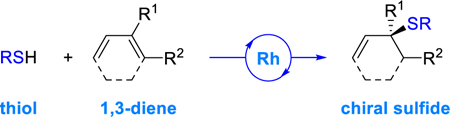
Abstract
We report a Rh-catalyzed hydrothiolation of 1,3-dienes, including petroleum feedstocks. Either secondary or tertiary allylic sulfides can be generated from the selective addition of a thiol to the more substituted double bond of a diene. The catalyst tolerates a wide range of functional groups, and the loading can be as low as 0.1 mol%. This method constitutes the first enantioselective hydrothiolation of 1,3-dienes.
Graphical Abstract
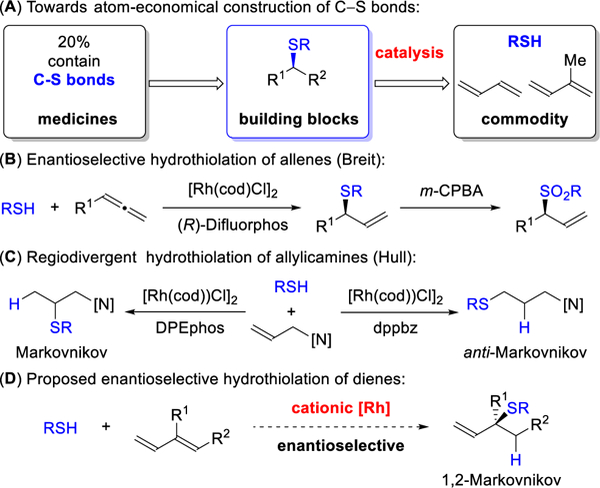
The pursuit of catalysts capable of forging carbon-sulfur linkages is a valuable goal, as molecules essential to life, from metabolites to macromolecules, contain sulfur atoms.1 In addition, approximately 20% of all FDA approved drugs are organosulfur compounds.2 The direct addition of a thiol to a double bond represents an attractive and atom-economical3 approach for generating C-S bonds.4 Inspired by this challenge, we chose to focus on the hydrothiolation of conjugated dienes, which are readily available and include commodity chemicals, like butadiene and isoprene (Figure 1).5 One previous hydrothiolation of 1,3-dienes was reported by He, where the application of Au catalysts resulted in racemic mixtures.6 By using Rh-catalysis, Breit pioneered an enantioselective hydrothiolation of allenes7 and Hull achieved a regiodivergent addition to allylic amine8. As a complement to these strategies, we herein communicate that Rh-catalysts generate allylic sulfides from 1,3-dienes, in a regio- and enantioselective fashion, thus allowing petroleum feedstocks to be transformed into enantioenriched building blocks.9
Figure 1.
Inspiration for hydrothiolation of 1,3-dienes.
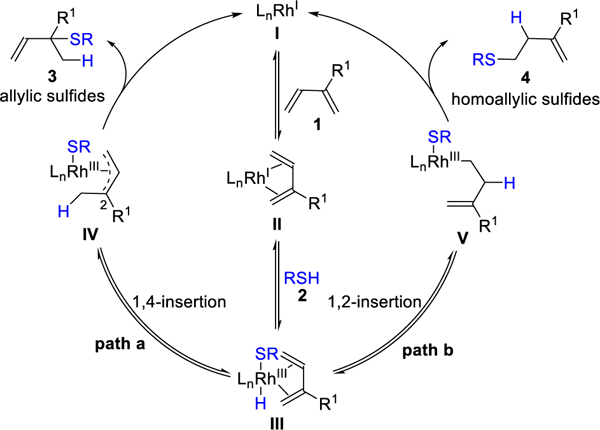
While asymmetric hydroamination of dienes has been demonstrated,10 thiols are more nucleophilic and acidic than amines, thus providing distinct challenges and opportunities for hydrofunctionalization.11, 12 In this study, we focus on Rh complexes I, which we imagined could bind and activate the diene 1 by η4-coordination (Figure 2). The resulting olefin-complex II undergoes oxidative addition to a thiol 2 to yield III. From III, Rh-hydride insertion can occur via 1,4 or 1,2-insertion, wherein rhodium adds to the less-hindered position of the diene. In path a, Rh-hydride insertion provides a Rh-π-allyl IV after 1,4-insertion. Because reductive elimination tends to favor branched products,13 we reasoned IV would yield tertiary allylic sulfides 3.14 In path b, 1,2-insertion provides V and reductive elimination gives homoallyic sulfides 4.
