Abstract
Background
Vegetation succession is one of the major driving processes of grassland degradation. Stoichiometry significantly contributes to vegetation dynamics. However, a knowledge gap exists in how soil nutrients and root enzymes influence the stoichiometric ratio to affect vegetation dynamics.
Methods
To address these questions, we selected a dominant species (Leymus chinensis (Trin.) Tzvel.) and a degraded-dominant species (Artemisia frigida Willd.) under different management regimes (enclosure and grazing) on the Inner Mongolia steppe. We measured (i) plant nutrient concentrations, (ii) root enzymes and (iii) soil nutrients to investigate how the selected plant species responded to grazing.
Results
The results show that: (i) N and P concentrations and the C:N:P ratio in different organs are significantly affected by grazing, and there is variation in the plant species’ response. Grazing significantly increased N and P in the leaves and stems of L. chinensis and the stems and roots of A. frigida. (ii) Grazing significantly increased the activities of glutamine synthase but decreased the activities of acid phosphatase in L. chinensis. The nitrate reductase and acid phosphatase activities significantly increased in A. frigida under grazing conditions. (iii) Grazing decreased the total nitrogen, total phosphorus, and available nitrogen, but increased the available phosphorus in the soil.
Conclusion
We conclude that A. frigida is better adapted to grazing than L. chinensis, possibly because of its relatively increased stem and root growth, which enhance population expansion following grazing. Conversely, L. chinensis showed increased leaf and stem growth, but suffered nutrient and biomass loss as a result of excessive foraging by livestock, which severely affected its ability to colonize. Root enzymes coupled with soil nutrients can regulate plant nutrients and stoichiometric ratios as an adaptive response to grazing. Thus, we demonstrated that stoichiometric ratios allow species to better withstand grazing disturbances. This study provides a new understanding of the mechanisms involved in grazing-resistance within a plant-soil system.
Keywords: Nutrient, Stoichiometry, Leymus chinensis, Artemisia frigida, Enzyme
Introduction
Grasslands are the largest terrestrial ecosystem in the world and play an extremely important role in the production of food and ecological services for humans. However, continuous overgrazing of grasslands leads to degradation. This not only diminishes productivity, diversity and soil quality but also affects economic growth and ecological sustainability (Pulido et al., 2016; Wang et al., 2017). The Inner Mongolia typical steppe, a major component of the eastern Eurasian temperate steppe and an important production base for animal husbandry in China, faces similar challenges (Li et al., 2012); this is true particularly in areas where the steppe is dominated by L. chinensis, which has high palatability, high nutritive value, and high primary productivity (Li et al., 2008). Leymus chinensis steppes have degraded into A. frigida communities because of long-term grazing during the past several decades (Li, Li & Ren, 2005) and consequently have become characterized by dwarf plants with low productivity. Previous studies have demonstrated that plant adaption to animal grazing depends on plant growth and reproductive characteristics (De Jong & Lin, 2017; Li, Li & Ren, 2005), palatability (Vesk & Westoby, 2001), nutrient use strategies (Hamilton & Frank, 2001), tolerance (regrowth potential after herbivory) (Strauss & Agrawal, 1999) and defence strategies (physical and chemical defence substances) (Zhang et al., 2014). These adaptive characteristics may be attributed to differences in N and P concentrations and the stoichiometric ratios in the plant tissues of different species because of the association of the nutrients with plant growth and ecosystem functions (Elser et al., 2010; Yu et al., 2010).
Ecological stoichiometry is the study of the balance between multiple elements in ecological interactions (Elser et al., 2000a; Elser et al., 2000b). Using this approach, patterns of plant responses to their chemical environment can be well understood. Plant stoichiometry shows why subordinate species withstand drought perturbations (Mariotte, Canarini & Dijkstra, 2017), the trade-off between competitive ability and grazing susceptibility (Branco et al., 2010), and the response of plant species to global-change-driven alterations in resource availability (Yu et al., 2015). The correlation between C:N:P, plant growth, and ecosystem functions (Elser et al., 2010; Yu et al., 2010) is supported by the hypothesis that increasing allocation to P-rich ribosomal RNA supports faster growth rates (Matzek & Vitousek, 2009), and its corollary is related to nutrient use strategies (De Deyn, Cornelissen & Bardgett, 2008) and chemical defence (Royer et al., 2013). The stoichiometric ratio affects the competitive abilities of species under grazing, for example, fast-growing species (competitors) were dominant in a fertilized pasture under low grazing pressure and slow-growing species (tolerant) were relatively abundant in unfertilized grazing systems (Hill et al., 2005). This variation driven by grazing in producer stoichiometry, in turn, can regulate grazing. Earlier reports have shown that animal grazing increased plant N and P concentrations and decreased C:N and C:P ratios on the whole (Bai et al., 2012; Heyburn et al., 2017). However, the rate of nutrient uptake enhanced plant competitiveness but also increased their nutritional quality for herbivores (Branco et al., 2010). Therefore, the stoichiometric ratio responses of plant species to livestock grazing remain latent. Most stoichiometric studies usually focus on the leaf because of its pivotal role in controlling N and P in the carbon obtained (He et al., 2006). However, relatively limited research has been conducted on stems and roots, even though they can serve as nutrient reservoirs that store excess nutrients absorbed from the soil and support the use of N and P in leaves (Cernusak, Winter & Turner, 2010; Yan et al., 2016). In contrast to leaves, the sensitivity of stems and roots to various environments have been demonstrated by woody species in greenhouse studies (Schreeg et al., 2014) and marsh plants along coastlines (Minden & Kleyer, 2014). More importantly, plants respond to grazing by varying the N and P concentrations in leaves, stems and roots as a consequence of changes in the structure and functions of the whole plant (Matzek & Vitousek, 2009). Hence, N and P concentrations and the stoichiometric ratio of stems and roots in response to grazing require further study.
A plant’s C:N:P stoichiometry can be strongly influenced by the environment, despite an organism’s considerable capacity to maintain their body stoichiometry within ranges (Sardans, Rivas-Ubach & Peñuelas, 2012). Soil nutrients can affect the stoichiometric ratios of plants; for example, the C, N and P contents of leaves and litter have been shown to be positively related to soil C, N and P contents (Ordoñez et al., 2009; Yang, Liu & An, 2018). Many studies have found that grazing can alter soil nutrient availability, which is strongly related to dung and urine deposition from grazing animals (Guo et al., 2017), litter decomposition rates (Semmartin et al., 2004), mineralization and nutrient cycling, etc. Moreover, grazing promotes greater accumulation of soil organic carbon (SOC) depending on the root system biomass, which in turn promotes more root biomass, fine root exudates and microbial biomass (Wilson et al., 2018). Previous studies have shown how animal grazing can increase N availability in soils, with an attendant significant decrease in plant above- and below-ground C:N ratios and a subsequent increase in plants below ground N:P ratios (Bai et al., 2012; Zheng et al., 2012). However, unpredictable changes in C:N:P ratios have also been reported, indicating that plant stoichiometry may not be simply related to soil nutrient availability (Heyburn et al., 2017). Therefore, key knowledge gaps exist regarding how long-term grazing influences plant stoichiometry, and whether or not changes in C:N:P ratios in plant tissues might be related to predictable changes in soil C, N, and P contents and storage; this study sought to fill these gaps. Notably, nutrient uptake rates may be regulated by inducible enzymes synthesized by plants. Niklas & Edward (2005) found that plants can adjust N concentration by releasing a certain amount of ribulose bisphosphate carboxylase/oxygenase (Rubisco) in photosynthetic organs. Nitrate reductase and glutamine synthase are two important enzymes in plant nitrogen metabolism (Zioni, Vaadia & Lips, 1971) and serve as the rate-limiting enzymes in the biochemical pathway for nitrogen assimilation. Phosphatase enzymes are responsible for the release of P from organic P-esters, which is one of the P-acquisition strategies (Rejmánková & Macek, 2008). However, little is known regarding the regulation of root enzymes as a grazing response in different plants.
