 | (FLE) TS | 18, 6 | 86.7% in one;88.6% in other |
| Handforth et al. (143) | 45, 46 | Open label | 2 | P | 26–32 | 50% and 33% |
| Lee et al. (79) (& ATN) | 20 | Open label | 3 | DIA, SGTC, TS | 18, 30, (1 loss) | 49.1% (20–71.4%) |
| Vesper et al. (144) | 39 | Open label | 1 | (PME), GTC, MYO | 12 | 50% MYO,100% GTC |
| Wille et al. (131) (& VIM) | 32 | Open label | 5 | (PME), GTC, MYO | 24 (12–42) | 30–100% |
| Capecci et al. (145) | 35, 30 | Open label | 2 | PM, GTC, DA, CPS, AA | 48, 18 | 65% in one and 0% in other |
| CB | ||||||
| Cooper et al. (20) | 29 | Open label | 15 | CPS, SGTC, GTC, MYO, TA | 27 | 10/15 “improved” |
| Van Buren et al. (19) | 27 | Double blind, cross over | 5 | CPS, SGTC, GTC, MYO | 15–21 range | No significant reduction |
| Levy et al. (146) | 29 | Open label | 6 | GTC | 7–20 range | 2/6 RR |
| Bidznski et al. (147) | NR | Open label | 14 | NR | 10–16 days | 13/14 “improved”; 1/14 no change |
| Wright et al. (148) | 30 | Clinical trial (Double blind, cross over) | 12 | GTC, DA, A, MYO, CPS | 6 blind | No statistically significant; 11/12 patients felt it helped |
| Davis et al. (149) | NR | Open label | 27 | Spastic seizures | 17 years | 23/27 improved; 4/27 no improvement |
| Chkhenkeli et al. (93) (& HCN, CDN) | 21–40 range | Single blind | 11 of 54 | GTC, CPS, SGTC, TS, PM | ≤18 | 5/11 seizure free;5/11 “worthwhile improvement”;1/11 no improvement* |
| Velasco et al. (135) | 26 | Clinical trial (Double blind, cross over) | 5 | GTC, TS, DA, MYO, AA | 24 (3 blind) | 67% (ON) vs. 7% (OFF);76% (62–89%) GTC; 57% (24–90%) TS |
| HCN | ||||||
| Chkhenkeli et al. (124) | NR | Open label | 57 | NR | NR | Unclear |
| Chkhenkeli et al. (93) (& CDN) | NR | Open label | 38 of 54 | GTC, CPS, SGTC, TS, PM | ≤18 | 21/38 Seizure free;14/38 “worthwhile improvement”;3/38 no improvement* |
| CZI | ||||||
| Franzini et al. (139) (& pHT) | 26 | Open label | 2 | (RS)SPS, SE | 6, 48 | 85% in one, andremission of SE in other;2/2 RR |
| Anderson et al. (150) | 20 | Open label | 3 | (NSPM)GTC, MYO, TA | 4.3 years (3–6) | 3/3 “improved” |
| pHT | ||||||
| Franzini et al. (139) | 20, 36 | Open label | 2 | DA, MYO, CPS | 9, 60 | 75% in one and 80% in other;2/2 RR |
| Benedetti et al. (141) | 21 | Open label | 5 | SPS, CPS, GTC, AA, DA, | 5 years | 89.6% (25–100%);5/5 RR |
| NA | ||||||
| Schmitt et al. (151) | 42 | Open label | 5 | SPS, CPS, GTC | 6 | 37.5% median; no significant changes in mean frequencies;2/5 RR of DS |
| Kwoski et al. (152) | 37 | Clinical trial (double blind, cross over) | 4 | SPS, CPS, SGTC | 15 (6 blind) | 17.2% (ON) vs. −1,6 (OFF) of DS at 28 days;3/4 RR of DS |
| FORNIX | ||||||
| Koubeissi et al. (26) | 41 | Open label | 7 | (MTLE), SPS, CPS | 1–9 days | Seizure odd reduced by 92% for day 1–2 |
Seizure types: SPS, Simple partial seizure; CPS, Complex partial seizure; TA, Typical Absence; GTC, Generalized tonic-clonic; SGTC, Secondarily generalized; SE, Status epilepticus; DA, Drop attack: atonic; AA, Atypical absence; MYO, Myoclonic; PM, Partial motor; TS, Tonic; RS, Rassmusen syndrome; NSPM, North Sea Progressive Myoclonic Epilepsy.
Targets: CB, Cerebellum; HCN, Head of caudate nucleus; CZI, Caudal zona incerta; pHT, Posterior hypothalamus; NA, Nucleus accumbens; CDN, Cerebellar dentate nucleus.
Seizure onset: Bi, Bilateral; Uni, Unilateral; MTLE, medial temporal lobe epilepsy.
Outcomes: RR: Responders rate (Seizure reduction ≥50%); DS: Disabling seizures (CPS+ GTC).
Other: NR, Not report.
Engel Classification (93).
Adverse Effects
There are well-known side effects and potential complications associated with DBS, which have been mainly elucidated by the literature regarding DBS in the treatment of movement disorders (153). The overall complication rate for DBS surgeries in patients with PD was 7%, which included mechanical complications (3%), hemorrhage or infarction (1%), lead removal (1%), hematoma (0.4%), and infection (0.4%) (154). Comparatively limited information is available regarding the specific complications of DBS in patients with epilepsy. The most common stimulation-related side effects in the SANTE studies were mainly those expected from implanted electrodes, including stimulation-related paresthesias (22.7%), implant site pain (20.9%), implant site infection (12.7%), and subclinical bleeding around electrodes (22). Other known complications include: wound infection; lead or extension fracture; erosion; lead tract fibrosis; electrode migration; external interference with other devices; equipment infection; pain; transient worsening or new seizures; and dizziness (59); skin complications [such as abrasions, ulcerations and aseptic necrosis (155)]; hardware discomfort; ineffective product (85, 156); and peri-electrode edema (157) that may produce disorientation, gait instability, headache, seizure or acute confusion (155).
Psychiatric Side Effects
Depression and Suicide
A large variability in the use of diagnostic scales measuring depression is noted in the existing literature regarding DBS. It is therefore difficult to directly compare the results of the studies. Anterior thalamic DBS was initially reported to be associated with higher rates of memory deficits and depression (158); however, 5-year (85) and 7-year follow-up (156) of the SANTE study population found no significant deterioration in cognition or depression scores. Randomized multicenter and observational studies have shown improvement in depression and anxiety scores after DBS in patients with PD (159, 160). However, higher prevalence of depression after STN-DBS has also been reported (158, 161). PD patients treated with STN-DBS were also found to have a higher suicide rate, in a paper published a decade ago. Suicidal behavior was frequently associated with postoperative depression and altered impulse regulation (162, 163). A recent randomized controlled trial did not find a direct association between suicide and DBS (164). This may be indicative of improved patient selection criteria in recent years. This is further complicated by the fact that suicide after DBS has occurred not only with different anatomic targets (particularly with thalamic and GPi stimulation) but also with other diseases (dystonia and essential tremor) (165). Although the evidence is contradictory and scarce in epilepsy, all patients should be carefully screened for suicide risk as part of the presurgical workup for DBS surgery. Additionally, patients should be monitored closely for depression and suicidality post-operatively.