Figure 2.
Proposed Rh-catalyzed hydrothiolation of 1,3-dienes.
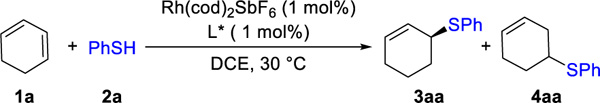
With this hypothesis in mind, we chose cyclohexadiene (1a) as the model substrate because its symmetric structure minimizes the number of isomers possible. We studied the coupling of 1a and thi-ophenol (2a) using different bidentate phosphine ligands in the presence of Rh(cod)2SbF6 (Table 1). With the JosiPhos (L1), DuPhos (L2), and BPE (L3) ligands, we observed a mixture of the allylic and homoallylic sulfides. In contrast, the BINAP family afforded excellent regioselectivity for the allylic sulfide 3aa (>20:1 rr) in high yields and enantioselectivity (>91% yields, and >85:15 er). With (S)-Tol-BINAP ligand (L5), we lowered the catalyst loading to 0.1 mol% and isolated (S)-sulfide 3aa15 on gram scale (1.2 g, 95% yield, 99:1 er).
Table 1.
Ligand Effects on Asymmetric Hydrothiolation of 1aa
 |
|---|
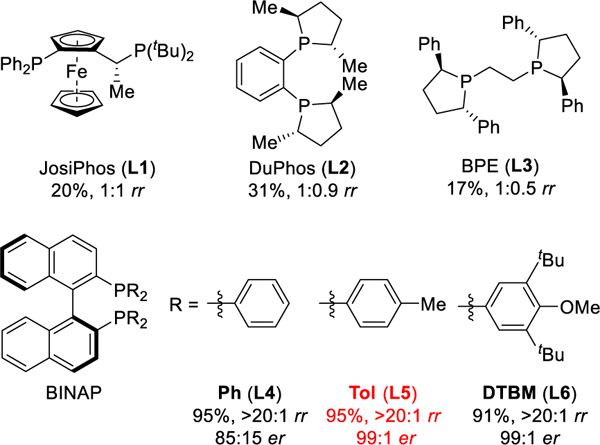 |
Reaction conditions: 1a (0.2 mmol), 2a (0.1 mmol), Rh(cod)2SbF6 (1 mol%), ligand (1 mol%), DCE (0.2 mL), 3 h. Isolated yield. Regioselectivity ratio (rr) is the ratio of 3aa to 4aa, which is determined by 1H NMR analysis of reaction mixture. Enantioselectivity determined by chiral SFC.
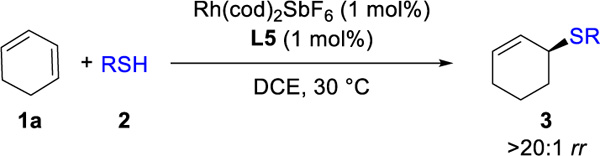
Table 2 showcases the scope of this method with eighteen different thiols and 1a, using catalyst Rh(L5). High reactivity (3ab–3as, 49–99%), enantioselectivity (96:4–>99:1 er), and regioselectivity (18:1 –>20:1 rr) are observed with both aliphatic and aromatic thiol partners. Tertiary thiols (such as tert-butylthiol and triphenylmethanethiol) are unreactive thus far, presumably due to steric hindrance. This method is compatible with heteroarene (3ao,16 3as), hydroxyl (3aq), carboxyl (3ar), amino (3ad, 3ae), and ester groups (3aj).
Table 2.
Hydrothiolation of 1a with Various Thiolsa
 |
|---|
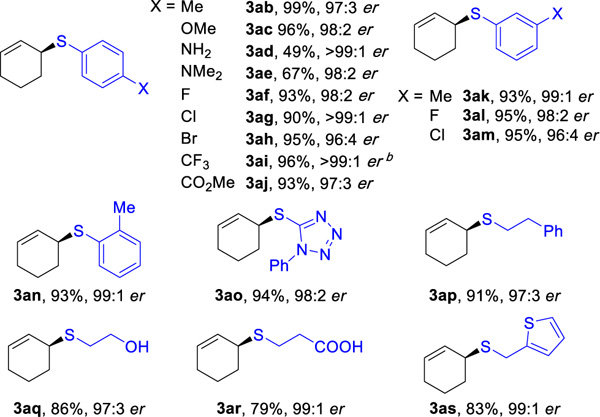 |
Reaction conditions: 1a (0.4 mmol), 2 (0.2 mmol), Rh(cod)2SbF6 (1 mol%), L5 (1 mol%), DCE (0.4 mL), 30 °C, 5 h. Isolated yields. Regioselectivity ratio (rr) is the ratio of 3 to 4, which is determined by 1H NMR analysis of reaction mixture. Enantioselectivity determined by chiral SFC.
