SUMMARY
SETTING:
A post-hoc exploratory analysis of a randomized, open-label clinical trial that enrolled 8053 participants from the United States, Canada, Brazil, and Spain.
OBJECTIVE:
To assess factors associated with noncompletion of study follow-up (NCF) in a 33-month latent tuberculous infection treatment trial, PREVENT TB.
DESIGN:
Participants were randomized to receive 3 months of weekly directly observed therapy vs. 9 months of daily self-administered therapy. NCF was defined as failing to be followed for at least 993 days (33 months) from enrollment. Possible factors associated with NCF were analyzed using univariate and multivariate regression via Cox proportional hazard model.
RESULTS:
Of 7061 adults selected for analysis, 841 (11.9%) did not complete study follow-up. Homelessness, young age, low education, history of incarceration, smoking, missing an early clinic visit, receiving isoniazid only, and male sex were significantly associated with NCF. Similar results were found in the North American region (United States and Canada) only. In Brazil and Spain, the only significant factor was missing an early clinic visit.
CONCLUSIONS:
Study subjects at higher risk for NCF were identified by characteristics known at enrollment or in early follow-up. Evaluation of follow-up in other trials might help determine whether the identified factors consistently correlate with retention.
Keywords: LTBI, loss to follow-up, adherence, compliance, retention
RESUME
CONTEXTE:
Une analyse post hoc exploratoire d’un essai clinique randomisé ouvert qui a enrfôlé 8053 participants des Etats-Unis, du Canada, du Brésil et d’Espagne.
OBJECTIF:
Evaluer les facteurs associés au non achévement du suivi de l’étude (NCF) dans un essai thérapeutique de 33 mois de l’infection tuberculeuse latente, l’étude PREVENT TB.
SCHÉMA:
Les participants ont été randomisés pour recevoir 3 mois de traitement hebdomadaire sous observation directe contre 9 mois de traitement quotidien auto-administré. Le NCF a été défini comme un abandon du suivi, qui durait au moins 993 jours (33 mois) à partir de l’enrôlement. Les facteurs éventuels associés au NCF ont été; analysés par régression univariée et multivariée via le modéle de risque proportionnel de Cox.
RESULTATS:
Sur 7061 adultes sélectionnés pour l’analyse, 841 (11,9%) n’ont pas terminé le suivi de l’étude. Le fait d’être sans domicile fixe, le jeune âge, un faible niveau d’éducation, des antécedents d’incarcération, le tabagisme, une absence lors des premiéres consultations, avoir reçu de l’isoniazide seul et le sexe masculin ont été significativement associés au NCF. Des résultats similaires ont été constatés seulement en Amérique du Nord (Etats-Unis et Canada). Au Brésil et en Espagne, le seul facteur significatif a été l’absence à une des premierés consultations.
CONCLUSION:
Les sujets de l’étude à risque plus élevé de NCF ont été identifiés par des caractéristiques connues lors de l’enrôlement ou lors d’un suivi précoce. L’évaluation du suivi dans d’autres essais pourrait contribuer à déterminer si les facteurs identifiés sont corréles de maniére régulière avec la rétention.
RESUMEN
MARCO DEREFERENCIA:
Un análisis a posteriori de un ensayo clínico aleatorizado sin anonimato, en el cual participaron 8053 personas de Estados Unidos, Canadá, Brasil y España.
OBJETIVO:
Evaluar los factores que se asocian con el incumplimiento de la integridad del seguimiento del estudio (NCF) en un ensayo clínico de 33 meses sobre el tratamiento de la infección tuberculosa latente (PREVENT TB).
MÉTODO:
Se randomizaron los participantes para recibir un tratamiento semanal directamente observado durante 3 meses o un tratamiento diario autoadministrado durante 9 meses. Se definió el NCF como la falta de un seguimiento mínimo de 993 días (33 meses) a partir de la inscripción en el estudio. Se examinaron los posibles factores asociados con el NCF mediante análisis de regresión univariante y multivariante, con un modelo de riesgos instantáneos proporcionales de Cox.
RESULTADOS:
De los 7061 adultos escogidos para el análisis, 841 no completaron el seguimiento del estudio (11,9%). Los factores que se asociaron de manera significativa con el NCF fueron la falta de domicilio, la juventud, el bajo grado de instrucción, el antecedente de encarcelamiento, el tabaquismo, la ausencia a una de las consultas iniciales, la administración exclusiva de isoniazida y el sexo masculino. Estos resultados se observaron solo en la región de Norteamérica (Estados Unidos y Canadá). En el Brasil y España, el único factor importante fue haber faltado a una de las consultas iniciales.
CONCLUSIÓN:
Fue posible reconocer a los participantes con riesgo de NCF a partir de características que se conocían en el momento de la inclusión en el estudio o durante la fase inicial del seguimiento. La evaluación del seguimiento de otros ensayos clínicos podría contribuir a determinar si los factores destacados en el presente estudio se correlacionan de manera constante con la permanencia en los estudios.
AN IMPORTANT GOAL of every clinical trial is to achieve completion of follow-up, which is especially challenging in studies with long follow-up, such as those required for tuberculosis (TB). Failure to retain participants results in smaller sample size, fewer outcomes, and a potentially under-powered study. It can also introduce bias, and impair the validity and generalizability of the results.1–5
Extensive research has focused on factors associated with non-completion of treatment. However, less research has been devoted to reasons for noncompletion of follow-up (NCF). Such information is particularly important for trials of preventive treatment for TB, in which endpoints (e.g., development of TB) are rare and follow-up is lengthy.
Differences among participants who do vs. those who do not complete the protocol-defined follow-up period may include demographic, clinical, social, and behavioral characteristics.6,7 Among 1075 participants enrolled in a TB treatment trial at US and Canadian sites, factors associated with NCF were cultural or linguistic and lack of stable housing.1 In a 3-year TB prevention trial in Botswana among human immunodeficiency virus (HIV) infected adults, men were less likely to complete treatment and follow-up.2
The purpose of the present analysis was to identify factors associated with NCF in a 33-month clinical trial, the PREVENT TB study.
STUDY POPULATION AND METHODS
We performed a post-hoc exploratory analysis based on a plan developed after completion of the PREVENT TB trial, a Phase III open-label, randomized trial of latent tuberculous infection (LTBI) treatment. Participants infected with Mycobacterium tuberculosis who were at high risk of developing TB were enrolled from June 2001 through February 2008 at 28 sites in low (the United States, Canada, and Spain) and high (Brazil) TB burden countries.8,9 Participants aged <18 years were excluded from this analysis because completion of follow-up might reflect characteristics of the parents rather than the child.
Participants were assigned to either 12 directly observed doses of once-weekly isoniazid (INH) (900 mg) and rifapentine (RPT) (900 mg) (3HP-DOT) or 9 months of daily self-administered INH (300 mg) (9HSAT), following unrestricted randomization, stratified by enrolling site and by participant HIV status. Within household clusters of close contacts, randomization was performed for the first participant enrolled; subsequent participants from the same household received the same regimen. Participants were evaluated monthly during treatment. Those receiving the 3HP-DOT regimen had scheduled visits for evaluation at weeks 4, 8, and 12, while participants receiving the 9H-SAT regimen had nine monthly visits during treatment. All participants were evaluated every 3 months after completion or discontinuation of treatment until month 21, then every 6 months until month 33 of the trial. The assessment at each visit included weight, signs and symptoms of TB, any anti-tuberculosis drug received, and new diagnosis or hospitalization.10
Adherence to the assigned regimen was assessed at each visit: by observed treatment records for 3HPDOT, and by pill count and self-report for 9H-SAT. This trial compared the effectiveness of the combined regimen with that of 9H-SAT after participants had been followed for 33 months from enrollment. Enrollment started at 22 North American sites in 2001; two sites were added in 2002 and one each in 2003 and 2008. Non-North American sites (in Brazil and Spain) were added in 2003 and 2004.
All participants provided written informed consent. Institutional review boards at the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, and at the participating clinical sites, approved the study protocol.
Factors possibly associated with NCF were organized as demographic (age, sex, race, ethnicity, country of origin, education, unemployment, homelessness, history of incarceration, and need for an interpreter), clinical (body mass index, HIV status, cirrhosis [by self-report], methadone treatment, and potential for pregnancy [defined below]), social (alcohol consumption [defined below], injection drug use ever, and current smoking at the time of enrollment), behavioral (missing an early clinic visit [defined below]), regimen assigned, and enrolling site. Potential for pregnancy was categorized as confirmed pregnancy, participants for whom pregnancy was not possible (women aged >51 years or men), or women of reproductive age (between 18 and 51 years of age). Alcohol use was defined as an affirmative response to a question asking whether the participant ever drank alcoholic beverages. Alcohol abuse was defined as a score of 72 on the CAGE questionnaire.11 Participants were considered to have missed an early visit on the 3HP-DOT regimen if they missed at least one of the first three observed doses, followed by receiving an observed dose at any time during the treatment period or, on the 9H-SAT regimen, if they missed at least one of the first three monthly visits, followed by a monthly visit at any time during the treatment period.
Non-completion of a 3HP-DOT or 9H-SAT regimen was defined as not taking at least 11 of 12 doses within 10–16 weeks for 3HP-DOT or at least 240 of 270 doses within 35–52 weeks for 9H-SAT. Participants were encouraged to continue study follow-up after completion or discontinuation of treatment. Non-completion of study follow-up was defined as failing to be followed for at least 993 days (33 months [equal to 9-month regimen plus 2 years of follow-up]) from enrollment, if TB or death did not occur before that time. Follow-up evaluations were conducted by telephone, e-mail, regular mail, or in person. Participants who were followed by e-mail or regular mail received a questionnaire with standard study follow-up questions. Follow-up continued for participants who moved or were incarcerated. An in-person visit was scheduled if the participant reported symptoms of TB at any time during the study, or reported symptoms of an adverse event within 60 days after the last study drug dose, and for the final study visit. Participants who completed at least 993 days of follow-up, developed TB, or died, were considered completers of follow-up. The analysis was conducted using SAS for Windows, Version 9.3 (Statistical Analysis System, Cary, NC, USA).
Proportions of NCF were calculated for all potential factors outlined. Age was stratified above and below the median age of the study population. We evaluated clinical and demographic characteristics of the combined cohorts, the North American (United States and Canada) and non-North American (Brazil and Spain) cohorts, and each cohort separately. We calculated hazard ratios (HRs), 95% confidence intervals (CIs), and P values for potential factors associated with NCF. The proportional hazard assumption was evaluated for each factor using a Kaplan-Meier graph, and by performing the proportionality test of binomial variables. We evaluated collinearity of all factors with P ⩽ 0.2 in the univariate analyses using the proc reg procedure in SAS (condition index around 10). Interaction terms were selected based on expert opinion (Appendix).* A final Cox proportional hazard model was defined in which backward elimination was performed at P < 0.05.
