Abstract
The use of natural deep eutectic solvents (NADES) as multifunctional solvents for limonene bioprocessing was reported. NADES were used for the extraction of limonene from orange peel wastes, as solvent for the chemoenzymatic epoxidation of limonene, and as sacrificial electron donor for the in situ generation of H2O2 to promote the epoxidation reaction. The proof‐of‐concept for this multifunctional use was provided, and the scope and current limitations of the concept were outlined.
Keywords: chemoenzymatic cascades, choline oxidase, limonene, lipase, natural deep eutectic solvents
Transforming the feedstock of today's chemical industry from fossil resources to renewable ones is a major challenge on the way to a biobased society. Especially the utilization of non‐edible agricultural waste products is getting more and more attention. Orange peels, for example, are an attractive source for terpenoids, particularly limonene, at large scale. During the production of orange juice only half the orange is used, leaving 8–20 million tons of residue per year. This orange peel waste is mostly burned, dumped in landfills, or used as animal feed, resulting in environmental and financial cost.1 Limonene, however, is a very versatile and promising platform chemical.2 Possible non‐flavor and fragrance applications range from cleansing agents to polymer building blocks. Particularly, mono‐ and di‐epoxides of limonene (and their mixtures) are versatile polymer building blocks.3 The traditional extraction of limonene from orange peels, however, is a very energy‐consuming process, which negatively impacts the overall eco‐efficiency of the process.2 Additionally, the extraction with synthetic hydrophobic solvents such as hexane is questionable from an environmental point of view. Alternatively, natural deep eutectic solvents (NADES) have been shown in previous studies to be an attractive alternative for the extraction and dissolution of natural products.4
To transform limonene into a building block for polymers, for example, it needs to be functionalized. Recently, Mihovilovic and co‐workers, for example, reported an elegant multi‐enzyme cascade to transform limonene into carvolactone as a potential building block for polyesters.5 Moreover, epoxidation of one or both C=C double bonds adds functionality into limonene, thus preparing it for further processing. In this respect, chemoenzymatic C=C epoxidation reactions are garnering increasing interest.6 Instead of stoichiometric amounts of reactive and instable peracids (with concomitant formation of significant amounts of the corresponding acids as wastes) for the Prilezhaev reaction, the chemoenzymatic route uses carboxylic acids only in catalytic amounts and generates the reactive peracid with H2O2 in situ catalyzed by hydrolases. H2O2, however, is a known inactivator of enzymes, thereby challenging the robustness (and practical feasibility) of the chemoenzymatic Prilezhaev reaction.7 We envisioned using choline chloride (ChCl)‐based NADES to alleviate this challenge. ChCl‐based NADES have been shown to stabilize lipases in the presence of H2O2.8 More importantly, choline can be oxidized by the enzyme Choline oxidase (ChOx)9 to the (likewise NADES‐forming)10 betaine while producing two equivalents of H2O2.
Overall, we propose the use of ChCl‐based NADES to serve three purposes at the same time: (1) as solvent for the extraction from waste orange peels, (2) as reaction medium for the chemoenzymatic epoxidation reaction, and (3) as sacrificial electron donor for the in situ generation of H2O2 to promote the chemoenzymatic epoxidation reaction (Scheme 1).
Scheme 1.

Biocatalytic cascade epoxidation of limonene. a) Direct oxidation of limonene into limonene epoxide enabled by peracids. b) Formation of peracids catalyzed by lipase with H2O2. c) Formation of H2O2 enabled by ChOx‐catalyzed choline chloride oxidation in NADES and O2 reduction.
In a first set of experiments we evaluated a series of ChCl‐based NADES for their efficiency in extracting limonene from orange peels (Figure 1 a). Especially the NADES ChCl‐Pro‐H2O (i.e., choline chloride/1,2‐propanediol/H2O in an equimolar ratio) and ChCl‐EG (i.e., choline chloride/ethylene glycol in an equimolar ratio) yielded the highest extraction efficiencies for limonene. Up to 17.8 mg limonene was extracted per g orange peel by treating them with NADES system (4 mL) at 40 °C for 24 h. GC analysis confirmed a very high purity of the limonene solution (Figure S4 in the Supporting Information). Hence, the extraction efficiency of the NADES system falls into the same order of magnitude as previously reported ionic liquid‐based extraction systems.5 It is worth mentioning that the amount of limonene extracted significantly varied in between different charges and depending on the storage conditions of the oranges (Figure 1 b). As a result, limonene concentrations between 11.5 and 47.8 mm were obtained. Compared to conventional extraction solvents such as ethyl acetate or n‐hexane, ChCl‐Pro‐H2O yielded approximately 82 and 86 % limonene, respectively (Figure S5 in the Supporting Information). Owing to its convincing extraction efficiency, we used ChCl‐Pro‐H2O for all further experiments.