In this study, we hypothesized that plant species have different abilities to take up nutrients under unbalanced N and P availability, and that might support plant adaptation to grazing. Thus, we studied the effects of grazing on C, N, and P contents and stoichiometry in the leaves, stems, and roots of two plant species (L. chinensis and A. frigida). Leymus chinensis is a xeric rhizomatous perennial grass, 0.4–0.9 m in height, that dominates the steppe zone of the Inner Mongolian Plateau, and it is also found in northeast China. Artemisia frigida is a semi-shrub that grows on sandy and gravelly soil of the Inner Mongolia Autonomous Region, in the northern part of China and is the dominant species of degraded grassland communities. Specifically, we address three questions: (1): How do the C, N and P concentrations and their stoichiometric ratios in the leaves, stems, and roots of two plant species respond to grazing? (2): How do plants mediate root enzymatic activities in response to grazing? (3): How does grazing induce soil nutrients to moderate species adaption?
Materials and Methods
Study sites
The study was conducted at the Inner Mongolia Grassland Ecosystem Research Station (43°38′N, 116°42′E), located in the Xilin River Basin, Xilinhaote, Inner Mongolia Autonomous Region, China. The soil is dark chestnut with a 20–30 cm thick humus layer and a calcic layer below 50–60 cm depth. The region is within a temperate-semiarid climate, with an annual mean temperature of approximately 0 °C and annual precipitation of approximately 350 mm. The precipitation fluctuates from 180 to 550 mm, 60–80% of which falls during the summer season of June to August. The growing season lasts from early April to late September for perennial plant species, whereas annual plants usually germinate in early July following the rains (Li et al., 2016).
Experimental design and measurements
The experiment was conducted at the peak of plant biomass accumulation during the middle of August. Two adjacent plots were selected to reduce the impact of climate factors. The first was an enclosure plot that has been excluded from sheep grazing for 34 years (from 1983 until the year of sampling), which was used as the control plot (C). The second was a grazing plot (G) that has been subjected to continuous free grazing with a stocking rate of approximately 9 sheep equivalent (SE) ha−1 year−1 with the same duration as the enclosure plot. Grazing begins in early June and ends during mid-October. The two plots have never been fertilized or mowed during the management (Fig. 1). To decrease the spatial variation and soil heterogeneity, we selected the sampling areas 50 × 400 m in size for the control sites and 20 × 400 m in size for the grazing sites. Subsequently, we randomly chose five subplots in each sampling area (0.2 ha and 0.1 ha for the control and grazing plots, respectively) set at intervals of at least 10 m. This study adopts pseudo-replication and a space-for-time substitution limitation (Hurlbert, 1984).
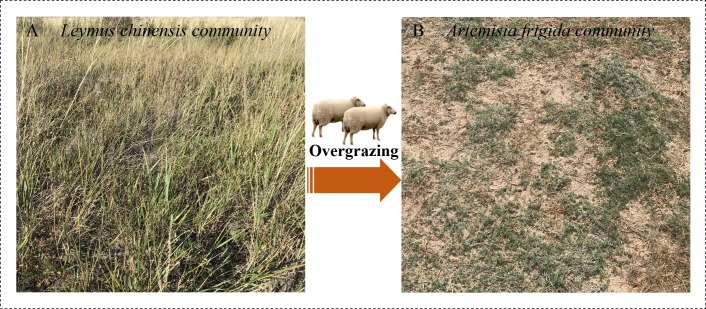
Figure 1. Two communities in Inner Mongolia steppe.
A typical community of Leymus chinensis located in Xilinhaote, Inner Mongolia Autonomous Region, China. The forms degenerate to B, Artemisia frigida community due to long-term grazing as used in this study. Photo credit: Wenjing Ma.
Following the principles of replication and randomization, there were five replicates for L. chinensis and A. frigida within each subplot; and these were combined and mixed to create one composite sample for each species. Taking L. chinensis as an example, we first selected and marked this species, then carefully uprooted the soil core of the whole plant using a shovel (20 cm depth) after removing impurities such as litter and stones. Roots were cautiously removed from the matrix to minimize root loss, repeatedly washed until they were fully exposed and subsequently rinsed with distilled water. Prior to drying, some fresh root samples near the tips (<0.5 mm in diameter for L. chinensis and <1 mm in diameter for A. frigida) were excised and placed into a 10 ml centrifuge tube and immediately frozen in liquid nitrogen before being taken to the laboratory for storage in a −80 °C cryogenic refrigerator for enzymatic analysis. Soil samples were taken at 0–20 cm depth in each of the soil cores following plant sampling. The plant samples were taken to the laboratory for analysis.
In the laboratory, the plant samples (L. chinensis and A. frigida) were separated into leaves, stems (containing leaf sheath) and roots (no seeds at the time of sampling), and oven-dried at 65 °C for 48 h to determine the dry matter content. The samples were milled (XL-02A, Xulang, China) and subsequently divided into two parts for determination of C, N and P concentrations in the leaves, stems, and roots. The first part was used to analyse the C concentration using the K2Cr2O7–H2SO4 oxidation method (Yu et al., 2012a). The second part was digested with acid and used to determine the N and P concentrations. Total nitrogen (N) was measured using the Kjeldahl method, and total phosphorus (P) was analysed using the molybdenum blue colourimetric method (Lü et al., 2015).
Soil samples were air-dried at room temperature for two weeks after removing fine roots and stones. The soil samples were ground, homogenized, and passed through a 2 mm mesh sieve. The total N and P contents of the soil samples were determined as described for the plant samples (Yang et al., 2017). The available nitrogen in the soil was measured using a micro-diffusion technique after alkaline hydrolysis. The soil available phosphorus was analysed using molybdenum antimony blue colourimetry after extraction with NaHCO3 (Fu et al., 2000).
The frozen root samples were analysed after 50 days of sampling. The frozen root samples were ground in a freezer grinder (JXFSTPRP-CL, Jingxin, China) to form slurries, and the activities of nitrate reductase (NR), glutamine synthase (GS) and acid phosphatase (ACP) were measured. The activities of NR and GS were assayed according to a test described by Sakar et al. (2010) and (Yu et al., 2012b) respectively. The activity of ACP was determined according to the method described by Wei et al. (2017).
Statistical analysis
Data were analysed using SPSS 19.0 (SPSS, Chicago, IL, USA). Before applying parametric tests, we tested for the normality and homogeneity of the variances for all data; variables were transformed (ln, sin, cos), if necessary, to meet the assumptions of the analysis of variance (ANOVA). One-way ANOVA was performed to compare the plant nutrient stoichiometry, soil nutrients and root enzymatic activities of the two plant species (L. chinensis vs A. frigida). Duncan’s multiple range test was used to determine the difference in means at a 0.05 probability level. The effects of treatment and species on the C, N and P concentrations and stoichiometric ratios of the leaves, stems and roots of the plant species were tested using two-way ANOVA. All resulting figures were prepared using Origin 8.5.
Results
Differences in nutrient concentrations and stoichiometry of leaves, stems, and roots in the two plant species
We examined the responses of the C, N and P concentrations in the leaves, stems, and roots of two plant species at the control and grazing sites (Tables 1 and 2). The C concentrations in the leaves, stems, and roots were unchanged in both species except that grazing increased the C concentrations in the leaves of A. frigida. Treatment × species did not significantly affect the C concentration in each organ. Comparatively, the highest N concentration was observed in the leaves of L. chinensis in the grazing plot. N concentration in each organ was not affected by treatment × species except for the leaf. Grazing significantly increased the N concentration in the leaves and stems, but not in the roots of L. chinensis. For A. frigida, the N in the leaves did not change, but the N concentration in the stems and roots increased at the grazing site, recording a 42.04% and 27.94% increase respectively. There was no significant difference in the P concentration in the leaves between the control and grazing plots for the two species. Treatment × species affected the P concentration in the stems and roots. The P concentration in the stem fractions recorded a higher P concentration at the grazing sites for both species. However, the P concentration was significantly higher for the roots of A. frigida under the grazing treatment, but no difference was observed for the same element in the roots of L. chinensis.