Apathy
Apathy has been frequently reported as a possible adverse effect of STN-DBS, however the existing literature is controversial. Some authors have found significant worsening of apathy scores 3–6 months after surgery (166). They hypothesized a direct influence of STN-DBS on the limbic system by diffusion of stimulus to the medial limbic compartment of STN. Recently, a metanalysis assessing apathy following bilateral DBS of the STN in PD concluded that the reduction of dopaminergic medication after surgery may be the cause of worsening apathy in this patient population (167). Other authors have failed to find significant differences in apathy prevalence or severity between surgical and non-surgical patients (168). On the contrary, some publications have stated positive psychiatric side effects of DBS (169). Results of STN stimulation in patients with PD in a Polish study confirmed the positive effects of stimulation on drive and ability to feel pleasure. The authors demonstrated improvement of mood, sleep and apathy following the first month after initiation of stimulation, which was seen independently of improvements in motor symptoms (170).
Cognitive Deterioration
Cognitive decline reported after DBS mainly affects frontal subcortical cognitive functions, such as verbal fluency, processing speed, attention, learning, and working memory (171). Worse cognitive outcomes after surgery remained unchanged, regardless of DBS settings or “on” and “off” motor states, suggesting the cause might be related to lead trajectory or location (172). Some psychiatric side effects have been also described, such as psychosis (for example, delusions of marital infidelity resembling Othello syndrome), and impulse control disorders, such as binge eating, hypersexuality, hypomania, and secondary increase in body weight (155).
Other Side Effects
Other less mentioned and more infrequently encountered, but nonetheless important complications include post-surgical headache, instability and gait disorders with falls (170, 173), as well as speech disorders (dysarthria, intelligibility, pitch variation in speech, worsening hypophonia, stuttering, and speech articulation problems) (155, 174). When the device is less frequently used, the complications are higher, for instance, bilateral cerebellar stimulation has been associated with re-operation in 60% and serious complications in 20% of the patients (136).
Epilepsy Comorbidities
People with epilepsy in the general population have a two to five-fold risk of somatic comorbid conditions compared to people without epilepsy. Thus, there is a clear need for an integrated approach in patients with epilepsy, especially in those with DRE who are good candidates for electrical stimulation. The process of localizing DBS targets is undergoing continuous evolution. The clinical effects of DBS are likely due to the activation of complex, widespread neuronal networks, directly and indirectly influenced by the stimulation of a single isolated target. The delivery of such stimulation may aid in the discovery of strategically combined targets for electrical stimulation to treat additional neurological, psychiatric, cognitive and somatic disorders. Computational modeling (experimental and clinical), engineering designs, and neuroimaging techniques play a critical role in this process (175).
Depression
Depression is the most common comorbidity of epilepsy, affecting between 10% to 60% of patients with seizures (176). Different targets have been used in patients with refractory major depressive disorder, such as subcallosal cingulate gyrus (Brodmann area 25), ventral capsule/ventral striatum, medial forebrain bundle, and the nucleus accumbens. The overall effect sizes have shown a significant reduction in Hamilton depression rating scale scores after DBS stimulation in these four regions (177). Selected patients with refractory depression and DRE could benefit from stimulation of these targets.
Obesity
There is some evidence that obesity may be more common in people with epilepsy than in the general population (178). The pathophysiology of obesity is complex, involving both altered patterns of eating and satiety, as well as compulsive behavior surrounding food intake. Proposed stimulation targets to treat obesity therefore include the hypothalamus and nucleus accumbens (179). To date, experience with DBS for the treatment of obesity is limited. Although surgery has been proven to be safe, no definitive conclusions can be made as to whether it is effective. There is an opportunity to study this further in patients with epilepsy and obesity, especially in those with gelastic seizures secondary to hypothalamic hamartomas. Further work is needed to address target selection, the kinetics of DBS, and the ideal stimulation parameters in this population.
Psychogenic Non-epileptic Seizures
Psychogenic non-epileptic seizures (PNES) are one of the most common differential diagnoses of epilepsy. PNES are involuntary episodes of any combination of altered movement, sensation, or awareness that bears resemblance to epileptic seizures, but are not accompanied by epileptiform electrical discharges (180). Unfortunately, PNES are an exclusion criterion for DBS candidates in most of the epilepsy centers. There are few reports of the experience with psychogenic movements disorders and DBS. This literature suggests that DBS does not produce side effects in patients with psychogenic disorders. One patient with psychogenic parkinson-like disorder underwent HFS of the STN for approximately 5 years without side effects, prior to the device being turned off (181). Similarly, authors from Bethesda described two cases of psychogenic dystonia who underwent DBS in the GPi, after initially being thought to have organic dystonia (182). DBS did not lead to any benefits or side effects for these patients. These reports further highlight the safety of DBS in neuronal tissue.
Limitations
Many of the available publications are non-randomized, unblinded, uncontrolled small studies. Therefore, the evidence is susceptible to major biases:
Regression to mean: patients are typically implanted when there is high seizure burden. In all chronic diseases, phases of high activity and lower activity are observed, thus seizure burden may have returned to baseline without any therapy.
Placebo effect: even in some controlled studies, there is no “sham-surgery” arm that would allow for estimation of the effect of an invasive procedure on the subjective seizure assessment.
High expectations of both investigator and patients, resulting highly suggestible results.
As well, in most studies, concomitant drug changes were allowed, but were usually not reported; therefore, the effect of stimulation cannot be accurately measured. Finally, stimulation paradigms were frequently chosen not on a pathophysiological basis, but rather on convenience and/or chance: e.g., the 50 Hz and pulse in STN stimulus is based on previously chosen paradigms for PD treatment. There are usually four or five different stimulation variables that are freely combined, resulting in hundreds of different combinations. Further investigations are necessary to solve these limitations in this promissory area.
Future Directions
The scope of DBS is increasing rapidly in parallel with the understanding of brain circuitry dysfunction in many pathologies (183). Investigators are only just beginning to realize the full potential of this growing field (184). The future of DBS depends on technological advances in the area: the focus should be the improvement of clinical knowledge as well as improved practicality (smaller size devices, increased battery life, greater tolerance and safety profiles, improved software). Further understanding of the mechanisms underlying cerebral circuits in epilepsy and its comorbidities is required, in order to further define specific criteria and predictors in the selection of patients who could benefit from DBS. This will be only possible investing resources in basic research, essential as it forms the foundation upon which translational research and clinical trials are built on. The poor results of DBS treatment in some patients as reported in the literature, may be mitigated in the future with improvement in patient selection, better target identification, and the development of more effective stimulation paradigms, such as closed-loop stimulation (184).
The identification of optimal targets for specific subgroups of patients, including patients with comorbidities, especially those with psychiatric disorders, is crucial for further development (185). Basic science studies have been fundamental in advancing the understanding of the complexity of alterations in neuronal networks and continue to provide valuable information for researchers and clinicians, allowing for further clinical developments, particularly in the identification of these practical targets. Modern methodologies such as EEG-fMRI studies are allowing for the delineation of epileptogenic neuronal networks, which is increasingly accepted over the classic concept of the epileptogenic zone (186). Analysis of epileptic neuronal networks will help guide treatments in a more precise way, hopefully resulting in more efficacious treatments.
Advances in surgical procedures may include the implementation of new electrodes to treat a single symptom synergistically or multiple symptoms at once, which would additionally allow for the establishment of different stimulation parameters for each electrode contact.
