Abstract
Background.
Negative affective interference with executive cognition is associated with emotion dysregulation and behavioral dyscontrol in BPD, including a diathesis to suicidal and self-injurious behavior. While clinically well described, the neural basis of affective interference with central executive network function, and resulting suicidal behavior is poorly understood.
Method.
In an fMRI study, 23 BPD suicide attempters completed an affectively modified Continuous Performance Task(X-CPT), in which targets and distractors were rendered on Negative, Positive and Neutral Ekman faces, with a Distorted image as a behavioral baseline. Responses to targets were contextualized by the affective context of the face. Lethality Rating Scale scores(LRS) were modeled as the primary regressor of interest on activation peaks, with HamD scores covaried.
Results.
In the Negative vs. Neutral contrast, LRS scores were inversely related to activation in the ACC, parietal precuneus, BG and OFC, with no positive relationships. Results were similar in the Negative vs Positive contrast. In the Neutral vs. Positive contrast, activations were much less extensive, with mixed positive and negative relationships. Contextualizing responses based on the effects of valence decreased participant’s ability to distinguish between targets and distracters; however, no differences were observed between valence contexts. fMRI-estimated effects were not confounded by differences in behavioral sensitivity across contexts.
Limitations.
In this female-only sample, possible gender differences were not addressed.
Conclusions.
With negative affective interference, increased lethality of suicidal behavior in BPD predicted diminished neural activation in areas critical to executive cognitive function. Therapies diminishing affective interference may reduce risk of suicidal behavior.
Keywords: negative affective interference, cognitive function, suicidal behavior, borderline personality disorder
Hypersensitivity to emotional stressors and emotion dysregulation are core characteristics of patients with Borderline Personality Disorder (BPD) (Gunderson and Lyons-Ruth, 2008; Linehan, 1993; Putnam and Silk, 2005; Sanislow et al. 2002). In clinical settings, negative life events, such as perceived rejection, or loss of relationship, precipitate episodes of affective instability and behavioral disinhibition, characterized by impulsive aggression, suicidal and self-injurious behavior (Brodsky,et al., 2006, Yen et al. 2004). Twenty-four hour ambulatory monitoring of BPD subjects reveals heightened affective instability compared to control subjects, with sudden, large mood swings from positive to negative moods, and frequent swings from anxiety to sadness and anger (Ebner-Priemer et al. 2007, 2008). In laboratory settings, patients with BPD experience affect more intensely and for longer periods of time when confronted with negative stressors compared to healthy control subjects (Jacob et al. 2008; Levine, et al., 1997). They are more sensitive to facial expressions compared to controls, especially angry faces, but also misconstrue neutral expressions as fearful (Schulze et al. 2013).
fMRI studies reveal that BPD subjects demonstrate increased amygdala activity in response to negative emotional stimuli, whether by negative Ekman faces (Donegan et al., 2003; Herpertz, et al., 2001; Minzenberg, et al., 2007), aversive IAPS scenes (Hazlett et al. 2012), or cue words recalling adverse life events (Beblo et al. 2006; Mitchell, et al., 2014, for review). In addition, BPD subjects show greater amygdala responses to repeated exposure to emotional pictures compared to control subjects, and a prolonged return to baseline, suggesting impaired habituation (Hazlett et al. 2012). When exposure to negative social images is repeated after a 3 day delay, BPD subjects show diminished habituation and hypersensitization to negative images, not found in healthy control subjects or comparison clinical subjects with Avoidant PD (Denny et al. 2018). In this study, hypersensitization to between-session repeated negative social stimuli in BPD subjects was expressed through hyper-activation of the neural salience network. (Diminished habituation and hypersensitization were also noted for neutral social images in BPD subjects, compared to controls, as BPD subjects responded to neutral images as though they were negative.)
The clinical relevance of emotion dysregulation lies in the disruption of executive cognitive functions such as directed attention, response inhibition, decision-making, planning and goal-directed behavior, needed to make adaptive responses to stressful events. For patients with BPD, the result is a vulnerability to impulsive, aggressive, suicidal and self-injurious behavior. While this sequence of events is well described clinically, the neural basis for diminished cognitive function due to affective interference with Central Executive Network (CEN) regulation in BPD is poorly understood. Negative emotional contexts interfere with neural processing in BPD. We have proposed that this affective interference results from an interaction of affective arousal with the underlying neurobiology of temperamental traits, such as impulsivity and aggressiveness (Soloff et al. 2017). Impulsivity and aggressiveness (or impulsive-aggression), are associated with high lethality suicide attempts and suicide completion in individuals with BPD (Chesin, Jeglic and Stanley, 2010, McGirr et al. 2007, 2009). The lethality of suicide attempts in BPD may be related, in part, to the combined effects of negative affective arousal, impulsivity and aggressiveness on neural networks involved in executive function (Soloff, White and Diwadkar, 2014). This study will specifically assess the effects of negative affective arousal on cognitive performance in BPD suicide attempters as a function of the lethality of their suicidal behavior.
Imaging studies of suicidal subjects across diagnoses report changes in structure and function of “the suicidal brain” in orbitofrontal and dorsolateral parts of the prefrontal cortex, changes related to decision-making, problem-solving and fluency (van Heeringen, Bijttebier and Godfrin, 2011; Desmyter, van Heeringen and Audenaert, 2011). Among BPD suicide attempters, structural MRI studies demonstrate a negative relationship between degree of medical lethality and grey matter volumes across multiple fronto-temporal-limbic regions associated with these executive functions (Soloff, White and Diwadker, 2014). In this fMRI study, we postulate that medical lethality of past suicidal behavior in BPD subjects will be related to the effects of negative affective interference on neural networks specifically related to cognitive task performance.
In this study, we measure the effects of affectively valenced stimuli on neural processing during a Continuous Performance Task (X-CPT) in BPD subjects with histories of suicidal behavior. Our analyses focus on assessing the relationship between medical lethality of suicidal behavior and neural processing.
In healthy subjects, neural processing of emotion regulation occurs simultaneously with emotion generation, with the prefrontal cortex (PFC) exercising tonic control over limbic arousal (Davidson et al. 2000, Gross and Thompson, 2006, Philips et al. 2008). In subjects with BPD, functional imaging studies demonstrate a decrease in activation, or cerebral blood flow, in fronto-limbic regulatory networks in response to emotionally aversive stimuli, such as recall of autobiographical accounts of abandonment experiences (Schmahl et al. 2003a), or episodes of self-injurious behavior (Kraus et al. 2010). A recent meta-analysis of 19 fMRI studies investigating the neural processing of negative stimuli in subjects with BPD reported functional hyperactivity of the left amygdala and posterior cingulate cortex, and blunted responsiveness of the bilateral dorsolateral PFC while processing negative (vs. neutral) emotional stimuli (Schulze, et al., 2016). The dorsolateral PFC is one of the neural “anchors” of the CEN, which mediates high level cognitive functions (Menon, 2011). Diminished functional connectivity between amygdala and frontal regulatory structures in BPD varies with the emotional valence of stimuli (Banks et al. 2007; Cullen et al. 2011; New et al., 2007; Soloff et al., 2017a). Limbic (“bottom-up”) hyperarousal in the face of diminished cortical (“top down”) activation may contribute to affective interference with the neural processing of cognitive tasks such as response inhibition and impulse control at times of emotional stress.
fMRI studies of response inhibition and impulse control in BPD often utilize standardized laboratory measures such as the Go No-Go, and Stop Signal tasks (Ruocco, et al., 2012). These standardized measures are pure assays for studying the cognitive responses of regions in the frontal and cingulate gyri. However, affective interference on cognitive processing and brain networks is an important framework for understanding emotional dysregulation. Studies which incorporate negative affective stimuli in their paradigms, such as “borderline-relevant” emotional words in an emotional linguistic Go No-Go test (Silbersweig et al.,2007), or aversive IAPS scenes in tests of defensive distancing (Koenigsberg et al.,2009), have demonstrated affective interference with neural processing during task performance in BPD compared to control subjects. In contrast, fMRI studies of response inhibition which use emotionally neutral paradigms generally fail to find activation differences between BPD and control subjects (van Eijk et al. 2015).