Table 1. Effects of treatment, species, and their interaction on the C, N and P concentrations and stoichiometric ratios of leaves, stems and roots of two plant species.
| Treatment | Species | Treatment * Species | ||||
|---|---|---|---|---|---|---|
| F | Sig | F | Sig | F | Sig | |
| Leaf C | 0.74 | 0.40 | 0.48 | 0.25 | 1.18 | 0.29 |
| Stem C | 1.95 | 0.18 | 0.70 | 0.41 | 2.27 | 0.15 |
| Root C | 0.20 | 0.66 | 10.69* | 0.00 | 0.04 | 0.84 |
| Leaf N | 30.94* | 0.00 | 0.00 | 0.98 | 22.68* | 0.00 |
| Stem N | 18.77* | 0.00 | 1.59 | 0.23 | 0.14 | 0.71 |
| Root N | 7.90* | 0.01 | 3.53 | 0.08 | 1.10 | 0.31 |
| Leaf P | 2.75 | 0.12 | 84.38* | 0.00 | 2.97 | 0.10 |
| Stem P | 130.54* | 0.00 | 0.08 | 0.79 | 8.15* | 0.01 |
| Root P | 14.92* | 0.00 | 9.31* | 0.01 | 13.24* | 0.00 |
| Leaf C:N | 18.81* | 0.00 | 0.26 | 0.62 | 31.40* | 0.00 |
| Stem C:N | 11.73* | 0.00 | 0.01 | 0.92 | 3.73 × 10−4 | 0.98 |
| Root C:N | 1.53 | 0.23 | 9.55* | 0.01 | 0.52 | 0.48 |
| Leaf C:P | 1.90 | 0.19 | 35.39* | 0.00 | 5.16* | 0.04 |
| Stem C:P | 96.15* | 0.00 | 0.05 | 0.83 | 6.54* | 0.02 |
| Root C:P | 3.80 | 0.07 | 11.65* | 0.00 | 6.72* | 0.02 |
| Leaf N:P | 7.31* | 0.02 | 82.24* | 0.00 | 5.16* | 0.04 |
| Stem N:P | 18.62* | 0.00 | 0.01 | 0.91 | 7.16* | 0.02 |
| Root N:P | 0.52 | 0.48 | 0.46 | 0.51 | 4.98* | 0.04 |
Notes.
Treatments are control plot and grazing plot. F-values and significance levels of two-way ANOVA are shown.
P < 0.05.
Table 2. C, N and P concentrations of leaves, stems and roots of two plant species in control plot and grazing plot.
| Leymus chinensis | Artemisia frigida | ||||
|---|---|---|---|---|---|
| Control | Grazing | Control | Grazing | ||
| C (g/kg) | Leaf | 292.18 ± 22.09a | 295.83 ± 25.78a | 302.24 ± 10.06b | 331.42 ± 6.15a |
| Stem | 221.12 ± 12.96a | 218.98 ± 16.54a | 198.90 ± 5.45a | 199.60 ± 3.99a | |
| Root | 203.99 ± 3.75a | 222.09 ± 14.53a | 181.51 ± 6.52a | 190.92 ± 3.61a | |
| N (g/kg) | Leaf | 18.50 ± 0.75b | 29.99 ± 1.54a | 23.83 ± 1.06a | 24.72 ± 0.95a |
| Stem | 10.30 ± 0.94b | 15.02 ± 1.37a | 9.42 ± 0.95b | 13.38 ± 0.58a | |
| Root | 8.88 ± 0.51a | 10.09 ± 0.58a | 9.45 ± 0.57b | 12.09 ± 0.97a | |
| P (g/kg) | Leaf | 0.48 ± 0.03a | 0.62 ± 0.06a | 0.94 ± 0.04a | 0.94 ± 0.09a |
| Stem | 0.45 ± 0.03b | 0.74 ± 0.03a | 0.35 ± 0.03b | 0.82 ± 0.04a | |
| Root | 0.46 ± 0.03a | 0.46 ± 0.03a | 0.44 ± 0.03b | 0.69 ± 0.04a | |
Notes.
Rows with different superscript are significantly different ( P < 0.05) for each plant species. Mean ± standard errors.
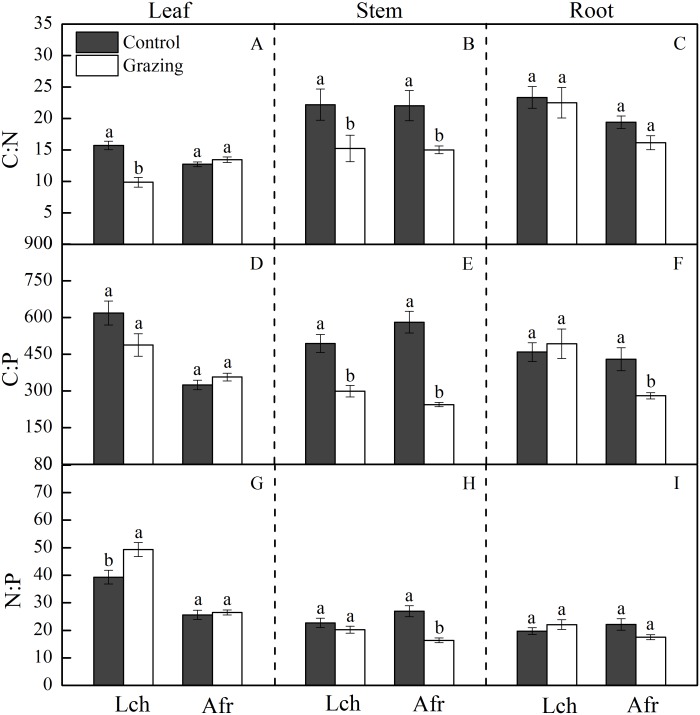
The C, N and P stoichiometric ratio in the leaves, stems, and roots of L. chinensis and A. frigida in response to grazing are shown in Table 1 and Fig. 2. Grazing significantly decreased the C:N ratio in the leaf and stem of L. chinensis, but no change was observed in the C:N ratio for the root. Similarly, the C:N ratio in the stem fraction of A. frigida decreased as a result of grazing, while that in the leaf and root of the plant remained unchanged. Treatment × species did not significantly affect the C:N ratio in each organ except the leaf. For L. chinensis, the stem C:P ratio significantly decreased as a result of grazing, while the C:P ratio in the leaf and root remain unchanged. For A. frigida, grazing reduced the C:P ratio in the stem and root but not the leaf. The C:P ratio in the leaf and root of L. chinensis were significantly higher than that of A. frigida, but that was not true for the stem. Treatment × species significantly affected the C:P ratio in each organ. The N:P ratio in the leaf of L. chinensis was significantly increased by grazing, and N:P ratio in the stem and root remained unchanged. Grazing significantly diminished N:P ratio in the stem and had no effect in the leaf and root of A. frigida. There was no significant difference in the N:P ratio of the stem and root for both species, while the N:P ratio in the leaf of L. chinensis was significantly higher than that of A. frigida. Treatment × species significantly affected the N:P ratio in each organ.
Figure 2. Effect of grazing on C, N and P stoichiometric ratios in the two plants.
The bars are mean ± standard errors. Error bars show the standard errors of five replicates. Lch is Leymus chinensis, Afr is Artemisia frigida. (A) is C:N ratios in the leaf, (B) is C:N ratios in the stem, (C) is C:N ratios in the root, (D) is C:P ratios in the leaf, (E) is C:P ratios in the stem, (F) is C:P ratios in the root, (G) is N:P ratios in the leaf, (H) is N:P ratios in the stem and (I) is N:P ratios in the root. Different letters indicate significant differences (P < 0.05) based on one-way ANOVA.
Grazing affects activities of root enzymes
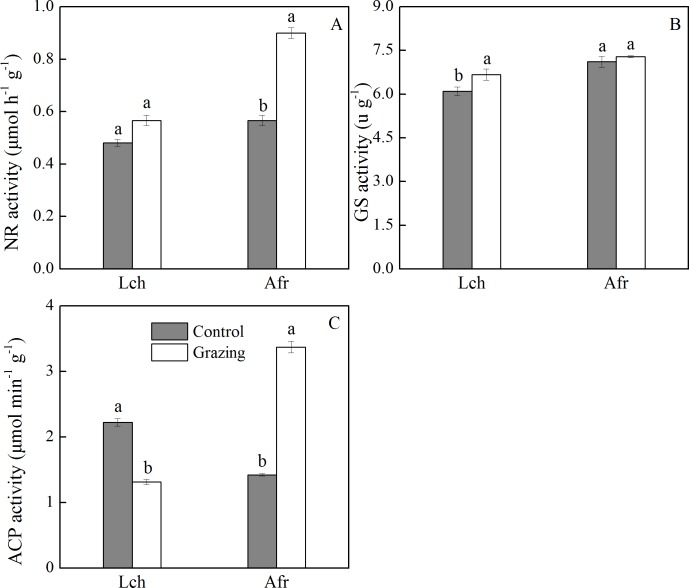
The effect of grazing on the activities of nitrate reductase, glutamine synthase, and acid phosphatase in the root of L. chinensis and A. frigida is shown in Fig. 3. The nitrate reductase activity of L. chinensis in the two plots showed no significant difference. The nitrate reductase activity of A. frigida in the grazing plot was significantly higher than it was in the control plot, as well as what was observed in the control and grazing plots of L. chinensis. The glutamine synthase activity of L. chinensis in the grazing plot was significantly higher compared to that in the control plot, while the glutamine synthase activity of A. frigida was not significantly different between the control and grazing plots. Grazing significantly decreased the acid phosphatase activity of L. chinensis (1.32 µmol/ (min g)) compared to that of the control plot (2.22 µmol/ (min g)). The acid phosphatase activity of A. frigida in the control plot was lower than that recorded in the grazing plot; the values were 1.42 µmol/ (min g) and 3.37 µmol/ (min g) respectively.