Finally, optogenetics rests on the use of genetically-encoded, light sensitive proteins, such as opsins, to modulate neuronal activity, intracellular signaling pathways, or gene expression with spatial, directional, temporal, and cell-type specificity (187). Epilepsy is one the disorders that has been widely explored in this regard. The ability to inhibit or activate different neurons with high specificity and resolution has turned it into an exciting research tool and a possible therapeutic intervention targeting neurons with abnormal activity (188). Studies in several animal models have shown a reduction in seizure activity both electrographically and clinically (189–191). Even so, there are still several limitations that must be overcome prior to its application in humans, particularly the ~1000-fold difference in brain volume between the rodent models and the much larger human neuronal circuits that makes optogenetic control difficult.
Conclusion
DBS is one of the most remarkable interventions in the history of functional neurosurgery. Whether it will significantly improve the outcomes of DRE patients remains to be seen. DBS tends to have better results in patients with generalized epilepsy, although it has been used with success in some patients with focal-onset seizures. The best DBS targets for each epileptic syndrome, as well as the optimal combination of stimulation variables for each target remains speculative, however ATN stimulation has been performed in the highest number of patients and with the most rigorous study protocol allowing it to be recommended over the other targets. Results in hippocampal and frontal stimulation are suboptimal and should be reserved only for patients in whom resective procedures are contraindicated. Surgery should be recommended before potential hippocampal or frontal stimulation is considered. Targets, such as the CN and cerebellar nuclei, need more exploration. There is a need for large, well-designed randomized control trials to validate and optimize the efficacy and safety of DBS.
Author Contributions
NZ and FS conceived and designed the analysis. NZ and LL initiated the collaborative project, collected the data, performed the analysis and wrote the paper. JO-H and LL monitored data collection, cleaned the data, and drafted the paper. AC and JT-Z revised the paper.
Conflict of Interest Statement
JT-Z receives grants from the University of Saskatchewan, the Royal University Hospital Foundation in Saskatoon, Saskatchewan, and the Saskatchewan Health Research Foundation. AC receives grants from the Royal University Hospital Foundation in Saskatoon, Saskatchewan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Jobst BC, Darcey TM, Thadani VM, Roberts DW. Brain stimulation for the treatment of epilepsy. Epilepsia. (2010) 51 (Suppl. 3):88–92. 10.1111/j.1528-1167.2010.02618.x [DOI] [PubMed] [Google Scholar]
- 2.Nagel SJ, Najm IM. Deep brain stimulation for epilepsy. Neuromodulation. (2009) 12:270–80. 10.1111/j.1525-1403.2009.00239.x [DOI] [PubMed] [Google Scholar]
- 3.DeLong M, Wichmann T. Deep brain stimulation for movement and other neurologic disorders. Ann N Y Acad Sci. (2012) 1265:1–8. 10.1111/j.1749-6632.2012.06608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpern C, Hurtig H, Jaggi J, Grossman M, Won M, Baltuch G. Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord. (2007) 13:1–16. 10.1016/j.parkreldis.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Schiefer TK, Matsumoto JY, Lee KH. Moving forward: advances in the treatment of movement disorders with deep brain stimulation. Front Integr Neurosci. (2011) 5:69. 10.3389/fnint.2011.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey S, Sarma N. Deep brain stimulation: current status. Neurol India. (2015) 63:9–18. 10.4103/0028-3886.152623 [DOI] [PubMed] [Google Scholar]
- 7.French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. (2004) 62:1261–73. 10.1212/01.WNL.0000123695.22623.32 [DOI] [PubMed] [Google Scholar]
- 8.Halpern CH, Attiah MA, Tekriwal A, Baltuch GH. A step-wise approach to deep brain stimulation in mice. Acta Neurochir. (2014) 156:1515–21. 10.1007/s00701-014-2062-4 [DOI] [PubMed] [Google Scholar]
- 9.Schwalb JM, Hamani C. The history and future of deep brain stimulation. Neurotherapeutics. (2008) 5:3–13. 10.1016/j.nurt.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horsley V, Clarke RH. The structure and functions of the cerebellum examined by a new method. Brain. (1908) 31:45–124. 10.1093/brain/31.1.45 [DOI] [Google Scholar]
- 11.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic apparatus for operations on the human brain. Science. (1947) 106:349–50. 10.1126/science.106.2754.349 [DOI] [PubMed] [Google Scholar]
- 12.Albe Fessard D, Arfel G, Guiot G, Derome P, Dela H, Korn H, et al. Characteristic electric activities of some cerebral structures in man. Ann Chirurg. (1963) 17:1185–214. [PubMed] [Google Scholar]
- 13.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. (1973) 29:158–61. 10.1001/archneur.1973.00490270040005 [DOI] [PubMed] [Google Scholar]
- 14.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. (1989) 12:366–75. 10.1016/0166-2236(89)90074-X [DOI] [PubMed] [Google Scholar]
- 15.Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, et al. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. (1989) 32:213–26. 10.1016/0306-4522(89)90120-6 [DOI] [PubMed] [Google Scholar]
- 16.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Movement Disorders. (1991) 6:288–92. 10.1002/mds.870060404 [DOI] [PubMed] [Google Scholar]
- 17.Pollak P, Benabid AL, Gross C, Gao DM, Laurent A, Benazzouz A, et al. [Effects of the stimulation of the subthalamic nucleus in Parkinson disease]. Revue Neurol. (1993) 149:175–6. [PubMed] [Google Scholar]
- 18.Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. (1994) 35:1126–9; discussion 9–30. 10.1227/00006123-199412000-00016 [DOI] [PubMed] [Google Scholar]
- 19.Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. (1978) 48:407–16. 10.3171/jns.1978.48.3.0407 [DOI] [PubMed] [Google Scholar]
- 20.Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP. Chronic cerebellar stimulation in epilepsy: clinical and anatomical studies. Arch Neurol. (1976) 33:559–70. 10.1001/archneur.1976.00500080037006 [DOI] [PubMed] [Google Scholar]
- 21.Upton AR, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS. Evoked metabolic responses in the limbic-striate system produced by stimulation of anterior thalamic nucleus in man. Pacing Clin Electrophysiol. (1987) 10 (1 Pt 2):217–25. 10.1111/j.1540-8159.1987.tb05952.x [DOI] [PubMed] [Google Scholar]
- 22.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. (2010) 51:899–908. 10.1111/j.1528-1167.2010.02536.x [DOI] [PubMed] [Google Scholar]
- 23.Hamani C, Hodaie Lozano AM. Present and future of deep brain stimulation for refractory epilepsy. Acta Neurochir. (2005) 147:227 10.1007/s00701-004-0474-2 [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation. (2013) 16:10–24. 10.1111/j.1525-1403.2012.00501.x [DOI] [PubMed] [Google Scholar]