Elucidating the mechanisms of affective interference in BPD is an important framework for bridging clinical observations with neuroscience. Toward that end we have been studying the neural effects of affective interference with cognitive functioning during task performance in subjects with BPD. To do this, we have used fMRI paradigms focused on response inhibition and impulse control (e.g. Go No-Go), conflict monitoring, error detection, response inhibition, and goal maintenance (e.g. X-CPT), and episodic memory recall (e.g. old vs new recognition). Using affectively modified versions of the Go No-Go, X-CPT, and Episodic Memory tasks, we showed that negatively valenced stimuli, compared to neutral or positive stimuli, interfered with neural processing during these tasks (Soloff, et al., 2015). Core personality characteristics of BPD, such as trait impulsivity and aggressiveness, were shown to predict specific regional brain responses during response inhibition in the Affective Go No-Go task, suggesting an interaction between affective arousal and neural networks involved in these core temperaments (Soloff, et al., 2017b). Trait impulsivity and aggressiveness are clinically important because of their close association with suicidal behavior across diagnoses, including BPD and mood disorders (Chesin, et al., 2010; Mann et al., 1999; McGirr et al., 2009, 2007; Oquendo et al., 2004,). Negative emotional context had the most robust effects on the activation matrix, with modest effects on task performance in both BPD and control subjects.
To assess the clinical relevance of affective interference with neural processing in BPD, we examined the relationship between medical lethality of suicidal behavior and neural responses during performance of an affectively modified X-CPT task. The X-CPT task presents competing choices and engages executive functions of conflict monitoring, error detection, task maintenance and response inhibition (Botvinick, et al., 2001; Carter, et al.,1998). When negative affective stimuli (angry or sad Ekman faces) vs. positive or neutral stimuli are employed, subjects with BPD demonstrated increased activation of superior parietal cortex, anterior cingulate cortex (ACC), mid-orbital frontal cortex (mid-OFC) and hippocampus (HIP) with no areas of decreased activation relative to controls (Soloff et al., 2015). Based on our findings with trait impulsivity and aggressiveness, and the clinical relationship between these temperaments, and medical lethality in BPD, we hypothesized a robust relationship between lethality of suicidal behavior, as assessed by degree of medical damage, and affective interference with neural processing of the X-CPT under negative affective conditions compared to neutral and positive conditions. i.e. Affective interference with neural processing of CEN function mediates suicidal behavior in BPD.
2. Methods
2.1. Participants, Inclusion criteria
The study was approved by the University of Pittsburgh IRB. All subjects gave written informed consent. Female subjects were recruited from an ongoing longitudinal study of BPD, from psychiatric outpatient clinics, and by advertisement from the surrounding community. Subjects were screened for BPD with the International Personality Disorders Examination (IPDE), using a lifetime timeframe (Loranger, 1999). Those meeting criteria for probable or definite lifetime BPD on the IPDE were then assessed for current BPD on the Diagnostic Interview for Borderline Patients-Revised (DIB-R), which has a two-year time frame (Zanarini, et al.,1989). Inclusion required a score of 8 or greater for definite BPD. Inclusion diagnosis was re-confirmed for subjects drawn from the longitudinal study before the fMRI study. Co-morbidity on Axis I was determined by the Structured Clinical Interview for DSM-IV (SCID) (First, et al., 2005). Depressed mood was assessed using the Hamilton Rating Scale for Depression-24 item version (HamD) (Guy, 1976).
Severity of suicidal behavior was assessed using the Lethality Rating Scale score (LRS) for the most serious lifetime attempt (Oquendo, et al., 2003). The LRS assigns lethality scores in an ordinal continuum of severity of medical damage, from 0 (no medical damage) to 8 (death) for 8 discrete forms of suicidal behavior. Scores are anchored by descriptions of medical consequences in increasing degrees of severity. The LRS has been used as a continuous measure of medical lethality in prior imaging studies (Soloff et al., 2014). Immediately preceding the scan, all subjects had negative urine toxicology for drugs of abuse (MedTox), and negative pregnancy tests. Subjects on maintenance psychoactive medication were permitted to remain on their medication.
2.2. Exclusion criteria
Exclusion criteria included: 1.) A lifetime (past or current) Axis I diagnosis of schizophrenia, delusional (paranoid) disorder, schizoaffective disorder, any bipolar disorder (I, II, mixed, manic or depressed), or psychotic depression. 2). A current DSM IV diagnosis of Substance Dependence or any current drug and/or alcohol related CNS deficits. (A DSM-IV diagnosis of Substance Abuse was permitted so long as the subject had been totally abstinent for one week, showed no signs of withdrawal, and had a clean urine toxicology drug screen (MedTox) at the time of the scan. 3.) Clinical evidence of CNS pathology of any etiology, including acquired or developmental deficits or seizure disorder. 4.) Physical disorders or treatments with known psychiatric consequence (e.g. hypothyroidism, steroid medications). 5.) Borderline Mental Retardation (IQ <70 by WAIS). 6.) Standard exclusion criteria for MRI scans included the following: ferro-magnetic implants such as cardiac pacers, cochlear implants, aneurysm clips, history of metal in eyes or other ferromagnetic body artifacts; inability to fit in the scanner due to obesity, claustrophobia or inability to tolerate brief confinement in the scanner; inability to co-operate with instructions.
2.3. Imaging Specifications
Anatomical images were acquired on the 3.0T Siemens Trio system in the axial plane parallel to the AC-PC line using a 3D MPRAGE sequence (TE/TI/TR=3.29ms/900ms/2200ms, flip angle=9, isotropic 1mm3 voxel, 192 axial slices, matrix size=256×192). fMRI data were acquired in the axial plane using gradient echo EPI (TR=2000 ms, TE=30 ms, flip angle=70 deg, 30 slices, slice thickness=3.1 mm, 3 mm × 3 mm in-plane, matrix size=64×64).
2.4. fMRI paradigms.
The X-CPT is a continuous performance task that assesses contextual attention to context-relevant targets by increasing response competition between targets. X-CPT requires conflict resolution and inhibition of a prepotent response tendency, robustly activating the ACC (Botvinick, et al., 2001; Tana, et al., 2010) and other structures. To assess affective interference with neural processing during this task, the X-CPT was modified by inserting Ekman Faces into the standard Continuous Performance Test (Ekman and Friesen, 1979). Contextual responses to targets were dependent on the valence of Ekman faces on which potential targets (e.g. “X”), and distracters (e.g. “A”), were rendered. In other words, responses to targets (“X”) were contextualized by the affective context of the face (i.e. negative, positive, or neutral valenced faces). In a mixed block jittered design, affective context was signaled at the beginning of each block alerting subjects to the contextualization of potential targets (e.g., during “negative” blocks, “X” became a target only if rendered on a face with negative valence (Soloff et al. 2015)). Stimuli were presented for 1000 ms with a jittered ISI (250–750 ms, 250 ms increments). Two blocks (54 s/block) of positive, negative and neutral valence were employed. In addition, two blocks of Distorted Faces (as a behavioral baseline) and three rest blocks (30 s) were used. The length of the task was ~ 10 minutes.