18:1 rr.
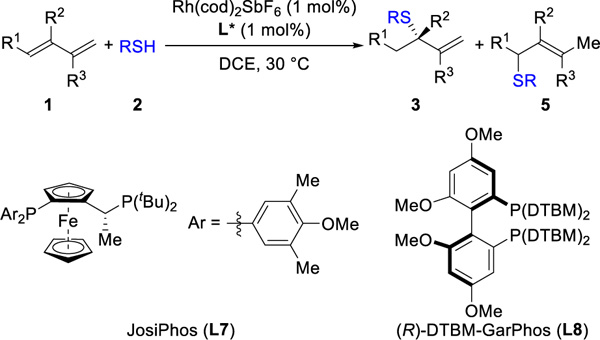
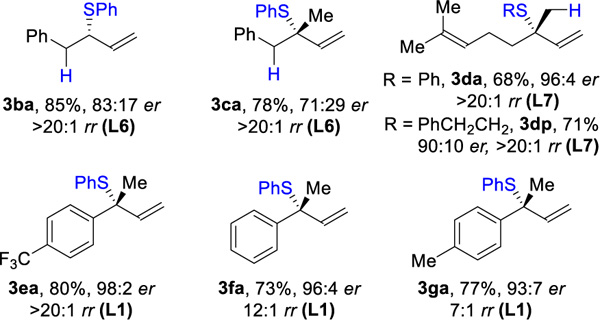
Next, we investigated hydrothiolation of unsymmetric 1,3-dienes (Table 3A). For 1-substituted (1b) and 1,2-disubstituted (1c) dienes, we found that a bulkier BINAP ligand (L6) afforded the best results (85% yield, 83:17 er and 78% yield 71:29 er, respectively). In contrast, the 2-substituted-1,3-dienes reacted poorly in the presence of BINAP ligands. In this case, the JosiPhos ligands provided a breakthrough. With L7, myrcene (3d) can be coupled with an aromatic thiol (3da, 68% yield, 96:4 er, >20:1 rr) and an aliphatic thiol (3dp, 71% yield, 90:10 er, >20:1 rr). 2-Aryl-1,3-dienes undergo hydrothiolation as well (3ea–3ga, 73–80%, 93:7–98:2 er); the presence of an electron-withdrawing group (3ea) on the phenyl ring exhibits higher regioselectivity (>20:1 rr) compared to electron-donating substituents (3ga, 7:1 rr).
Table 3.
Hydrothiolation of Various 1,3-Dienesa
 |
| (A) Hydrotholation of unsymmetric dienesb |
 |
| (B) Hydrotholation of feedstock dienes(> 20:1 rr) |
 |
Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), Rh(cod)2SbF6 (1 mol%), L (1 mol%), DCE (0.4 mL), 30 °C, 5 h. Isolated yields. Ligand used in parentheses. Regioselectivity ratio (rr) is the ratio of 3 to 5, which is determined by 1H NMR analysis of reaction mixture. Enantioselectivity de-termined by chiral SFC.
Using Rh(cod)2SbF6 (5 mol%), L (5 mol%), 15 h.