Figure 3. Effect of grazing on root enzymes of two species.
(A) Nitrate reductase activity; (B) glutamine synthase activity; (C) acid phosphatase activity. Lch is Leymus chinensis, Afr is Artemisia frigida. The results of one-way ANOVA for a comparison between the control and grazing for the species. Letters above the columns indicate significant differences (P < 0.05).
Grazing affects soil nutrients
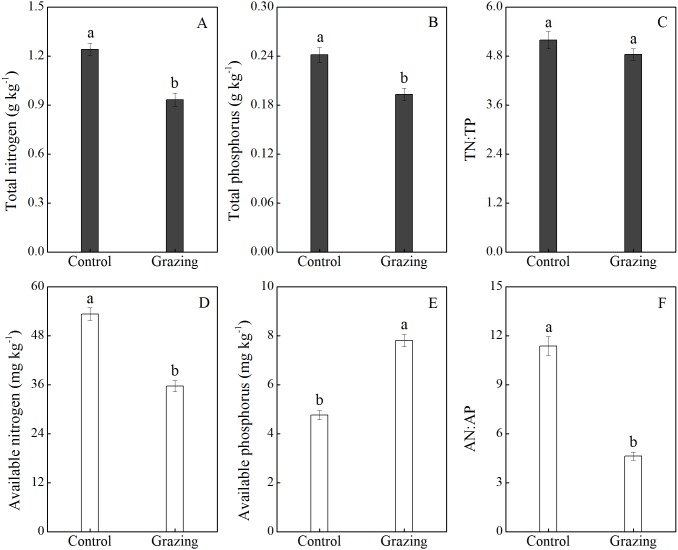
The effects of grazing on soil nutrients are shown in Fig. 4. Grazing decreased the soil total N and total P, with a mean reduction of 25.00% and 20.83% respectively. There was no significant difference in the ratio of soil total N to total P between the control plot (5.19) and the grazing plot (4.84). In addition, available nitrogen was higher in the control plot (53.30 mg/kg) than in the grazing plot (35.68 mg/kg). Conversely, available phosphorus was significantly higher in the grazing plot. Compared to the control plot, the available phosphorus increased by 63.87% in the grazing plot. The changes in available nitrogen and available phosphorus resulted in a decreased AN:AP ratio in the soil under the grazing treatment.
Figure 4. Effect of grazing on N and P nutrient and stoichiometric ratios in the soil.
(A) Total nitrogen; (B) total phosphorus; (C) the ratio of total nitrogen to total phosphorus; (D) available nitrogen; (E) available phosphorus; (F) the ratio of available nitrogen to available phosphorus. Different letters represent significant differences among treatments at a 95 % confidence interval.
Discussion
Implication of C, N and P concentrations and stoichiometry in different organs
Herbivore grazing may modify plant C, N and P concentrations and stoichiometry. Bai et al. (2012) showed that grazing increased N content in the leaves of plant species in three community types: meadow, typical and desert steppe. In this study, grazing enhanced N concentrations in the leaf of L. chinensis, but it did not in A. frigida. This finding supports that changes in N and P concentrations because of grazing are species-specific, as reported by Yang et al. (2017) that grazing did not change the N content of Stipa grandis but influenced the N content of Agropyron cristatum. Elsewhere, Li et al. (2010) also found that N and P concentrations for several species were not affected by grazing, but N and P concentrations in Poa pratensis were higher under grazing treatments than in control treatments. Most previous studies have focused on leaf nutrient concentrations and stoichiometry because of its role in obtaining carbon by controlling N and P. Zheng et al. (2012) discussed how stoichiometric changes in root tissues respond to grazing, with a consequent increase in N and P contents in the roots. Our data found that grazing enhanced N and P concentrations in the root tissue of A. frigida. Interestingly, we observed an increase in N and P concentrations in the stem of the two plant species, showing a sensitive response by this organ to grazing. These results agree with Minden & Kleyer (2014) and Schreeg et al. (2014) that the stem and belowground organs show a higher variation in elemental composition than the leaf in herbaceous, semi-shrub and marsh species. In addition, our result corroborates the evidence put forward by Zheng et al. (2012) that the leaf is not the only plant organ that responds to grazing, but the stem and root also show a response.
Plant growth requires proteins synthesis, which in turn demands large amounts of N and P for the ribosome; thus, fast-growing species are characterized by low C:P and N:P ratios (Elser et al., 2000b). Therefore, variations in stoichiometric ratios in plant organs have significant implications for the fundamental ecological process of a grazing response (Elser et al., 2010; Peñuelas et al., 2013). In our study, the increase in N and P concentrations in the leaf and stem suggest that both the leaf and stem growth of L. chinensis are positively influenced by grazing. This agrees with the result that L. chinensis responds to grazing by improving compensatory growth to reduce biomass loss (Wang et al., 2004). Another experiment by Niu, He & Lechowicz (2016) reported that plants in Tibetan alpine meadows increase foliar nutrients but tend to have lower leaf dry matter content, which accelerates growth and regrowth. However, it is noteworthy that compensatory growth is directly linked to grazing intensity. Zhao, Chen & Lin (2008) showed that L. chinensis exhibited over-compensatory growth at light and moderate clipping intensities (20% and 40% aerial mass removed) with a greater accumulated aboveground biomass; however, intense clipping (80% aerial mass removed) removed most of the aboveground tissues, which greatly reduced the growth of aboveground biomass and resulted in under-compensatory growth compared to that of the unclipped plants. Therefore, plants may not resist the damage caused by overgrazing if the amount of livestock foraging is more than the increase in biomass. However, A. frigida shows a different adaptive strategy that is devoid of change in leaf growth rate, but rather it uses an increase in its stem and root growth rates under grazing. Orians, Thorn & Gómez (2011), using a conceptual model for resource flow in plants, proposed that herbivore-induced export of nutrients from fine roots into stems and storage roots sequestered nutrients in tissues inaccessible to herbivores. This finding concurs with the results of Li, Li & Ren (2005) that grazing enhanced branch and indefinite root density of A. frigida as an adaptive strategy to grazing at a moderate stocking rate (4.0 sheep ha−1) in a rotational grazing system. In addition, the adaptation of A. frigida to grazing was supported by the consequent population expansion resulting from the rapid growth of the stem and root (Hou et al., 2009).