Figure 1.

a) Direct extraction of limonene from orange peel with different ChCl‐based NADES (molar ratios are given in parentheses). ChCl‐Pro‐H2O: choline chloride/1,2‐propanediol/H2O (1:1:1); ChCl‐EG: choline chloride/ethylene glycol (1:1); ChCl‐MA: choline chloride/malonic acid (1:1); ChCl‐Urea‐Gly: choline chloride/urea/glycerol (1:1:1); ChCl‐Xyl‐H2O: choline chloride/xylopyranose/H2O (5:2:5); ChCl‐Isosor: choline chloride/isosorbide (1:1); ChCl‐Sor : choline chloride/sorbitol (1:1); ChCl‐Gly: choline chloride/glycerol (1:2); ChCl‐Urea: choline chloride/urea (1:2); ChCl‐OA: choline chloride/oxalic acid (1:1). b) Direct extraction of limonene from different orange peels with ChCl‐Pro‐H2O NADES system. Reaction conditions: a) orange bought locally in Guangzhou in China with varied NADES system; orange peel (1 g) in a glass vial filled with NADES system (4 mL) for reaction, pH 7.0, 40 °C, 24 h, 500 rpm; b) use of ChCl‐Pro‐H2O NADES system with oranges bought globally; orange peel (1 g) in a glass vial filled with NADES system (4 mL) for reaction, pH 7.0, 40 °C, 24 h, 500 rpm.
For the envisaged H2O2 generation, the recently reported ChOx from Arthrobacter nicotianae was used.9 The enzyme was produced in recombinant Escherichia coli following the previously established protocol. Endogenous catalase was removed from the cell lysate through a chromatographic step (details on the expression and purification procedure are given in the Experimental Section). Overall, in 2 days, 90.1 mg essentially pure ChOx were obtained from a 1000 mL culture.
Next, we evaluated ChOx as H2O2 generation catalyst in the NADES system. It was found that ChOx could generate H2O2 in ChCl‐based NADES systems such as ChCl‐Pro‐H2O. A preliminary characterization of the parameters influencing the efficiency of the system revealed that the H2O2‐generation rate linearly depended on the ChOx concentration applied. The enzyme was most active under neutral to slightly alkaline conditions, at temperatures between 30 and 40 °C, and in NADES system/buffer ratios between 25 and 75 % (v/v) (Figure S7 in the Supporting Information).
Encouraged by these findings, we proceeded to assemble the complete reaction system to evaluate a range of different hydrolases as catalysts for the in situ formation of peracids (such as peroctanoic acid) to perform the Prilezhaev reaction on limonene (Table 1). In essence, all lipases tested gave very satisfactory results, accumulating 6–8 mm of the desired (di)epoxides.
Table 1.
The results from different enzymes.[a]
| Entry | Lipase | Forms of enzyme | Product conc. [mm] |
|---|---|---|---|
| 1 | Novo435 | immobilized enzyme | 8.69 |
| 2 | Lipozyme RMIM | immobilized enzyme | 8.12 |
| 3 | Lipozyme TLIM | immobilized enzyme | 7.46 |
| 4 | Lipolase 100 L | liquid enzyme | 7.01 |
| 5 | Palatase 20 000 L | liquid enzyme | 6.18 |
| 6 | G50 | powder enzyme | 6.18 |
| 7 | MAS1‐wt | liquid enzyme | 6.20 |
| 8 | MAS1‐H108A | liquid enzyme | 6.19 |
| 9 | MAJ1‐wt | liquid enzyme | 6.16 |
| 10 | AFLB‐wt | powder enzyme | 6.10 |
| 11 | AOL‐wt | liquid enzyme | 6.15 |
| 12 | AOL‐E | liquid enzyme | 5.97 |
[a] Reaction conditions: orange peel (1 g, orange bought locally in Guangzhou, China), octanoic acid (200 mm), ChOx (80 μm), in a glass vial filled with ChCl‐Pro‐H2O NADES system (4 mL) for reaction, pH 7.0, 40 °C, 24 h, 500 rpm. Entries 1–6 are commercial lipases, and entries 7–12 are house‐made lipases. Enzyme concentration in the reaction system was 80 μm.