Duration of retention was divided into three equal periods: 1–331, 332–662, and 663–993 days. Proportions of clinical and demographic characteristics in these three groups, as well as time of follow-up in person-months, were calculated separately.
We used Pearson’s correlation coefficient and a linear regression model to evaluate the relationship between the proportions of NCF and participants enrolled by site. Univariate and multivariate analyses of the North American cohort were also performed among the first participants enrolled of a cluster and among participants who completed at least 80% of the study follow-up (at least 793 days from enrollment). The latter was intended to evaluate whether early vs. late loss to follow-up differed between study regimens. Most sites provided compensation for study participants for the time and effort required to participate; however, the methods and amounts were too variable for a meaningful analysis of their influence on completion of study follow-up.
RESULTS
Of 8053 participants enrolled in the PREVENT TB trial, 992 were excluded from the analysis: 706 were aged <18 years, 259 were determined ineligible after enrollment, and 27 were unable to complete follow-up due to closure of the enrolling site. The remaining 7061 participants made up the cohort selected for analysis; 841 (11.9%) did not complete study follow-up. NCF was higher among participants who did not complete treatment (419/1601, 26.2%) than among those who completed the assigned regimen (422/5460, 7.7%) (P < 0.001) (Figure and Table 1). No clear violation of the proportional hazard assumption was found. The univariate analysis showed that receiving 9H-SAT (9H-SAT, 448/3418 [13.1%] vs. 3HP-DOT, 393/3643 [10.8%]), young age, male sex, Hispanic ethnicity, low education, history of incarceration, homelessness, alcohol abuse, injection drug use, current smoking at the time of enrollment, enrolling site, and missing an early clinic visit were associated with NCF. In a multivariate logistic regression analysis, after controlling for several factors, we found that the hazard of NCF after completion or discontinuation of LTBI treatment was 1.9 times higher among participants who experienced homelessness; 1.4 times higher among participants aged <37 years, those with <9th grade education, those with a history of incarceration, those who reported tobacco smoking at the time of enrollment, and those who missed an early clinic visit; and 1.2 times higher among participants receiving 9H-SAT than among those receiving 3HP-DOT, and among males. Enrolling site was statistically significant, indicating variability across the sites even after controlling for other factors (P < 0.001) (Table 2; interaction terms are evaluated in Appendix Table A.1).
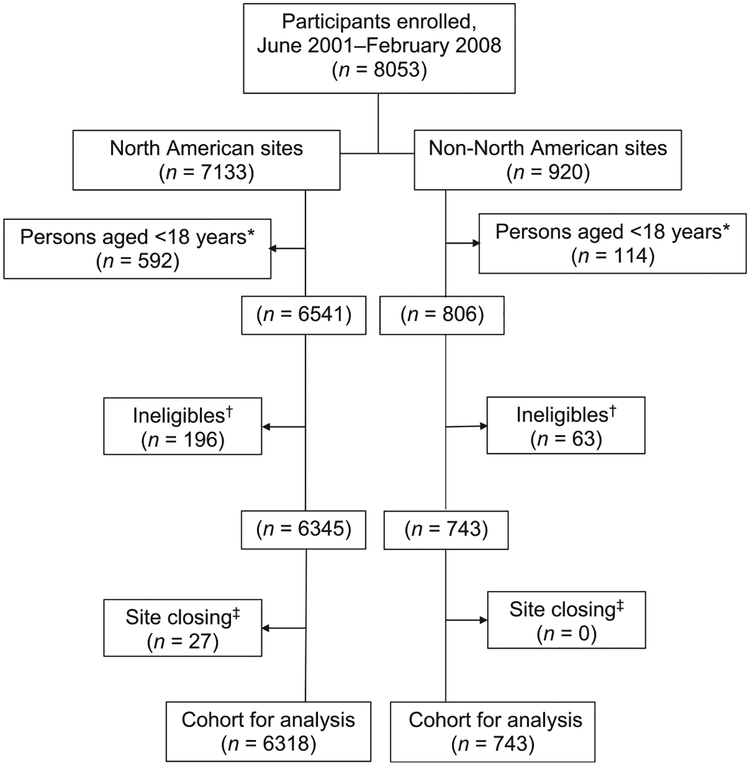
Figure.
Flow chart of participants evaluated for factors associated with non-completion of study follow-up in a 33-month Clinical Trial of Latent Tuberculosis Infection Treatment, the PREVENT TB Trial. This figure shows the number of participants who were enrolled in the trial, the groups that were excluded from the analysis, and the remaining cohort that was evaluated for factors associated with non-completion of study follow-up, by region. Inclusion criteria were as follows: 1) close contact with a culture-confirmed PTB patient (within 2 years before enrollment) and TST-positive; 2) recent conversion to TST positivity; 3) HIV infection plus TST-positive or recent close contact with a culture-confirmed PTB patient regardless of TST results; or 4) TST-positive with fibrotic changes on chest radiograph consistent with previously untreated TB. Exclusion criteria were as follows: 1) current confirmed TB; 2) suspected TB; 3) TB resistant to INH or RMP in the source case; 4) history of treatment for >14consecutive days with a rifamycin or >30 consecutive days with INH during the previous 2 years; 5) documented history of completing adequate treatment for active TB or latent M. tuberculosis infection in an HIV-negative person; 6) history of sensitivity/intolerance to INH or rifamycins, 7) serum AST >5 times the upper limit of normal if AST was determined, 8) pregnant or lactating females (not excluded in this cohort for analysis), 9) persons currently receiving or planning to receive HIV-1 therapy within 90 days of enrollment, or 10) weight <10.0 kg. PREVENT TB Trial reasons for ineligibility: source case INH/RMP-resistant (50%), source case culture-negative (31%), other (19%). This analysis includes participants from all enrolling sites: the United States, Canada, Brazil and Spain. * This analysis considers only adults aged ⩾ 18 years. †Microbiological and non-microbiological ineligibles. ‡Participants who did not have the opportunity to complete at least 993 days of study follow-up due to closure of enrolling site; clustering below and above 900 days: 59.3% (16/27) were followed in the trial between 687 and 889 days and 40.7% (11/27) between 915 and 991 days. PTB = pulmonary tuberculosis; TST = tuberculosis skin test; HIV = human immunodeficiency virus; INH = isoniazid; RMP = rifompicin; AST = aspartate immunotransferase.
Table 1.
Completion of follow-up status by completion of treatment in the North American and non-North American regions combined*
| Did not complete treatment (n = 1601) | Completed treatment (n = 5460) | |||
|---|---|---|---|---|
| Did not complete follow-up (n = 419) | Completed follow-up (n = 1182) | Did not complete follow-up (n = 422) | Completed follow-up (n = 5038) | |
| Regimen | n (%) | n (%) | n (%) | n (%) |
| 3HP-DOT (n = 3643)† | 155 (37.0) | 485 (41.0) | 238 (56.4) | 2765 (54.9) |
| 9H-SAT (n = 3418)† | 264 (63.0) | 697 (59.0) | 184 (43.6) | 2273 (45.1) |
| Total (n = 7061) | 419 (26.2)‡ | 1182 (73.8)‡ | 422 (7.7)§ | 5038 (92.3)§ |
Proportion of non-completion of follow-up between those who did not complete treatment (419/1601, 26.2%)vs. those who completed treatment (422/5460, 7.7%) was statistically significant (P < 0.001).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily selfadministered INH (maximum dose, 300 mg). Participants had the opportunity to continue study follow-up after completion or discontinuation of treatment.
The denominator for the percentage is all participants who did not complete treatment (n = 1601).
The denominator for the percentage is all participants who completed treatment (n =5460).
H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered therapy.
Table 2.
Univariate and multivariate analyses of factors associated with non-completion of study follow-up (n = 841) compared to those who completed study follow-up (n = 6220) in the North American and non-North American regions combined: Cox proportional hazard model (n= 7061)
| Non-completion of follow-up (%) | Multivariate | ||
|---|---|---|---|
| HR (95%CI) | aHR (95%CI) | ||
| Regimen* | |||
| 3HP-DOT (n = 3643) | 10.8 | Reference | Reference |
| 9H-SAT (n = 3418) | 13.1 | 1.2 (1.07–1.41) | 1.2 (1.04–1.37) |
| Age, years (median = 37) | |||
| <37 (n = 3413) | 13.5 | 1.3 (1.15–1.50) | 1.4 (1.21–1.62) |
| ⩾37 (n = 3648) | 10.4 | Reference | Reference |
| Sex | |||
| Female (n = 3194) | 10.4 | Reference | Reference |
| Male (n = 3867) | 13.2 | 1.3 (1.14–1.50) | 1.2 (1.01–1.36) |
| Race | |||
| White (n = 3530) | 13.2 | Reference | Reference |
| Black (n = 1642) | 12.8 | 0.98 (0.83–1.15) | 0.87 (0.72–1.05) |
| Asian (n = 888) | 7.4 | 0.5 (0.42–0.71) | 0.6 (0.45–0.80) |
| Other (n = 1001)† | 9.8 | 0.7 (0.58–0.90) | 0.9 (0.57–1.31) |
| Ethnicity | |||
| Non-Hispanic (n = 3668) | 10.9 | Reference | |
| Hispanic (n = 2650) | 14.1 | 1.3 (1.14–1.51) | |
| Brazil (n = 248) | 10.5 | ||
| Spain (n = 495) | 8.9 | ||
| HIV status | |||
| Negative (n = 3509) | 12.3 | Reference | |
| Positive (n = 206) | 12.1 | 1.0 (0.67–1.49) | |
| Unknown (n = 3346) | 11.5 | 0.9 (0.81–1.06) | |
| Country of origin | |||
| Non-US (n = 4542) | 12.1 | Reference | |
| US (n = 2519) | 11.5 | 1.0 (0.83–1.10) | |
| Educational level | |||
| ⩽8th grade (n = 1444) | 13.0 | 1.4(1.12–1.78) | 1.4 (1.05–1.73) |
| 9th grade, some college (n = 4384) | 12.3 | 1.3 (1.08–1.61) | 1.1 (0.92–1.40) |
| College or higher (n = 1233) | 9.4 | Reference | Reference |
| Incarceration‡ | |||
| No (n = 6665) | 11.5 | Reference | Reference |
| Yes (n = 396) | 18.7 | 1.7 (1.36–2.19) | 1.4 (1.03–1.82) |
| Unemployed | |||
| No (n = 6252) | 11.7 | Reference | Reference |
| Yes (n = 809) | 13.4 | 1.2 (0.95–1.43) | 0.8 (0.60–0.99) |
| Homeless§ | |||
| No (n = 6553) | 11.3 | Reference | Reference |
| Yes (n = 508) | 19.5 | 1.8 (1.49–2.26) | 1.9 (1.42–2.47) |
| Alcohol consumption¶ | |||
| No (n = 3306) | 11.1 | Reference | |
| Use (n = 3229) | 12.3 | 1.1 (0.97–1.29) | |
| Abuse (n = 526) | 14.8 | 1.4(1.10–1.80) | |
| Injection drug use | |||
| No (n = 6780) | 11.7 | Reference | |
| Yes (n = 281) | 16.4 | 1.4(1.07–1.95) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 6787) | 11.9 | Reference | |
| Yes (n = 274) | 11.3 | 1.0 (0.67–1.37) | |
| Current smoker | |||
| No (n = 4941) | 10.6 | Reference | Reference |
| Yes (n = 2120) | 14.9 | 1.5 (1.27–1.67) | 1.4 (1.16–1.59) |
| Methadone treatment | |||
| No (n = 6920) | 11.9 | Reference | |
| Yes (n = 141) | 11.4 | 1.0 (0.58–1.57) | |
| Potential for pregnancy | |||
| Not possible (n = 4447) | 12.5 | Reference | |
| Female (n = 2506) | 11.1 | 0.9 (0.75–1.0) | |
| Pregnancy (n = 108) | 8.3 | 0.6 (0.33–1.23) | |
| Enrolling site# | |||
| Missing clinic visit** | |||
| No (n = 6633) | 11.5 | Reference | Reference |
| Yes (n = 428) | 18.9 | 1.7 (1.37–2.17) | 1.4 (1.07–1.73) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 with the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was analyzed after a random selection of the reference value; overall P < 0.001.