A higher foliar C:N nutrient ratio allows for increased C storage per unit nutrient, i.e., high C:N and C:P ratios represent high utilization of N and P by plants (Elser et al., 2010; Peñuelas et al., 2013). Thus, species with slow growth and a conservative resource-use strategy may dominate nutrient-limited soils by enhancing resource use efficiency (with a minimal investment of ribosomes per unit protein synthesized). In contrast, under high-resource conditions where rapid growth and acquisitive resource-use strategy are a better competitive advantage, higher investment in ribosomes per unit of protein maximizes the speed of protein synthesis and therefore growth (Bai et al., 2012; Sardans & Peñuelas, 2013). For example, Minden & Kleyer (2014) found that species of a frequently inundated marsh (nutrient-rich) had a lower C:N ratio than that of a nutrient limiting marsh (nutrient-poor). The C:N ratio in the leaf of L. chinensis decreased but remained unchanged in the leaf of A. frigida, and this indicated that the latter possesses a conservative resource-use strategy under grazing. The importance of suitable leaf stoichiometry for dominance at grazing sites is also reflected in the chemical anti-herbivore defence (Endara & Coley, 2011). Royer et al. (2013) found that C-based secondary defensive compounds are significantly and positively correlated to C:N. The authors further noted that C:N ratio can be considered a good indicator of the secondary compound concentration in organs, particularly for those involved in chemical defence. The leaf C:N ratio of A. frigida is higher than that of L. chinensis indicating that A. frigida may be appropriate to prevent defoliation by herbivores because of its higher level of defensive substance. This is supported by Liu et al. (2015) who reported that moderate and severe mechanical damage rapidly increase the secondary metabolites of A. frigida and the main components of secondary metabolites are terpenoids, which can inhibit the rate and time of defoliation. Da Silveira Pontes et al. (2015) reported that herbivores preferred to eat tender leaves of fast-growing species with rich nitrogen rather than the leaves of slow-growing species, which are richer in complex carbon compounds not involved in photo assimilation. Therefore, leaf nutrients and stoichiometry maximize plant fitness in a particular environment, reflecting a different trade-off between growth and defensive ability (Sardans & Peñuelas, 2013). In our study, L. chinensis increased its growth rate to compensate for biomass loss, with a consequent limit in its defensive ability because of the increase of N in the leaf of L. chinensis, which improves its herbivore palatability. However, A. frigida may have a better capacity to either tolerate or avoid defoliation at the expense of leaf growth.
Root enzymes may regulate plant adaption to grazing
A plant stoichiometric ratio may be regulated by synthesized enzymes, with possible changes in the rate of nutrient uptake. The correlation between nitrogen and phosphorus availability with root and rhizosphere enzymes has been proved (Raynaud, Jaillard & Leadley Paul, 2008; Rejmánková & Macek, 2008). Nitrate reductase and glutamine synthase are two important enzymes in plant nitrogen metabolism. The first step of nitrate degradation occurs in the cytoplasm, where nitrate reductase converts nitrate to nitrite. Subsequently, ammonia and glutamine degraded by nitrite reductase form glutamine through glutamine synthase (Campbell & Kinghorn, 1990). Previous research has found that nitrification enzymatic activity is significantly influenced by the management regime (grazing and mowing) (Patra et al., 2006), suggesting that eubacterial structures and free-living N2-fixing communities are controlled by management, whereas the diversity of nitrate reducers and ammonia oxidizers is dependent on both management and plant species. In our study, observations showed that L. chinensis and A. frigida have different pathways of enhancing nutrient utilization to increase the N concentration. Leymus chinensis slightly improved the assimilation of ammonium nitrogen in its roots by increasing the GS activity but showed no increase in nitrate assimilation. Artemisia frigida increased nitrate nitrogen assimilation by greatly increasing NR activity. Acid phosphatases are important components of the response of plants to P limitation (Ciereszko et al., 2017). Acid phosphatase, an induced enzyme, catalyses organophosphorus (present both in soil and plant tissues) into small molecules of available phosphorus and functions in the processes of P uptake, transport and recycling (Ciereszko et al., 2017; Duff, Sarath & Plaxton, 1994). The increase in acid phosphatase activity under P-deficient conditions has been documented for various plant species, including wheat, lupine and clover, or sedges (Carex) (Ciereszko et al., 2017; Güsewell, 2017; Hunter & McManus, 1999). Our results indicate that grazing decreased the ACP activity in the root of L. chinensis suggesting that its growth is not restricted by phosphorus under grazing. The decrease in ACP activity may have been because the grazing increased available P in the soil, as a way of meeting the P required for the growth of L. chinensis. However, A. frigida increased the ACP activity when the soil available P increased, which indicated that A. frigida may require more P for its growth compared to that of L. chinensis. This result supports Liu, Tian & Zhang (2014) that A. frigida has a high physiological demand for phosphorus. In addition, it has great potential to hydrolyse and release organophosphorus in the soil for plant root growth, thereby increasing its colonization. As indicated by our results, A. frigida increased the N and P concentration by increasing the NR and ACP activities to regulate, which may have supported its adaptation to grazing by promoting the growth of the stems and roots.
Changes in soil nutrients induced by grazing
Grazing-induced soil properties can control the variation in C:N:P stoichiometry in plant tissues, which may be related to climate, steppe type, grazing intensity, and livestock species (Li et al., 2010; Wu et al., 2011). In our study, grazing decreased total N, total P, and available N while it increased available P in the soil. Long-term grazing leads to nitrogen loss in grassland ecosystems because of the cumulative effect of the continuous removal of soil total nitrogen from the ecosystem by foraging animals, although animals only use 10–15% of the N ingested and the remaining is excreted in urine and dung (Duff, Sarath & Plaxton, 1994). In addition, the N-fixation process is limited by the decrease in the number of leguminous plants. The effect of grazing on soil available N is mainly related to many complex factors and processes, such as plant litters, microorganisms, soil properties, litter decomposition, soil respiration, and N mineralization. This process is mainly caused by the selection of high-quality pasture by herbivores, which increases the abundance of inferior plants (lower N content or organic compounds in chemical defence), reduces the litter quality and slows the decomposition rate, thereby reducing the available nitrogen content in the soil (Abbasi & Adams, 2000). Grazing reduces the quantity and quality of litter, leading to a decreased input of soil organic matter pools and C supply from microorganisms (Golluscio et al., 2009), which in turn results in a negative effect on soil C and N cycles. Stable isotope tracers found that grazing decreased 15N recovery both in plant and microbial N pools but strongly promoted NO3− accumulation in soil thus negatively affecting potential ecosystem N retention (Wu et al., 2011). In addition, grazed areas may be subject to an additional loss of N-rich topsoil through wind erosion (Steffens et al., 2008). For phosphorus, the results from the research described here provide evidence that grazing reduces soil total P content. A study conducted on the effect of grazing on soil total P in a L. chinensis steppe after 19 years of grazing found that in the 0–60 cm soil layer, 24.9% of soil P stock was lost, mainly in the organic form (Sternberg et al., 2015). In this study, we observed that soil available phosphorus increased in the grazing plot compared to that of the control plot. This is consistent with Rui et al. (2012), who found that free grazing significantly increased APi (an inorganic form of phosphorus in the soil extracted by the extractant solution of 1 M ammonium chloride (NH4Cl)) and BPi (an inorganic form of phosphorus in the soil extracted by 0.5 M sodium bicarbonate (NaHCO3)) in the 0–10 and 10–20 cm soil layers respectively. In natural ecosystems, the decomposition of feces and urine excreted by herbivores is a faster pathway to release available P. Microbial mineralization of organic P is a key process of soil P cycling in unfertilized/natural ecosystems to produce available inorganic P for plants (Chen et al., 2004), which may be affected by grazing and a grazing-induced increase in soil temperature because of enhanced solar radiation. Therefore, grazing can stimulate microbial activity and have an impact on plant root exudation and mycorrhizal fungi, which can further stimulate the excretion of phosphatase and organic acids to release P (Oburger et al., 2009).
Previous studies have found that animal grazing increases N availability in soils with an attendant significant decrease in plants above- and below-ground C:N ratios, as well as an increase in plant belowground N:P ratios (Bai et al., 2012; Zheng et al., 2012). In our study, we observed that grazing decreased the soil available N but increased plant N, consistent with a previous report that grazing decreases soil available N (Chen et al., 2018). The decrease of available N in the soil increases the limiting effect of the element on the growth of L. chinensis. This concurs with Bai et al. (2014), who found that the growth of L. chinensis may be subject to N limitation during dry years. A nitrogen addition experiment conducted in a temperate climate and in a plot fenced to exclude livestock grazing showed a linear decrease in the biomass of A. frigida with an increase in N addition. This suggests that N has little effect on the growth of A. frigida (Fang et al., 2012). Therefore, the reduction in N available in the soil had less effect on A. frigida compared to that on L. chinensis. Notably, an increase in the available P in the soil as a result of animal grazing could increase the P in plant tissues. Since A. frigida is sensitive to phosphorus (Liu, Tian & Zhang, 2014), the increased available P in the soil had a more positive impact on the growth of A. frigida. In contrast, the decrease in available N in the soil inhibited the growth of L. chinensis. Therefore, changes in nutrient availability induced by grazing affected the nutrient concentration and stoichiometric ratios in plant tissues and the adaptation of the plants to grazing.