2.5. Image and fMRI data Analyses
Data were processed with Statistical Parametric Mapping (SPM12) using standardized methods. Serial correlations were corrected using an auto-regression (AR(1)) filter, with an expanded high-pass filter (256 s) applied to remove low frequency fluctuations. Realignment was performed to correct for head motion artifact. Normalization parameters, achieved after normalizing each subjects’ high-resolution anatomical image to the template image, were applied to each acquired EPI image. The resultant normalized images were resliced (8 mm3 voxels) and smoothed (8 mm FWHM). Data were modeled to assess the effects of block context. Epochs (Negative, Positive, Neutral) were modeled as separate regressors by convolving with the canonical hemodynamic response function. First level contrast structures were constructed to represent relative differences in activation with an emphasis on identifying activation to Negative relative to non-Negative and Neutral relative to Positive context. For each first-level model, the six motion parameters were modeled as regressors of no interest to model statistical artifacts associated with motion.
The three first-level contrasts of interest (Negative > Positive; Negative > Neutral and Neutral > Positive) were forwarded to separate second-level regression analyses. In each second level model, LRS score was modeled as the primary regressor of interest, with HamD scores modeled as a covariate. (Depressed mood may have a dampening effect on activation patterns (Soloff et al. 2017b). For this reason, HamD was a covariate in all analyses of the current study.)
Cluster level correction was performed using established methods (Ward, 2000). 104 Monte Carlo simulations based on the observed smoothness of the data were conducted to derive the minimum cluster extent to be deemed significant for a contiguous set of supra-threshold voxels. Cluster level correction (p<0.05) was applied to identify contiguous voxels in a priori anatomically defined regions of interest, signifying differences across groups (Maldjian, et al., 2003; Tzourio-Mazoyer et al., 2002). Cluster level corrections were applied in an anatomical mask of interest. The extensive mask included regions associated with emotion regulation, attention and memory, or areas reported to have structural deficits in BPD subjects in prior studies (Soloff,et al., 2008; Soloff, et al., 2012). These regions included the amygdala (AMY), hippocampus (HIP) and parahippocampus, the parietal lobe, the orbitofrontal cortex (OFC), the dorsal prefrontal cortex (dPFC), the anterior cingulate cortex (ACC) and the basal ganglia (BG).
2.6. Behavioral Data analysis
The behavioral data were analyzed for task-sensitivity (d’), defined by a cumulative metric based on correct performance (responding to targets; not responding to non-targets) and incorrect performance (not responding to targets; responding to non-targets)(MacMillan & Creelman, 2005). In repeated measures analyses, the effects of Condition on behavioral sensitivity were analyzed with Condition (Distorted, Negative, Neutral and Positive) as the single within-subjects factor, and age and LRS as covariates.
2.7. Effects of medication status.
Half of the sample (52.2%) was on established medication regimens, which remained unchanged throughout the assessment period, including the fMRI scan. Effects of medication status (yes/no) on response amplitude data from significance peaks, and on LRS data, were assessed by independent sample t tests.
3. Results
The sample included 23 female subjects, with ages ranging from 21 – 44 years (mean (s.d.) = 28.3 (7.3 yrs.). All subjects met current criteria for BPD at the time of the scan, and had histories of suicide attempts, with a mean of 2.8 (1.6) attempts per subject. The mean (s.d.) Lethality Rating Scale (LRS) score was 2.8 (1.8) for the most serious lifetime attempt. High lethality attempts (defined by an LRS score of 4 or greater) were reported by 30.4 % of attempters, while 69.6% had low lethality attempts (LRS score of 3 or less). Current MDD was diagnosed in 60.9% of subjects at the time of the scan, with mean (s.d.) HamD score of 18.3 (12.1). Very few subjects (n = 3) met criteria for a recent substance use disorder. A history of childhood abuse was reported by 73.9%, with sexual abuse in 56.5%. Half (52.2%) of subjects were taking psychoactive medications at the time of the scan, which included antidepressants (n=9), anxiolytics (n = 6), mood stabilizers (n = 6), neuroleptics (n = 3), and psychostimulants (n =1).
3.1. fMRI
We report our results in order, first reporting the regression results (negative or positive relationships) for contrasts for the Negative (relative to the Positive or Neutral) conditions, followed by the contrast for the Neutral (relative to the Positive) condition.
Negative context.
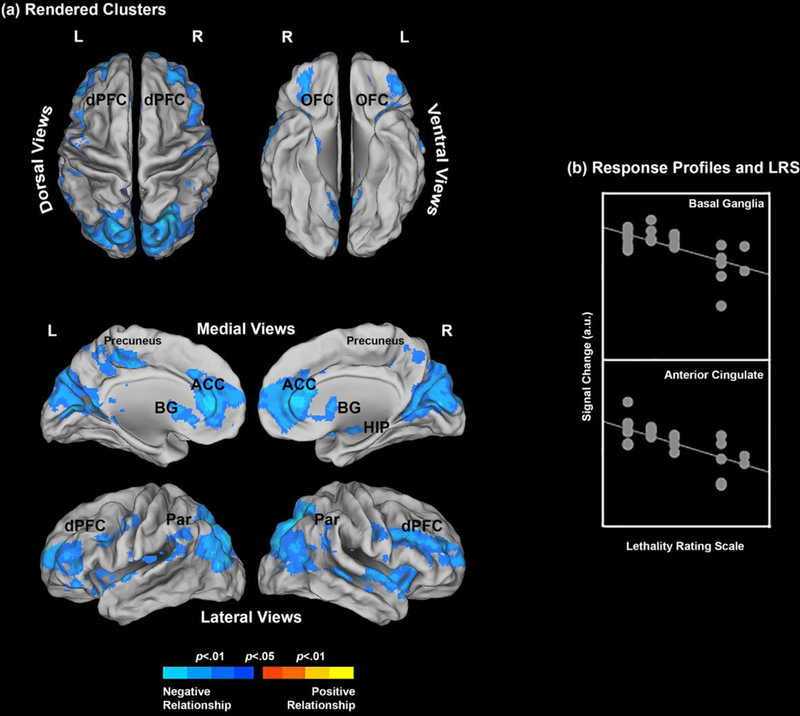
In the Negative vs. Neutral contrast (Neg vs. Neu), we observed a robust negative relationship between LRS scores and activation profiles. This effect was observed across multiple ROIs, including the ACC, parietal precuneus, BG and OFC (in order of cluster extent), and, to a lesser extent, the parietal cortex, dPFC, and HIP (Table 1). There were no areas with significant positive relationships (see Figure 1).
Table 1.