Missed at least one of the first three DOT visits for the 3HP-DOT regimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or monthly visit after the missing DOT/visit respectively (includes those who did not receive any study dose).
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment; HIV = human immunodeficiencyvirus.
From the total cohort, 6318 participants were enrolled at North American sites (United States and Canada) and 743 at non-North American sites (Brazil and Spain). Differences in clinical and demographic characteristics between regions are shown in Table 3.
Table 3.
Clinical and demographic characteristics of participants stratified by region of enrollment (n = 7061)
| Region of enrollment | ||
|---|---|---|
| North America (n = 6318) | Non-North America (n = 743) | |
| n (%) | n (%) | |
| Regimen | ||
| 3HP-DOT (n = 3643)* | 3254 (51.5) | 389 (52.4) |
| 9H-SAT (n = 3418)* | 3064 (48.5) | 354 (47.6) |
| Age, years (median = 37) | ||
| <37 (n = 3413) | 3048 (48.2) | 365 (49.1) |
| ⩾37 (n = 3648) | 3270 (51.8) | 378 (50.9) |
| Sex | ||
| Female (n = 3194) | 2838 (44.9) | 356 (47.9) |
| Male (n = 3867) | 3480 (55.1) | 387 (52.1) |
| Race | ||
| African (n = 1642) | 1642 (26) | — |
| Asian (n = 888) | 888 (14.1) | — |
| Other (n = 1001) | 258 (4.1) | 743 (100) |
| White (n = 3530) | 3530 (55.9) | — |
| Ethnicity | ||
| Hispanic (n = 2650) | 2650 (41.9) | — |
| Non-Hispanic (n = 3668) | 3668 (58.1) | — |
| Spain (n = 248) | — | 248 (33.4) |
| Brazil (n = 495) | — | 495 (66.6) |
| Country of birth | ||
| US (n = 2519) | 2519 (39.9) | — |
| Non-US (n = 4542) | 3799 (60.1) | 743 (100) |
| HIV status | ||
| Negative (n = 3509) | 3142 (49.7) | 367 (49.4) |
| Positive (n = 206) | 148 (2.3 | 58 (7.8) |
| Unknown (n = 3346) | 3028 (47.9) | 318 (42.8) |
| Body mass index, kg/m2 | ||
| <18.5 (n = 131) | 108 (1.7) | 23 (3.1) |
| 18.5–24.9 (n = 2146) | 1813 (28.7) | 333 (44.8) |
| 25–29.9 (n = 2503) | 2258 (35.7) | 245 (33) |
| ⩾30 (n = 2281) | 2139 (33.9 | 142 (19.1) |
| Educational level | ||
| ⩽8th grade (n = 1444) | 1145 (18.1) | 299 (40.2) |
| 9th grade, some college (n = 4384) | 3997 (63.3) | 387 (52.1) |
| College or higher (n = 1233) | 1176 (18.6) | 57 (7.7) |
| History of incarceration | ||
| No (n = 6665) | 5925 (93.8) | 740 (99.6) |
| Yes (n = 396) | 393 (6.2) | 3 (0.4) |
| Unemployment | ||
| No (n = 6252) | 5534 (87.6) | 718 (96.6) |
| Yes (n = 809) | 784 (12.4) | 25 (3.4) |
| Homelessness | ||
| No (n = 6553) | 5815 (92.0) | 738 (99.3) |
| Yes (n = 508) | 503 (8.0) | 5 (0.7) |
| Alcohol consumption | ||
| Use (n = 3229) | 2925 (46.3) | 304 (40.9) |
| Abuse (n = 526) | 484 (7.7) | 42 (5.7) |
| No (n = 3306) | 2909 (46.0) | 397 (53.4) |
| Injection drug use ever | ||
| No (n = 6780) | 6052 (95.8) | 728 (98.0) |
| Yes (n = 281) | 266 (4.2) | 15 (2.0) |
| Chronic liver disease | ||
| No (n = 6787) | 6068 (96.0) | 719 (96.8) |
| Yes (n = 274) | 250 (4.0) | 24 (3.2) |
| Current smoker | ||
| No (n = 4941) | 4394 (69.5) | 547 (73.6) |
| Yes (n = 2120) | 1924 (30.5) | 196 (26.4) |
| Potential for pregnancy | ||
| Female (n = 2506) | 2243 (35.5) | 263 (35.4) |
| Pregnancy (n = 108) | 105 (1.7) | 3 (0.4) |
| Not possible (n = 4447) | 3970 (62.8) | 477 (64.2) |
| Methadone use | ||
| No (n = 6920) | 6182 (97.8) | 738 (99.3) |
| Yes (n = 141) | 136 (2.2) | 5 (0.7) |
| Concomitant medications ⩾4 | ||
| No (n = 6069) | 5367 (84.9) | 702 (94.5) |
| Yes (n = 992) | 951 (15.1) | 41 (5.5) |
| Contact TB | ||
| No (n = 2198) | 2114(33.5) | 84(11.3) |
| Yes (n = 4863) | 4204 (66.5) | 659 (88.7) |
| TST conversion | ||
| No (n = 4749) | 4074 (64.5) | 675 (90.8) |
| Yes (n = 2312) | 2244 (35.5) | 68 (9.2) |
| Fibrosis | ||
| No (n = 6873) | 6133 (97.1) | 740 (99.6) |
| Yes (n = 188) | 185 (2.9) | 3 (0.4) |
| HIV/LTBI treatment | ||
| No (n = 6865) | 6178 (97.8) | 687 (92.5) |
| Yes (n = 196) | 140 (2.2) | 56 (7.5) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily selfadministered INH (maximum dose, 300 mg).
H, INH = isoniazid; P, RPT=rifapentine; DOT = directly observed therapy; SAT = self-administered treatment; HIV = human immunodeficiency virus; LTBI = latent tuberculous infection.
Among the 6318 participants enrolled at North American sites, 771 did not complete study follow-up (771/6318, 12.2%) (Appendix Table A.2). The univariate and multivariate analyses found similar results compared to the total trial cohort (Table 4; interaction terms are evaluated in Appendix Table A.3). Among 743 participants enrolled in non-North American sites, 70 did not complete study follow-up (70/743, 9.4%; Appendix Table A.4). The only factor significantly associated with NCF in the univariate and multivariate analyses was missing an early clinic visit (adjusted HR 6.1) (Table 5).
Table 4.
Univariate and multivariate analysis of factors associated with non-completion of study follow-up (n = 771) compared to those who completed study follow-up (n = 5547) in the North American region: Cox proportional hazard model (n = 6318)
| Non-completion of follow-up % | Univariate* HR (95%CI) | Multivariate aHR (95%CI) | |
|---|---|---|---|
| Regimen† | |||
| 3HP-DOT (n = 3254) | 11.2 | Reference | Reference |
| 9H-SAT (n = 3064) | 13.3 | 1.2 (1.05–1.39) | 1.2 (1.05–1.39) |
| Age, years (median = 37) | |||
| <37 (n = 3048) | 13.9 | 1.3 (1.14–1.52) | 1.4 (1.20–1.61) |
| ⩾37 (n = 3270) | 10.6 | Reference | Reference |
| Sex | |||
| Female (n = 2838) | 10.7 | Reference | |
| Male (n = 3480) | 13.5 | 1.3 (1.12–1.49) | |
| Race | |||
| White (n = 3530) | 13.2 | Reference | Reference |
| African American (n = 1642) | 12.8 | 1.0 (0.83–1.16) | 0.9 (0.78–1.14) |
| Asian (n = 888) | 7.4 | 0.5 (0.42–0.71) | 0.5 (0.39–0.70) |
| Other (n = 258)‡ | 10.9 | 0.8 (0.55–1.19) | 0.8 (0.55–1.26) |
| Ethnicity | |||
| Non-Hispanic (n = 3668) | 10.9 | Reference | |
| Hispanic (n = 2650) | 14.1 | 1.3 (1.13–1.50) | |
| HIV status | |||
| Negative (n = 3142) | 12.6 | Reference | |
| Positive (n = 148) | 12.2 | 1.0 (0.61–1.57) | |
| Unknown (n = 3028) | 11.8 | 0.9 (0.81–1.08) | |
| Country of origin | |||
| Non-US (n = 3799) | 12.7 | Reference | Reference |
| US (n = 2519) | 11.5 | 0.9 (0.79–1.05) | 0.6 (0.50–0.73) |
| Educational level | |||
| ⩽8th grade (n = 1145) | 14.4 | 1.6 (1.27–2.05) | |
| 9th grade, some college (n = 3997) | 12.5 | 1.4(1.12–1.70) | |
| College or higher (n = 1176) | 9.2 | Reference | |
| Incarceration§ | |||
| No (n = 5925) | 11.8 | Reference | Reference |
| Yes (n = 393) | 18.8 | 1.7 (1.34–2.16) | 1.4 (1.08–1.91) |
| Unemployed | |||
| No (n = 5534) | 12.0 | Reference | |
| Yes (n = 784) | 13.5 | 1.1 (0.93–1.41) | |
| Homeless¶ | |||
| No (n = 5815) | 11.6 | Reference | Reference |
| Yes (n = 503) | 19.5 | 1.8 (1.44–2.21) | 2.0 (1.50–2.58) |
| Alcohol consumption# | |||
| No (n = 2909) | 11.2 | Reference | |
| Use (n = 2925) | 12.6 | 1.1 (0.98–1.32) | |
| Abuse (n = 484) | 15.7 | 1.5 (1.15–1.90) | |
| Injection drug use | |||
| No (n = 6052) | 12.0 | Reference | |
| Yes (n = 266) | 16.2 | 1.4(1.02–1.89) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 6068) | 12.2 | Reference | |
| Yes (n = 250) | 12.0 | 1.0 (0.69–1.44) | |
| Current smoker | |||
| No (n = 4394) | 10.9 | Reference | Reference |
| Yes (n = 1924) | 15.3 | 1.5 (1.27–1.70) | 1.5 (1.28–1.77) |
| Methadone treatment | |||
| No (n = 6182 | 12.2 | Reference | |
| Yes (n = 136) | 11.8 | 1.0 (0.59–1.59) | |
| Potential for pregnancy | |||
| Not possible (n = 3970) | 12.7 | Reference | |
| Female (n = 2243) | 11.5 | 0.9 (0.77–1.03) | |
| Pregnancy (n = 105) | 8.6 | 0.6 (0.33–1.25) | |
| Enrolling site** | |||
| Missing an early clinic visit††‡‡ | |||
| No (n = 5906) | 11.8 | Reference | |
| Yes (n = 412) | 18.0 | 1.6 (1.24–2.0) | |
In univariate analysis, among 2498 non-US/Canada-born participants, 34 used the services of an interpreter, while 2464 did not; respectively 11.9% and 14.7% of those who did not complete study follow-up did not need and did need an interpreter; this difference was non-significant (HR 1.3, 95%CI 0.53–3.10, P = 0.58).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was analyzed after a random selection of the reference value; overall P < 0.001.