Conclusions
In conclusion, we contend that A. frigida is better adaptated to grazing than L. chinensis, possibly due to its increased stem and root growth, which enhances its population expansion and its capacity to avoid defoliation by herbivores given its higher level of defensive substance. However, L. chinensis increased its leaf growth but was subject to biomass loss as a result of excessive foraging by livestock, severely affecting its ability to colonize. We also found a sensitivity of the stems and roots of herbaceous vegetation to various environments. Root enzymes coupled with soil nutrients can regulate plant nutrients and stoichiometric ratios as an adaptive response to grazing. This result provides a new understanding of the mechanisms involved in grazing-resistance within a plant-soil system. Further studies are required to demonstrate how soil nutrients in the rhizosphere and enzymes in the roots and rhizosphere of plants respond to grazing.
Supplemental Information
NR is nitrate reductase, GS is glutamine synthase and ACP is acid phosphatase
N is nitrogen, P is phosphorus
Acknowledgments
We would like to thank Inner Mongolia University and the Inner Mongolia Grassland Ecosystem Research Station of the Chinese Academy of Science for providing the laboratory for sample processing. We are grateful to Shiming Tang and Qing Zhang, School of Ecology and Environment at Inner Mongolia University, for providing valuable suggestions.
Funding Statement
This work was supported by the Natural Science Foundation of Inner Mongolia (2015ZD02), National Natural Science Foundation of China (31502008, 41601269), Major Science and Technology Program of Inner Mongolia: The production system of grassland-livestock resources-saving and model of ecotone sustainable development, and Central Public-interest Scientific Institution Basal Research Fund (Y2018LM05). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yong Ding, Email: dingyong228@126.com.
Xiangyang Hou, Email: houxy16@vip.126.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wenjing Ma and Jin Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Saheed Olaide Jimoh conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Yujuan Zhang, Fenghui Guo, Yong Ding and Xiliang Li conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Xiangyang Hou conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available as Files S1 and S2. The raw data show two species’ different responses to grazing and how grazing affects soil nutrients. These data were used for statistical analysis to compare species A and species B.
References
- Abbasi & Adams (2000).Abbasi MK, Adams WA. Estimation of simultaneous nitrification and denitrification in grassland soil associated with urea-N using 15N and nitrification inhibitor. Biology and Fertility of Soils. 2000;31(1):38–44. doi: 10.1007/s003740050621. [DOI] [Google Scholar]
- Bai et al. (2014).Bai X, Cheng J, Zheng S, Zhan S, Bai Y. Ecophysiological responses of Leymus chinensis to nitrogen and phosphorus additions in a typical steppe. Chinese Journal of Plant Ecology. 2014;38:103–115. [Google Scholar]
- Bai et al. (2012).Bai Y, Wu J, Clark CM, Pan Q, Zhang L, Chen S, Wang Q, Han X. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. Journal of Applied Ecology. 2012;49(6):1204–1215. doi: 10.1111/j.1365-2664.2012.02205.x. [DOI] [Google Scholar]
- Branco et al. (2010).Branco P, Stomp M, Egas M, Huisman J. Evolution of nutrient uptake reveals a trade-off in the ecological stoichiometry of plant-herbivore interactions. American Naturalist. 2010;176(6):E162–E176. doi: 10.1086/657036. [DOI] [PubMed] [Google Scholar]
- Campbell & Kinghorn (1990).Campbell WH, Kinghorn JR. Functional domains of assimilatory nitrate reductases and nitrite reductases. Trends in Biochemical Sciences. 1990;15(8):315–319. doi: 10.1016/0968-0004(90)90021-3. [DOI] [PubMed] [Google Scholar]
- Cernusak, Winter & Turner (2010).Cernusak LA, Winter K, Turner BL. Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytologist. 2010;185(3):770–779. doi: 10.1111/j.1469-8137.2009.03106.x. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2004).Chen CR, Condron LM, Davis MR, Sherlock RR. Effects of plant species on microbial biomass phosphorus and phosphatase activity in a range of grassland soils. Biology and Fertility of Soils. 2004;40(5):313–322. doi: 10.1007/s00374-004-0781-z. [DOI] [Google Scholar]
- Chen et al. (2018).Chen H, Zhao X, Chen X, Lin Q, Li G. Seasonal changes of soil microbial C, N, P and associated nutrient dynamics in a semiarid grassland of north China. Applied Soil Ecology. 2018;128:89–97. doi: 10.1016/j.apsoil.2018.04.008. [DOI] [Google Scholar]
- Ciereszko et al. (2017).Ciereszko I, Balwicka H, Zebrowska E, Ciereszko I, Balwicka H, Zebrowska E. Acid phosphatases activity and growth of barley, oat, rye and wheat plants as affected by Pi deficiency. Open Plant Science Journal. 2017;10:110–122. [Google Scholar]
- Da Silveira Pontes et al. (2015).Da Silveira Pontes L, Maire V, Schellberg J, Louault F. Grass strategies and grassland community responses to environmental drivers: a review. Agronomy for Sustainable Development. 2015;35(4):1297–1318. doi: 10.1007/s13593-015-0314-1. [DOI] [Google Scholar]
- De Deyn, Cornelissen & Bardgett (2008).De Deyn GB, Cornelissen JHC, Bardgett RD. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters. 2008;11(5):516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- De Jong & Lin (2017).De Jong TJ, Lin T. How to quantify plant tolerance to loss of biomass? Ecology and Evolution. 2017;7(9):3080–3086. doi: 10.1002/ece3.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, Sarath & Plaxton (1994).Duff SMG, Sarath G, Plaxton WC. The role of acid phosphatases in plant phosphorus metabolism. Physiologia Plantarum. 1994;90(4):791–800. doi: 10.1111/j.1399-3054.1994.tb02539.x. [DOI] [Google Scholar]
- Elser et al. (2010).Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ. Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytologist. 2010;186(3):593–608. doi: 10.1111/j.1469-8137.2010.03214.x. [DOI] [PubMed] [Google Scholar]
- Elser et al. (2000a).Elser JJ, O’Brien WJ, Dobberfuhl DR, Dowling TE. The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. Journal of Evolutionary Biology. 2000a;13(5):845–853. doi: 10.1046/j.1420-9101.2000.00215.x. [DOI] [Google Scholar]
- Elser et al. (2000b).Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW. Biological stoichiometry from genes to ecosystems. Ecology Letters. 2000b;3(6):540–550. doi: 10.1046/j.1461-0248.2000.00185.x. [DOI] [Google Scholar]
- Endara & Coley (2011).Endara MJ, Coley PD. The resource availability hypothesis revisited: a meta-analysis. Functional Ecology. 2011;25(2):389–398. doi: 10.1111/j.1365-2435.2010.01803.x. [DOI] [Google Scholar]
- Fang et al. (2012).Fang Y, Xun F, Bai W, Zhang W, Li L. Long-term nitrogen addition leads to loss of species richness due to litter accumulation and soil acidification in a temperate steppe. PLOS ONE. 2012;7(10):e47369. doi: 10.1371/journal.pone.0047369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al. (2000).Fu B, Chen L, Ma K, Zhou H, Wang J. The relationships between land use and soil conditions in the hilly area of the loess plateau in northern Shaanxi, China. Catena. 2000;39(1):69–78. doi: 10.1016/S0341-8162(99)00084-3. [DOI] [Google Scholar]
- Golluscio et al. (2009).Golluscio RA, Austin AT, García Martínez GC, Gonzalez-Polo M, Sala OE, Jackson RB. Sheep grazing decreases organic carbon and nitrogen pools in the patagonian steppe: combination of direct and indirect effects. Ecosystems. 2009;12(4):686–697. doi: 10.1007/s10021-009-9252-6. [DOI] [Google Scholar]