13:1 rr
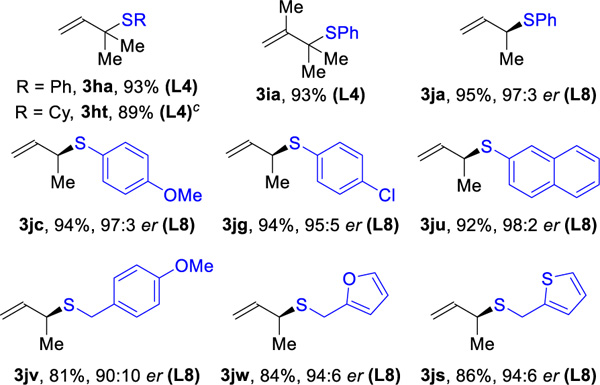
Isoprene and butadiene are petroleum feedstocks, produced on a million metric ton scale every year and used as monomers to make plastics.17 Hydrothiolation of isoprene (1h) with thiophenol and cyclohexanethiol gives the corresponding tertiary sulfides (3ha, 3ht), in >89% yield and >20:1 rr (Table 3B). A commercial diene, 2,3-dimethyl-1,3-butadiene (1i), transforms into the tertiary sulfide 3ia (93% yield, >20:1 rr). The construction of chiral products from butadiene remains a challenge that has inspired hydrohydroxyalkyation,5c cycloadditions18 and difunctionalizations19. To meet this challenge, we simply switched the ligand to DTMB-GarPhos (L8). With Rh(L8), high reactivity (81–95%) and regioselectivity (>20:1 rr) are achieved using both aliphatic and aromatic thiols. The products derived from aromatic thiols (3ja, 3jc, 3jg, 3ju) are obtained in higher enantioselectivities (95:5–98:2 er) than those from aliphatic thiols (3jv, 3jw, 3js, 90:10–94:6 er)
Aside from enantioselective examples, we examined the addition of a L-cysteine ester 2x to 1,3-cyclohexadiene (Figure 3). Either diastereomeric product, 3ax or 3ax’, can be generated with high diastereoselectivity (>20:1 dr), depending on the enantiomer of ligand L5 employed.
Figure 3.
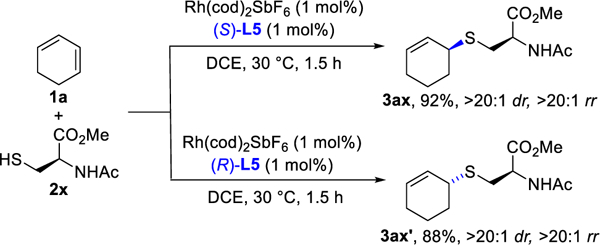
Catalyst-controlled diastereoselective hydrothiolation.
In principle, the coupling of a thiol and unsymmetrical diene (e.g., 2-phenyl-1,3-diene 1f) can result in up to eleven different isomers.20 In addition to stereoisomers, constitutional isomers may arise due to competing 1,2 versus 1,4-addition, as well as anti-Mar-kovnikov versus Markovnikov type selectivity. By using a cationic rhodium precatalyst, we obtain allylic sulfides with high chemo-, regio-, and enantio-control. The catalyst loading can be lowered to 0.1 mol% and many functional groups can be tolerated, including heteroarene, hydroxyl, carboxyl, amino, and ester groups. By choosing the appropriate phosphine ligand, we can transform a wide-range of dienes into chiral sulfides. The regiocontrol observed supports a mechanism distinct from what was previously proposed for related hydroaminations.5h,10a Further studies are warranted to elucidate the mechanism and develop access to other re-gioisomers.
Supplementary Material
ACKNOWLEDGMENT
Funding provided by UC Irvine, the National Institutes of Health (1R35GM127071) and the National Science Foundation (CHE-1465263). We thank Alexander Lu for help with initial studies and the Jarvo Lab for help with chiral SFC analysis.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data for all new compounds (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).For reviews on C–S bond formation, see: [Google Scholar]; (a) Kondo T; Mitsudo T-A Chem. Rev. 2000, 100, 3205. [DOI] [PubMed] [Google Scholar]; (b) Arisawa M; Ya-maguchi M PureAppl. Chem. 2008, 80, 993. [Google Scholar]; (c) Chauhan P; Ma-hajan S; Enders D Chem. Rev. 2014, 114, 8807. [DOI] [PubMed] [Google Scholar]; (d) Shen C; Zhang P; Sun Q; Bai S; Andy Hor TS; Liu X Chem. Soc. Rev. 2015, 44, 291. [DOI] [PubMed] [Google Scholar]; (e) Yu J-S; Huang H-M; Ding P-G; Hu X-S; Zhou F; Zhou J ACS Catal. 2016, 6, 5319. [Google Scholar]; (f) Qiao A; Jiang X Org. Biomol. Chem. 2017, 15, 1942 For review on enzymatic C–S bond formation, [DOI] [PubMed] [Google Scholar]; (g) see: Dunbar KL; Scharf DH; Litomska A; Hertweck C Chem. Rev. 2017, 117, 5521. [DOI] [PubMed] [Google Scholar]
- (2).(a) McGrath NA; Brichacek M; Njardarson JT J. Chem. Educ. 2010, 87, 1348. [Google Scholar]; (b) Scott KA; Njardarson JT Top. Curr. Chem. 2018, 376, 5. [DOI] [PubMed] [Google Scholar]
- (3).Trost BM Science 1991, 254, 1471. [DOI] [PubMed] [Google Scholar]
- (4).For enantioselective additions to Michael acceptors, see: ref. 1c.