Relationship between LRS scores and activation profiles for each contrast, with HamD covaried.
| Affective_CPT MNI Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t | Cluster extent | p (peak) |
| Neg vs Neu | ||||||
| Basal Ganglia | 22 | 14 | 19 | 3.93 | 2034 | <0.001 |
| Anterior Cingulate | −10 | 33 | 13 | 4.75 | 5022 | <0.001 |
| Hippocampus | 24 | −40 | 6 | 3.21 | 303 | 0.002 |
| Parahippocampus Orbitofrotal cortex | −39 | 15 | −15 | 3.95 | 1243 | <0.001 |
| Parietal cortex | 21 | −73 | 49 | 5.01 | 870 | <0.001 |
| Dorsal prefrontal cortex | 12 | 50 | 25 | 4.75 | 739 | <0.001 |
| Precuneus | 20 | −72 | 46 | 4.51 | 3811 | <0.001 |
| Neg vs Pos | ||||||
| Basal Ganglia | 12 | 15 | 4 | 4.57 | 2512 | <0.001 |
| Anterior Cingulate | −14 | 39 | 7 | 3.95 | 3069 | <0.001 |
| Hippocampus | 22 | −39 | 6 | 2.22 | 21 | 0.019 |
| Parahippoc ampus | - | - | - | - | - | - |
| Orbitofrotal cortex | 22 | 54 | −6 | 4.43 | 567 | <0.001 |
| Parietal cortex | 46 | −43 | 37 | 3.91 | 1784 | <0.001 |
| Dorsal prefrontal cortex | 52 | 36 | 18 | 5.06 | 630 | <0.001 |
| Precuneus | 8 | −64 | 37 | 4.57 | 3292 | <0.001 |
| Neu vs Pos | ||||||
| Basal Ganglia | −4 | 10 | 12 | 3.23 | 216 | 0.002 |
| Anterior Cingulate | - | - | - | - | - | - |
| Hippocampus | - | - | - | - | - | - |
| Parahippoc ampus | - | - | - | - | - | - |
| Orbitofrotal cortex | 24 | 48 | −14 | 2.83 | 56 | 0.005 |
| Parietal cortex | 44 | −58 | 49 | 3.56 | 747 | 0.001 |
| Dorsal prefrontal cortex | 51 | 36 | 18 | 2.68 | 278 | 0.007 |
| Precuneus | 10 | −64 | 34 | 3.27 | 310 | 0.002 |
Figure 1.
(a) The clusters resulting from the underlying regression model show where the Lethality Rating Scale significantly predicts brain activation profiles. Results are depicted for the Negative > Neutral contrast (see Methods and Results) and the clusters are projected to dorsal, ventral, medial and lateral cortical surfaces. Only negative relationships were observed; That is, an increase in suicidality predicted a decrease in activation profiles under the Negative > Neutral contrast. The form of this relationship is explicated in (b) for the basal ganglia and the anterior cingulate (see Table 1 for peaks) where signal change is plotted against the LRS.
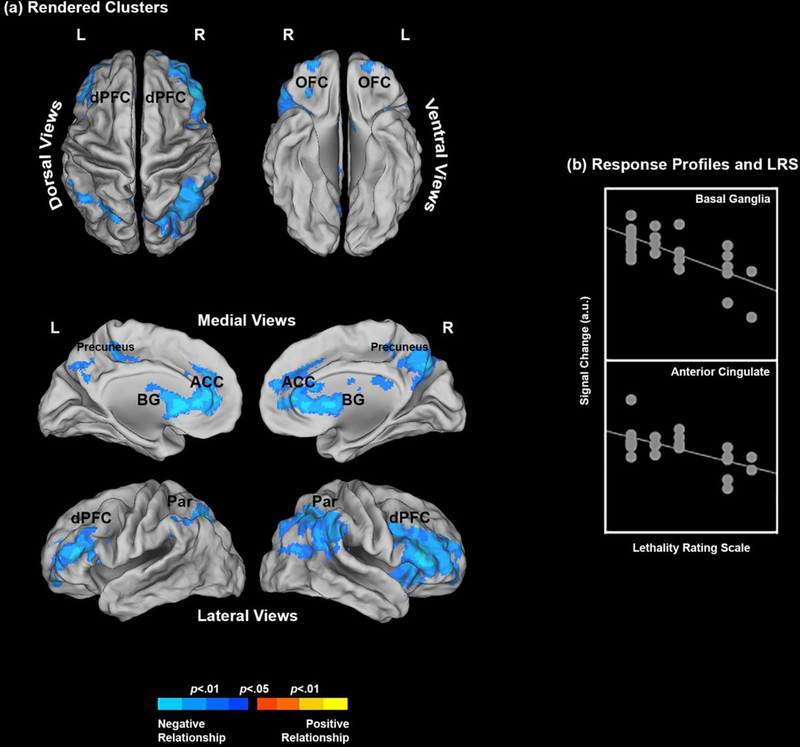
The results were highly comparable in the Negative vs Positive contrast (Neg vs. Pos). Degree of medical lethality (LRS) was negatively related to activation in the parietal precuneus, ACC, BG, and parietal cortex (in order of cluster extent), with lesser areas of activation noted in dPFC, OFC and HIP. As in the Neg. vs. Neu. contrast, there were no areas with significant positive relationships (Figure 2).
Figure 2.
(a) The clusters resulting from the underlying regression model show where the Lethality Rating Scale significantly predicts brain activation profiles. Results are depicted for the Negative > Positive contrast (see Methods and Results) and the clusters are projected to dorsal, ventral, medial and lateral cortical surfaces. Only negative relationships were observed; That is, an increase in suicidality predicted a decrease in activation profiles under the Negative > Positive contrast. The form of this relationship is explicated in (b) for the basal ganglia and the anterior cingulate (see Table 1 for peaks) where signal change is plotted against the LRS.
Neutral context.
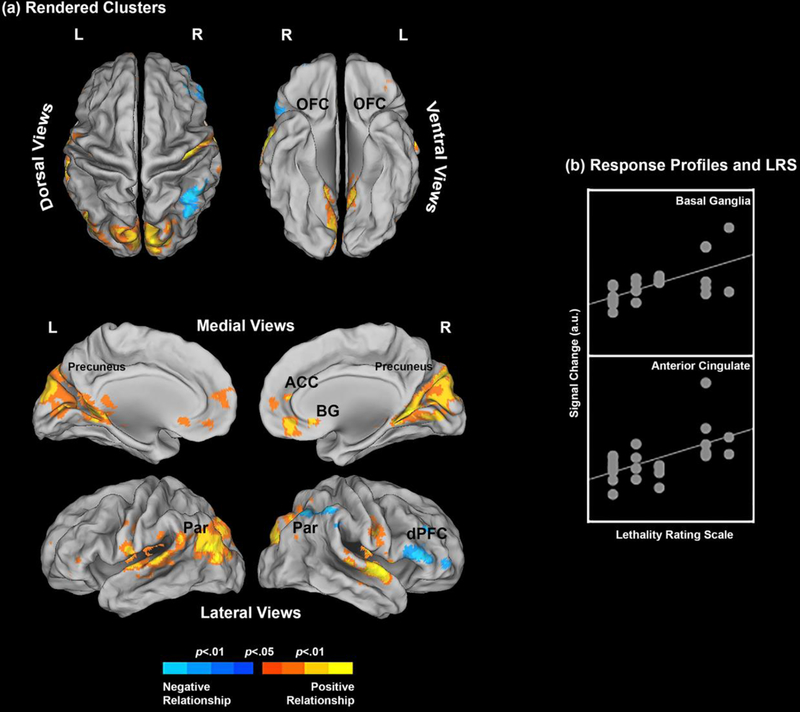
Result for the Neutral (relative to Positive) contrast were equivocal, with areas of both negative and positive relationships with LRS noted. However, compared to the negative affective conditions described above, correlations with LRS scores in the positive affective condition, were far less extensive (Table 1). A negative relationship was noted between LRS scores and activation in the parietal cortex and precuneus, and a positive relationship in the ACC and precuneus (Figure 3).
Figure 3.