Missing at least one of the first three DOT visits for the 3HP-DOT regimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or monthly visit after the missing DOT/visit respectively (includes those who did not receive any study dose).
The relation between missing an early clinic visit and history of incarceration or homelessness was non-significant. Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR=hazard ratio; CI =confidence interval; aHR=adjusted HR; H, INH =isoniazid; P, RPT=rifapentine; DOT=directly observed therapy; SAT =self-administered treatment; HIV = human immunodeficiency virus.
Table 5.
Univariate and multivariate analysis of factors associated with non-completion of study follow-up (n = 70) compared to those who completed study follow-up (n = 643) in the Non-North American region (n = 743): Cox proportional hazard model
| Characteristic | Non-completion of follow-up % | Univariate* HR (95%CI) | Multivariate aHR (95%CI) |
|---|---|---|---|
| Regimen† | |||
| 3HP-DOT (n = 389) | 7.7 | Reference | |
| 9INH-SAT (n = 354) | 11.3 | 1.5 (0.93–2.40) | |
| Age, years (median = 37) | |||
| <37 (n = 365) | 10.4 | 1.3 (0.78–2.0) | |
| ⩾37 (n = 378) | 8.5 | Reference | |
| Sex | |||
| Female (n = 356) | 7.9 | Reference | |
| Male (n = 387) | 10.9 | 1.4 (0.87–2.27) | |
| HIV status | |||
| Negative (n = 367) | 9.8 | Reference | |
| Positive (n = 58) | 12.1 | 1.2 (0.56–2.81) | |
| Unknown (n = 318) | 8.5 | 0.9 (0.53–1.43) | |
| Educational level | |||
| ⩽ 8th grade (n = 299) | 7.7 | 0.5 (0.24–1.17) | |
| 9th grade, some college (n = 387) | 10.1 | 0.7 (0.32–1.46) | |
| College or higher (n = 57) | 14.0 | Reference | |
| Incarceration‡ | |||
| No (n = 740) | 9.5 | Reference | |
| Yes (n = 3) | 0.0 | <0.001 (<0.001->999) | |
| Unemployed | |||
| No (n = 718) | 9.5 | Reference | |
| Yes (n = 25) | 8.0 | 0.9 (0.21–3.52) | |
| Homeless§ | |||
| No (n = 738) | 9.4 | Reference | |
| Yes (n = 5) | 20.0 | 2.3 (0.32–16.81) | |
| Alcohol consumption¶ | |||
| No (n = 397) | 10.1 | Reference | |
| Use (n = 304) | 9.2 | 0.9 (0.57–1.48) | |
| Abuse (n = 42) | 4.8 | 0.5 (0.12–1.96) | |
| Injection drug use | |||
| No (n = 728) | 9.2 | Reference | |
| Yes (n = 15) | 20.0 | 2.3 (0.71–7.20) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 719) | 9.6 | Reference | |
| Yes (n = 24) | 4.2 | 0.4 (0.06–2.96) | |
| Current smoker | |||
| No (n = 547) | 8.8 | Reference | |
| Yes (n = 196) | 11.2 | 1.3 (0.77–2.12) | |
| Methadone treatment | |||
| No (n = 738) | 9.5 | Reference | |
| Yes (n = 5) | 0.0 | <0.001 (<0.001->999.9) | |
| Potential for pregnancy | |||
| Not possible (n = 477) | 10.7 | Reference | |
| Female (n = 263) | 7.2 | 0.7 (0.39–1.11 | |
| Pregnancy (n = 3) | 0.0 | <0.001 (<0.001->999.9) | |
| Enrolling site | |||
| Spain (n = 248) | 3.5 | 1.18 (0.72–1.91) | |
| Brazil (n = 495) | 8.9 | Reference | |
| Missing clinic visit¶ | |||
| No (n = 727) | 8.7 | Reference | Reference |
| Yes (n = 16) | 43.8 | 6.1 (2.80–13.34) | 6.1 (2.80–13.34) |
In univariate analysis, only 5 participants required the services of an interpreter among participants enrolled at non-US/Canada sites (n = 743). The association between need for an interpreter and non-completion of follow-up was not significant (P = 0.98).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily selfadministered INH (maximum dose, 300 mg).
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 on the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Missing at least one of the first three DOT visits for the 3HP-DOT regimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or monthly visit after the missing DOT/visit, respectively (includes those who did not receive any study dose). No statistically significant interactions resulted between missing an early clinic visit and factors with P ⩽ 0.2 in univariate analysis.
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT =self-administered treatment; HIV = human immunodeficiency virus.
In most cases, it was not possible to determine the reason for NCF (Table 6). No major differences in clinical and demographic characteristics were found among participants who discontinued follow-up within the first 331 days, 332–662 days, or 663–993 days. However, the percentage of participants who discontinued follow-up during all three intervals was higher among participants allocated to the 9HSAT regimen (Appendix Table A.5).
Table 6.
Reasons for non-completion of study follow-up by region and by regimen
| North-America (n = 771) | Non North-America (n = 70) | |||
|---|---|---|---|---|
| 3HP-DOT* (n = 363) | 9H-SAT* (n = 408) | 3HP-DOT* (n = 30) | 9H-SAT* (n = 40) | |
| Reason | n (%) | n (%) | n (%) | n (%) |
| Withdrew consent (n = 139) | 58 (16.0) | 72 (17.6) | 6 (20.0) | 3(7.5) |
| Developed active TB (n = 5)† | 3 (0.83) | 2 (0.49) | — | — |
| Refusal of further follow-up (n = 7) | 4 (1.1) | 3 (0.74) | — | — |
| Lost to follow-up (n = 410)‡ | 187 (51.5) | 194 (47.5) | 11 (36.7) | 18 (45.0) |
| Other (n = 147) | 56 (15.4) | 76 (18.6) | 7 (23.3) | 8 (20.0) |
| Missing (n = 133) | 55 (15.2) | 61 (15.0) | 6 (20.0) | 11 (27.5) |
| Total (n = 841) | 363 (47.1) | 408 (52.8) | 30 (42.9) | 40 (57.1) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Classified as 3HP-DOT probable clinical TB in adult (n=2), spine disease (discitis) not characteristic of TB spondylitis (n = 1)and 9H-SAT probable clinical TB in adult (n = 2).
3 participants in the 3HP group and 4 in the 9H group died after month 33, and were lost at the last follow-up evaluation. They were therefore added to the lost to follow-up category.
H, INH = isoniazid; P, RPT = rifapentine; DOT = directlyobserved therapy; SAT = self-administered treatment.
No association was found between NCF proportion and the number of participants enrolled by site (Pearson’s correlation coefficient = −0.15; P = 0.44, R2 = 0.02) (Appendix Table A.6). The proportion of NCF by enrolling site, stratified by regimen, did not show any pattern among high enrolling sites (Appendix Table A.7).
Among 5228 participants who were the first enrolled in a cluster in the North American region, 1247 did not complete treatment and 3981 did complete treatment; 330 (26.5%) participants not completing treatment and 320 (8.0%) participants completing treatment did not complete study follow-up (P < 0.001) (Appendix Table A.8). Results of univariate and multivariate analyses were similar to those shown in Tables 2 and 4 (Appendix Table A.9).
In the sensitivity analysis evaluating failure to complete at least 80% of follow-up, among participants enrolled in the North American sites, 1481 did not complete treatment and 4837 completed treatment; 321 (21.7%) treatment non-completers and 185 (3.8%) treatment completers did not complete study follow-up (P < 0.001) (Appendix Table A.10). Results of univariate and multivariate analysies were similar to those shown on Tables 2 and 4 (Appendix Table A.11).
In this study, collinearity was not found among factors that resulted in P ⩽ 0.2 in the univariate analysis.
DISCUSSION
Our study found that in a large (n = 7061) clinical trial of LTBI treatment requiring lengthy follow-up, the proportion that did not complete at least 993 days follow-up after enrollment was 11.9% (North American sites 12.2%, non-North American sites 9.4%). This proportion is similar to the 10.2% NCF rate found in a clinical trial in the United States and Canada with 2 years of post-TB treatment follow-up.1 Retention is especially challenging for an LTBI treatment trial, considering the asymptomatic nature of the condition being treated.12
The analyses conducted in two separate regions showed different results. Differences in sociodemographic characteristics as well as the small sample size of the non-North American cohort, with the small number of observations for some factors, might explain these differences. The HRs of some factors (e.g., young age, male sex, homelessness, injection drug use, and smoking) were >1, but were not statistically significant.