- (5).For select hydrofunctionalizations of dienes, see: [Google Scholar]; (a) Löber O; Kawatsura M; Hartwig JF J. Am. Chem. Soc. 2001, 123, 4366. [DOI] [PubMed] [Google Scholar]; (b) Page JP; RajanBabu TV J. Am. Chem. Soc. 2012, 134, 6556. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zbieg JR; Yamaguchi E; McInturff EL; Krische MJ Science 2012, 336, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Park BY; Montgomery TP; Garza VJ; Krische MJ J. Am. Chem. Soc. 2013, 135, 16320. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Saini V; O’Dair M; Sigman MS J. Am. Chem. Soc. 2015, 137, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nguyen KD; Herkommer D; Krische MJ J. Am. Chem. Soc. 2016, 138, 14210. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Marcum JS; Roberts CC; Manan RS; Cervarich TN; Meek SJ J. Am. Chem. Soc. 2017, 139, 15580. [DOI] [PubMed] [Google Scholar]; (h) Yang X-H; Lu A; Dong VM J. Am. Chem. Soc. 2017, 139, 14049. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Gui Y-Y; Hu N; Chen X-W; Liao L-L; Ju T; Ye J-H; Zhang Z; Li J; Yu D-GJ Am. Chem. Soc. 2017, 139, 17011. [DOI] [PubMed] [Google Scholar]; (j) Adamson NJ; Wilbur KCE; Malcolmson SJ J. Am. Chem. Soc. 2018, 140, 2761. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Liu Y; Fiorito D; Mazet C Chem. Sci. 2018, 9, 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Schmidt VA; Rose Kennedy C; Bezdek MJ; Chirik PJ J. Am. Chem. Soc. 2018, 140, 3443 For reviews see:, [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Hydrofunctionalization. In Topics in Organometallic Chemistry; Ananikov VP, Tanaka M, Eds.; Springer: Berlin, 2014; Vol. 343. [Google Scholar]; (n) McNeill E; Ritter T Acc. Chem. Res. 2015, 48, 2330. [DOI] [PubMed] [Google Scholar]
- (6).This Au-catalyzed hydrothiolation provides racemic isomers resulting from 3,4-Markovnikov selectivity, see: Brouwer C; Ra-haman R; He C Synlett 2007, 11, 1785. [Google Scholar]
- (7).(a) Pritzius AB; Breit B Angew. Chem. Int. Ed. 2015, 54, 3121. [DOI] [PubMed] [Google Scholar]; (b) Pritzius AB; Breit B Angew. Chem. Int. Ed. 2015, 54, 15818. [DOI] [PubMed] [Google Scholar]
- (8).Kennemur JL; Kortman GD; Hull KL J. Am. Chem. Soc. 2016, 138, 11914. [DOI] [PubMed] [Google Scholar]
- (9).For the use ofthiols as ligands, see: [Google Scholar]; (a) Mellah M; Voituriez A; Schulz E Chem. Rev. 2007, 107, 5133 For use in enantiore-tentive rearrangements see:, [DOI] [PubMed] [Google Scholar]; (b) Schaumann E Top. Curr. Chem. 2007, 274, 1 For use in cross-coupling see:, [Google Scholar]; (c) Modha SG; Mehta VP; Van der Eycken EK Chem. Soc. Rev. 2013, 42, 5042. [DOI] [PubMed] [Google Scholar]
