Abstract
The looming concern of antimicrobial resistance (AMR) has prompted the government of many countries of the world to act upon and come up with the guidelines, comprehensive recommendations and policies concerning prudent use of antibiotics and containment of AMR. However, such initiatives from countries with high incidence of antibiotic-resistant bacteria in food animals are still in infancy. This review highlights the existing global policies on antibiotics use in food animals along with details of the various Indian policies and guidelines. In India, in spite of availability of integrated policies for livestock, poultry and aquaculture sector, uniform regulations with coordinated initiative are needed to formulate strict policies regarding antimicrobial use both in humans and animals. In an attempt to create effective framework to tackle the AMR, the Indian Council of Medical Research initiated a series of dialogues with various stakeholders and suggested various action points for urgent implementation. This review summarizes the recommendations made during the various consultations. The overarching aim of this review is to clearly delineate the action points which need to be carried out urgently to regulate the antibiotic use in animals.
Keywords: Antibiotics, antimicrobial resistance, food animals, India, Indian Council of Medical Research, Policy
Introduction
The use of antibiotics in healthy/farm animals is one of the neglected causes for the global antimicrobial resistance (AMR) upsurge. The recent debates and discussions on the undue routine use of antibiotics have necessitated the immediate interconnected action from government and society. The absence of consensus on standard policies, both, international and national veterinary domain due to inadequate research, uncharacterized risk impact and polarized stakeholder interests have collectively led to this area being neglected for a long time. Globally, there are now awareness and acceptance of the fact that coordinated actions are required to reduce the advent and dispersal of AMR, as isolated interventions may provide only limited impact. Here we discuss the initiatives taken at the international and national levels to address the excessive use of antimicrobials in the production of food and food animals and the major gaps which need to be addressed.
Global scenario on antibiotic use in animals
The widespread exploitation of antibiotics in healthy food animals has amplified the extent of AMR globally1. Antibiotic consumption in animals is not limited to their therapeutic use. These are used for metaphylaxis (administration of antimicrobials to animals when perceived to be in contact with animals diagnosed with disease), for prophylaxis (mass administration of antimicrobials to animals to prevent disease when risk is established) and as antibiotic growth promoters (AGPs; administration of antimicrobials to animals to boost feed efficiency and increase weight gain, e.g. avilamycin, bambermycin, efrotomycin and ionophores)2. Global data show that high consumption of antibiotics in the animal health sector is as growth promoters than for treatment purpose. This is primarily to meet the growing demand of protein-rich food in countries where growing economies are leading to higher demand of nutritious food of animal origin. Food and Agriculture Organization (FAO) has demonstrated this gradual but sustained growth in volume of meat produced3.
There is limited evidence on the association between the antibiotic usage and prevalence of antibiotic-resistant (ABR) bacteria as well as their dissemination into the surrounding environments. The commensal bacteria present in livestock can acquire ABR genes and may accelerate the cross-sectoral ABR problem by transmission of resistant microorganisms to humans through livestock products, crop culture, aquaculture products and environment4,5. A study from seven European countries has documented correlation between the usage of eight classes of antibiotics and the occurrence of ABR Escherichia coli in pigs, poultry and cattle6.
Globally, various studies have documented the incidence of ABR bacteria in food animals and their food products, such as methicillin-resistant Staphylococcus aureus (MRSA)7, ABR Campylobacter spp.8, colistin-resistant E. coli9,10, multidrug-resistant (MDR) Acinetobacter baumannii and Pseudomonas aeruginosa11, carbapenem-resistant12 and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (e.g. E. coli, Salmonella spp., Shigella spp. and Klebsiella spp.)12,13. The ABR incidence is more in developing Asian and African countries14. A report on antibiotic usage in food animals by the ‘World Health Organization (WHO) - Advisory Group on Integrated Surveillance of AMR’ pointed out the increasing resistance to fluoroquinolones, macrolides, third- and fourth-generation cephalosporins and vancomycin15. This report emphasizes on the high risk of global ABR spread as most of the developing nations constitute a large proportion of exporters of food animals and their products. Studies from Europe (Denmark, Norway, Sweden and Switzerland) have documented the positive impact of reduced usage of antibiotics in food animals on the levels of ABR in bacteria16,17,18,19. However, the resulting benefits of reduced ABR are challenging to associate with human health as total biomass production of food animal raised for consumption surpasses the human biomass20. This scenario also proliferates the advent of new resistance mutation in bacteria of animal origin21.
The analysis of global trends of antibiotic consumption has highlighted the high usage of antibiotics in veterinary sector than human sector15,22,23. Globally, in food animals, the total utilization of antimicrobials was valued at about 131,000 tonnes in 2013 and is expected to reach at about 200,000 tons by 203021. An increase in both human population and global prosperity is main contributing factor in this projected augmentation of antimicrobial use in food animals. The analysis of consumption quantity of different antibiotics shows a substantial variation across the countries. As per 2010 global estimate, the maximum amount of antibiotics was consumed by China in livestock followed by the USA, Brazil, Germany and India24. In 2013, China (23%) and the USA (13%) held the highest shares in consumption of antibiotics for food animal production21, with India (4%) at the fourth position. The current trends predict India to be the next largest shareholder in gross antimicrobial usage in food animals. The presence of antimicrobial residues in food animal products has been reported from India which indicates the unregulated and extensive use of antibiotics25.