- 25.Durand D. Electrical stimulation can inhibit synchronized neuronal activity. Brain Res. (1986) 382:139–44. 10.1016/0006-8993(86)90121-6 [DOI] [PubMed] [Google Scholar]
- 26.Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. (2013) 74:223–31. 10.1002/ana.23915 [DOI] [PubMed] [Google Scholar]
- 27.Mirski MA, Fisher RS. Electrical stimulation of the mammillary nuclei increases seizure threshold to pentylenetetrazol in rats. Epilepsia. (1994) 35:1309–16. 10.1111/j.1528-1157.1994.tb01803.x [DOI] [PubMed] [Google Scholar]
- 28.Mirski MA, Ferrendelli JA. Interruption of the mammillothalamic tract prevents seizures in guinea pigs. Science. (1984) 226:72–4. 10.1126/science.6433485 [DOI] [PubMed] [Google Scholar]
- 29.Lega BC, Halpern CH, Jaggi JL, Baltuch GH. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiol Dis. (2010) 38:354–60. 10.1016/j.nbd.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 30.Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, et al. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. (2017) 93:194–210. 10.1016/j.neuron.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detre JA, Alsop DC, Aguirre GK, Sperling MR. Coupling of cortical and thalamic ictal activity in human partial epilepsy: demonstration by functional magnetic resonance imaging. Epilepsia. (1996) 37:657–61. 10.1111/j.1528-1157.1996.tb00630.x [DOI] [PubMed] [Google Scholar]
- 32.Mondragon S, Lamarche M. Suppression of motor seizures after specific thalamotomy in chronic epileptic monkeys. Epilepsy Res. (1990) 5:137–45. 10.1016/0920-1211(90)90030-Y [DOI] [PubMed] [Google Scholar]
- 33.Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. (1998) 80:1439–55. 10.1152/jn.1998.80.3.1439 [DOI] [PubMed] [Google Scholar]
- 34.Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. (2004) 123:299–336. 10.1016/j.neuroscience.2003.08.051 [DOI] [PubMed] [Google Scholar]
- 35.Polack P-O, Charpier S. Intracellular activity of cortical and thalamic neurones during high-voltage rhythmic spike discharge in Long-Evans rats in vivo. J Physiol. (2006) 571:461–76. 10.1113/jphysiol.2005.100925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. (2014) 11:508–26. 10.1007/s13311-014-0279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiken S, Nambu A. Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci. (2014) 8:33. 10.3389/fnsys.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladas TP, Chiang CC, Gonzalez-Reyes LE, Nowak T, Durand DM. Seizure reduction through interneuron-mediated entrainment using low frequency optical stimulation. Exp Neurol. (2015) 269:120–32. 10.1016/j.expneurol.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. (2009) 63:508–22. 10.1016/j.neuron.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. (1975) 98:417–40. 10.1016/0006-8993(75)90364-9 [DOI] [PubMed] [Google Scholar]
- 41.Montgomery EB. Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol. (2006) 117:2691–702. 10.1016/j.clinph.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 42.Kang G, Lowery M. Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson's disease: a simulation study. Front Comput Neurosci. (2014) 8:32. 10.3389/fncom.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. (2010) 11:227–38. 10.1038/nrn2803 [DOI] [PubMed] [Google Scholar]
- 44.Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MG, Denys D, Reynolds BA, et al. Deep brain stimulation and the role of astrocytes. Mol Psychiatry. (2012) 17:124–31. 10.1038/mp.2011.61 [DOI] [PubMed] [Google Scholar]
- 45.Tawfik VL, Chang SY, Hitti FL, Roberts DW, Leiter JC, Jovanovic S, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. (2010) 67:367–75. 10.1227/01.NEU.0000371988.73620.4C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. (2017) 13:548. 10.1038/nrneurol.2017.105 [DOI] [PubMed] [Google Scholar]
- 47.Jahanshahi A, Schonfeld LM, Lemmens E, Hendrix S, Temel Y. In vitro and in vivo neuronal electrotaxis: a potential mechanism for restoration? Mol Neurobiol. (2014) 49:1005–16. 10.1007/s12035-013-8575-7 [DOI] [PubMed] [Google Scholar]
- 48.Kadar E, Lim LW, Carreras G, Genis D, Temel Y, Huguet G. High-frequency stimulation of the ventrolateral thalamus regulates gene expression in hippocampus, motor cortex and caudate-putamen. Brain Res. (2011) 1391:1–13. 10.1016/j.brainres.2011.03.059 [DOI] [PubMed] [Google Scholar]
- 49.Vedam-Mai V, Gardner B, Okun MS, Siebzehnrubl FA, Kam M, Aponso P, et al. Increased precursor cell proliferation after deep brain stimulation for Parkinson's disease: a human study. PLoS ONE. (2014) 9:e88770. 10.1371/journal.pone.0088770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergey GK. Neurostimulation in the treatment of epilepsy. Exp Neurol. (2013) 244:87–95. 10.1016/j.expneurol.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 51.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. (2015) 84:810–7. 10.1212/WNL.0000000000001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. (2014) 11:553–63. 10.1007/s13311-014-0280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. (2011) 77:1295–304. 10.1212/WNL.0b013e3182302056 [DOI] [PubMed] [Google Scholar]
- 54.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. (2014) 55:432–41. 10.1111/epi.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gionfriddo MR, Greenberg AJ, Wahegaonkar AL, Lee KH. Pathways of translation: deep brain stimulation. Clin Transl Sci. (2013) 6:497–501. 10.1111/cts.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth A, Buttrick SS, Cajigas I, Jagid JR, Ivan ME. Accuracy of frame-based and frameless systems for deep brain stimulation: a meta-analysis. J Clin Neurosci. (2018) 57:1–5. 10.1016/j.jocn.2018.08.039 [DOI] [PubMed] [Google Scholar]
- 57.Cukiert A, Cukiert CM, Burattini JA, Lima AM. Seizure outcome after hippocampal deep brain stimulation in a prospective cohort of patients with refractory temporal lobe epilepsy. Seizure. (2014) 23:6–9. 10.1016/j.seizure.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 58.Lim SN, Lee ST, Tsai YT, Chen IA, Tu PH, Chen JL, et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. (2007) 48:342–7. 10.1111/j.1528-1167.2006.00898.x [DOI] [PubMed] [Google Scholar]
- 59.Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. (2018) 59:273–90. 10.1111/epi.13964 [DOI] [PubMed] [Google Scholar]
- 60.Edwards CA, Kouzani A, Lee KH, Ross EK. Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin Proc. (2017) 92:1427–44. 10.1016/j.mayocp.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 61.Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. (2002) 43:603–8. 10.1046/j.1528-1157.2002.26001.x [DOI] [PubMed] [Google Scholar]
- 62.Kotagal P. Neurostimulation: vagus nerve stimulation and beyond. Semin Pediatr Neurol. (2011) 18:186–94. 10.1016/j.spen.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 63.Osorio I, Overman J, Giftakis J, Wilkinson SB. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. (2007) 48:1561–71. 10.1111/j.1528-1167.2007.01044.x [DOI] [PubMed] [Google Scholar]
- 64.Lee KJ, Shon YM, Cho CB. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact Funct Neurosurg. (2012) 90:379–85. 10.1159/000339991 [DOI] [PubMed] [Google Scholar]