Amongst the hydrolases tested, lipase B from Candida antarctica (CalB, used as commercial preparation Novo435) excelled regarding its activity. We therefore continued our investigations with this lipase preparation. Figure 2 shows a representative time course of the complete reaction system (Scheme 1). Continuous product formation for at least 2 days was observed. Quite expectedly, first the monoepoxide was formed, later followed by some accumulation of the diepoxide product. After 2 days, a total of 8.72 mm limonene epoxides (6.13 mm 2 and 2.59 mm 3) was obtained. Notably, similar results were obtained by using other orange peels as limonene source (Figure 3), even though the absolute limonene content varied significantly depending on the origin of the orange starting material.
Figure 2.
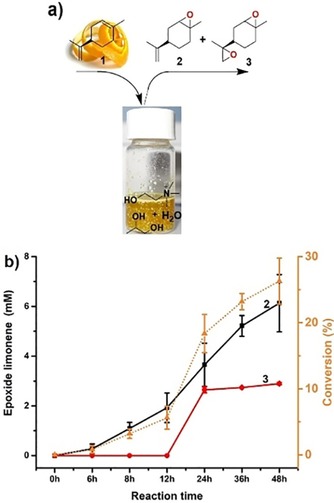
a) Schematic illustration of limonene (1) extraction from orange peel and enzymatic conversion into monoepoxide (2) and diepoxide (3). b) Time course of the limonene epoxidation formation. Reaction conditions: orange peel (1 g, orange bought locally in Guangzhou in China), octanoic acid (100 mm), Novo435 (100 mg), ChOx (40 μm), in a glass vial filled with ChCl‐Pro‐H2O NADES system (4 mL) for reaction, pH 7.5, 40 °C, 500 rpm. (▴): limonene conversion; (▪): limonene monoepoxide concentration; (•): limonene diepoxide concentration.
Figure 3.
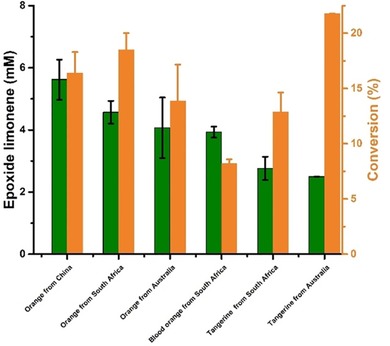
Limonene epoxides obtained from different orange peels by using the combined extraction/epoxidation system (Scheme 1). Reaction conditions: orange peel (1 g), octanoic acid (100 mm), Novo435 (100 mg), ChOx (40 μm), in a glass vial filled with ChCl‐Pro‐H2O NADES system (4 mL) for reaction, pH 7.5, 24 h, 40 °C, 500 rpm, (▪): limonene epoxide concentration, (▪): limonene conversion.
To gain further insight into the factors influencing the overall efficiency of the cascade reaction, we systematically varied some reaction parameters such as temperature, pH, and H2O2‐generation rate (as ChOx concentration). As shown in Figure 4, raising the reaction temperature above 30 °C had a negative effect on the reaction, which we attribute to a poorer long‐term stability of the oxidase at elevated temperatures. However, because the extraction efficiency of limonene from the orange peels increased with temperature, we chose 40 °C as a compromise for all further experiments. Future solutions to this issue may include a two‐step procedure and/or application of more thermotolerant enzyme mutants. Quite expectedly, the epoxide formation rate was the highest at pH 7, which also constitutes the optimal pH for both biocatalysts used. Increasing the octanoic acid concentration positively influenced the overall productivity. Admittedly, so far, octanoic acid is not catalytic yet, which may to some extent be owing to a competing ester formation between the diol and octanoic acid. Increasing the ChOx concentration also increased the overall epoxidation rate, suggesting that the H2O2‐dependent peracid formation reaction may be overall rate‐limiting, thereby explaining the relatively poor conversion of the overall system after 24 h of approximately 33 % (11.3 mm limonene epoxides from ≈34.3 mm limonene). Indeed, a control experiment, in which stoichiometric amounts of H2O2 were added externally (under otherwise identical conditions), resulted in near‐full conversion (93 %) within 12 h (Figure S6 in the Supporting Information).