The inclusion of persons experiencing homelessness in TB clinical trials is important because they are disproportionally affected by TB. They represent a challenge for recruitment, initiation and completion of treatment, and for retention in research settings, due to unstable housing, food insecurity, and high prevalence of chronic mental health conditions.1,13,14 Data from the PREVENT TB study, North American region, showed that the proportion of non-completion of LTBI treatment not associated with adverse events was 57% higher in participants with history of homelessness before enrollment than in participants with no such history (P < 0.001).15 The same participants analyzed in this study showed a high rate of failure of retention to the end of the trial (19.5%). Given the complexity of working with people who have unstable housing, appropriate management is needed to maximize adherence in research settings.16,17
Self-reported smoking at the time of enrollment is also associated with both non-completion of LTBI treatment15 and NCF. Other risk factors are also associated with smoking: among 2120 participants who reported smoking at enrollment, 14% had a history of incarceration and 18% had experienced homelessness. Although a smoking cessation program might not succeed during the relatively short treatment phase, it might be feasible during a lengthy follow-up trial.18
The analyses performed indicate that missing an early clinic visit is a factor significantly associated with NCF after completion or discontinuation of LTBI treatment. This result is consistent with a separate analysis that indicated that participants who missed an early clinic visit for reasons other than an adverse event, but who returned at least once later, were more likely to discontinue the LTBI treatment regimen.15 Focused interventions to increase both completion of treatment and retention to the end of the trial might target participants who miss an early visit.
Incentives in clinical trials may be useful for recruitment and retention of participants.4,19–21 An effort was made to analyze the influence of compensation provided by enrolling sites to participants of this trial; however, a meaningful analysis of influence on completion of follow-up was not possible due to the high variability among methods and amounts of compensation.
In conclusion, clinical trials require retention through both treatment and follow-up phase to be successful. Evaluation of follow-up in other trials might help determine whether the identified factors in this study consistently correlate with NCF.
Acknowledgements
The study investigators and coordinators thank M Chen (Centers for Disease Control and Prevention [CDC]), N Shang (CDC), and B Stewart (CDC) for statistical guidance; N Scott (CDC, CDC Foundation) for data management; E Sizemore, whose detailed and tireless work during the trial helped to maximize completion rates; and all study participants.
RNM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the CDC, Atlanta, GA, USA. Sanofi, Paris, France, donated the rifapentine (RPT) used in this study, and donated over US$2.5 million to the CDC Foundation to supplement available US federal funding for RPT research. Details on the uses of these funds are available. Sanofi did not participate in study design, data collection, analysis, or interpretation; writing of this manuscript; or in the decision to submit this manuscript for publication. In addition to Sanofi contributions, no other disclosures have been reported.
The PREVENT TB study (Tuberculosis Trials Consortium Study 26) was registered at ClinicalTrials.gov (NCT00023452).
Potential conflicts of interest: RNM is employed by CDC Foundation, which receives funds for RPT research from Sanofi; TRS is a member of the data safety monitoring board for a clinical trial sponsored by Otsuka, Tokyo, Japan. The other authors declare no conflict of interest.
APPENDIX
Table A.1.
Univariate and multivariate analyses of factors associated with non-completion of study follow-up (n = 841) compared to those who completed study follow-up (n = 6220) in the North American and non-North American regions combined (n = 7061): Cox proportional hazard model
| Non-completion of follow-up % | Univariate HR (95%CI) | Multivariate aHR (95%CI) | |
|---|---|---|---|
| Regimen* | |||
| 3HP-DOT (n = 3643) | 10.8 | Reference | Reference |
| 9H-SAT (n = 3418) | 13.1 | 1.2 (1.07–1.41) | |
| Age, years (median = 37) | |||
| <37 (n = 3413) | 13.5 | 1.3 (1.15–1.50) | 1.4 (1.21–1.61) |
| ⩾37 (n = 3648) | 10.4 | Reference | Reference |
| Sex | |||
| Female (n = 3194) | 10.4 | Reference | |
| Male (n = 3867) | 13.2 | 1.3 (1.14–1.50) | |
| Race | |||
| White (n = 3530) | 13.2 | Reference | Reference |
| Black (n = 1642) | 12.8 | 0.98 (0.83–1.15) | 0.9 (0.72–1.03) |
| Asian (n = 888) | 7.4 | 0.5 (0.42–0.71) | 0.6 (0.44–0.77) |
| Other (n = 1001)† | 9.8 | 0.7 (0.58–0.90) | 0.8 (0.55–1.27) |
| Ethnicity | |||
| Non-Hispanic (n = 3668) | 10.9 | Reference | |
| Hispanic (n = 2650) | 14.1 | 1.3 (1.14–1.51) | |
| Brazil (n = 248) | 10.5 | ||
| Spain (n = 495) | 8.9 | ||
| HIV status | |||
| Negative (n = 3509) | 12.3 | Reference | |
| Positive (n = 206) | 12.1 | 1.0 (0.67–1.49) | |
| Unknown (n = 3346) | 11.5 | 0.9 (0.81–1.06) | |
| Country of origin | |||
| Non-US (n = 4542) | 12.1 | Reference | |
| US (n = 2519) | 11.5 | 1.0 (0.83–1.10) | |
| Educational level | |||
| ⩽8th grade (n = 1444) | 13.0 | 1.4(1.12–1.78) | |
| 9th grade, some college (n = 4384) | 12.3 | 1.3 (1.08–1.61) | |
| College or higher (n = 1233) | 9.4 | Reference | |
| Incarceration‡ | |||
| No (n = 6665) | 11.5 | Reference | |
| Yes (n = 396) | 18.7 | 1.7 (1.36–2.19) | |
| Unemployed | |||
| No (n = 6252) | 11.7 | Reference | |
| Yes (n = 809) | 13.4 | 1.2 (0.95–1.43) | |
| Homeless§ | |||
| No (n = 6553) | 11.3 | Reference | Reference |
| Yes (n = 508) | 19.5 | 1.8 (1.49–2.26) | 1.7 (1.32–2.23) |
| Alcohol consumption¶ | |||
| No (n = 3306) | 11.1 | Reference | |
| Use (n = 3229) | 12.3 | 1.1 (0.97–1.29) | |
| Abuse (n = 526) | 14.8 | 1.4(1.10–1.80) | |
| Injection drug use | |||
| No (n = 6780) | 11.7 | Reference | |
| Yes (n = 281) | 16.4 | 1.4(1.07–1.95) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 6787) | 11.9 | Reference | |
| Yes (n = 274) | 11.3 | 1.0 (0.67–1.37) | |
| Current smoker | |||
| No (n = 4941) | 10.6 | Reference | Reference |
| Yes (n = 2120) | 14.9 | 1.5 (1.27–1.67) | 1.4 (1.17–1.62) |
| Methadone treatment | |||
| No (n = 6920) | 11.9 | Reference | |
| Yes (n = 141) | 11.4 | 1.0 (0.58–1.57) | |
| Potential for pregnancy | |||
| Not possible (n = 4447) | 12.5 | Reference | |
| Female (n = 2506) | 11.1 | 0.9 (0.75–1.0) | |
| Pregnancy (n = 108) | 8.3 | 0.6 (0.33–1.23) | |
| Enrolling site# | |||
| Missing clinic visit** | |||
| No (n = 6633) | 11.5 | Reference | Reference |
| Yes (n = 428) | 18.9 | 1.7 (1.37–2.17) | 1.4 (1.07–1.72) |
| Interaction terms | |||
| IDU × regimen | |||
| 3HP-DOT: IDU vs. no IDU | 0.6 (0.38–0.81) | ||
| 9H-SAT: IDU vs. no IDU | 1.7 (0.95–2.96) | ||
| Sex × alcohol | |||
| Female: abuse vs. no alcohol | 0.3 (0.12–0.89) | ||
| Male: abuse vs. no alcohol | 1.2 (0.91–1.68) | ||
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 with the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was analyzed after a random selection of the reference value; overall P < 0.001.
Missing at least one of the first three DOT visits for the 3HP-DOTregimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or a monthly visit after the missing DOT/visit, respectively (includes those who did not receive any study dose).
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT =self-administered treatment; HIV = human immunodeficiency virus.
Table A.2.
Completion of follow-up status by completion of treatment in the North American region (n = 6318)
| Did not complete treatment (n = 1481) | Completed treatment (n = 4837) | |||
|---|---|---|---|---|
| Did not complete follow-up* (n = 385) | Completed follow-up (n = 1096) | Did not complete follow-up* (n = 386) | Completed follow-up (n = 4451) | |
| Regimen | n (%) | n (%) | n (%) | n (%) |
| 3HP-DOT (n = 3254)† | 141 (36.6) | 450 (41.1) | 222 (57.5) | 2441 (54.8) |
| 9H-SAT (n = 3064)† | 244 (63.4) | 646 (58.9) | 164 (42.5) | 2010 (45.2) |
| Total (n = 6318) | 385 (26.0)‡ | 1096 (74.0)‡ | 386 (8.0)§ | 4451 (92.0)§ |
Participants had the opportunity to continue study follow-up after completion or discontinuation of treatment; non-completion of follow-up = (385 + 386)/6318 = 12.2%. The difference in the proportion of non-completion of follow-up between those who did not complete treatment (385/1481, 26.0%) vs. those who completed treatment (386/4837, 7.7%) was statistically significant (P < 0.001).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
The denominator for the percentage is all participants who did not complete treatment (n = 1481).
The denominator for the percentage is all participants who completed treatment (n = 4837).
H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment.
Table A.3.