(a) The clusters resulting from the underlying regression model show where the Lethality Rating Scale significantly predicts brain activation profiles. Results are depicted for the Neutral > Positive contrast (see Methods and Results) and the clusters are projected to dorsal, ventral, medial and lateral cortical surfaces. Here, a mixture of complementary negative and positive relationships were observed. That is, an increase in suicidality predicted a decrease in activation profiles under the Neutral > Positive contrast in some regions (dPFC, parietal), but predicted an increase in other regions (e.g., basal ganglia and anterior cingulate). The form of the positive relationship is explicated in (b) for the basal ganglia and the anterior cingulate (see Table 1 for peaks) where signal change is plotted against the LRS.
3.2. Behavioral analyses.
Equipment error resulted in the logging of responses for only sixteen of the 23 participants in the sample. In repeated measures analyses, the effects of condition on behavioral sensitivity were analyzed with Condition (Distorted, Negative, Neutral and Positive) as the single within-subjects factor and age and LRS as covariates. These analyses revealed a significant effect of Condition, F3,39=6.53, p<.001, MSe=.234. The moderate effect (Partial η2=.33) was driven by a decrease in task-sensitivity between the Distorted and each of the other contexts (p’s <.001, pair-wise Least Significant Difference tests). This decrease indicates that contextualizing responses based on the effects of valence decreased participant’s ability to distinguish between targets and distracters. However, no differences were observed between valence contexts (Negative, Neutral, Positive; p’s>.10), allowing us to infer that fMRI-estimated effects were not confounded with differences in behavioral sensitivity across contexts. We also conducted individual regression analyses on behavioral sensitivity for each of the affective contexts, using parametric (Pearson’s) and non-parametric (Spearman’s ρ) statistics with the Lethality Rating Scare score as the predictor variable. None of the six analyses were significant (p’s>.4), evidence that any fMRI estimated effects from the regression analyses were not confounded with effects of the LRS on behavioral sensitivity. As behavioral performance was optimized, we fully expected imaging results to be uncoupled from behavioral performance.
3.3. Medication effects.
The sample (n=23) was divided by current use of psychotropic medication to examine medication effects on fMRI profiles. There were 12 current medication users compared to 11 med-free subjects. Response amplitude data (shown in Figs 1–3) from significant peaks (Table 1) and LRS data were submitted to independent sample t tests (28 independent tests). No significant effects were observed (all ts < 1.2, all ps > .25). As in our previous studies, medication usage was not a significant factor affecting our fMRI results.
4. Discussion
Negative affective interference with executive cognition mediates emotion dysregulation and behavioral dyscontrol in BPD, creating the diathesis to suicidal and self-injurious behavior. In the current study, we related changes in neural processing of an affectively valenced X-CPT task to a clinical measure of medical lethality among suicide attempters with BPD. As expected, the most robust effects occurred under negative affective conditions, where increased degrees of medical lethality were associated with diminished activation in ACC and the parietal precuneus. These effects suggest a greater loss of “top down” inhibitory control among BPD suicide attempters as the severity of medical lethality increases. These speculations are supported by the fact that in the negative affective context, there was an absence of any positive correlations between degree of medical lethality and activation metrics.
The relationship between degree of medical lethality and activation metrics in the neutral affective context was more nuanced, with areas of both negative and positive correlation. However, areas of correlation, both negative and positive, were much less extensive than under negative affective conditions. Positive correlations between medical lethality and activation metrics, most notably in the ACC, may reflect compensatory efforts at enhanced “top down” control in response to negative affective interference associated with suicidal behavior. We previously reported a positive correlation between trait impulsivity and activation in both dACC and OFC in an affective Go No-Go task under negative affective conditions, suggesting enhanced engagement of “top down” inhibitory controls to compensate for the subjects’ temperamental impulsivity. The opposite effect was noted in healthy controls (Soloff et al. 2017). A reciprocal effect which increased cognitive control and suppressed emotional expression would be an adaptive response during a suicidal crisis (Drevets and Raichle, 1998).
Suicidal behavior in BPD and other psychiatric disorders is closely associated with temperamental traits of impulsivity and aggressiveness (Brodsky et al., 1997; Mann et al., 1999; McGirr et al., 2007, 2009, Oquendo et al., 2004). We have previously reported that trait impulsivity and behavioral aggression mediated regional brain responses in subjects with BPD during an affectively valenced test of response inhibition and motor impulsiveness (the Go No-Go task) (Soloff et al., 2017b). Under negative affective conditions, behavioral aggression, assessed by the Brown-Goodwin Lifetime History of Aggression (Brown and Goodwin, 1986), had a negative effect on activation, with no positive effects. Increased levels of lifetime aggression were associated with diminished activation in areas which mediate behavioral inhibition (OFC), social decision-making (BG), and recall of episodic memory (HIP). The similarity to effects of medical lethality among BPD suicide attempters on the Affective X-CPT task may reflect the close relationship of behavioral aggression to suicidal behavior in BPD and across diagnoses (McGirr et al., 2007, 2009, Oquendo,et al., 2004, Soloff et al., 2012, 2014).
Under negative affective conditions, increased degrees of medical lethality were associated with diminished activation in areas regulating both executive cognition (e.g. ACC), and task-related functions (e.g. parietal precuneus). In healthy subjects, the X-CPT robustly activates the dACC, a region widely involved in executive regulation of affect and behavior through conflict monitoring, error detection, response inhibition, and reward-based decision-making (Botvinik et al., 2001, 2004, 2007; Carter et al.,1998; Marsh et al., 2007; Shenhav et.al., 2016; Sheth et al., 2012). A theoretical model unifying the functions of conflict monitoring and decision-making holds that the dACC makes strategic adjustments in cognitive control dependent upon the overall expected value of control, i.e. a reward-based decision process (Botvinick, 2007, Shenhav, et al. 2016). The robust inverse relationship between medical lethality of suicide attempts and activation of ACC under negative affective conditions strongly suggests a down-regulation of executive control in BPD subjects at times of negative emotional duress.
The observed effects are also generally consistent with the “triple network model of psychopathology” (Menon, 2011; 2018). In this model, the dACC is an anchoring neural node of the salience network (SN), tasked with detecting and evaluating external and internal events, allocating attention, and co-ordinating behavioral responses with the central executive network (CEN) in rule-based problem solving and goal directed actions. The CEN is anchored in the dorsolateral PFC, and posterior parietal cortex, which are also inversely related to medical lethality of suicidal behavior in the current study (Menon, 2011). (We discuss the relationship of our results to this model in further detail below).
Degree of medical lethality was inversely related to activation of the parietal precuneus, a region which processes task-relevant functions, particularly spatial attention. Posterior parietal cortex and parietal precuneus are also part of the CEN, facilitating recall of spatial details of images and perceptual decision making required to execute the X-CPT (Cavanna and Trimble, 2006).
In the negative affective context, increased degrees of medical lethality were also associated with diminished activation of the basal ganglia (BG), which are involved in attention and reward-based decision-making (Herrero et al. 2002, Voytek and Knight, 2010). Structural abnormalities in the BG have been associated with suicidal behavior (Vang et al., 2010), and with impulsive decision-making (“delay discounting”) among depressed elders who have attempted suicide compared to non-suicidal depressed elders (Dombrovski et al., 2012). Structural abnormalities in striatum are thought to contribute to suicidal behavior through impulsive decision-making
Menon (2011) has proposed an influential neural model for behavioral deficits in psychiatric disorders that is based on abnormal functional integration and aberrant connectivity in critical brain networks. In this model, behavioral manifestations of psychiatric disorders may be attributed to: a) weak intrinsic connectivity between neural nodes, b) abnormal recruitment of brain nodes not typically part of the CEN, or, c) impaired access to salient task-relevant stimuli. Each of these attributes may apply to the effects of negative affective interference on neural processing of cognitive tasks in BPD subjects:
Limbic hyperarousal and diminished fronto-limbic inhibitory function has been previously demonstrated in BPD subjects exposed to negative affective stimuli in diverse paradigms (Kraus et al., 2010; Kraus-Utz et al., 2014; Minzenberg et al., 2007; Schmahl et al., 2003a; Silbersweig et al., 2007). In addition, BPD subjects have been shown to have diminished or aberrant functional connectivity between amygdala and regions of the frontal cortex, including ACC (New et al. 2007, Soloff et al., 2017a).