- 65.Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French JA, Blum DE, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. (2004) 45:346–54. 10.1111/j.0013-9580.2004.01304.x [DOI] [PubMed] [Google Scholar]
- 66.Andrade DM, Zumsteg D, Hamani C, Hodaie M, Sarkissian S, Lozano AM, et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. (2006) 66:1571–3. 10.1212/01.wnl.0000206364.19772.39 [DOI] [PubMed] [Google Scholar]
- 67.Voges BR, Schmitt FC, Hamel W, House PM, Kluge C, Moll CK, et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia. (2015) 56:e99–e103. 10.1111/epi.13045 [DOI] [PubMed] [Google Scholar]
- 68.Mottonen T, Katisko J, Haapasalo J, Tahtinen T, Kiekara T, Kahara V, et al. Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: delineation using 3 T MRI and intraoperative microelectrode recording. Neuroimage Clin. (2015) 7:823–9. 10.1016/j.nicl.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaseja H. Expanding the therapeutic spectrum of anterior thalamic nucleus deep brain stimulation in intractable epilepsy: a postulation. Epilepsy Behav. (2015) 43:46–7. 10.1016/j.yebeh.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 70.Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. (2005) 481:127–44. 10.1002/cne.20348 [DOI] [PubMed] [Google Scholar]
- 71.Gorji A, Mittag C, Shahabi P, Seidenbecher T, Pape HC. Seizure-related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiol Dis. (2011) 43:266–74. 10.1016/j.nbd.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 72.Banerjee PK, Snead OC, 3rd. Thalamic mediodorsal and intralaminar nuclear lesions disrupt the generation of experimentally induced generalized absence-like seizures in rats. Epilepsy Res. (1994) 17:193–205. 10.1016/0920-1211(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 73.Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. (2015) 56:114–24. 10.1111/epi.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Velasco F, Velasco M, Jimenez F, Velasco AL, Brito F, Rise M, et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. (2000) 47:295–304; discussion 5. 10.1097/00006123-200008000-00007 [DOI] [PubMed] [Google Scholar]
- 75.Velasco AL, Velasco F, Jimenez F, Velasco M, Castro G, Carrillo-Ruiz JD, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. (2006) 47:1203–12. 10.1111/j.1528-1167.2006.00593.x [DOI] [PubMed] [Google Scholar]
- 76.Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. (1992) 33:841–51. 10.1111/j.1528-1157.1992.tb02192.x [DOI] [PubMed] [Google Scholar]
- 77.Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. (2013) 54:1823–33. 10.1111/epi.12352 [DOI] [PubMed] [Google Scholar]
- 78.Sussman N. M., Goldman H. W., Jackel R. A., Kaplan L., Callanan M., Berger J., et al. Anterior thalamic stimulation in medically refractory epilepsy. Part II: Preliminary clinical results. Epilepsia. (1988) 29:677. [Google Scholar]
- 79.Lee KJ, Jang KS, Shon YM. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir Suppl. (2006) 99:87–91. 10.1007/978-3-211-35205-2_17 [DOI] [PubMed] [Google Scholar]
- 80.Andrade DM, Hamani C, Lozano AM, Wennberg RA. Dravet syndrome and deep brain stimulation: seizure control after 10 years of treatment. Epilepsia. (2010) 51:1314–6. 10.1111/j.1528-1167.2009.02408.x [DOI] [PubMed] [Google Scholar]
- 81.Oh YS, Kim HJ, Lee KJ, Kim YI, Lim SC, Shon YM. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. (2012) 21:183–7. 10.1016/j.seizure.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 82.Van Gompel JJ, Klassen BT, Worrell GA, Lee KH, Shin C, Zhao CZ, et al. Anterior nuclear deep brain stimulation guided by concordant hippocampal recording. Neurosurg Focus. (2015) 38:E9 10.3171/2015.3.FOCUS1541 [DOI] [PubMed] [Google Scholar]
- 83.Piacentino M, Durisotti C, Garofalo PG, Bonanni P, Volzone A, Ranzato F, et al. Anterior thalamic nucleus deep brain Stimulation (DBS) for drug-resistant complex partial seizures (CPS) with or without generalization: long-term evaluation and predictive outcome. Acta Neurochir. (2015) 157:1525–32; discussion 32. 10.1007/s00701-015-2498-1 [DOI] [PubMed] [Google Scholar]
- 84.Lehtimaki K, Mottonen T, Jarventausta K, Katisko J, Tahtinen T, Haapasalo J, et al. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. (2016) 9:268–75. 10.1016/j.brs.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 85.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. (2015) 84:1017–25. 10.1212/WNL.0000000000001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krishna V, King NK, Sammartino F, Strauss I, Andrade DM, Wennberg RA, et al. Anterior nucleus deep brain stimulation for refractory epilepsy: insights into patterns of seizure control and efficacious target. Neurosurgery. (2016) 78:802–11. 10.1227/NEU.0000000000001197 [DOI] [PubMed] [Google Scholar]
- 87.Franco A, Pimentel J, Campos AR, Morgado C, Pinelo S, Ferreira AG, et al. Stimulation of the bilateral anterior nuclei of the thalamus in the treatment of refractory epilepsy: two cases of subcortical band heterotopia. Epileptic Disord. (2016) 18:426–30. 10.1684/epd.2016.0878 [DOI] [PubMed] [Google Scholar]
- 88.Valentin A, Selway RP, Amarouche M, Mundil N, Ughratdar I, Ayoubian L, et al. Intracranial stimulation for children with epilepsy. Eur J Paediatr Neurol. (2017) 21:223–31. 10.1016/j.ejpn.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 89.Nora T, Heinonen H, Tenhunen M, Rainesalo S, Järvenpää S, Lehtimäki K, et al. Stimulation induced electrographic seizures in deep brain stimulation of the anterior nucleus of the thalamus do not preclude a subsequent favorable treatment response. Front Neurol. (2018) 9:66 10.3389/fneur.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piacentino M, Beggio G, Zordan L, Bonanni P. Hippocampal deep brain stimulation: persistent seizure control after bilateral extra-cranial electrode fracture. Neurol Sci. (2018) 39:1431–5. 10.1007/s10072-018-3444-9 [DOI] [PubMed] [Google Scholar]
- 91.Jarvenpaa S, Rosti-Otajarvi E, Rainesalo S, Laukkanen L, Lehtimaki K, Peltola J. Executive functions may predict outcome in deep brain stimulation of anterior nucleus of thalamus for treatment of refractory epilepsy. Front Neurol. (2018) 9:324. 10.3389/fneur.2018.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Velasco F, Velasco M, Ogarrio C, Fanghanel G. Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: a preliminary report. Epilepsia. (1987) 28:421–30. 10.1111/j.1528-1157.1987.tb03668.x [DOI] [PubMed] [Google Scholar]
- 93.Chkhenkeli SA, Sramka M, Lortkipanidze GS, Rakviashvili TN, Bregvadze E, Magalashvili GE, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. (2004) 106:318–29. 10.1016/j.clineuro.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 94.Cukiert A, Burattini JA, Cukiert CM, Argentoni-Baldochi M, Baise-Zung C, Forster CR, et al. Centro-median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure. (2009) 18:588–92. 10.1016/j.seizure.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 95.Valentin A, Nguyen HQ, Skupenova AM, Agirre-Arrizubieta Z, Jewell S, Mullatti N, et al. Centromedian thalamic nuclei deep brain stimulation in refractory status epilepticus. Brain Stimul. (2012) 5:594–8. 10.1016/j.brs.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 96.Son BC, Shon YM, Choi JG, Kim J, Ha SW, Kim SH, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. (2016) 94:187–97. 10.1159/000446611 [DOI] [PubMed] [Google Scholar]