- (10).(a) Yang X-H; Dong VM J. Am. Chem. Soc. 2017, 139, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Adamson NJ; Hull E; Malcolmson SJ Am. Chem. Soc. 2017, 139, 7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For select transition-metal catalyzed hydrothiolation of alkynes, see: [Google Scholar]; (a) Kuniyasu H; Ogawa A; Sato K-I; Ryu I; Kambe N; Sonoda N J. Am. Chem. Soc. 1992, 114, 5902. [Google Scholar]; (b) Ogawa A; Ikeda T; Kimura K; Hirao T J. Am. Chem. Soc.1999, 121, 5108. [Google Scholar]; (c) Cao C; Fraser LR; Love JA J. Am. Chem. Soc. 2005, 127, 17614. [DOI] [PubMed] [Google Scholar]; (d) Yang J; Sabarre A; Fraser LA; Patrick BO; Love JA J. Org. Chem. 2009, 74, 182. [DOI] [PubMed] [Google Scholar]; (e) Di Giuseppe A; Castarlenas R; Pérez-Torrente JJ; Crucianelli M; Polo V; Sancho R; Lahoz FJ; Oro LA J. Am. Chem. Soc. 2012, 134, 8171. [DOI] [PubMed] [Google Scholar]
- (12).For select transition-metal catalyzed hydrothiolation of alkenes, see: [Google Scholar]; (a) Tamai T; Fujiwara K; Higashimae S; Nomoto A; Ogawa A Org. Lett. 2016, 18, 2114. [DOI] [PubMed] [Google Scholar]; (b) Cabrero-Antonino JR; Leyva-Perez A; Corma A Adv. Synth. Catal. 2012, 354, 678. [Google Scholar]; (c) Tamai T; Ogawa AJ Org. Chem. 2014, 79, 5028. [DOI] [PubMed] [Google Scholar]; (d) Yi H; Song C; Li Y; Pao C-W; Lee J-F; Lei A Chem. Eur. J. 2016, 22, 18331. [DOI] [PubMed] [Google Scholar]
- (13).(a) Chen Q-A; Chen Z; Dong VM J. Am. Chem. Soc. 2015, 137, 8392. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cruz FA; Dong VMJ Am. Chem. Soc. 2017, 139, 1029 For reviews see:, [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Koschker P; Breit B Acc. Chem. Res. 2016, 49, 1524. [DOI] [PubMed] [Google Scholar]; (d) Haydl AM; Breit B; Liang T; Krische MJ Angew. Chem. Int. Ed. 2017, 56, 11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).For review on enantioselective synthesis of tertiary allylic sulfides, see: ref 1e.
- (15).The absolute configuration of 3aa was assigned as S in accordance with: Gais H-J; Böhme A J. Org. Chem. 2002, 67, 1153. [DOI] [PubMed] [Google Scholar]
- (16).The corresponding sulfone has been used in cross-coupling reactions, see: Merchant RR; Edwards JT; Qin T; Kruszyk MM; Bi C; Che G; Bao D-H; Qiao W; Sun L; Collins MR; Fadeyi OO; Gallego GM; Mousseau JJ; Nuhant P; Baran PS Science 2018, 360, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).For reviews, see: [Google Scholar]; (a) Ezinkwo GO; Tretjakov VF; Taly-shinky RM; Ilolov AM; Mutombo TA Catal. Sustainable Energy 2013, 1, 100. [Google Scholar]; (b) Makshina EV; Dusselier M; Janssens W; Degrève J; Jacobs PA; Sels BF Chem. Soc. Rev. 2014, 43, 7917. [DOI] [PubMed] [Google Scholar]
- (18).(a) Corey EJ; Shibata T; Lee TW J. Am. Chem. Soc. 2002, 124, 3808. [DOI] [PubMed] [Google Scholar]; (b) Ryu DH; Corey EJ J. Am. Chem. Soc. 2003, 125, 6388. [DOI] [PubMed] [Google Scholar]; (c) Yeung Y-Y; Hong S; Corey EJ J. Am. Chem. Soc. 2006, 128, 6310. [DOI] [PubMed] [Google Scholar]; (d) Hayashi Y; Samanta S; Gotah H; Ishikawa H Angew. Chem. Int. Ed. 2008, 47, 6634. [DOI] [PubMed] [Google Scholar]
- (19).(a) Li X; Meng F; Torker S; Shi Y; Hoveyda AH Angew. Chem. Int. Ed. 2016, 55, 9997. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xiong Y; zhang GJ Am. Chem. Soc. 2018, 140, 2735. [DOI] [PubMed] [Google Scholar]
- (20).See Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