Global policies on antibiotic use in animals
Acknowledging the problem of unregulated use of antibiotics in food animals and consequent AMR, various organizations, viz. WHO, FAO, World Organisation for Animal Health, formerly called Office International des Epizooties (OIE), European Union (EU), European Centre for Disease Prevention and Control (ECDC), European Medicines Agency (EMA) and governments across the globe, have committed to develop guidelines and policies to limit the antibiotic use based on scientific evidence. The WHO has endorsed ‘One Health approach’ to address the problem of ABR globally through alliance between WHO, FAO and OIE referred as ‘Tripartite Alliance’. The WHO published the ‘Global Action Plan (GAP) on AMR’ in 2015 in partnership with its tripartite partners26. The GAP focuses on the set of strategies which include improving sanitation, hygiene and awareness about AMR, strengthening surveillance, promoting research, reducing infection incidence and optimizing the use of antibiotics along with improved interventions. The Tripartite Alliance further supports the multi-sectoral retorts to food safety hazards and risks from zoonosis and provides guidance to reduce these risks26. The FAO has also launched its ‘AMR Strategy’ to implement the WHO's GAP in the food and agricultural sectors27. In continuation with these efforts, OIE has evaluated animal health systems that detailed the lack of apt legislation to regulate various provisions related to the import, production, distribution and antimicrobials usage in 110 out of 130 countries28. The OIE has emphasized on the implementation of veterinary legislation and regulations endorsing judicial use of ‘Veterinary Critically Important Antimicrobial Agents, Veterinary Highly Important Antimicrobial Agents and Veterinary Important Antimicrobial Agents’28,29. The WHO has also ranked antimicrobials as per importance to human medicine, aiming to curtail the antimicrobials usage in food-producing animals and reserve certain classes of drugs for human use15. In Europe, Veterinary Committee on Antimicrobial Susceptibility Testing (Vet CAST) has provided the susceptibility testing guidelines on bacteria of animal origin and zoonotic bacteria as per the framework of European Committee on Antimicrobial Susceptibility Testing (EUCAST)30.
Many countries (Japan, USA and Colombia) particularly in the EU (Denmark, The Netherlands and Sweden) have implemented national targets to reduce antibiotic usage, ban on use and re-labelling of antimicrobials for livestock feed, benchmarks for antibiotics at farm level and antibiotic stewardship (e.g. NORM-VET, NethMap-MARAN, JVARM, DANMAP, FDA, NethMap-MARAN and SWEDRES-SVARM). Table I summarizes national resistance surveillance programmes across the world. The information driven from these national programmes has been instrumental in shaping local policies on antibiotic usage in food animals. However, the majority of low- and middle-income countries have neither mechanism in place to supervise the antibiotic consumption and subsequent residue testing in food animals and their products nor the national guidelines or programmes. The government of India has also highlighted the need of a strict regulatory framework and policies in the country to restrict antimicrobials usage in livestock (e.g. chicken, cattle and pigs) and their food produce31.
Table I.
National resistance surveillance programmes across the world
| Country | Year | Programme | Actions | Website |
|---|---|---|---|---|
| The Netherlands | 2002 | NethMap-MARAN | To get detailed trends on exposure of farm animals to antibiotics. To monitor national data on overall antibiotic sales and usage of antibiotics per animal species. |
https://www.wur.nl/en/Research-Results/Projects-and-programmes/MARAN-Antibiotic-usage/Introduction.htm |
| Sweden | 2000 | SVARM | Data on antimicrobials usage in relation to changes in animal populations and of animal health. To gather knowledge on AMR among bacteria (indicator, pathogen, and zoonotic) from animals. Provide a basis for recommendations on appropriate choice of therapy in veterinary medicine. |
https://www.sva.se/en/antibiotics/svarm-resistance-monitoring |
| Norway | 2000 | NORM-VET | One health and combat threats to humans, animals and the environment. To monitor AMR in the veterinary sector and food production area. To study association between antimicrobial usage and occurrence of resistance.To set policies, assess risks and evaluate interventions from data. |
https://www.vetinst.no/overvaking/antibiotikaresistens-norm-vet |
| Denmark | 1995- | DANMAP | Included entire supply chain by implementing ‘farm to fork to sickbed’ surveillance. Collection of antibiotic consumption data from hospitals, PHC and drugstores. Usage data of veterinary medicine in food and companion animals by veterinarians, pharmacies and feed mills. |
https://www.danmap.org/about-danmap |
| Canada | 2006 | CIPARS | Monitor antimicrobial consumption trend and resistance pattern in certain bacterial spp. from human, animal and different sources of food. Facilitate the impact assessment of antimicrobial use on public health. Data usage for outbreak investigations, post-approval monitoring of veterinary drugs and research. |
https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars.html |
| USA | 1996 | NARMS | A collaborative programme that monitor antibiotic susceptibility changes in enteric bacteria found from ailing people (CDC), retail meats (FDA), and food animals (USDA). Assist FDA to make decisions related to approval of safe and effective antimicrobial drugs for animals. |
https://www.fda.gov/animal-veterinary/antimicrobial-resistance/national-antimicrobial-resistance-monitoring-system |