Any model for emotion dysregulation and behavioral dyscontrol in BPD must also account for the role of heritable temperamental traits such as impulsivity and aggressiveness. Impulsivity and behavioral aggression in BPD (and other PDs) are associated with discrete structural, metabolic, and functional abnormalities believed to mediate these traits (Goodman et al., 2011; Hazlett et al., 2005; Krause-Utz et al., 2014; Niedfeld et al., 2013; Sala et al., 2011; Schmahl et al. 2003b; Schmahl and Bremner, 2006; Siever et al.,1999; Siever, 2008; Soloff et al., 2000, 2003, 2007, 2008, 2014; Sprung et al., 2002; Tebartz van Elst et al., 2003; Zetzsche et al., 2007). We have previously demonstrated inverse relationships between a measure of behavioral aggression, brain activation and behavioral performance on an Affective Go No-Go task under negative affective conditions in BPD (Soloff et al., 2017b). These relationships suggest interference with CEN function by brain regions which mediate temperamental aggressiveness, and are not part of the CEN. How the temperamental traits of impulsivity and aggressiveness interfere with neural processing of executive function is not well defined, but may be considered as examples of “abnormal recruitment” under the triple network model.
Finally, an inverse relationship between medical lethality and activation of the parietal precuneus under negative affective conditions suggests impaired processing of task-relevant visual/spatial stimuli.
4.1. Clinical Relevance
The current study is, to our knowledge, the first to demonstrate an association between the severity of medical damage in suicide attempters with BPD and affective interference with neural processing of executive functions. We previously suggested that the diathesis to suicidal behavior in BPD lies, in part, in the pre-existing structural, metabolic and functional abnormalities which characterize the neurobiology of trait impulsivity and aggressiveness (Soloff et al., 2012, 2014). Emotion dysregulation, behavioral dyscontrol, and, ultimately, suicidal behavior, may result when negative affect interferes with executive cognitive function, in interaction with the pre-existing neurobiology of temperamental traits. In terms of network models, degree of medical lethality is related to diminished functional connectivity between SN and CEN (resulting in a loss of “top down“ control), and interference with CEN by networks mediating temperaments such as trait impulsivity and aggressiveness.
4.2. Limitations
Half (52.2%) of our subjects were taking psychoactive medications at the time of the study. In prior studies we found no significant differences between medicated and non-medicated BPD subjects in signal change analyses across multiple fMRI paradigms (e.g. affectively valenced Go No-Go, X-CPT, and Episodic Memory Recall tasks) (Soloff et al. 2015). Our null results may be attributable to reduced power due to small sample sizes. Our results are consistent with other fMRI studies of BPD subjects (Beblo et al., 2006; Buchheim et al., 2008; Donegan et al., 2003), though not all (Silbersweig et al. 2007), that found no significant effects (or minimal effects) of medication use on fMRI results.
This study of female subjects with BPD does not address possible gender differences in the relationship of suicidal behavior to brain activation. Male BPD subjects demonstrate more externalizing behaviors than females, including impulsive-aggression and anti-social acts. Female BPD subjects typically have more internalizing symptom expression, such as depression, anxiety, and self-injury (Johnson et al., 2003). These differences could be reflected in differing patterns of neural activation in relation to suicidal behavior. Since 75% of clinically diagnosed BPD patients are female, accruing a sufficiently large male BPD attempter sample to address gender effects was not possible at this time.
4.3. Conclusions
fMRI studies demonstrate how negative emotional stimuli interfere with neural processing of cognitive task performance in healthy subjects (Goldstein et al., 2007), and those with BPD, resulting in diminished “top down” frontal cortical control in the face of “bottom-up” limbic hyperarousal (Koenigsberg et al., 2009; Silbersweig et al., 2007; Wingenfeld et al., 2009). Within neural networks, competition may exist for limited resources needed to process both cognitive function and negative affect simultaneously (Pessoa, 2009). Increased cognitive control requires reciprocal emotional suppression (Drevets and Raichle, 1998, Mayberg et al.1999). The failure of this adaptive process in BPD is expressed clinically in symptoms of emotion dysregulation, and a diathesis to impulsive aggression and suicidal behavior.
Functional imaging studies inform clinical practice precisely because they reveal neural activations associated with symptom presentation, and thereby suggest mechanisms mediating symptom improvement. For example, psychotherapies which enhance emotion regulation and behavioral control in BPD, such as Dialectal Behavior therapy (DBT) and Transference–Focused Psychotherapy (TFP), increase neural activation in areas related to frontal, “top down” cognitive control and decrease activation in limbic areas associated with emotional reactivity (Goodman et al. 2014; Perez et al. 2016; Schnell and Herpertz, 2007). This framework linking neuroscience with clinical practice can be extended to the search for functional bio-markers in suicidality. We consider our work a step in this direction.
Highlights.
Negative affect interferes with cognitive function, mediating emotion dysregulation, impulsive-aggression and suicidal behavior.
23 BPD suicide attempters completed an affective X-CPT paradigm, modified to include Ekman faces.
For each affective condition, Lethality Rating Scale (LRS) scores were regressed on neural activations, with HamD co-varied.
Under negative affective conditions, there was a robust negative relationship between LRS scores and activation in ACC, parietal precuneus, basal ganglia and OFC, with no positive relationships.
Affective conditions decreased task-sensitivity, with no differences between affects.
With negative affective interference, increased lethality of suicidal behavior in BPD predicted diminished neural activation in areas related to executive cognitive function.