- Guo et al. (2017).Guo Y, Liu L, Zheng L, Yu F, Song M, Zhang X. Long-term grazing affects relationships between nitrogen form uptake and biomass of alpine meadow plants. Plant Ecology. 2017;218(9):1035–1045. doi: 10.1007/s11258-017-0746-6. [DOI] [Google Scholar]
- Güsewell (2017).Güsewell S. Regulation of dauciform root formation and root phosphatase activities of sedges (Carex) by nitrogen and phosphorus. Plant and Soil. 2017;415(1–2):57–72. doi: 10.1007/s11104-016-3142-4. [DOI] [Google Scholar]
- Hamilton & Frank (2001).Hamilton EW, Frank DA. Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology. 2001;82(9):2397–2402. doi: 10.1890/0012-9658(2001)082[2397:CPSSMA]2.0.CO;2. [DOI] [Google Scholar]
- He et al. (2006).He J, Fang J, Wang Z, Guo D, Flynn DFB, Geng Z. Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia. 2006;149(1):115–122. doi: 10.1007/s00442-006-0425-0. [DOI] [PubMed] [Google Scholar]
- Heyburn et al. (2017).Heyburn J, McKenzie P, Crawley MJ, Fornara DA. Effects of grassland management on plant C:N:P stoichiometry: implications for soil element cycling and storage. Ecosphere. 2017;8(10):e01963. doi: 10.1002/ecs2.1963. [DOI] [Google Scholar]
- Hill et al. (2005).Hill JO, Simpson RJ, Wood JT, Moore AD, Chapman DF. The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition. Australian Journal of Agricultural Research. 2005;56(10):1027–1039. doi: 10.1071/AR04279. [DOI] [Google Scholar]
- Hou et al. (2009).Hou M, Wang M, Li Z, Yang N, Chen H. Effect of grazing intensity on characteristics of vegetative propagation of three plants in the stipa breviflora steppe. Journal of Inner Mongolia Agriculture University. 2009;30:71–75. [Google Scholar]
- Hunter & McManus (1999).Hunter DA, McManus MT. Comparison of acid phosphatases in two genotypes of white clover with different responses to applied phosphate. Journal of Plant Nutrition. 1999;22(4–5):679–692. doi: 10.1080/01904169909365663. [DOI] [Google Scholar]
- Hurlbert (1984).Hurlbert SH. Pseudoreplication and the design of ecological field experiments. Ecological Monographs. 1984;54(2):187–211. doi: 10.2307/1942661. [DOI] [Google Scholar]
- Li et al. (2010).Li C, Hao X, Willms WD, Zhao M, Han G. Effect of long-term cattle grazing on seasonal nitrogen and phosphorus concentrations in range forage species in the fescue grassland of southwestern Alberta. Journal of Plant Nutrition and Soil Science. 2010;173(6):946–951. doi: 10.1002/jpln.200900243. [DOI] [Google Scholar]
- Li et al. (2016).Li X, Hou X, Ren W, Baoyin T, Liu Z, Badgery W, Li Y, Wu X, Xu H. Long-term effects of mowing on plasticity and allometry of Leymus chinensis in a temperate semi-arid grassland, China. Journal of Arid Land. 2016;8(6):899–909. doi: 10.1007/s40333-016-0005-z. [DOI] [Google Scholar]
- Li, Li & Ren (2005).Li J, Li Z, Ren J. Effect of grazing intensity on clonal morphological plasticity and biomass allocation patterns of Artemisia frigida and Potentilla acaulis in the Inner Mongolia steppe. New Zealand Journal of Agricultural Research. 2005;48(1):57–61. doi: 10.1080/00288233.2005.9513631. [DOI] [Google Scholar]
- Li et al. (2012).Li S, Verburg PH, Lv S, Wu J, Li X. Spatial analysis of the driving factors of grassland degradation under conditions of climate change and intensive use in Inner Mongolia, China. Regional Environmental Change. 2012;12(3):461–474. doi: 10.1007/s10113-011-0264-3. [DOI] [Google Scholar]
- Li et al. (2008).Li Y, Wang W, Liu Z, Jiang S. Grazing gradient versus restoration succession of Leymus chinensis (Trin.) Tzvel. grassland in Inner Mongolia. Restoration Ecology. 2008;16(4):572–583. doi: 10.1111/j.1526-100X.2007.00332.x. [DOI] [Google Scholar]
- Liu et al. (2015).Liu M, Jia L, Zhang H, Zang X, Zhang R, Gao Y. Mechanical damage on secondary metabolites from Artemisia frigida leaves. Journal of Zhejiang A & F University. 2015;32:845–852. [Google Scholar]
- Liu, Tian & Zhang (2014).Liu N, Tian Q, Zhang W. Comparison of adaptive strategies to phosphorus-deficient soil between dominant species Artemisia frigida and Stipa krylovii in typical steppe of Nei Mongol. Chinese Journal of Plant Ecology. 2014;38:905–915. [Google Scholar]
- Lü et al. (2015).Lü X, Freschet GT, Kazakou E, Wang Z, Zhou L, Han X. Contrasting responses in leaf nutrient-use strategies of two dominant grass species along a 30-yr temperate steppe grazing exclusion chronosequence. Plant and Soil. 2015;387(1–2):69–79. doi: 10.1007/s11104-014-2282-7. [DOI] [Google Scholar]
- Mariotte, Canarini & Dijkstra (2017).Mariotte P, Canarini A, Dijkstra FA. Stoichiometric N:P flexibility and mycorrhizal symbiosis favour plant resistance against drought. Journal of Ecology. 2017;105(4):958–967. doi: 10.1111/1365-2745.12731. [DOI] [Google Scholar]
- Matzek & Vitousek (2009).Matzek V, Vitousek PM. N:P stoichiometry and protein: RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecology Letters. 2009;12(8):765–771. doi: 10.1111/j.1461-0248.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- Minden & Kleyer (2014).Minden V, Kleyer M. Internal and external regulation of plant organ stoichiometry. Plant Biology. 2014;16(5):897–907. doi: 10.1111/plb.12155. [DOI] [PubMed] [Google Scholar]
- Niklas & Edward (2005).Niklas KJ, Edward DC. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. American Journal of Botany. 2005;92(8):1256–1263. doi: 10.3732/ajb.92.8.1256. [DOI] [PubMed] [Google Scholar]
- Niu, He & Lechowicz (2016).Niu K, He J, Lechowicz MJ. Grazing-induced shifts in community functional composition and soil nutrient availability in Tibetan alpine meadows. Journal of Applied Ecology. 2016;53(5):1554–1564. doi: 10.1111/1365-2664.12727. [DOI] [Google Scholar]
- Oburger et al. (2009).Oburger E, Kirk GJD, Wenzel WW, Puschenreiter M, Jones DL. Interactive effects of organic acids in the rhizosphere. Soil Biology and Biochemistry. 2009;41(3):449–457. doi: 10.1016/j.soilbio.2008.10.034. [DOI] [Google Scholar]
- Ordoñez et al. (2009).Ordoñez JC, Van Bodegom PM, Witte J-PM, Wright IJ, Reich PB, Aerts R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography. 2009;18(2):137–149. doi: 10.1111/j.1466-8238.2008.00441.x. [DOI] [Google Scholar]
- Orians, Thorn & Gómez (2011).Orians CM, Thorn A, Gómez S. Herbivore-induced resource sequestration in plants: why bother? Oecologia. 2011;167:1–9. doi: 10.1007/s00442-011-1968-2. [DOI] [PubMed] [Google Scholar]
- Patra et al. (2006).Patra AK, Abbadie L, Clays-Josserand A, Degrange V, Grayston SJ, Guillaumaud N, Loiseau P, Louault F, Mahmood S, Nazaret S, Philippot L, Poly F, Prosser JI, Roux XL. Effects of management regime and plant species on the enzyme activity and genetic structure of N-fixing, denitrifying and nitrifying bacterial communities in grassland soils. Environmental Microbiology. 2006;8(6):1005–1016. doi: 10.1111/j.1462-2920.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas et al. (2013).Peñuelas J, Poulter B, Sardans J, Ciais P, Van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nature Communications. 2013;4 doi: 10.1038/ncomms3934. Article 3934. [DOI] [PubMed] [Google Scholar]
- Pulido et al. (2016).Pulido M, Schnabel S, Lavado Contador JF, Lozano-Parra J, González F. The impact of heavy grazing on soil quality and pasture production in rangelands of SW spain. Land Degradation & Development. 2016;29(2):219–230. doi: 10.1002/ldr.2501. [DOI] [Google Scholar]
- Raynaud, Jaillard & Leadley Paul (2008).Raynaud X, Jaillard B, Leadley Paul W. Plants may alter competition by modifying nutrient bioavailability in rhizosphere: a modeling approach. The American Naturalist. 2008;171(1):44–58. doi: 10.1086/523951. [DOI] [PubMed] [Google Scholar]