| Finland | 2002 | FINRES-VET | Monitor antimicrobials usage to treat animals. Resistance trends to antimicrobials in pets and certain food-producing animals. Analysis of AMR prevalence and appearance of new resistance phenotypes. |
https://www.ruokavirasto.fi/en/farmers/animal-husbandry/animal-medication/monitoring-of-antibiotic-resistance/ |
| Australia | 2000-2004 | Programme for AMR in Bacteria of Animal Origin Pilot Surveillance | Assessed resistance prevalence to important antimicrobials in key bacteria (Escherichia coli, Enterococcus spp., and Campylobacter spp.) found in caecum of food-producing animals | http://www.agriculture.gov.au/animal/health/amr/antimicrobial-resistance-bacteria-animal-origin |
| France | 2001 | ONERBA- RESAPATH | Veterinarians provide data as a part of their routine clinical services. Monitor resistance to antimicrobials in pathogenic bacteria. Collection and genetic characterization of resistant isolates of interest. Technical support to local laboratories Contribute data for comparative analysis between humans and animals. |
https://resapath.anses.fr/ |
| Germany | 2001 | GERM-Vet | Trends of susceptibility to antimicrobial in pathogens (bacterial) of animal origin. Formulate the coordinating and decision-making measures for veterinarians. | http://www.bvl.bund.de |
| Japan | 1999 | JVARM | Monitor AMR in bacteria from food producer animals. Collect data on antimicrobials usage in animals. Monitor the antimicrobials usefulness in food-producing animals. Endorse judicious use of antimicrobials to tackle public health issue. |
http://www.maff.go.jp/nval/tyosa_kenkyu/taiseiki/monitor/e_index.html |
NethMap-MARAN, Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands; SVARM, Swedish Veterinary Antimicrobial Resistance Monitoring; NORM-VET, Norwegian Surveillance System for Antimicrobial Drug Resistance; DANMAP, Danish Integrated Antimicrobial Resistance Monitoring and Research Programme; CIPARS, Canadian Integrated Programme for Antimicrobial Resistance Surveillance; NARMS, National Antimicrobial Resistance Monitoring System; FINRES-VET, The Finnish Veterinary Antimicrobial Resistance Monitoring and Consumption of Antimicrobial Agents; JVARM, The Japanese Veterinary Antimicrobial Resistance Monitoring System; ONERBA, National Observatory of the Epidemiology of Bacterial Resistance to Antibiotics; RESAPATH, French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin; VARSS, Veterinary Antimicrobial Resistance and Sales Surveillance; GERM-Vet, German National Antibiotic Resistance Monitoring; AMR, antimicrobial resistance; PHC, primary healthcare centre; ITAVARM, Italian Veterinary Antimicrobial Resistance Monitoring; CDC, Centers for Disease Control and Prevention; FDA, Food and Drug Administration; USDA, United States Department of Agriculture
Indian scenario on antibiotic use in animals
India has high burden of infectious diseases both in humans and animals. Factors such as poor nutrition, sanitation and overcrowding are some of the leading causes for the occurrence of infectious diseases. India has a population of cattle (191 million), buffalo (109 million), sheep (65 million), goats (135 million), pigs (10 million) and more than 700 million poultry that produce almost 75 billion eggs every year32. Poultry production system demands excessive use of antimicrobials, mostly as growth promoters. There is enough literature stating the use of antibiotics and report of its resistance development in various livestock sectors, including poultry and aquaculture in India. Amongst the several bacterial species affecting the poultry, Staphylococcus, Pasteurella multocida and other bacteria have shown 100 per cent resistance to some drugs33,34. In the North-East region, P. multocida isolates (n=72) from swine showed 70 per cent resistance to amikacin, streptomycin, penicillin-G and vancoymcin34. Suresh et al35 reported Salmonella in 7.7 per cent of total 492 eggs in south India and found 100 per cent resistance to ampicillin, neomycin, polymyxin-B and tetracycline. A study conducted in various districts of Chhattisgarh evaluated the prevalence of Salmonella (7%) in 200 chicken meat samples. These isolates were reported to be highly susceptible to ciprofloxacin, 96.8 per cent to gentamicin and 93.7 per cent resistant to erythromycin36. Recently, a survey was carried out on 18 poultry farms of Punjab to analyze resistance of E. coli isolates (n=1556) against 11 antimicrobials37. The results of this study demonstrated a high prevalence of ESBL-producing Enterobacteriaceae in poultry farms (87 and 42% in broilers and layers, respectively). A high prevalence of E. coli resistant to nalidixic acid (86.1%) and around 45 per cent to various antibiotics such as tetracycline, ciprofloxacin and ampicillin were observed37. A study conducted in Hyderabad on Helicobacter pullorum isolates from both free-range and broiler chickens reported the resistant profiles against various antibiotics, viz. fluoroquinolones, cephalosporins, sulphonamides and macrolides38. Ruban et al39 reported that 66.7 per cent chicken meat of retail outlets harboured MRSA.
Amongst livestock, about 20-30 per cent isolates of S. aureus from milk samples from cow and buffalo affected with mastitis were found to be resistant to gentamicin, tetracycline, erythromycin and lincomycin40. S. aureus in bovine and swine samples showed higher resistance to penicillin (94.6%) and ciprofloxacin (56.7%), while resistance to vancomycin was not found41. Vancomycin-resistant S. aureus isolates were reported from milk samples of both cow and goat from West Bengal42. Similarly, emerging trends of MDR ESBL-producing E. coli and other Gram-negative bacteria were observed from cattle and poultry sector in Odisha43 and from cattle suffering from subclinical mastitis in West Bengal44.