Figure 4.
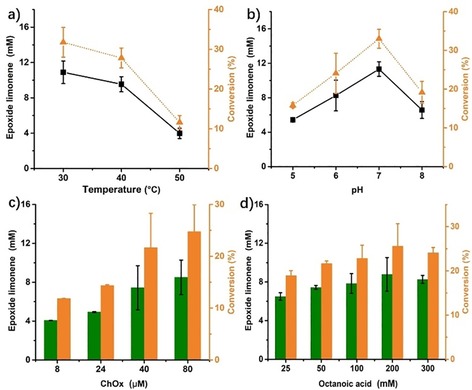
Characterization of the influence of some reaction parameters on the epoxide formation. General conditions: oranges bought locally in Guangzhou in China, orange peel (1 g) in a glass vial filled with ChCl‐Pro‐H2O NADES system (4 mL), octanoic acid (200 mm), Novo435 (100 mg), 24 h, 500 rpm. a) 40 μm ChOx, pH 7.0, b) 80 μm ChOx, 40 °C, c) 40 °C, d) 40 μm ChOx, 40 °C. In a) and b): (▪): limonene oxide concentration, (▴): limonene epoxide conversion. In c) and d): (▪): limonene oxide concentration, (▪): limonene epoxide conversion.
Overall, we have demonstrated a new enzymatic cascade approach to oxidize limonene from agricultural wastes to the corresponding epoxide products. The NADES used in this study served three purposes at the same time: 1) as extraction medium for limonene from orange peel wastes, 2) as solvent for the chemoenzymatic epoxidation reaction, and 3) as source of reducing equivalents to promote the ChOx‐mediated reductive activation of O2 to produce H2O2.
Admittedly, the system in its present form is still far away from being economically applicable. But we are convinced that this proof‐of‐concept study will inspire others to evaluate NADES as multipurpose solvents/reagents to promote H2O2‐dependent reactions (e.g., for the oxidation/oxyfunctionalization of natural product)s. Envisaging preparative applicability, higher loadings of the H2O2‐generating ChOx are beneficial but probably not very attractive from an economical point of view. More active natural variants and/or engineered mutants of the ChOx used here appear more promising. Also, hydrolases (either from natural or man‐made diversity) exhibiting higher affinities towards H2O2 and the carboxylic acids (i.e., lower K M values) are currently being evaluated by some of us.
Experimental Section
Chemical reagents and materials
All chemicals were purchased from Sigma–Aldrich, TCI, or Aladdin in the highest purity available and used without further purification. Novo435 (Lipozyme CalB on resin), Palatase 20 000 L, Lipozyme RMIM, Lipolase 100 L, Lipozyme TLIM, G50 were purchased from Guangzhou Mingyao Trading Co.,Ltd (Guangzhou, China); the source (microbial) and activity of these enzymes are listed in Table S1 in the Supporting Information. Water was purified with a Millipore (Bedford, MA) Milli‐Q water system. Fresh orange was purchased from a whole‐sale fruit market in Guangzhou (Guangdong, China). The orange peels were treated with a conventional crusher; if not used immediately, the thus‐obtained material was stored at 4 °C until further use.
Experimental set‐up and operating conditions
An Agilent 7890B GC system (Agilent Technologies, Palo Alto, CA, USA) was used together with an Agilent J&W CP‐Sil 88 GC column (Agilent Technologies, Palo Alto, CA, USA: 60 m length×0.25 mm inner diameter×0.20 μm film thickness). The method was as follows: injector temperature: 250 °C; split mode: 30:1; detector temperature: 280 °C; GC oven temperature program: initial 50 to 200 °C at a ramp rate of 10 °C min−1, hold for 3 min, from 200 to 230 °C at a ramp rate of 5 °C min−1, then hold for 2 min. The retention times were: 8.95 min for (R)‐(+)‐limonene; 12.88 and 13.03 min for (+)‐limonene monoepoxide; 18.78, 18.84, and 19.02 min for limonene diepoxide.
Preparation of NADES
ChCl‐based NADES were prepared following previously reported thermal treatment protocols.4, 10b In short, all NADES components were mixed and heated to 80 °C for 2 h while agitating rigorously. In all cases, colorless liquids (at room temperature) were obtained.