- Rejmánková & Macek (2008).Rejmánková E, Macek P. Response of root and sediment phosphatase activity to increased nutrients and salinity. Biogeochemistry. 2008;90(2):159–169. doi: 10.1007/s10533-008-9242-3. [DOI] [Google Scholar]
- Royer et al. (2013).Royer M, Larbat R, Le Bot J, Adamowicz S, Robin C. Is the C:N ratio a reliable indicator of C allocation to primary and defence-related metabolisms in tomato? Phytochemistry. 2013;88:25–33. doi: 10.1016/j.phytochem.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Rui et al. (2012).Rui Y, Wang Y, Chen C, Zhou X, Wang S, Xu Z, Duan J, Kang X, Lu S, Luo C. Warming and grazing increase mineralization of organic P in an alpine meadow ecosystem of Qinghai-Tibet Plateau, China. Plant and Soil. 2012;357(1–2):73–87. doi: 10.1007/s11104-012-1132-8. [DOI] [Google Scholar]
- Sakar et al. (2010).Sakar FS, Arslan H, Kirmizi S, Güleryüz G. Nitrate reductase activity (NRA) in Asphodelus aestivus Brot. (Liliaceae): distribution among organs, seasonal variation and differences among populations. Flora-orphology, Distribution, Functional Ecology of Plants. 2010;205(8):527–531. doi: 10.1016/j.flora.2009.12.015. [DOI] [Google Scholar]
- Sardans & Peñuelas (2013).Sardans J, Peñuelas J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Global Ecology and Biogeography. 2013;22(4):494–507. doi: 10.1111/geb.12015. [DOI] [Google Scholar]
- Sardans, Rivas-Ubach & Peñuelas (2012).Sardans J, Rivas-Ubach A, Peñuelas J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspectives in Plant Ecology, Evolution and Systematics. 2012;14(1):33–47. doi: 10.1016/j.ppees.2011.08.002. [DOI] [Google Scholar]
- Schreeg et al. (2014).Schreeg LA, Santiago LS, Wright SJ, Turner BL. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology. 2014;95(8):2062–2068. doi: 10.1890/13-1671.1. [DOI] [PubMed] [Google Scholar]
- Semmartin et al. (2004).Semmartin M, Aguiar MR, Distel RA, Moretto AS, Ghersa CM. Litter quality and nutrient cycling affected by grazing-induced species replacements along a precipitation gradient. Oikos. 2004;107(1):148–160. doi: 10.1111/j.0030-1299.2004.13153. [DOI] [Google Scholar]
- Steffens et al. (2008).Steffens M, Kölbl A, Totsche KU, Kögel-Knabner I. Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (P.R. China) Geoderma. 2008;143(1–2):63–72. doi: 10.1016/j.geoderma.2007.09.004. [DOI] [Google Scholar]
- Sternberg et al. (2015).Sternberg M, Golodets C, Gutman M, Perevolotsky A, Ungar ED, Kigel J, Henkin Z. Testing the limits of resistance: a 19-year study of Mediterranean grassland response to grazing regimes. Global Change Biology. 2015;21(5):1939–1950. doi: 10.1111/gcb.12866. [DOI] [PubMed] [Google Scholar]
- Strauss & Agrawal (1999).Strauss SY, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evolution. 1999;14(5):179–185. doi: 10.1016/S0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Vesk & Westoby (2001).Vesk PA, Westoby M. Predicting plant species’ responses to grazing. Journal of Applied Ecology. 2001;38(5):897–909. doi: 10.1046/j.1365-2664.2001.00646.x. [DOI] [Google Scholar]
- Wang et al. (2017).Wang Z, Deng X, Song W, Li Z, Chen J. What is the main cause of grassland degradation? A case study of grassland ecosystem service in the middle-south Inner Mongolia. Catena. 2017;150:100–107. doi: 10.1016/j.catena.2016.11.014. [DOI] [Google Scholar]
- Wang et al. (2004).Wang Z, Li L, Han X, Dong M. Do rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecologica. 2004;26(3):255–260. doi: 10.1016/j.actao.2004.08.007. [DOI] [Google Scholar]
- Wei et al. (2017).Wei K, Bao H, Huang S, Chen L. Effects of long-term fertilization on available P, P composition and phosphatase activities in soil from the Huang-Huai-Hai Plain of China. Agriculture Ecosystems & Environment. 2017;237:134–142. doi: 10.1016/j.agee.2016.12.030. [DOI] [Google Scholar]
- Wilson et al. (2018).Wilson CH, Strickland MS, Hutchings JA, Bianchi TS, Flory SL. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Global Change Biology. 2018;24(7):2997–3009. doi: 10.1111/gcb.14070. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2011).Wu H, Dannenmann M, Fanselow N, Wolf B, Yao Z, Wu X, Brüggemann N, Zheng X, Han X, Dittert K. Feedback of grazing on gross rates of N mineralization and inorganic N partitioning in steppe soils of Inner Mongolia. Plant and Soil. 2011;340(1):127–139. doi: 10.1007/s11104-010-0575-z. [DOI] [Google Scholar]
- Yan et al. (2016).Yan Z, Guan H, Han W, Han T, Guo Y, Fang J. Reproductive organ and young tissues show constrained elemental composition in Arabidopsis thaliana. Annals of Botany. 2016;117(3):431–439. doi: 10.1093/aob/mcv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang Z, Baoyin T, Minggagud H, Sun H, Li FY. Recovery succession drives the convergence, and grazing versus fencing drives the divergence of plant and soil N/P stoichiometry in a semiarid steppe of Inner Mongolia. Plant and Soil. 2017;420(1–2):303–314. doi: 10.1007/s11104-017-3404-9. [DOI] [Google Scholar]
- Yang, Liu & An (2018).Yang Y, Liu B, An S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena. 2018;166:328–338. doi: 10.1016/j.catena.2018.04.018. [DOI] [Google Scholar]
- Yu et al. (2010).Yu Q, Chen Q, Elser JJ, He N, Wu H, Zhang G, Wu J, Bai Y, Han X. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecology Letters. 2010;13(11):1390–1399. doi: 10.1111/j.1461-0248.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2012b).Yu X, Wang B, Zhang C, Xu W, He J, Zhu L, Wang S. Effect of root restriction on nitrogen levels and glutamine synthetase activity in ‘Kyoho’ grapevines. Scientia Horticulturae. 2012b;137:156–163. doi: 10.1016/j.scienta.2012.01.025. [DOI] [Google Scholar]
- Yu et al. (2015).Yu Q, Wilcox K, Pierre KL, Knapp AK, Han X, Smith MD. Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology. 2015;96(9):2328–2335. doi: 10.1890/14-1897.1. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2012a).Yu Q, Wu H, He N, Lü X, Wang Z, Elser JJ, Wu J, Han X. Testing the growth rate hypothesis in vascular plants with above- and below-ground biomass. PLOS ONE. 2012a;7(3):e32162. doi: 10.1371/journal.pone.0032162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang R, Zhang W, Zuo Z, Li R, Wu J, Gao Y. Inhibition effects of volatile organic compounds from Artemisia frigida Willd. on the pasture grass intake by lambs. Small Ruminant Research. 2014;121(2–3):248–254. doi: 10.1016/j.smallrumres.2014.06.001. [DOI] [Google Scholar]
- Zhao, Chen & Lin (2008).Zhao W, Chen S, Lin G. Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecology. 2008;196(1):85–99. doi: 10.1007/s11258-007-9336-3. [DOI] [Google Scholar]
- Zheng et al. (2012).Zheng S, Ren H, Li W, Lan Z. Scale-dependent effects of grazing on plant C: N: P stoichiometry and linkages to ecosystem functioning in the Inner Mongolia grassland. PLOS ONE. 2012;7(12):e51750. doi: 10.1371/journal.pone.0051750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioni, Vaadia & Lips (1971).Zioni AB, Vaadia Y, Lips SH. Nitrate uptake by roots as regulated by nitrate reduction products of the shoot. Physiologia Plantarum. 1971;24(2):288–290. doi: 10.1111/j.1399-3054.1971.tb03493.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NR is nitrate reductase, GS is glutamine synthase and ACP is acid phosphatase
N is nitrogen, P is phosphorus
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available as Files S1 and S2. The raw data show two species’ different responses to grazing and how grazing affects soil nutrients. These data were used for statistical analysis to compare species A and species B.