Several antibiotics such as oxytetracycline, ampicillin, enrofloxacin, ciprofloxacin and sparfloxacin and other drugs have been used for prophylaxis and treatment and to improve larval survival in aquaculture. In one study, 90 isolates of Vibrio spp. and seven isolates of Aeromonas spp. isolated from ponds and hatcheries on the East coast were found to be resistant to ampicillin (100%), and almost >50 per cent isolates showed resistance to ciprofloxacin, ceftriaxone, kanamycin and furazolidone45. Further, different Aeromonas spp. obtained from fish and water samples of north India, diseased catfish and from fish of retails markets were resistant to a variety of antibiotics including colistin45,46,47,48. Vibrio harveyi isolates from shrimp in Tamil Nadu were reported to be resistant to ciprofloxacin, penicillin, rifampicin and vancomycin49.
Policies on antibiotics use in food animals in India
India ranks second in aquaculture production globally50. Hence, it is imperative to limit the prophylactic antibiotics usage in aquaculture and focus on the correct guidelines for the implementation. Excessive use of antibiotics in animals leads to accumulation of antibiotic residues in food chain. The absence of national estimates on quantum of the problem and guidelines on antibiotics usage in farm animals and AMR in India has further fuelled the problem.
In the absence of any uniform policy about the antibiotics usage in animals in India, prophylactic use of antibiotic in poultry production and in livestock is common. Table II summarizes the recommendations by various organizations to address the use of antibiotics and ABR in the food animal sector. In 2007, the Bureau of Indian Standards of Poultry Feed recommended to stop the use of antibiotics with systemic action as AGPs in feed51. The National Policy on Containment of AMR (2011) has not laid much emphasis on AMR pattern from animals52. In 2012-2017, National Programme on Containment of AMR gave main emphasis on laboratory-based AMR surveillance system but with no concrete timelines53. In 2013, National Livestock Policy encouraged States for careful use of antibiotics54. In 2013, Drug Controller General of India (DCGI) made it obligatory to mention the labels of antibiotics with appropriate withdrawal periods meant for animal use, and if not validated, then it should be set for at least seven days for egg or milk, for poultry meat (28 days) and fish meat (500 degree days)55. In June 2014, Department of Animal Husbandry, Dairying and Fisheries (DAHDF) requested States to advise veterinarians for the careful use of antibiotics and ban antibiotics mixing in feed56. They also proposed the necessary veterinary supervision during antibiotics use on animals and for tracking the antibiotic usage. It was also advised that licensed antibiotics should be supplied to registered users.
Table II.
Recommendations by organizations to address the use of antibiotics and antibiotics resistance in livestock, poultry and fisheries sector
| Year | Organizations | Recommendations/proposals |
|---|---|---|
| Poultry/livestock | ||
| 2007 | BIS-Poultry feed recommendation51 | Stop use of antibiotic growth promoters with systemic action in poultry feed such as chloramphenicol, doxycycline, tetracycline, nitrofurazone and furazolidone. Stop usage of gut acting antibiotics in five years. |
| 2011 | National Policy on containment of AMR52 | Action plan includes enforcement and enhancement of regulatory provision on antimicrobials usage in poultry sector, along with necessary labelling in food. Promote rational use of drugs via education and managerial and regulatory strategy. Advocate the strengthening of diagnostics for AMR monitoring. |
| 2012-2017 | National Programme on Containment of AMR (http://dghs.gov.in/WriteReadData/userfiles/file/National_Programme_on_Containment_of_Anti_Microbial_Resistance.pdf) | Aimed at laboratory-based AMR surveillance system. Strengthen infection control guidelines. Generate awareness among healthcare providers. |
| 2013 | Drug Controller General of India: Drugs and Cosmetics Act, 19405 https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=Mjcx | Directed the drug manufacturers about administration of antibiotics and their withdrawal period for food-producing animals. Compulsory labelling of withdrawal periods on antibiotics intended for animal use; If this period is not validated, then use following period ‘egg or milk (seven days), poultry meat (28 days), fish meat (500 days)’. |
| 2013 | The National Livestock Policy (http://dahd.nic.in/sites/default/filess/NLP%202013%20Final11.pdf) | Encouraged States to promote judicious use of antibiotics; however, barely touched antibiotic resistance issue. |
| 2014 | Department of Animal Husbandry, Dairying and Fisheries, Circular to States (http://www.farmer.gov.in/dadf/Advisories/Advisory_on_use_of_antibiotics_in_food_producing_animals.pdf) | Requested States to advise veterinarians and feed manufacturers about judicious antibiotics use for treatment of ailing food-producing animals, and to stop antibiotics use in feed. |
| 2015 | FSSAI (http://old.fssai.gov.in/Portals/0/Pdf/Draft_Meat_Poultry_Comments.pdf) | Suggested veterinary supervision during antibiotics use on animals. Feed for meat producing animals or birds should not include meat, blood meal, bone tissues except milk and milk products. Suggested different slaughterhouse for poultry and livestock animals.Strictly ban AGPs in poultry and meat. Supply licensed antibiotics for tracking antibiotic use. |
| 2017-2022 | National Policy on Containment of AMR (http://www.searo.who.int/india/topics/antimicrobial_resistance/nap_amr.pdf) | To provide better awareness and AMR understanding by various trainings, learning and communication. Effective surveillance. Emphasis on infection prevention and control. Primarily focuses on resistance in bacteria. |
| Aquaculture | ||
| 2011 | FSSAI (http://fsdaup.gov.in/writereaddata/images/pdf/act-and-rules/fss-regulation/Food-safety-and-standards-contaminats-toxins-and-residues-regulation-2011.pdf) | Tolerance limit for four antibiotics (tetracycline, oxytetracycline, trimethoprim and oxolinic acid) was set for seafoods (shrimps, prawns) and a variety of fish and related products. Ban of several antibiotics as well as other pharmacologically active substances. |
BIS, Bureau of Indian Standards; AMR, Antimicrobial Resistance; FSSAI, Food Safety and Standard Authority of India; AGPs, Antibiotic growth promoters
The Food Safety and Standards Authority of India (FSSAI) in 2015 issued guidelines to limit the use of antibiotics in meat and meat products; however, these are still under the process of implementation57. It is proposed that feed for meat-producing animals or birds should not include meat, blood meal and bone tissues except milk and milk products. The FSSAI has also suggested different slaughterhouse for poultry and livestock animals, and there should be slaughter of only those animals that are allowed as per the Food Safety and Standards (Food Product Standards and Food Additives) regulations, 201157. The FSSAI in November 2017 has invited comments from all stakeholders and general public to fix a tolerance limit of antibiotics and veterinary drugs in meat and meat products. Recently, National Action Plan on AMR (2017-2021) has identified six strategic priorities under NAP-AMR31. Their main focus areas are to develop better awareness and AMR understanding by providing training, strengthening education, reducing the incidence of infection, promoting investments and strengthening India's leadership on AMR.