Acknowledgements
This work was supported by the National Institutes of Mental Health (MH 048063, PHS). Additional support (VAD) was provided by a Career Development Chair from Wayne State University, the Charles H. Gershenson Distinguished Faculty Fellowship from Wayne State University, the Lyckaki-Young Fund from the State of Michigan, the Prechter Family Bipolar Foundation, the Children’s Hospital of Michigan Foundation, the Children’s Research Center of Michigan, the Cohen Neuroscience Endowment, and the Dorsey Endowment. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIMH or other funding sources, which had no role in the conduct of the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure
Declarations of Interest: none
References
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL, 2007. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci 2(4), 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T, Driessen M, Mertens M, Wingenfeld K, Piefke M, Rullkoetter N, Silva-Saavedra A, Mensebach C, Reddemann L, Rau H, Markowitsch HJ, Wulff H, Lange W, Berea C, Ollech I, Woermann FG, 2006. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychol. Med 36, 845–856. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, 2007. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, and Behavioral Neuroscience 7(4), 356–366. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Carter CS, Braver TS, Barch DM, & Cohen JD, 2001. Conflict monitoring and cognitive control. Psychol. Rev 108(3), 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS, 2004. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci 8(12), 539–54. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Groves SA, Oquendo MA, Mann JJ, Stanley B, 2006. Interpersonal precipitants and suicide attempts in borderline personality disorder. Suicide Life Threat. Behav 36, 313–322. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Malone KM, Ellis SP, Dulit RA, Mann JJ, 1997. Characteristics of borderline personality disorder associated with suicidal behavior. Am. J. Psychiatry 154, 1715–1719. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, 1986. Cerebrospinal fluid correlates of suicide attempts and aggression. Ann. N.Y. Acad. Sci 487, 175–188. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Kircher T, Martius P, Pokorny D, Ruchsow M, Spitzer M, Walter H, 2008. Neural correlates of attachment trauma in borderline personality disorder: A functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging 163, 223–235. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD, 1998. Anterior cingulate cortex, error detection, and online monitoring of performance. Science 280, 747–749. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR, 2006. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129 (Pt 3), 564–583. [DOI] [PubMed] [Google Scholar]
- Chesin MS, Jeglic EL, Stanley B, 2010. Pathways to high lethality suicidal attempts in individuals with borderline personality disorder. Arch. Suicide Res 14, 342–362. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Vizuela N, Thomas KM, Han GJ, Lim KO, Camchong J, Meuller BA, Bell CH, Heller MD, Schulz SC, 2011. Amygdala functional connectivity in young women with borderline personality disorder. Brain Connect. 1(1), 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, 1999. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci 3(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Denny BT, Fan J, Fels S, Galitzer H, Schiller D, Koenigsberg HW, 2018. Sensitization of the neural salience network to repeated emotional stimuli following initial habituation in patients with borderline personality disorder. Am. J.Psychiatry 175:7, 657–664. Doi: 10.1176/appi.ajp.2018.17030367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter S, van Heeringen C, Audenaert K,2011. Structural and functional neuroimaging studies of the suicidal brain. Prog.Neuropsychopgharmacol.Biol.Psychiatry 35,796–808. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H, 2012. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol. Med 42 (6), 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashen TH, Wexler BE, 2003. Amygdala hyper-reactivity in borderline personality disorder: Implications for emotional dysregulation. Biol. Psychiatry 54(11), 1284–1293. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME, 1998. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implication for interactions between emotion and cognition. Cogn.Emot 12, 353–385 [Google Scholar]
- Ebner-Priemer UW, Kuo J, Kleindienst N, Welch SS, Reisch T, Reinhard I, Lieb K, Linehan MM, Bohus M, 2007. State affective instability in borderline personality disorder assessed by ambulatory monitoring. Psychol. Med 37(7), 961–970. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Schlotz W, Kleindienst N, Rosenthal MZ, Detterer L, Linehan MM, Bohus M, 2008. Distress and affective dysregulation in patients with borderline personality disorder: A psychophysiological ambulatory monitoring study. J. Nerv. Ment. Dis 196(4), 314–320. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV,1979. Pictures of facial affect. Consulting Psychologists; Palo Alto, CA. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2005. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 4/2005 revision). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Goldstein M, Brendel G, Teuscher O, Pan H, Epstein J, Beutel M, Yang Y, Thomas K, Levy K, Silverman M, Clarkin J, Posner M, Kernberg O, Styern E, Silbersweig D, 2007. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no-go fMRI study. NeuroImage 36, 1026–1040. [DOI] [PubMed] [Google Scholar]
- Goodman M, Carpenter D, Tang C, Goldstein K, Avedon J, Hernandez M, Mascitelli KA, Blair NJ, New AS, Triebwasser J, Siever LJ, Hazlett EA, 2014. Dialectical behavior therapy alters emotion regulation and amygdala activity in patients with borderline personality disorder. J.Psychiatr. Res 57, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS, 2011. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. J.Psychiatr. Res 45(6), 803–807. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA, 2006. Conceptual foundations In Gross JJ (Ed.), Handbook of Emotion Regulation, Guilford Press, New York: pp.5–18. [Google Scholar]
- Gunderson JG, Lyons-Ruth K, 2008. BPD’s interpersonal hypersensitivity phenotype: A gene-environment-developmental model. J. Pers. Disord 22(1), 22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W, 1976. ECDEU assessment manual of psychopharmacology-revised. Rockville, MD: DHEW Publ. No. ADM 76–338. [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS, 2005. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol. Psychiatry 58, 614–623. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, Haznedar MM, Myerson D, Goodman M, Siever LJ, Chu KW, 2012. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol. Psychiatry 72, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM, 2002. Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst 18,386–404. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krinks T, Erberich SG, Willmes K, Thron A, Sass H, 2001. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biol. Psychiatry 50, 292–298. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Guenzler C, Zimmermann S, Scheel CN, Rusch N, Leonhart R, Nerb J, Lieb K, 2008. Time course of anger and other emotions in women with borderline personality disorder: A preliminary study. J. Behav. Ther. Exp. Psychiatry 39, 391–402. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Shea MT, Yen S, Battle CI, Zlotnick C, Sanislow CA, Grilo CM, Skodol AE, Bender DS, McGlashen TH, Gunderson JG, Zanarini MC,2003. Gender differences in borderline personality disorder: findings from the Collaborative Longitudinal Personality Disorders study. Compr. Psychiatry 44(4), 284–292. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner K, Liu X, Guise KG, Pizzarello S, Dorantes C, Guerreri S, Tecuta L, Goodman M, New A, Siever LJ, 2009. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: A study of patients with borderline personality disorder. Biol. Psychiatry 66(9), 854–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Valerius G, Seifritz E, Ruf M, Bremner JD, Bohus M, Schmahl C, 2010. Script-driven imagery of self-injurious behavior in patients with borderline personality disorder: a pilot fMRI study. Acta Psychiatr. Scand 121, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A, Winter D, Niedtfeld I, Schmahl C, 2014. The latest neuroimaging findings in borderline personality disorder. Curr. Psychiatry Rep 16, 438. [DOI] [PubMed] [Google Scholar]
- Levine D, Marziali E, Hood J,1997. Emotion processing in borderline personality disorders. J. Nerv. Ment. Dis 185, 240–246. [DOI] [PubMed] [Google Scholar]
- Linehan MM, 1993. Cognitive-Behavioral Treatment of Borderline Personality Disorder. Guilford Press, New York. [Google Scholar]
- Loranger AW, 1999. International Personality Disorder Examination DSM-IV and ICD-10 Interviews. Psychological Assessment Resources, Inc., Lutz, FL. [Google Scholar]
- Macmillan NA, Creelman CD, 2005. Detection theory: A user’s guide. Mahwah,N.J: Lawrence Erlbaum Associates. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19 (3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM (1999). Toward a clinical model of suicidal behavior in psychiatric patients. Am.J. Psychiatry 156, 181–189. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Vythilingam M, Busis S, Blair RJ, 2007. Response options and expectations of reward in decision-making: the differential roles of dorsal and rostral anterior cingulate cortex. Neuroimage 35(2), 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis J, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT, 1999. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682. [DOI] [PubMed] [Google Scholar]