- 97.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. (2001) 345:311–8. 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- 98.Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. (2006) 66:1490–4. 10.1212/01.wnl.0000209300.49308.8f [DOI] [PubMed] [Google Scholar]
- 99.Katsumori H, Minabe Y, Osawa M, Ashby CR Jr. Acute effects of various GABA receptor agonists and glutamate antagonists on focal hippocampal seizures in freely moving rats elicited by low-frequency stimulation. Synapse. (1998) 28:103–9. [DOI] [PubMed] [Google Scholar]
- 100.Rashid S, Pho G, Czigler M, Werz MA, Durand DM. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia. (2012) 53:147–56. 10.1111/j.1528-1167.2011.03348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen N, Gao Y, Yan N, Liu C, Zhang JG, Xing WM, et al. High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience. (2014) 256:370–8. 10.1016/j.neuroscience.2013.10.059 [DOI] [PubMed] [Google Scholar]
- 102.Wyckhuys T, Raedt R, Vonck K, Wadman W, Boon P. Comparison of hippocampal Deep Brain Stimulation with high (130Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Res. (2010) 88:239–46. 10.1016/j.eplepsyres.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 103.Lian J, Bikson M, Sciortino C, Stacey WC, Durand DM. Local suppression of epileptiform activity by electrical stimulation in rat hippocampus in vitro. J Physiol. (2003) 547 (Pt 2):427–34. 10.1113/jphysiol.2002.033209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto J, Ikeda A, Satow T, Takeshita K, Takayama M, Matsuhashi M, et al. Low-frequency electric cortical stimulation has an inhibitory effect on epileptic focus in mesial temporal lobe epilepsy. Epilepsia. (2002) 43:491–5. 10.1046/j.1528-1157.2002.29001.x [DOI] [PubMed] [Google Scholar]
- 105.Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. (2007) 48:1551–60. 10.1111/j.1528-1167.2007.01005.x [DOI] [PubMed] [Google Scholar]
- 106.McLachlan RS, Pigott S, Tellez-Zenteno JF, Wiebe S, Parrent A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia. (2010) 51:304–7. 10.1111/j.1528-1167.2009.02332.x [DOI] [PubMed] [Google Scholar]
- 107.Vonck K, Sprengers M, Carrette E, Dauwe I, Miatton M, Meurs A, et al. A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int J Neural Syst. (2013) 23:1250034. 10.1142/S0129065712500347 [DOI] [PubMed] [Google Scholar]
- 108.Cukiert A, Cukiert CM, Burattini JA, Mariani PP, Bezerra DF. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: a prospective, controlled, randomized, double-blind study. Epilepsia. (2017) 58:1728–33. 10.1111/epi.13860 [DOI] [PubMed] [Google Scholar]
- 109.Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. (2007) 48:1895–903. 10.1111/j.1528-1167.2007.01181.x [DOI] [PubMed] [Google Scholar]
- 110.Boex C, Seeck M, Vulliemoz S, Rossetti AO, Staedler C, Spinelli L, et al. Chronic deep brain stimulation in mesial temporal lobe epilepsy. Seizure. (2011) 20:485–90. 10.1016/j.seizure.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 111.Schulze-Bonhage A, Dennig D, Wagner K, Cordeiro JG, Carius A, Fauser S, et al. Seizure control resulting from intrahippocampal depth electrode insertion. J Neurol Neurosurg Psychiatry. (2010) 81:352–3. 10.1136/jnnp.2009.180075 [DOI] [PubMed] [Google Scholar]
- 112.Ge Y, Hu W, Liu C, Zhang JG, Meng FG. Brain stimulation for treatment of refractory epilepsy. Chin Med J. (2013) 126:3364–70. 10.3760/cma.j.issn.0366-6999.20131163 [DOI] [PubMed] [Google Scholar]
- 113.Velasco AL, Velasco M, Velasco F, Menes D, Gordon F, Rocha L, et al. Subacute and chronic electrical stimulation of the hippocampus on intractable temporal lobe seizures: preliminary report. Arch Med Res. (2000) 31:316–28. 10.1016/S0188-4409(00)00064-3 [DOI] [PubMed] [Google Scholar]
- 114.Velasco M, Velasco F, Velasco AL, Boleaga B, Jimenez F, Brito F, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. (2000) 41:158–69. 10.1111/j.1528-1157.2000.tb00135.x [DOI] [PubMed] [Google Scholar]
- 115.Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. (2002) 52:556–65. 10.1002/ana.10323 [DOI] [PubMed] [Google Scholar]
- 116.Vonck K, Boon P, Claeys P, Dedeurwaerdere S, Achten R, Van Roost D. Long-term deep brain stimulation for refractory temporal lobe epilepsy. Epilepsia. (2005) 46 (Suppl. 5):98–9. 10.1111/j.1528-1167.2005.01016.x [DOI] [PubMed] [Google Scholar]
- 117.Velasco AL, Velasco F, Velasco M, Jimenez F, Carrillo-Ruiz JD, Castro G. The role of neuromodulation of the hippocampus in the treatment of intractable complex partial seizures of the temporal lobe. Acta Neurochir Suppl. (2007) 97 (Pt 2):329–32. 10.1007/978-3-211-33081-4_36 [DOI] [PubMed] [Google Scholar]
- 118.Bondallaz P, Boex C, Rossetti AO, Foletti G, Spinelli L, Vulliemoz S, et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure. (2013) 22:390–5. 10.1016/j.seizure.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 119.Tyrand R, Seeck M, Spinelli L, Pralong E, Vulliemoz S, Foletti G, et al. Effects of amygdala-hippocampal stimulation on interictal epileptic discharges. Epilepsy Res. (2012) 99:87–93. 10.1016/j.eplepsyres.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 120.Jin H, Li W, Dong C, Wu J, Zhao W, Zhao Z, et al. Hippocampal deep brain stimulation in nonlesional refractory mesial temporal lobe epilepsy. Seizure. (2016) 37:1–7. 10.1016/j.seizure.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 121.Lim SN, Lee CY, Lee ST, Tu PH, Chang BL, Lee CH, et al. Low and high frequency hippocampal stimulation for drug-resistant mesial temporal lobe epilepsy. Neuromodulation. (2016) 19:365–72. 10.1111/ner.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Halpern CH, Samadani U, Litt B, Jaggi JL, Baltuch GH. Deep brain stimulation for epilepsy. Neurotherapeutics. (2008) 5:59–67. 10.1016/j.nurt.2007.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sramka M, Chkhenkeli SA. Clinical experience in intraoperational determination of brain inhibitory structures and application of implanted neurostimulators in epilepsy. Stereotact Funct Neurosurg. (1990) 54–55:56–9. 10.1159/000100190 [DOI] [PubMed] [Google Scholar]
- 124.Chkhenkeli SA, Chkhenkeli IS. Effects of therapeutic stimulation of nucleus caudatus on epileptic electrical activity of brain in patients with intractable epilepsy. Stereotact Funct Neurosurg. (1997) 69 (Pt 2):221–4. 10.1159/000099878 [DOI] [PubMed] [Google Scholar]
- 125.Gale K. Role of the substantia nigra in GABA-mediated anticonvulsant actions. Adv Neurol. (1986) 44:343–64. [PubMed] [Google Scholar]
- 126.Pereira EA, Green AL, Stacey RJ, Aziz TZ. Refractory epilepsy and deep brain stimulation. J Clin Neurosci. (2012) 19:27–33. 10.1016/j.jocn.2011.03.043 [DOI] [PubMed] [Google Scholar]