Univariate and multivariate analysis of factors associated with non-completion of study follow-up (n = 771) compared to those who completed study follow-up (n = 5547) in the North American region: Cox proportional hazard model (n = 6318)
| Non-completion follow-up % | Univariate* HR (95%CI) | Multivariate with interaction terms aHR (95%CI) | |
|---|---|---|---|
| Regimen† | |||
| 3HP-DOT (n = 3254) | 11.2 | Reference | Reference |
| 9H-SAT (n = 3064) | 13.3 | 1.2 (1.05–1.39) | 1.2 (1.01–1.36) |
| Age, years (median = 37) | |||
| <37 (n = 3048) | 13.9 | 1.3 (1.14–1.52) | |
| ⩾37 (n = 3270) | 10.6 | Reference | |
| Sex | |||
| Female (n = 2838) | 10.7 | Reference | |
| Male (n = 3480) | 13.5 | 1.3 (1.12–1.49) | |
| Race | |||
| White (n = 3530) | 13.2 | Reference | Reference |
| African American (n = 1642) | 12.8 | 1.0 (0.83–1.16) | 1.0 (0.83–1.29) |
| Asian (n = 888) | 7.4 | 0.5 (0.42–0.71) | 0.6 (0.39–0.77) |
| Other (n = 258)‡ | 10.9 | 0.8 (0.55–1.19) | 0.8 (0.55–1.27) |
| Ethnicity | |||
| Non-Hispanic (n = 3668) | 10.9 | Reference | |
| Hispanic (n = 2650) | 14.1 | 1.3 (1.13–1.50) | |
| HIV status | |||
| Negative (n = 3142) | 12.6 | Reference | |
| Positive (n = 148) | 12.2 | 1.0 (0.61–1.57) | |
| Unknown (n = 3028) | 11.8 | 0.9 (0.81–1.08) | |
| Country of origin | |||
| Non-US (n = 3799) | 12.7 | Reference | |
| US (n = 2519) | 11.5 | 0.9 (0.79–1.05) | |
| Educational level | |||
| ⩾8th grade (n = 1145) | 14.4 | 1.6 (1.27–2.05) | |
| 9th grade some college (n = 3997) | 12.5 | 1.4 (1.12–1.70) | |
| College or higher (n = 1176) | 9.2 | Reference | |
| Incarceration§ | |||
| No (n = 5925) | 11.8 | Reference | |
| Yes (n = 393) | 18.8 | 1.7 (1.34–2.16) | |
| Unemployed | |||
| No (n = 5534) | 12.0 | Reference | |
| Yes (n = 784) | 13.5 | 1.1 (0.93–1.41) | |
| Homeless¶ | |||
| No (n = 5815) | 11.6 | Reference | Reference |
| Yes (n = 503) | 19.5 | 1.8 (1.44–2.21) | 2.1 (1.59–2.75) |
| Alcohol consumption# | |||
| No (n = 2909) | 11.2 | Reference | |
| Use (n = 2925) | 12.6 | 1.1 (0.98–1.32) | |
| Abuse (n = 484) | 15.7 | 1.5 (1.15–1.90) | |
| Injection drug use | |||
| No (n = 6052) | 12.0 | Reference | |
| Yes (n = 266) | 16.2 | 1.4 (1.02–1.89) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 6068) | 12.2 | Reference | |
| Yes (n = 250) | 12.0 | 1.0 (0.69–1.44) | |
| Current smoker | |||
| No (n = 4394) | 10.9 | Reference | Reference |
| Yes (n = 1924) | 15.3 | 1.5 (1.27–1.70) | 1.5 (1.30–1.78) |
| Methadone treatment | |||
| No (n = 6182) | 12.2 | Reference | |
| Yes (n = 136) | 11.8 | 1.0 (0.59–1.59) | |
| Potential for pregnancy | |||
| Not possible (n = 3970) | 12.7 | Reference | |
| Female (n = 2243) | 11.5 | 0.9 (0.77–1.03) | |
| Pregnancy (n = 105) | 8.6 | 0.6 (0.33–1.25) | |
| Enrolling site** | |||
| Missing an early clinic visit†† | |||
| No (n = 5906) | 11.8 | Reference | Reference |
| Yes (n = 412) | 18.0 | 1.6 (1.24–2.0) | 1.3 (1.004–1.65) |
| Interaction terms | |||
| Country of birth × age | |||
| US: age <37 years vs. ⩾37 years | 1.7 (1.35–2.20) | ||
| Non-US: age <37 years vs. ⩾37 years | 1.2 (1.0–1.45) | ||
| Incarceration × ethnicity | |||
| Incarceration: Hispanic vs. non-Hispanic | 2.1 (1.19–3.56) | ||
| No incarceration: Hispanic vs. non-Hispanic | 1.1 (0.84–1.38) | ||
In univariate analysis, among 2498 non-US/Canada-born participants, 34 used the services of an interpreter, while 2464 did not; respectively 11.9% and 14.7% of those who did not complete study follow-up did not need and did need an interpreter; this difference was non-significant (HR 1.3, 95%CI 0.53–3.10, P = 0.58).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 with the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was analyzed after a random selection of the reference value; overall P < 0.001.
Missing at least one of the first three DOT visits for the 3HP-D0T regimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or a monthly visit after the missing DOT/visit, respectively (includes those who did not receive any study dose). No significant interaction resulted between missing an early clinic visit and history of incarceration or homelessness.
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR=hazard ratio; CI =confidence interval; aHR=adjusted HR; H, INH =isoniazid; P, RPT=rifapentine; DOT=directly observed therapy; SAT =self-administered treatment; HIV = human immunodeficiency virus.
Table A.4.
Completion of follow-up status by completion of treatment in the non-North American region (n = 743)*
| Did not complete treatment (n = 120) | Completed treatment (n = 623) | |||
|---|---|---|---|---|
| Did not complete follow-up (n = 34) | Completed follow-up (n = 86) | Did not complete follow-up (n = 36) | Completed follow-up (n = 587) | |
| Regimen | n (%) | n (%) | n (%) | n (%) |
| 3HP-DOT (n = 389)† | 14 (41.2) | 35 (40.7) | 16 (44.4) | 324 (55.2) |
| 9H-SAT (n = 354)† | 20 (58.8) | 51 (59.3) | 20 (55.6) | 263 (44.8) |
| Total (n = 743) | 34 (28.3)‡ | 86 (71.7)‡ | 36 (5.8)§ | 587 (94.2)§ |
Participants had the opportunity to continue study follow-up after completion or discontinuation of treatment. Non-completion of follow-up = (34 + 36)/743 = 9.4%. The proportion of non-completion of follow-up between those who did not complete treatment (34/120, 28.3%) vs. those who completed treatment (36/623, 5.8%) was statistically significant (P < 0.001).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
The denominator for the percentage is all participants who did not complete treatment (n = 120).
The denominator for the percentage is all participants who completed treatment (n = 623)
H, INH = isoniazid; P, RPT =rifapentine; DOT = directly observed therapy; SAT = self-administered treatment.
Table A.5.
Clinical and demographic characteristics of participants who did not complete study follow-up, by number of days of follow-up in the trial
| NCF within the first 331 days (n = 352) | NCF between 332 and 662 days (n = 167) | NCF between 663 and 993 days (n = 322) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Person-month | 1214 | 2892 | 9538 |
| Regimen* | |||
| 3HP-DOT | 168 (47.7) | 78 (46.7) | 145 (45.7) |
| 9H-SAT | 184 (52.3) | 89 (53.3) | 175 (54.4) |
| Age, years, median [IQR] | 35 [26–45] | 35 [26–45] | 36 [26–45] |
| Male sex | 228 (64.8) | 112 (67.1) | 170 (52.8) |
| Race | |||
| White | 186 (52.8) | 96 (57.5) | 185 (57.5) |
| African American | 95 (27.0) | 42 (25.2) | 73 (22.7) |
| Asian | 33 (9.4) | 14 (8.4) | 19 (5.9) |
| Other† | 38 (10.8) | 15 (9.0) | 45 (14.0) |
| Ethnicity | |||
| Hispanic | 146 (41.5) | 80 (47.9) | 147 (45.7) |
| Non-Hispanic | 180 (51.1) | 78 (46.7) | 140 (43.5) |
| Spain | 9 (2.6) | 4 (2.4) | 13 (4.0) |
| Brazil | 17 (4.8) | 5 (3.0) | 22 (6.8) |
| HIV status | |||
| Positive | 11 (3.1) | 4 (2.34) | 10 (3.1) |
| Negative | 167 (47.4) | 87 (52.1) | 179 (55.6) |
| Unknown | 174 (49.4) | 76 (45.5) | 133 (41.3) |
| Country of birth | |||
| US | 114 (32.4) | 55 (32.9) | 121 (37.6) |
| Non-US | 238 (67.6) | 112 (67.1) | 201 (62.4) |
| Body mass index, median [IQR] | 27 [24–30] | 27 [24–31] | 27 [24–31] |
| Education | |||
| ⩽8th grade | 81 (23.0) | 37 (22.2) | 70 (21.7) |
| 9th grade, some college | 216 (61.4) | 110 (65.9) | 211 (65.5) |
| College or higher | 55 (15.6) | 20 (12.0) | 41 (12.7) |
| History of incarceration | 33 (9.4) | 17 (10.2) | 24 (7.5) |
| Unemployed | 43 (12.2) | 24 (14.4) | 41 (12.7) |
| Homeless | 42 (11.9) | 28 (16.8) | 29 (9.0) |
| Alcohol consumption | |||
| No | 147 (41.8) | 60 (35.9) | 160 (49.7) |
| Use | 169 (48.0) | 83 (49.7) | 144 (44.7) |
| Abuse | 36 (10.2) | 24 (14.4) | 18 (5.6) |
| Injection drug use | 22 (6.3) | 5 (3.0) | 19 (5.9) |
| Current smoker | 137 (38.9) | 76 (45.5) | 103 (32.0) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily selfadministered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
NCF=non-completion of follow-up; H, INH=isoniazid; P, RPT=rifapentine; DOT=directly observed therapy; SAT=self-administered treatment; IQR=interquartile range: HIV = human immunodeficiency virus.
Table A.6.
Proportion of non-completion of study follow-up by site, sorted by number of participants enrolled*
| Non-completion of follow-up | Participants enrolled (n = 7062) | |
|---|---|---|
| Site | n (% enrollment) | n (%) |
| G | 145 (12.6) | 1,153 (16.3) |
| R | 69 (13.4) | 515 (7.3) |
| Y | 50 (10.0) | 502 (7.1) |
| P | 44 (8.9) | 495 (7.0) |
| AA | 37 (10.3) | 359 (5.1) |
| B | 69 (20.8) | 331 (4.7) |
| K | 48 (15.0) | 319 (4.5) |
| I | 14 (4.6) | 306 (4.3) |
| F | 25 (8.5) | 294 (4.2) |
| E | 18 (6.3) | 288 (4.1) |
| L | 18 (6.5) | 276 (3.9) |
| Q | 26 (10.5) | 248 (3.5) |
| M | 26 (11.8) | 221 (3.1) |
| C | 17 (8.2) | 208 (2.9) |
| O | 35 (17.8) | 197 (2.8) |
| T | 10 (5.3) | 188 (2.7) |
| J | 17 (9.8) | 173 (2.4) |
| V | 23 (13.5) | 170 (2.4) |
| Z | 13 (8.3) | 157 (2.2) |
| N | 18 (15.1) | 119 (1.7) |
| D | 37 (32.2) | 115 (1.6) |
| W | 25 (22.3) | 112 (1.6) |
| U | 29 (30.5) | 95 (1.3) |
| H | 3 (3.5) | 85 (1.2) |
| X | 11 (19.3) | 57 (0.8) |
| S | 2 (4.4) | 45 (0.6) |
| A | 12 (36.4) | 33 (0.5) |
| KK | 0 | 1 (0.0) |
No association between proportions of non-completion of study follow-up and number of participants enrolled by site: Pearson’s correlation coefficient = −0.15, P = 0.44, R2 = 0.02.