Preparation of ChOx
ChOx was isolated from the soil bacterium Arthrobacter nicotianae grown on choline as sole carbon source. A basic biochemical characterization of purified ChOx (M w=59.1 kDa) revealed a pH optimum of 7.4 and activity over a broad temperature range (15–70 °C). Its specific activity towards choline chloride was determined as (4.70±0.12) U mg−1 [K M=(1.51±0.09) mm].9
Production and isolation of recombinant ChOx enzyme was conducted in Escherichia coli according to the method previously reported with minor modification.9 The cultivation protocol was as follows: (1) Overnight culture (ONC): lysogeny broth [LB, 25 mL, yeast extract 0.5 % (w/v), peptone 1 % (w/v), NaCl 0.5 % (w/v)] supplemented with Kanamycin (50 μg mL−1) in a 100 mL Erlenmeyer flask was incubated at 30 °C und 200 rpm. (2) Main culture: The main culture consisted of 400 mL of the same culture medium in a 1 L Erlenmeyer flask, inoculated with the corresponding volume of the ONC constituting a start OD600 of 0.1. The main culture was incubated at 30 °C, 200 rpm for approximately 2.5– 4 h to reach an OD600 value of 0.8–1.1. (3) Induction: After reaching the desired OD600, the main culture was cooled to 20 °C before being induced with isopropyl β‐D‐1‐thiogalactopyranoside (IPTG, 0.05 mm). (4) Harvest: The harvest of the culture took place 5 h after induction. Then, the cell pellet was collected by centrifugation (4000 rcf, 20 min, 4 °C) followed by supersonic treatment.
Protein purification
ChOx was purified by using an GE Chromatography system (Biorad). At first, the crude enzyme was injected into a HisPrepTMFF16/10 (Code28‐9365‐51) column at a flow rate of 5 mL min−1. After 5‐fold column volume equilibrated by washing buffer A (20 mm sodium phosphate buffer, 500 mm NaCl, pH 7.5), the retained protein was eluted by a linear elution with elution buffer B (20 mm sodium phosphate buffer, 500 mm NaCl, 500 mm imidazole, pH 7.5). In the linear elution, the flow rate of the mixed buffers was 2 mL min−1, and the whole course of elution time (elution buffer B gradient increased from 0 to 100 %) was 100 min. We obtained the target protein (59.1 kDa) when the elution gradient of elution buffer B increased from 40 to 60 %. After elution, the target protein was desalted by column HiPrepTM26/10 (Code17‐5087‐01) with 20 mm sodium phosphate buffer (pH 7.5) at a flow rate of 5 mL min−1. The purified protein was stored at 4 °C.
Chemoenzymatic epoxidation reaction
All reactions were performed in a 20 mL glass vial submerged in a thermostatic oil bath for temperature control. First, NADES (2.0 mL) and buffer (2.0 mL, NaPi, pH 7.0, 60 mm) were added into the reaction vessel to form 4 mL NADES system. Unless mentioned otherwise, orange peel (1 g) was then added into the reaction vessel. Octanoic acid (200 mm), ChOx (40 μm), and Novo435 (100 mg) were then added into the reaction vessel. The reaction vial was closed, and we used a balloon full of O2 to supply O2 for the reaction. The homemade experimental setup is shown in Figure S2 in the Supporting Information.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC0311104), National Outstanding Youth Science Foundation of China (31725022), International Collaboration Base for Molecular Enzymology and Enzyme Engineering (2017A050503001), State Key Laboratory of Pulp and Paper Engineering (Project Number: 2017‐04‐SKLPPE), and also supported by the 111 Project (B17018). F.H. gratefully acknowledges funding by the European Research Commission (ERC consolidator grant, No.648026), the European Union (H2020‐BBI‐PPP‐2015‐2‐1‐720297), and the Netherlands Organisation for Scientific Research (VICI grant, No. 724.014.003).
Y. Ma, P. Li, Y. Li, S. J.-P. Willot, W. Zhang, D. Ribitsch, Y. H. Choi, R. Verpoorte, T. Zhang, F. Hollmann, Y. Wang, ChemSusChem 2019, 12, 1310.
Contributor Information
Prof. Dr. Frank Hollmann, Email: F.Hollmann@tudelft.nl.
Prof. Dr. Yonghua Wang, Email: yonghw@scut.edu.cn
References
- 1. Rezzadori K., Benedetti S., Amante E. R., Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar]
- 2. Ciriminna R., Lomeli-Rodriguez M., Demma Carà P., Lopez-Sanchez J. A., Pagliaro M., Chem. Commun. 2014, 50, 15288–15296. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Hauenstein O., Agarwal S., Greiner A., Nat. Commun. 2016, 7, 11862; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Bähr M., Bitto A., Mülhaupt R., Green Chem. 2012, 14, 1447–1454. [Google Scholar]
- 4.