- McGirr A, Alda M, Seguin M, Cabot S, Lesage A, Turecki G, 2009. Familial aggregation of suicide explained by Cluster B traits: A three-group family study of suicide controlling for major depression. Am. J. Psychiatry 166 (10), 1124–1134. [DOI] [PubMed] [Google Scholar]
- McGirr A, Paris J, Lesage A, Renaud J, Turecki G, 2007. Risk factors for suicide completion in borderline personality disorder: A case-control study of Cluster B comorbidity and impulsive aggression. J. Clin. Psychiatry 68, 721–729. [DOI] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, 2018. The Triple Network Model, Insight, and Large-Scale Brain Organization in Autusm. Biol. Psychiatry 84(4):236–238. doi: 10.1016/j.biopsych.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ, 2007. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: An event-related fMRI study. Psychiatry Res. Neuroimaging 155, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AE, Dickens GL, Picchioni MM, 2014. Facial emotion processing in borderline personality disorder: A systematic review and meta-analysis. Neuropsychol. Rev 24, 166–184. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ, 2007. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology 32 (7), 1629–1640. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I, Schulze I, Krause-Utz A, Demirakca T, Bohus M, Schmahl C, 2013. Voxel-based morphometry in women with borderline personality disorder with and without comorbid post-traumatic stress disorder. PLoS One 8(6), e65824. doi: 10.1371/journal.pone.0065824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ, 2004. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am. J. Psychiatry 161, 1433–1441. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ 2003. Risk factors for suicidal behavior. The utility and limitations of research instruments In: First MB(Ed.) Standardized Evaluation in Clinical Practice. Review of Psychiatry 8,103–130. American Psychiatric Publishing; Washington,D.C. [Google Scholar]
- Perez DL, Vago DR, Pan H, Root J, Teuscher O, Fuchs BH, Leung L, Epstein J, Cain NM, Clarkin JF, Lenzenweger MF, Kernberg OF, Levy KN, Silbersweig DA, Stern E, 2016. Frontolimbic neural circuit changes in emotional processing and inhibitory control associated with clinical improvement following Transference-Focused Psychotherapy in borderline personality disorder. Psychiatry Clin.Neurosci 70 (1), 51–61. doi: 10.1111/pcn.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, 2009. How do emotion and motivation direct executive control? Trends Cogn. Sci 13 (4), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 13(9), 829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KM, Silk KR, 2005. Emotion dysregulation and the development of borderline personality disorder. Dev. Psychopathol 17(4), 899–925. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF, 2013. Neural correlates of negative emotionality in borderline personality disorder: An activation-likelihood-estimation meta-analysis. Biol. Psychiatry 73, 153–160. [DOI] [PubMed] [Google Scholar]
- Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E, Gambini F, Isola M, De Bona M, Rambaldelli G, d’Allio G, Barale F, Zappoli F, Brambilla P, 2011. Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. J. Affect. Disord 131(1–3), 417–421. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Grilo CM, Morey LC, Bender DS, Skodol AE, Gunderson JG, Shea MT, Stout RL, Zanarini MC, McGlashan TH, 2002. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: findings from the Collaborative Longitudinal Personality Disorders Study. Am. J. Psychiatry 159(2), 284–290. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Bremner JD, 2006. Neuroimaging in borderline personality disorder. J.Psychiatr. Res 40, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Vermetten E, Sanislow C, McGlashan TH, Bremner JD, 2003a. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biol. Psychiatry 54, 142–151. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD, 2003b. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. Neuroimaging 122, 193–198. [DOI] [PubMed] [Google Scholar]
- Schmahl C, Bremner JD, 2006. Neuroimaging in borderline personality disorder. Journal of Psychiatric Research, 40, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K, Herpertz SC, (2007). Effects of dialectic-behavior-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. J. Psychiatr. Res 41, 837–847. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Köppen D, Herpertz SC, 2013. Enhanced detection of emotional facial expressions in borderline personality disorder. Psychopathology 46(4), 217–224. [DOI] [PubMed] [Google Scholar]
- Schulze L,Schmahl C, Niedtfeld I, 2016. Neural correlates of disturbed emotion processing in borderline personality disorder: A multi-modal meta-analysis. Biol. Psychiatry 79(2), 97–106. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM, 2016. Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci 19(10), 1286–1291. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskander EN, 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioral adaption. Nature 488(7410), 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, 2008. Neurobiology of aggression and violence. Am. J. Psychiatry 165, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum M, New AS, Spiegel-Cohen J, Wei T, Hazlett E, Sevin E, Nunn M, Mitropoulou M, 1999. d,l fenfluramine response in impulsive personality disorder assessed with [18-F] fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology, 20, 413–423. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E, 2007. Failure of fronto-limbic inhibitory function in the context of negative emotion in borderline personality disorder. Am. J. Psychiatry 164, 1832–1841. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Burgess A, Ramaseshan K, Chowdury A, Diwadkar VA, 2017b. Impulsivity and aggression mediate regional brain responses in borderline personality disorder: an fMRI study. Psychiatry Res. Neuroimaging 260, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Ramaseshan K, Burgess A, Diwadkar VA, 2017a. Hypermodulation of brain networks by the amygdala among women with borderline personality disorder: Network signatures of affective interference during cognitive processing, J.Psychiatr. Res doi: 10.1016/j.jpsychires.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D, 2003. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Res. Neuroimaging 123, 153–163. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM, 2000. Fenfluramine-activated FDG study of borderline personality disorder. Biol. Psychiatry 47, 540–547. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Nutche J, Goradia D, Diwadkar V, 2008. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. Neuroimaging 164, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH, 2007. 5HT2A receptor binding is increased in borderline personality disorder. Biol. Psychiatry 62, 580–587. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA, 2012. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J. Psychiatr. Res 46, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White RA, Diwadkar VA, 2014. Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Res. Neuroimaging 222, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA, 2015. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Res. 233, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung L, Shaw RB, Koenigsberg H, Platholi J, Silverman J, Siever LJ, 2002. Blunted prefrontal cortical 18-fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Arch.Gen.Psychiatry 59, 621–629. [DOI] [PubMed] [Google Scholar]
- Tana MG, Montin E, Cerutti S, Bianchi AM, 2010. Exploring cortical attentional systems by using fMRI during a Continuous Perfomance Test. Comput. Intell. Neurosci 329213, doi: 10.1155/2010/329213. PMID:20011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D, 2003. Frontolimbic brain abnormalities in patients with borderline personality disorder. A volumetric MRI study. Biol. Psychiatry 54(2), 163–171. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic parcellation of the MNI MRI single subject brain. NeuroImage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- van Eijk J, Sebastian A, Krause-Utz A, Cackowski S, Demirakca T, Biedermann SV, Lieb K, Bohus M, Schmahl C, Ende G, Tuscher O, 2015. Women with borderline personality disorder do not show altered BOLD responses during response inhibition. Psychiatry Res. Neuroimaging 234, 378–389.. [DOI] [PubMed] [Google Scholar]
- van Heeringen C, Bijttebier S, Godfrin K, 2011. Suicidal brains: A review of functional and structural brain studies in association with suicidal behavior. Neuroscience and Biobehavioral Reviews 35, 688–698. [DOI] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Benz L, van Westen D, Lindstrom MB, 2010. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatric Res. Neuroimaging 183,177–179. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT, 2010. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc. Natl. Acad. Sci. USA 107, 18167–18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD, 2000. Simultaneous inference for fMRI data AFNI 3dDeconvolve Documentation. Medical College of Wisconsin, Milwaukee. [Google Scholar]
- Wingenfeld K, Rullkoetter N, Mensebach C, Beblo T, Mertens M, Kreisel S, Toepper M, Driessen M, Woermann FG, 2009. Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology 34(4), 571–586. [DOI] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, McGlashan TH, Zanarini MC, Morey LC, 2004. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. Am. J. Psychiatry 161, 1296–1298. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Gunderson JG, Frankenburg FR, Chauncey DL, 1989. The Revised Diagnostic Interview for Borderlines: Discriminating BPD from other Axis II Disorders. J.Pers. Disord 3, 10–18. [Google Scholar]
- Zetzsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Munchhausen E, Tabrizi S, Leinsinger G, Born C, Reiser M, Moller H-J, Meisenzahl EM, 2007. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Res. 154, 157–170. [DOI] [PubMed] [Google Scholar]