- 127.Benabid AL, Koudsie A, Benazzouz A, Vercueil L, Fraix V, Chabardes S, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson's disease. Extension to new indications such as dystonia and epilepsy. J Neurol. (2001) 248 (Suppl. 3):III37–47. 10.1007/PL00007825 [DOI] [PubMed] [Google Scholar]
- 128.Benabid AL, Minotti L, Koudsie A, de Saint Martin A, Hirsch E. Antiepileptic effect of high-frequency stimulation of the subthalamic nucleus (corpus luysi) in a case of medically intractable epilepsy caused by focal dysplasia: a 30-month follow-up: technical case report. Neurosurgery. (2002) 50:1385–91; discussion 91–2. 10.1227/00006123-200206000-00037 [DOI] [PubMed] [Google Scholar]
- 129.Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. (2002) 4 (Suppl. 3):S83–93. [PubMed] [Google Scholar]
- 130.Loddenkemper T, Pan A, Neme S, Baker KB, Rezai AR, Dinner DS, et al. Deep brain stimulation in epilepsy. J Clin Neurophysiol. (2001) 18:514–32. 10.1097/00004691-200111000-00002 [DOI] [PubMed] [Google Scholar]
- 131.Wille C, Steinhoff BJ, Altenmuller DM, Staack AM, Bilic S, Nikkhah G, et al. Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood–report of five cases. Epilepsia. (2011) 52:489–96. 10.1111/j.1528-1167.2010.02884.x [DOI] [PubMed] [Google Scholar]
- 132.Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R. Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology. (2004) 63:907–9. 10.1212/01.WNL.0000137419.85535.C7 [DOI] [PubMed] [Google Scholar]
- 133.Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol. (2015) 77:1027–49. 10.1002/ana.24399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis R. Cerebellar stimulation for seizure control. In: Lozano AM, Gildenberg RR, Tasker RR, editors. Textbook of Stereotactic And Functional Neurosurgery. 1st ed. Heidelberg: Springer-Verlag; (2009). p. 2823–37. 10.1007/978-3-540-69960-6_168 [DOI] [Google Scholar]
- 135.Velasco F, Carrillo-Ruiz JD, Brito F, Velasco M, Velasco AL, Marquez I, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. (2005) 46:1071–81. 10.1111/j.1528-1167.2005.70504.x [DOI] [PubMed] [Google Scholar]
- 136.Kellinghaus C, Loddenkemper T. Double-blind, randomized controlled study of bilateral cerebellar stimulation. Epilepsia. (2006) 47:1247. 10.1111/j.1528-1167.2006.00598_4.x [DOI] [PubMed] [Google Scholar]
- 137.van Rijckevorsel K, Abu Serieh B, de Tourtchaninoff M, Raftopoulos C. Deep EEG recordings of the mammillary body in epilepsy patients. Epilepsia. (2005) 46:781–5. 10.1111/j.1528-1167.2005.45704.x [DOI] [PubMed] [Google Scholar]
- 138.Khan S, Wright I, Javed S, Sharples P, Jardine P, Carter M, et al. High frequency stimulation of the mamillothalamic tract for the treatment of resistant seizures associated with hypothalamic hamartoma. Epilepsia. (2009) 50:1608–11. 10.1111/j.1528-1167.2008.01995.x [DOI] [PubMed] [Google Scholar]
- 139.Franzini A, Messina G, Marras C, Villani F, Cordella R, Broggi G. Deep brain stimulation of two unconventional targets in refractory non-resectable epilepsy. Stereotact Funct Neurosurg. (2008) 86:373–81. 10.1159/000175800 [DOI] [PubMed] [Google Scholar]
- 140.Blik V. Electric stimulation of the tuberomamillary nucleus affects epileptic activity and sleep-wake cycle in a genetic absence epilepsy model. Epilepsy Res. (2015) 109:119–25. 10.1016/j.eplepsyres.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 141.Benedetti-Isaac JC, Torres-Zambrano M, Vargas-Toscano A, Perea-Castro E, Alcala-Cerra G, Furlanetti LL, et al. Seizure frequency reduction after posteromedial hypothalamus deep brain stimulation in drug-resistant epilepsy associated with intractable aggressive behavior. Epilepsia. (2015) 56:1152–61. 10.1111/epi.13025 [DOI] [PubMed] [Google Scholar]
- 142.Shon YM, Lee KJ, Kim HJ, Chung YA, Ahn KJ, Kim YI, et al. Effect of chronic deep brain stimulation of the subthalamic nucleus for frontal lobe epilepsy: subtraction SPECT analysis. Stereotact Funct Neurosurg. (2005) 83:84–90. 10.1159/000086867 [DOI] [PubMed] [Google Scholar]
- 143.Handforth A, DeSalles AA, Krahl SE. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. (2006) 47:1239–41. 10.1111/j.1528-1167.2006.00563.x [DOI] [PubMed] [Google Scholar]
- 144.Vesper J, Steinhoff B, Rona S, Wille C, Bilic S, Nikkhah G, et al. Chronic high-frequency deep brain stimulation of the STN/SNr for progressive myoclonic epilepsy. Epilepsia. (2007) 48:1984–9. 10.1111/j.1528-1167.2007.01166.x [DOI] [PubMed] [Google Scholar]
- 145.Capecci M, Ricciuti RA, Ortenzi A, Paggi A, Durazzi V, Rychlicki F, et al. Chronic bilateral subthalamic stimulation after anterior callosotomy in drug-resistant epilepsy: long-term clinical and functional outcome of two cases. Epilepsy Res. (2012) 98:135–9. 10.1016/j.eplepsyres.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 146.Levy LF, Auchterlonie WC. Chronic cerebellar stimulation in the treatment of epilepsy. Epilepsia. (1979) 20:235–45. 10.1111/j.1528-1157.1979.tb04800.x [DOI] [PubMed] [Google Scholar]
- 147.Bidzinski J, Bacia T, Ostrowski K, Czarkwiani L. Effect of cerebellar cortical electrostimulation on the frequency of epileptic seizures in severe forms of epilepsy. Neurol Neurochir Pol. (1981) 15:605–9. [PubMed] [Google Scholar]
- 148.Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. (1984) 47:769–74. 10.1136/jnnp.47.8.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davis R, Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg. (1992) 58:200–8. 10.1159/000098996 [DOI] [PubMed] [Google Scholar]
- 150.Anderson DG, Németh AH, Fawcett KA, Sims D, Miller J, Krause A. Deep brain stimulation in three related cases of north sea progressive myoclonic epilepsy from South Africa. Mov Disord Clin Pract. (2017) 4:249–53. 10.1002/mdc3.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmitt FC, Voges J, Heinze HJ, Zaehle T, Holtkamp M, Kowski AB. Safety and feasibility of nucleus accumbens stimulation in five patients with epilepsy. J Neurol. (2014) 261:1477–84. 10.1007/s00415-014-7364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kowski AB, Voges J, Heinze HJ, Oltmanns F, Holtkamp M, Schmitt FC. Nucleus accumbens stimulation in partial epilepsy–a randomized controlled case series. Epilepsia. (2015) 56:e78–82. 10.1111/epi.12999 [DOI] [PubMed] [Google Scholar]
- 153.Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. (2004) 3:111–8. 10.1016/S1474-4422(03)00664-1 [DOI] [PubMed] [Google Scholar]
- 154.Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol. (2014) 10:261–70. 10.1038/nrneurol.2014.59 [DOI] [PubMed] [Google Scholar]
- 155.Rossi M, Bruno V, Arena J, Cammarota A, Merello M. Challenges in PD patient management after DBS: a pragmatic review. Mov Disord Clin Pract. (2018) 5:246–54. 10.1002/mdc3.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Troster AI, Meador KJ, Irwin CP, Fisher RS. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. (2017) 45:133–41. 10.1016/j.seizure.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 157.Saitoh T, Enatsu R, Mikami T, Suzuki Y, Kanno A, Kitagawa M, et al. Peri-electrode edema after deep brain stimulation. J Clin Neurosci. (2019) 59:29–31. 10.1016/j.jocn.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 158.Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. (2017) 7:Cd008497. 10.1002/14651858.CD008497.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: a randomised, multicentre study. The Lancet Neurology. (2008) 7:605–14. 10.1016/S1474-4422(08)70114-5 [DOI] [PubMed] [Google Scholar]