For seafoods, regulations of antibiotics use and other pharmacologically active substances were set by the FSSAI in 2011. These guidelines were set as per ‘The Prevention of Food Adulteration Rules, 1995-Part XVIII: Antibiotic and other Pharmacologically Active Substances’52. As per their policy, there was prescribed tolerance limit [mg/kg (ppm)] for the use of antibiotics namely, tetracycline (0.1), trimethoprim (0.05), oxytetracycline (0.1) and oxolinic acid (0.3) for seafoods with shrimps, prawns or other fish variety and products of fish. In addition to this, 'several antibiotics and other pharmacologically active substances, namely nitrofurantoin, chloramphenicol, neomycin, nalidixic acid, sulphamethoxazole, Aristolochia spp. and preparations thereof, chloroform, chlorpromazine, colchicine, dapsone, dimetridazole, metronidazole, ronidazole, ipronidazole, other nitroimidazoles, clenbuterol, diethylstilbestrol, sulphonamide drugs (except approved sulphadimethoxine, sulphabromomethazine and sulphamethoxypyridazine), fluoroquinolones and glycopeptides are prohibited’52.
Since selection pressure is one of the well-known contributors to AMR, limiting inappropriate antimicrobial use remains a major concern. For instance, colistin is widely used as a growth promoter in the production of food animals in India specifically in poultry sector58. Colistin sulphate, an antibiotic used as a growth promoter, is easily available in the market without the need for prescription. Besides poultry, these antibiotic-based feed supplements are also used in aquaculture. Due to extensive use of colistin in the veterinary sector, there is an urgent need for regulations to ban the use of colistin in feed. The reason why colistin is drawing attention towards AMR is because it is the last resort antibiotic in humans for carbapenem-resistant cases, and as per the WHO, it is under the list of ‘highest priority’ critically important antimicrobial for human medicine59. Hence, in the updated list of essential medicines 2017, colistin has been put in reserve category. After carbapenem-resistant cases, colistin-resistant cases among Gram-negative bacteria are also being reported from hospitals in India60,61,62. This needs to be done urgently because of documented dangerous spread of colistin-resistant bacteria from China to several other countries in the recent past9. In Enterobacteriaceae, a study showed the appearance of transferable colistin resistance which was due to extensive use of this antibiotic in China for pigs’ rearing. The resistance was first reported in 2016 by Liu et al9 and has also been found in different parts of India63. This implies a serious warning to protect global health by restricting or phasing out use of such critical drugs. The extensive use of such antibiotics in organized poultry farms in India has also been shown to culminate in emergence of widespread resistance to several pathogens63.
Besides colistin, the antibiotics that should be reserved exclusively for human use are fourth- and fifth-generation cephalosporins, polymyxins (both colistin and polymyxin B), carbapenems and oxazolidinones (linezolid), fosfomycin aztreonam, tigecycline and daptomycin59. It is therefore, imperative to initiate an appropriate action over these reserved groups of antibiotics to ensure compliance, and if not, then this may lead to greater emergence of resistance.
Major gaps of antimicrobial resistance (AMR) study
• Non uniform policy
• Lack of epidemiological and standardization studies in veterinary sector to capture reliable data
• Lack of veterinary surveillance of antimicrobial resistance and antimicrobial use
• Lack of awareness among farmers and veterinary professionals
Initiatives of Indian Council of Medical Research (ICMR) on AMR in food animals
In spite of availability of several policies and guidelines on antibiotics use in animals, livestock, poultry and fisheries, the antimicrobial use in these settings remains high and unregulated. Implementation of the guidelines is a major challenge and requires urgent and comprehensive efforts (Box).
In view of global emphasis to regulated use of antibiotics in animals sector, The Indian Council of Medical Research (ICMR) in collaboration with Indian Council of Agriculture Research (ICAR) has initiated a project in 2017 to understand the gaps in the antimicrobial susceptibility testing and to fill those gaps by carrying out capacity building activities using the standardized methodology. The core emphasis of the project is to develop standard operating procedures for antimicrobial susceptibility testing for veterinary microbiology laboratories and identify available capacities and the gap areas. The project envisages developing capacity in AMR in veterinary sector for providing important linkages with human sector. In June 2017, the ICMR organized a meeting of all the relevant stakeholders to discuss the possibility of reserving a few classes of drugs for human use. The group made the following observations:
-
(i).