Table A.7.
Proportion of non-completion of study follow-up by site and by regimen
| 3HP-DOT* | 9H-SAT* | |||
|---|---|---|---|---|
| Non-completion of follow-up | Enrollments (n = 3644) | Non-completion of follow-up | Enrollments (n = 3418) | |
| Enrolling site | n (% of enrollments) | n (%) | n (% of enrollments) | n (%) |
| G | 73 (12.5) | 584 (16.0) | 72 (12.7) | 569 (16.6) |
| R | 36 (13.6) | 265 (7.3) | 33 (13.2) | 250 (7.3) |
| B | 34 (20.6) | 165 (4.5) | 35 (21.1) | 166 (4.9) |
| Y | 26 (10.4) | 250 (6.9) | 24 (9.5) | 252 (7.4) |
| P | 19 (7.8) | 245 (6.7) | 25 (10.0) | 250 (7.3) |
| D | 17 (33.3) | 51 (1.4) | 20 (31.3) | 64 (1.9) |
| O | 16 (18.2) | 88 (2.4) | 19 (17.4) | 109 (3.2) |
| M | 14 (10.1) | 138 (3.8) | 12 (14.6) | 82 (2.4) |
| U | 14 (31.1) | 45 (1.2) | 15 (30.0) | 50 (1.5) |
| E | 13 (7.4) | 176 (4.8) | 5 (4.5) | 112 (3.3) |
| AA | 13(7.5) | 174 (4.8) | 24 (13.0) | 185 (5.4) |
| J | 12 (13.2) | 91 (2.5) | 5(6.1) | 82 (2.4) |
| F | 11 (6.6) | 166 (4.6) | 14 (10.9) | 128 (3.7) |
| N | 11 (16.7) | 66 (1.8) | 7(13.2) | 53(1.6) |
| Q | 11 (7.6) | 144 (4.0) | 15 (14.4) | 104 (3.0) |
| W | 11 (22.4) | 49 (1.3) | 14 (22.2) | 63 (1.8) |
| K | 10 (6.0) | 168 (4.6) | 38 (25.2) | 151 (4.4) |
| V | 9 (9.4) | 96 (2.6) | 14 (18.9) | 74 (2.2) |
| C | 8 (8.1) | 99 (2.7) | 9 (8.3) | 109 (3.2) |
| L | 8(5.6) | 142 (3.9) | 10 (7.5) | 134 (3.9) |
| I | 7 (4.5) | 155 (4.3) | 7 (4.6) | 151 (4.4) |
| Z | 7 (9.6) | 73 (2.0) | 6(7.1) | 84 (2.5) |
| A | 6 (30.0) | 20 (0.5) | 6 (46.2) | 13 (0.4) |
| X | 3 (10.7) | 28 (0.8) | 8 (27.6) | 29 (0.8) |
| T | 2 (2.2) | 93 (2.6) | 8 (8.4) | 95 (2.8) |
| H | 1 (2.1) | 48 (1.3) | 2 (5.4) | 37 (1.1) |
| S | 1 (4.3) | 23 (0.6) | 1 (4.5) | 22 (0.6) |
| KK | 0 | 1 (0.0) | 0 | 0 |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment.
Table A.8.
Completion of follow-up status by completion of treatment among first participants enrolled in a cluster in the North American region (n = 5228)*
| Did not complete treatment (n = 1247) | Completed treatment (n = 3981) | |||
|---|---|---|---|---|
| Did not complete follow-up (n = 330) | Completed follow-up (n = 917) | Did not complete follow-up (n = 320) | Completed follow-up (n = 3661) | |
| Regimen† | n (%) | n (%) | n (%) | n (%) |
| 3HP-DOT (n = 2590) | 117 (35.5) | 366 (39.9) | 178 (55.6) | 1929 (52.7) |
| 9H-SAT (n = 2638) | 213 (64.6) | 551 (60.1) | 142 (44.4) | 1732 (47.3) |
| Total (n = 5228) | 330 (26.5)‡ | 917 (73.5)‡ | 320 (8.0)§ | 3661 (92.0)§ |
Participants had the opportunity to continue study follow-up after completion or discontinuation of treatment. The proportion of non-completion of follow-up between those who did not complete treatment (26.5%) vs. those who completed treatment (8.0%) was statistically significant (P < 0.001).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
The denominator is all participants who did not complete treatment (n = 1247).
The denominator is all participants who completed treatment (n = 3981).
H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment.
Table A.9.
Univariate and multivariate analyses of factors associated with non-completion of study follow-up (n = 650) compared to those who completed study follow-up (n = 4578) among the first participants enrolled in a cluster in the North American region: Cox proportional hazard model (n = 5228)
| Non-completion of follow-up % | Univariate HR (95%CI) | Multivariate aHR (95%CI) | |
|---|---|---|---|
| Regimen* | |||
| 3HP-DOT (n = 2590) | 11.4 | Reference | Reference |
| 9H-SAT (n = 2638) | 13.5 | 1.2 (1.02–1.39) | |
| Age, years (median = 37) | |||
| <37 (n = 2447) | 14.8 | 1.5 (1.24–1.69) | 1.6 (1.33–1.85) |
| ⩾37 (n = 2781) | 10.4 | Reference | Reference |
| Sex | |||
| Female (n = 2407) | 11.1 | Reference | |
| Male (n = 2821) | 13.6 | 1.3 (1.08–1.48) | |
| Race | |||
| White (n = 2826) | 13.7 | Reference | Reference |
| Black (n = 1487) | 12.7 | 0.9 (0.79–1.12) | 1.0 (0.80–1.21) |
| Asian (n = 722) | 7.5 | 0.5 (0.40–0.71) | 0.5 (0.37–0.70) |
| Other (n = 193)† | 10.4 | 0.8 (0.48–1.18) | 0.8 (0.48–1.27) |
| Ethnicity | |||
| Non-Hispanic (n = 3247) | 10.9 | Reference | |
| Hispanic (n = 1981) | 15.0 | 1.4 (1.20–1.63) | |
| HIV status | |||
| Negative (n = 2729) | 12.8 | Reference | |
| Positive (n = 146) | 12.3 | 1.0 (0.61–1.58) | |
| Unknown (n = 2353) | 12.0 | 0.9 (0.81–1.11) | |
| Country of origin | |||
| Non-US (n = 2966) | 13.3 | Reference | Reference |
| US (n = 2262) | 11.3 | 0.8 (0.72–0.99) | 0.6 (0.50–0.77) |
| Educational level | |||
| ⩽8th grade (n = 799) | 16.4 | 1.9 (1.46–2.46) | 1.5 (1.11–1.96) |
| 9th grade, some college (n = 3320) | 12.6 | 1.4 (1.15–1.78) | 1.1 (0.91–1.44) |
| College or higher (n = 1109) | 9.0 | Reference | Reference |
| Incarceration‡ | |||
| No (n = 4890) | 12.0 | Reference | Reference |
| Yes (n = 338) | 18.9 | 1.7 (1.29–2.16) | 1.5 (1.09–2.0) |
| Unemployed | |||
| No (n = 4568) | 12.2 | Reference | |
| Yes (n = 660) | 13.8 | 1.1 (0.92–1.43) | |
| Homeless§ | |||
| No (n = 4813) | 11.9 | Reference | Reference |
| Yes (n = 415) | 19.0 | 1.7 (1.33–2.14) | 1.8 (1.37–2.48) |
| Alcohol consumption¶ | |||
| No (n = 2302) | 11.8 | Reference | |
| Use (n = 2539) | 12.5 | 1.1 (0.91–1.26) | |
| Abuse (n = 387) | 16.3 | 1.5 (1.11–1.93) | |
| Injection drug use | |||
| No (n = 4984) | 12.3 | Reference | |
| Yes (n = 244) | 14.8 | 1.2 (0.87–1.71) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 5008) | 12.5 | Reference | |
| Yes (n = 220) | 11.8 | 1.0 (0.65–1.42) | |
| Current smoker | |||
| No (n = 3611) | 11.1 | Reference | Reference |
| Yes (n = 1617) | 15.3 | 1.4 (1.23–1.68) | 1.5 (1.27–1.80) |
| Methadone treatment | |||
| No (n = 5095) | 12.4 | Reference | |
| Yes (n = 133) | 12.0 | 1.0 (0.59–1.59) | |
| Potential for pregnancy | |||
| Not possible (n = 3225) | 12.9 | Reference | |
| Female (n = 1923) | 11.8 | 0.9 (0.76–1.05) | |
| Pregnancy (n = 80) | 7.5 | 0.6 (0.25–1.24) | |
| Enrolling site# | |||
| Missing clinic visit** | |||
| No (n = 4880) | 12.0 | Reference | Reference |
| Yes (n = 349) | 19.5 | 1.7 (1.32–2.19) | 1.5 (1.14–1.91) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 on the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was anaylsed after a random selection of the refereence value; overall P < 0.001.
Missing at least one of the first three DOT visits for the 3HP-DOT regimen and at least one of the three monthly clinic visits for the 9H-SATregimen, followed by a DOT or monthly visit after the missing DOT/visit, respectively (includes those who did not receive any study dose). No statistically significant interactions resulted between missing an early clinic visit and factors with P ⩽ 0.2 in univariate analysis.
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment; HIV = human immunodeficiency virus.
Table A.10.
Partial completion (80%) of study follow-up by completion of treatment in the North American region (n = 6318)*
| Did not complete treatment (n = 1481) | Completed treatment (n = 4837) | |||
|---|---|---|---|---|
| Did not complete follow-up | Completed follow-up | Did not complete follow-up | Completed follow-up | |
| Regimen† | (n = 321) | (n = 1160) | (n = 185) | (n = 4652) |
| 3HP-DOT (n = 3254) | 122 (38.0) | 469 (40.4) | 122 (65.9) | 2541 (54.6) |
| 9H-SAT (n = 3064) | 199 (62.0) | 691 (59.6) | 63 (34.1) | 2111 (45.4) |
| Total (n = 6318) | 321 (21.7)‡ | 1160 (78.3)‡ | 185 (3.8)§ | 4652 (96.2)§ |
Participants had the opportunity to continue with the study follow-up after completion or discontinuation of treatment. The proportion of non-completion of 80% of follow-up between those who did not complete treatment (21.7%) vs. those who completed (3.8%) was statistically significant (P < 0.001).
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
The denominator is all participants who did not complete treatment (n = 1481).