- 160.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2004) 75:834–9. 10.1136/jnnp.2002.009803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Strutt AM, Simpson R, Jankovic J, York MK. Changes in cognitive-emotional and physiological symptoms of depression following STN-DBS for the treatment of Parkinson's disease. Eur J Neurol. (2012) 19:121–7. 10.1111/j.1468-1331.2011.03447.x [DOI] [PubMed] [Google Scholar]
- 162.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. (2008) 131 (Pt 10):2720–8. 10.1093/brain/awn214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Soulas T, Gurruchaga JM, Palfi S, Cesaro P, Nguyen JP, Fenelon G. Attempted and completed suicides after subthalamic nucleus stimulation for Parkinson's disease. J Neurol Neurosurg Psychiatry. (2008) 79:952–4. 10.1136/jnnp.2007.130583 [DOI] [PubMed] [Google Scholar]
- 164.Weintraub D, Duda JE, Carlson K, Luo P, Sagher O, Stern M, et al. Suicide ideation and behaviours after STN and GPi DBS surgery for Parkinson's disease: results from a randomised, controlled trial. J Neurol Neurosurg Psychiatry. (2013) 84:1113–8. 10.1136/jnnp-2012-304396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: a meta-analysis of ten years' experience. Movement Disorders. (2007) 22:1722–8. 10.1002/mds.21551 [DOI] [PubMed] [Google Scholar]
- 166.Drapier D, Drapier S, Sauleau P, Haegelen C, Raoul S, Biseul I, et al. Does subthalamic nucleus stimulation induce apathy in Parkinson's disease? J Neurol. (2006) 253:1083–91. 10.1007/s00415-006-0177-0 [DOI] [PubMed] [Google Scholar]
- 167.Wang Y, Li Y, Zhang X, Xie A. Apathy following bilateral deep brain stimulation of subthalamic nucleus in Parkinson's disease: a meta-analysis. Parkinson Dis. (2018) 2018:7. 10.1155/2018/9756468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hindle Fisher I, Pall HS, Mitchell RD, Kausar J, Cavanna AE. Apathy in patients with Parkinson's disease following deep brain stimulation of the subthalamic nucleus. CNS Spectrums. (2016) 21:258–64. 10.1017/S1092852916000171 [DOI] [PubMed] [Google Scholar]
- 169.Castelli L, Perozzo P, Zibetti M, Crivelli B, Morabito U, Lanotte M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson's disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol. (2006) 55:136–44. 10.1159/000093213 [DOI] [PubMed] [Google Scholar]
- 170.Antosik-Wojcinska A, Swiecicki L, Dominiak M, Soltan E, Bienkowski P, Mandat T. Impact of STN-DBS on mood, drive, anhedonia and risk of psychiatric side-effects in the population of PD patients. J Neurol Sci. (2017) 375:342–7. 10.1016/j.jns.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 171.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. (2009) 65:586–95. 10.1002/ana.21596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Voon V, Howell NA, Krack P. Psychiatric considerations in deep brain stimulation for Parkinson's disease. Handb Clin Neurol. (2013) 116:147–54. 10.1016/B978-0-444-53497-2.00012-7 [DOI] [PubMed] [Google Scholar]
- 173.Antosik-Wojcinska AZ, Swiecicki L, Bienkowski P, Mandat T, Soltan E. Othello syndrome after STN DBS—psychiatric side-effects of DBS and methods of dealing with them. Psychiatria Polska. (2016) 50:323–7. 10.12740/PP/34042 [DOI] [PubMed] [Google Scholar]
- 174.Morello A, Beber BC, Fagundes VC, Cielo CA, Rieder CRM. Dysphonia and dysarthria in people with Parkinson's disease after subthalamic nucleus deep brain stimulation: effect of frequency modulation. J Voice. (2018) 3:12 10.1016/j.jvoice.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 175.Sweet JA, Pace J, Girgis F, Miller JP. Computational modeling and neuroimaging techniques for targeting during deep brain stimulation. Front Neuroanatomy. (2016) 10:71. 10.3389/fnana.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blaszczyk B, Czuczwar SJ. Epilepsy coexisting with depression. Pharmacol Rep. (2016) 68:1084–92. 10.1016/j.pharep.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 177.Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuro-Psychopharmacol Biol Psychiatry. (2018) 82:224–32. 10.1016/j.pnpbp.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 178.Hinnell C, Williams J, Metcalfe A, Patten SB, Parker R, Wiebe S, et al. Health status and health-related behaviors in epilepsy compared to other chronic conditions—A national population-based study. Epilepsia. (2010) 51:853–61. 10.1111/j.1528-1167.2009.02477.x [DOI] [PubMed] [Google Scholar]
- 179.Franco R, Fonoff ET, Alvarenga P, Lopes AC, Miguel EC, Teixeira MJ, et al. DBS for obesity. Brain Sci. (2016) 6:21. 10.3390/brainsci6030021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reuber M, Rawlings GH. Nonepileptic seizures - subjective phenomena. Handb Clin Neurol. (2016) 139:283–96. 10.1016/B978-0-12-801772-2.00025-4 [DOI] [PubMed] [Google Scholar]
- 181.Langevin J-P, Skoch JM, Sherman SJ. Deep brain stimulation of a patient with psychogenic movement disorder. Surg Neurol Int. (2016) 7 (Suppl. 35):S824-S6. 10.4103/2152-7806.194063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ramos VFML, Pillai AS, Lungu C, Ostrem J, Starr P, Hallett M. Intraoperative neurophysiology in deep brain surgery for psychogenic dystonia. Ann Clin Transl Neurol. (2015) 2:707–10. 10.1002/acn3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lozano AM, Lipsman N, Bergman H. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. (2019) 15:148–60. 10.1038/s41582-018-0128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pycroft L, Stein J, Aziz T. Deep brain stimulation: an overview of history, methods, and future developments. Brain Neurosci Adv. (2018) 2:17 10.1177/2398212818816017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Clair AH, Haynes W, Mallet L. Recent advances in deep brain stimulation in psychiatric disorders. F1000 Res. (2018) 7:699. 10.12688/f1000research.14187.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Laufs H. Functional imaging of seizures and epilepsy: evolution from zones to networks. Curr Opin Neurol. (2012) 25:194–200. 10.1097/WCO.0b013e3283515db9 [DOI] [PubMed] [Google Scholar]
- 187.Christenson Wick Z, Krook-Magnuson E. Specificity, versatility, and continual development: the power of optogenetics for epilepsy research. Front Cell Neurosci. (2018) 12:151 10.3389/fncel.2018.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tonnesen J, Kokaia M. Epilepsy and optogenetics: can seizures be controlled by light? Clin Sci. (2017) 131:1605–16. 10.1042/CS20160492 [DOI] [PubMed] [Google Scholar]
- 189.Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. (2012) 4:161ra52. 10.1126/scitranslmed.3004190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. (2013) 16:64–70. 10.1038/nn.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. (2014) 1:e.2014. 10.1523/ENEURO.0005-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]