Regulation of crucial antibiotics of human use: The antibiotics which are designated to be ‘critically important’ or ‘last line’ antibiotics for humans,59 viz polymyxins (colistin), glycopeptides (vancomycin), fluoroquinolones and fourth- and fifth-generation cephalosporins (carbapenems) should not be used for treatment in food-producing animals.
-
(ii).
Antibiotics exclusively for animal treatment: Antibiotics such as penicillin, tetracyclines, cephalosporins, quinolones, sulphonamides and aminoglycosides are crucial for treatment in animals and cannot be spared. Glycopeptides and carbapenems are used in pets.
-
(iii).
Standardization of diagnostic methods: There is a dearth in diagnostic methods for detection of AMR in animals which again need to be standardized and synchronized for their uniform use in all the veterinary laboratories across the country.
-
(iv).
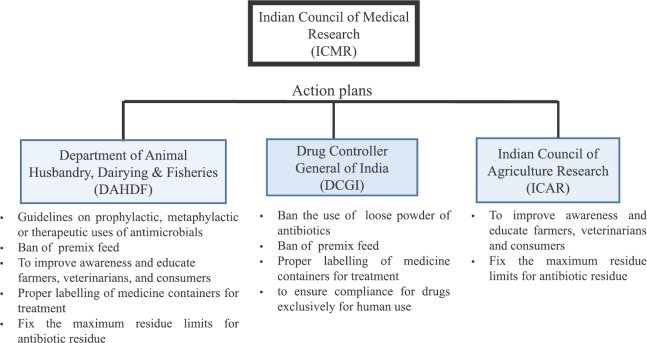
Research areas: There is a need to carry out research studies on transmission dynamics to determine spread of resistant isolates between animals, humans and fisheries. The Group suggested action points for urgent implementation of antimicrobials use in food animals with the help of the Department of Animal Husbandry, Dairying and Fisheries (DAHDF), DCGI and ICAR (Figure).
Figure.
A schematic outline of action points for antibiotic use in food-producing animals.
Conclusions
There is now ample evidence available from other countries to suggest that the use of antimicrobials in animal sector creates drug-resistant pathogens which pose potential threat to humans. There is also evidence to support that stewardship in animals helps improve resistance profiles. India needs to formulate strict policies regarding antimicrobial use both in humans and animals. In line with the suggestions of the WHO, it will be in national interest to regulate the use of antibiotics where unavoidable and restrict unnecessary use. With no new candidates in the pipeline, it will essential to reserve certain classes of antibiotics for human use and those antibiotics should not be used in animals at all.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Drivers, dynamics and epidemiology of antimicrobial resistance in animal production. Rome: FAO; 2016. Food and Agriculture Organization of the United Nations. [Google Scholar]
- 2.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34:S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 3.Food outlook, biannual report on global food markets. Rome: FAO; 2018. Food and Agriculture Organization of the United Nation. [Google Scholar]
- 4.Marshall BM, Levy SB. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev. 2011;24:718–33. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen HK. Antibiotic resistance gene discovery in food-producing animals. Curr Opin Microbiol. 2014;19:25–9. doi: 10.1016/j.mib.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J Antimicrob Chemother. 2014;69:827–34. doi: 10.1093/jac/dkt443. [DOI] [PubMed] [Google Scholar]
- 7.Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. MBio. 2012;3 doi: 10.1128/mBio.00305-11. pii: e00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewnetu D, Mihret A. Prevalence and antimicrobial resistance of Campylobacter isolates from humans and chickens in Bahir Dar, Ethiopia. Foodborne Pathog Dis. 2010;7:667–70. doi: 10.1089/fpd.2009.0433. [DOI] [PubMed] [Google Scholar]
- 9.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes MR, Moura Q, Sartori L, Silva KC, Cunha MP, Esposito F, et al. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.17.30214. pii: 30214. [DOI] [PubMed] [Google Scholar]
- 11.Al Bayssari C, Dabboussi F, Hamze M, Rolain JM. Emergence of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii in livestock animals in Lebanon. J Antimicro Chemother. 2015;70:950–1. doi: 10.1093/jac/dku469. [DOI] [PubMed] [Google Scholar]
- 12.Fischer J, Rodríguez I, Schmoger S, Friese A, Roesler U, Helmuth R, et al. Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J Antimicrob Chemother. 2012;67:1793–5. doi: 10.1093/jac/dks108. [DOI] [PubMed] [Google Scholar]
- 13.Boonyasiri A, Tangkoskul T, Seenama C, Saiyarin J, Tiengrim S, Thamlikitkul V, et al. Prevalence of antibiotic resistant bacteria in healthy adults, foods, food animals, and the environment in selected areas in Thailand. Pathog Glob Health. 2014;108:235–45. doi: 10.1179/2047773214Y.0000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Founou LL, Founou RC, Essack SY. Antimicrobial resistance in food chain: A developing country perspective. Front Microbiol. 2016;7:1881. doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collignon PJ, Conly JM, Andremont A, McEwen SA, Aidara-Kane A World Health Organization Advisory Group. Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISAR) World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin Infect Dis. 2016;63:1087–93. doi: 10.1093/cid/ciw475. [DOI] [PubMed] [Google Scholar]
- 16.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F, et al. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–9. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerlin P, Wissing A, Aarestrup FM, Frey J, Nicolet J. Antimicrobial growth promoter ban and resistance to macrolides and vancomycin in enterococci from pigs. J Clin Microbiol. 2001;39:4193–5. doi: 10.1128/JCM.39.11.4193-4195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grave K, Jensen VF, Odensvik K, Wierup M, Bangen M. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev Vet Med. 2006;75:123–32. doi: 10.1016/j.prevetmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Jensen HH, Hayes DJ. Impact of Denmark's ban on antimicrobials for growth promotion. Curr Opin Microbiol. 2014;19:30–6. doi: 10.1016/j.mib.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I, et al. The weight of nations: An estimation of adult human biomass. BMC Public Health. 2012;12:439. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, et al. Reducing antimicrobial use in food animals. Science. 2017;357:1350–2. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–50. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 23.ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals - Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017;15:4872. doi: 10.2903/j.efsa.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112:5649–54. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JP, Gupta U, et al. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Global action plan on antimicrobial resistance. WHO. 2015. [accessed on November 14, 2017]. Available from: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf .
- 27.The FAO Action Plan on Antimicrobial Resistance 2016-2020. Supporting the food and agriculture sectors in implementing the global action plan on antimicrobial resistance to minimize the impact of antimicrobial resistance. Rome: FAO; 2016. Food and Agriculture Organization of the United Nations. [Google Scholar]
- 28.World Organization for Animal Health (OIE) OIE annual report on the use of antimicrobial agents in animals. Better understanding of the global situation. France: OIE; 2016. [Google Scholar]
- 29.World Organization for Animal Health (OIE) OIE list of antimicrobial agents of veterinary importance. France: OIE; 2015. [Google Scholar]
- 30.The European Committee on Antimicrobial Susceptibility Testing - EUCAST. [accessed on January 2, 2018]. Available from: http://www.eucast.org/ast_of_veterinary_pathogens/
- 31.Government of India. National action plan on antimicrobial resistance, 2017-2021. [accessed on January 2, 2018]. Available from: http://www.searo.who.int/india/topics/antimicrobial_resistance/nap_amr.pdf .
- 32.Kaur G, Brar YS, Kothari DP. Potential of livestock generated biomass: Untapped energy source in India. Energies. 2017;10:847. [Google Scholar]
- 33.Dhanarani TS, Shankar C, Park J, Dexilin M, Kumar RR, Thamaraiselvi K, et al. Study on acquisition of bacterial antibiotic resistance determinants in poultry litter. Poult Sci. 2009;88:1381–7. doi: 10.3382/ps.2008-00327. [DOI] [PubMed] [Google Scholar]
- 34.Shivachandra SB, Kumar AA, Biswas A, Ramakrishnan MA, Singh VP, Srivastava SK, et al. Antibiotic sensitivity patterns among Indian strains of avian Pasteurella multocida. Trop Anim Health Prod. 2004;36:743–50. doi: 10.1023/b:trop.0000045950.35070.7f. [DOI] [PubMed] [Google Scholar]
- 35.Suresh T, Hatha AAM, Sreenivasan D, Sangeetha N, Lashmanaperumalsamy P. Prevalence and antimicrobial resistance of Salmonella enteritidis and other Salmonellas in the eggs and egg-storing trays from retail markets of Coimbatore, South India. Food Microbiol. 2006;23:294–9. doi: 10.1016/j.fm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Naik VK, Shakya S, Patyal A, Gade NE, Bhoomika Isolation and molecular characterization of Salmonella spp. from chevon and chicken meat collected from different districts of Chhattisgarh, India. Vet World. 2015;8:702–6. doi: 10.14202/vetworld.2015.702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brower CH, Mandal S, Hayer S, Sran M, Zehra A, Patel SJ, et al. The prevalence of extended-spectrum beta-lactamase-producing multidrug-resistant Escherichia coli in poultry chickens and variation according to farming practices in Punjab, India. Environ Health Perspect. 2017;125:077015. doi: 10.1289/EHP292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qumar S, Majid M, Kumar N, Tiwari SK, Semmler T, Devi S, et al. Genome dynamics and molecular infection epidemiology of multidrug-resistant Helicobacter pullorum isolates obtained from broiler and free-range chickens in India. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.02305-16. pii: e02305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruban SW, Babu RN, Abraham RJJ, Senthilkumar TMA, Kumaraswamy P, Rao VA, et al. Prevalence of panton valentine leukocidin (PVL) gene in methicillin resistant Staphylococcus aureus isolated from market samples of chicken meat. Int J Cur Microbiol Appl Sci. 2017;6:2459–66. [Google Scholar]
- 40.Kumar R, Yadav BR, Singh RS. Antibiotic resistance and pathogenicity factors in Staphylococcus aureus isolated from mastitic Sahiwal cattle. J Biosci. 2011;36:175–88. doi: 10.1007/s12038-011-9004-6. [DOI] [PubMed] [Google Scholar]
- 41.Zehra A, Singh R, Kaur S, Gill JPS. Molecular characterization of antibiotic-resistant Staphylococcus aureus from livestock (bovine and swine) Vet World. 2017;10:598–604. doi: 10.14202/vetworld.2017.598-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya D, Banerjee J, Bandyopadhyay S, Mondal B, Nanda PK, Samanta I, et al. First report on vancomycin-resistant Staphylococcus aureus in bovine and caprine milk. Microb Drug Resist. 2016;22:675–81. doi: 10.1089/mdr.2015.0330. [DOI] [PubMed] [Google Scholar]
- 43.Kar D, Bandyopadhyay S, Bhattacharyya D, Samanta I, Mahanti A, Nanda PK, et al. Molecular and phylogenetic characterization of multidrug resistant extended spectrum beta-lactamase producing Escherichia coli isolated from poultry and cattle in Odisha, India. Infect Genet Evol. 2015;29:82–90. doi: 10.1016/j.meegid.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Das A, Guha C, Biswas U, Jana PS, Chatterjee A, Samanta I, et al. Detection of emerging antibiotic resistance in bacteria isolated from subclinical mastitis in cattle in West Bengal. Vet World. 2017;10:517–20. doi: 10.14202/vetworld.2017.517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaseeharan B, Ramasamy P, Murugan T, Chen JC. In vitro susceptibility of antibiotics against Vibrio spp. and Aeromonas spp. isolated from Penaeus monodon hatcheries and ponds. Int J Antimicrob Agents. 2005;26:285–91. doi: 10.1016/j.ijantimicag.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Rathore G, Swaminathan TR, Abidi R, Mahanta PC, Kapoor D. Isolation and characterization of motile aeromonads from aquatic environment. Ind J Fish. 2005;52:241–8. [Google Scholar]