The denominator is all participants who completed treatment (n = 4837).
H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment.
Table A.11.
Univariate and multivariate analyses of factors associated with non-completion of study follow-up (n = 506) compared to those who completed 80% of study follow-up (n = 5812) in the North American region: Cox proportional hazard model (n = 6318)
| Non-completion of follow-up % | Univariate HR (95%CI) | Multivariate aHR (95%CI) | |
|---|---|---|---|
| Regimen* | |||
| 3HP-DOT (n = 3254) | 7.5 | Reference | |
| 9H-SAT (n = 3064) | 8.6 | 1.1 (0.96–1.37) | |
| Age, years (median = 37) | |||
| <37 (n = 3048) | 9.4 | 1.4(1.18–1.68) | 1.5 (1.28–1.86) |
| ⩾37 (n = 3270) | 6.7 | Reference | Reference |
| Sex | |||
| Female (n = 2838) | 6.1 | Reference | |
| Male (n = 3480) | 9.6 | 1.6 (1.33–1.92) | |
| Race | |||
| White (n = 3530) | 8.4 | Reference | Reference |
| Black (n = 1642) | 8.7 | 1.1 (0.86–1.28) | 1.0 (0.79–1.26) |
| Asian (n = 888) | 5.5 | 0.7 (0.48–0.88) | 0.6 (0.42–0.82) |
| Other (n = 258)† | 7.4 | 0.9 (0.55–1.39) | 0.8 (0.51–1.39) |
| Ethnicity | |||
| Non-Hispanic (n = 3668) | 7.4 | Reference | |
| Hispanic (n = 2650) | 8.9 | 1.2 (1.01–1.43) | |
| HIV status | |||
| Negative (n = 3142) | 7.9 | Reference | |
| Positive (n = 148) | 7.4 | 1.0 (0.52–1.74) | |
| Unknown (n = 3028) | 8.2 | 1.0 (0.87–1.24) | |
| Country of origin | |||
| Non-US (n = 3799) | 8.7 | Reference | Reference |
| US (n = 2519) | 7.0 | 0.8 (0.67–0.96) | 0.6 (0.44–0.71) |
| Educational level | |||
| ⩽8th grade (n = 1145) | 9.6 | 1.6 (1.19–2.17) | |
| 9th grade, some college (n = 3997) | 8.1 | 1.4 (1.05–1.75) | |
| College or higher (n = 1176) | 6.0 | Reference | |
| Incarceration‡ | |||
| No (n = 5925) | 7.6 | Reference | |
| Yes (n = 393) | 13.7 | 1.9 (1.41–2.48) | 1.4 (1.04–2.02) |
| Unemployed | |||
| No (n = 5534) | 7.9 | Reference | |
| Yes (n = 784) | 8.8 | 1.1 (0.87–1.45) | |
| Homeless§ | |||
| No (n = 5815) | 7.5 | Reference | Reference |
| Yes (n = 503) | 14.3 | 2.0 (1.54–2.54) | 2.1 (1.55–2.95) |
| Alcohol consumption¶ | |||
| No (n = 2909) | 6.7 | Reference | |
| Use (n = 2925) | 8.6 | 1.3 (1.07–1.56) | |
| Abuse (n = 484) | 12.2 | 1.9 (1.40–2.51) | |
| Injection drug use | |||
| No (n = 6052) | 7.9 | Reference | |
| Yes (n = 266) | 9.8 | 1.3 (0.85–1.86) | |
| Chronic liver disease (cirrhosis) | |||
| No (n = 6068) | 8.1 | Reference | |
| Yes (n = 250) | 6.0 | 0.7 (0.45–1.25) | |
| Current smoker | |||
| No (n = 4394) | 6.8 | Reference | Reference |
| Yes (n = 1924) | 10.8 | 1.6 (1.37–1.95) | 1.6 (1.31–1.96) |
| Methadone treatment | |||
| No (n = 6182) | 8.0 | Reference | |
| Yes (n = 136) | 7.4 | 0.9 (0.49–1.73) | |
| Pregnancy | |||
| Not possible (n = 3970) | 9.0 | Reference | Reference |
| Female (n = 2243) | 6.6 | 0.7 (0.59–0.87) | 0.8 (0.65–0.97) |
| Pregnancy (n = 105) | 2.9 | 0.3 (0.10–0.97) | 0.3 (0.1–0.98 |
| Enrolling site# | |||
| Missing clinic visit** | |||
| No (n = 5906) | 7.7 | Reference | Reference |
| Yes (n = 412) | 12.6 | 1.7 (1.25–2.22) | 1.4 (1.03–1.85) |
3HP-DOT = 3 months of directly observed once-weekly RPT (maximum dose, 900 mg) plus INH (maximum dose, 900 mg); 9H-SAT = 9 months of daily self-administered INH (maximum dose, 300 mg).
Includes North American Indian and other participants in the United States and Canada.
History of living in a correctional institution for ⩾1 month prior to enrollment.
History of homelessness or living in a shelter or single room occupancy for ⩾6 months prior to enrollment.
Use: affirmative response to a question asking whether the participant ever drank alcoholic beverages. Abuse: score of ⩾2 on the CAGE (Cut down, Annoyed, Guilty, Eye-opener) questionnaire.
Enrolling site was analyzed after a random selection of the reference value; overall P < 0.001.
Missing at least one of the first three DOT visits for the 3HP-DOT regimen and at least one of the three monthly clinic visits for the 9H-SAT regimen, followed by a DOT or a monthly visit after the missing DOT/visit, respectively (includes those who did not receive any study dose).
Note: Body mass index was explored, but due to a lack of statistical significance and lack of a meaningful association with the outcome, it was not included in the table/analyses.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; H, INH = isoniazid; P, RPT = rifapentine; DOT = directly observed therapy; SAT = self-administered treatment; HIV = human immunodeficiency virus.
Table A.12.
Interaction terms evaluated in the multivariate analyses
| Regimen × age |
| Regimen × sex |
| Regimen × race |
| Regimen × ethnicity |
| Regimen × country of birth (US vs. non-US) |
| Regimen × education |
| Regimen × incarceration |
| Regimen × unemployed |
| Regimen × homelessness |
| Regimen × alcohol use/abuse |
| Regimen × injection drug use |
| Regimen × smoking |
| Regimen × reproduction |
| Regimen × missing a clinic visit |
| Sex × age |
| Sex × race |
| Sex × incarceration |
| Sex × unemployed |
| Sex × homelessness |
| Sex × alcohol use/abuse |
| Sex × injection drug use |
| Sex × missing a clinic visit |
| Race × incarceration |
| Race × unemployment |
| Race × homelessness |
| Race × missing a clinic visit |
| Ethnicity × age |
| Ethnicity × sex |
| Ethnicity × country of birth (US vs. non-US) |
| Ethnicity × education |
| Ethnicity × incarceration |
| Ethnicity × unemployed |
| Ethnicity × homeless |
| Ethnicity × injection drug use |
| Ethnicity × smoking |
| Ethnicity × potential for reproduction |
| Ethnicity × missing a clinic visit |
| Country of birth × age |
| Country of birth × sex |
| Country of birth × incarceration |
| Country of birth × unemployment |
| Country of birth × homeless |
| Unemployed × alcohol use/abuse |
| Unemployed × missing a clinic visit |
| Homeless × missing a clinic visit |
| Injection drug use × missing a clinic visit |
| Injection drug use × homelessness |
| Smoking × age |
| Missing a clinic visit × age |
Footnotes
[A version in Spanish of this article is available from the Editorial Office in Paris and from the Union website www.theunion.org]
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2017/00000021/00000003/art000 …‥
References
- 1.Conwell DS, Mosher A, Khan A, et al. Factors associated with loss to follow-up in a large tuberculosis treatment trial (TBTC Study 22). Contemp Clin Trials 2007; 28: 288–294. [DOI] [PubMed] [Google Scholar]
- 2.Gust DA, Mosimaneotsile B, Mathebula U, et al. Risk factors for non-adherence and loss to follow-up in a three-year clinical trial in Botswana. PLOS ONE 2011; 6: e18435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akl EA, Briel M, You JJ, et al. LOST to follow-up Information in Trials (LOST-IT): a protocol on the potential impact. Trials 2009; 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein SL, Feldman J. Incentives to participate in clinical trials: practical and ethical considerations. Am J Epidemiol 2015; 33: 1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Calfas KJ, Marshall SJ, et al. Clinical trial management of participant recruitment, enrollment, engagement, and retention in the SMART study using a Marketing and Information Technology (MARKIT) model. Contem Clin Trials 2015; 42: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol 1997; 50: 385–391. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 8.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 1999; 282: 677–686. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Global tuberculosis report, 2015. WHO/HTM/TB/2015.22. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 10.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365: 2155–2166. [DOI] [PubMed] [Google Scholar]
- 11.Shayesta D, Kopec JA. The CAGE Questionnaire for alcohol misuse: a review of reliability and validity studies. Clin Invest Med 2007; 30: 33–41. [DOI] [PubMed] [Google Scholar]
- 12.Brueton VC, Tierney J, Stenning S, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013; 12: MR000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks SM, Taylor Z, Burrows NR, Qayad MG, Miller B. Hospitalization of homeless persons with tuberculosis in the United States. AmericanJ Public Health 2000; 90: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyamathi A, Stein JA, Schumann A, Tyler D. Latent variable assessment of outcomes in a nurse-managed intervention to increase latent tuberculosis treatment completion in homeless adults. Health Psychol 2007; 26: 68–76. [DOI] [PubMed] [Google Scholar]
- 15.Moro RN, Borisov AS, Saukkonen J, et al. Factors associated with noncompletion of latent tuberculosis infection treatment: experience from the PREVENT TB Trial in the United States and Canada. Clin Infect Dis 2016; 62: 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulsky JP, Pilote L, Hahn JA, et al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Arch Intern Med 2000; 160: 697–702. [DOI] [PubMed] [Google Scholar]
- 17.Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. A trial to improve adherence to referral. Arch Intern Med 1996; 156: 161–165. [PubMed] [Google Scholar]
- 18.Siddiqi K, Lee ACK. An integrated approach to treat tobacco addiction in countries with high tuberculosis incidence. Trop Med Int Health 2009; 14: 420–428. [DOI] [PubMed] [Google Scholar]
- 19.Lutge EE, Wiysonge CS, Knight SE, Sinclair D, Volmink J. Incentives and enablers to improve adherence in tuberculosis. Cochrane Database Syst Rev 2015; 9: CD007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower P, Brueton V, Gamble C, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014; 15:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally competent strategies for recruitment and retention of African American populations into clinical trials. Clin Transl Sci 2015; 8: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]