- 4a. Choi Y. H., van Spronsen J., Dai Y., Verberne M., Hollmann F., Arends I. W. C. E., Witkamp G.-J., Verpoorte R., Plant Physiol. 2011, 156, 1701–1705; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. van den Bruinhorst A., Kouris P., Timmer J. M. K., de Croon M. H. J. M., Kroon M. C., Nat. Prod. Chem. Res. 2016, 4, 1000242. [Google Scholar]
- 5. Oberleitner N., Ressmann A. K., Bica K., Gartner P., Fraaije M. W., Bornscheuer U. T., Rudroff F., Mihovilovic M. D., Green Chem. 2017, 19, 367–371. [Google Scholar]
- 6.
- 6a. Björkling F., Godtfredsen S. E., Kirk O., J. Chem. Soc. Chem. Commun. 1990, 1301–1303; [Google Scholar]
- 6b. Warwel S., Klaas M. R. G., J. Mol. Catal. B 1995, 1, 29–35; [Google Scholar]
- 6c. Kotlewska A. J., van Rantwijk F., Sheldon R. A., Arends I., Green Chem. 2011, 13, 2154–2160; [Google Scholar]
- 6d. Ranganathan S., Zeitlhofer S., Sieber V., Green Chem. 2017, 19, 2576–2586; [Google Scholar]
- 6e. Ranganathan S., Sieber V., React. Chem. Eng. 2017, 2, 885–895; [Google Scholar]
- 6f. Ranganathan S., Tebbe J., Wiemann L., Sieber V., Proc. Biochem. 2016, 51, 1479–1485; [Google Scholar]
- 6g. Wiemann L. O., Faltl C., Sieber V., Z. Naturforsch. B 2012, 67, 1056–1060; [Google Scholar]
- 6h. Meyer-Wassewitz J., Hohmann D., Ansorge-Schumacher M. B., Kraume M., Drews A., Bioche. Eng. J. 2017, 126, 68–77; [Google Scholar]
- 6i. Meyer J., Horst A. E. W., Steinhagen M., Holtmann D., Ansorge-Schumacher M. B., Kraume M., Drews A., Eng. Life Sci. 2017, 17, 759–767; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6j. Meyer J., Holtmann D., Ansorge-Schumacher M. B., Kraume M., Drews A., Biochem. Eng. J. 2017, 118, 34–40. [Google Scholar]
- 7. Törnvall U., Orellana-Coca C., Hatti-Kaul R., Adlercreutz D., Enzyme Microb. Technol. 2007, 40, 447–451. [Google Scholar]
- 8.
- 8a. Zhou P., Wang X., Zeng C., Wang W., Yang B., Hollmann F., Wang Y., ChemCatChem 2017, 9, 934–936; [Google Scholar]
- 8b. Zhou P., Wang X., Yang B., Hollmann F., Wang Y., RSC Adv. 2017, 7, 12518–12523; [Google Scholar]
- 8c. Lan D., Wang X., Zhou P., Hollmann F., Wang Y., RSC Adv. 2017, 7, 40367–40370. [Google Scholar]
- 9.
- 9a. Ribitsch D., Winkler S., Gruber K., Karl W., Wehrschütz-Sigl E., Eiteljörg I., Schratl P., Remler P., Stehr R., Bessler C., Mußmann N., Sauter K., Maurer K. H., Schwab H., Appl. Microbiol. Biotechnol. 2010, 87, 1743–1752; [DOI] [PubMed] [Google Scholar]
- 9b. Ribitsch D., Karl W., Wehrschütz-Sigl E., Tutz S., Remler P., Weber H. J., Gruber K., Stehr R., Bessler C., Hoven N., Sauter K., Maurer K. H., Schwab H., Appl. Microbiol. Biotechnol. 2009, 81, 875–886. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Zahrina I., Mulia K., Yanuar A., Nasikin M., J. Mol. Struct. 2018, 1158, 133–138; [Google Scholar]
- 10b. Dai Y., van Spronsen J., Witkamp G.-J., Verpoorte R., Choi Y. H., Anal. Chim. Acta 2013, 766, 61–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