- 47.Jayavignesh V, Sendesh KK, Bhat AD. Biochemical characterization and cytotoxicity of the Aeromonas hydrophila isolated from Catfish. Arch Appl Sci Res. 2011;3:85–93. [Google Scholar]
- 48.Kaskhedikar M, Chhabra D. Multiple drug resistance in Aeromonas hydrophila isolates of fish. Vet World. 2010;3:76–7. [Google Scholar]
- 49.Stalin N, Srinivasan P. Molecular characterization of antibiotic resistant Vibrio harveyi isolated from shrimp aquaculture environment in the South East coast of India. Microb Pathog. 2016;97:110–8. doi: 10.1016/j.micpath.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 50.World aquaculture 2010: FAO fisheries and aquaculture technical paper. Rome: FAO; 2011. Food and Agriculture Organization of the United Nations (FAO) [Google Scholar]
- 51.New Delhi: 2007. Indian Standard, Poultry Feeds - Specifications. Bureau of Indian Standards (BIS) 5th Revision IS1374. [Google Scholar]
- 52.National policy for containment of antimicrobial resistance. New Delhi: MoHFW; 2011. Directorate General of Health Services. Ministry of Health & Family Welfare. [Google Scholar]
- 53.Government of India. National programme on containment of anti-microbial resistance. [accessed on January 2, 2018]. Available from: https://dghs.gov.in/WriteReadData/userfiles/file/National_Programme_on_Containment_of_Anti_Microbial_Resistance.pdf .
- 54.Department of Animal Husbandry, Dairying & Fisheries. National livestock policy. New Delhi: Ministry of Agriculture, Government of India; 2013. [accessed on January 2, 2018]. Available from: http://dahd.nic.in/sites/default/filess/NLP%202013%20Final11.pdf . [Google Scholar]
- 55.Directorate General of Health Services. Sub role 3A of rule 97 of Drugs and Cosmetics rules 1945 regarding withdrawal period. [accessed on January 2, 2018]. Available form: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=Mjcx .
- 56.Advisory on use of antibiotics in food producing animals. New Delhi: Ministry of Agriculture, Government of India; 2014. [accessed on January 2, 2018]. Department of Animal Husbandry, Dairying & Fisheries. http://www.farmer.gov.in/dadf/Advisories/Advisory_on_use_of_antibiotics_in_food_producing_animals.pdf) [Google Scholar]
- 57.Food safety and Standards Authority of India. Comments with regard to placing of meat and poultry products on the Indian market. New Delhi: Controller of Publications, Ministry of Health and Family Welfare, Government of India; 2015. [accessed on January 2, 2018]. Available from: http://old.fssai.gov.in/Portals/0/Pdf/Draft_Meat_Poultry_Comments.pdf . [Google Scholar]
- 58.Davies M, Walsh TR. A colistin crisis in India. Lancet Infect Dis. 2018;18:256–7. doi: 10.1016/S1473-3099(18)30072-0. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. The Selection and Use of Essential Medicines. Report of the WHO Expert Committee on Selection and Use of Essential Medicines, (including the 20th WHO Model List of Essential Medicines and the 6th WHO Model List of Essential Medicines for Children); WHO Technical Report Series 1006. Geneva: WHO; 2017. [Google Scholar]
- 60.Arjun R, Gopalakrishnan R, Nambi PS, Kumar DS, Madhumitha R, Ramasubramanian V, et al. A study of 24 patients with colistin-resistant gram-negative isolates in a tertiary care hospital in South India. Indian J Crit Care Med. 2017;21:317–21. doi: 10.4103/ijccm.IJCCM_454_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaur A, Gandra S, Gupta P, Mehta Y, Laxminarayan R, Sengupta S, et al. Clinical outcome of dual colistin- and carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A single-center retrospective study of 75 cases in India. Am J Infect Control. 2017;45:1289–91. doi: 10.1016/j.ajic.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Manohar P, Shanthini T, Ayyanar R, Bozdogan B, Wilson A, Tamhankar AJ, et al. The distribution of carbapenem- and colistin-resistance in gram-negative bacteria from the Tamil Nadu region in India. J Med Microbiol. 2017;66:874–83. doi: 10.1099/jmm.0.000508. [DOI] [PubMed] [Google Scholar]
- 63.Center for Disease Dynamics Economics & Policy. Antibiotic use and resistance in food animals: Current policy and recommendations. [accessed on January 2, 2018]. Available form: https://www.cddep.org/wpcontent/uploads/2017/06/india_abx_report-2.pdf .