Abstract
Background:
Dysregulation of glucocorticoid receptors has been implicated in addiction and stress-related disorders. FKBP5 is a co-chaperone of the glucocorticoid receptor and regulates receptor sensitivity. While FKBP5 is known to be involved in mood- and stress-related disorders, less is known regarding FKBP5 and cocaine abuse. This study investigated the regulation of FKBP5 expression in the extended amygdala and paraventricular nucleus of the hypothalamus, regions important in the control of stress-responses and HPA axis function, following chronic and acute cocaine administration.
Methods:
Adult male and female rats received saline or cocaine three times per day for 1 or 14 days. Brain tissue was collected 30 minutes, 24 hours, 48 hours, 7 days or 14 days following the final injection. FKBP5 mRNA was measured by qRT-PCR in the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST) and paraventricular nucleus (PVN).
Results:
FKBP5 mRNA levels were significantly elevated as a result of chronic cocaine administration in both males and females in the PVN and BNST 30 minutes and 24 hours after the final injection. In females, FKBP5 was also elevated in the CeA. Following acute cocaine, FKBP5 gene expression was unaltered except for elevated levels in the BNST of females at 24 hours of withdrawal.
Conclusions:
These results demonstrate that FKBP5 mRNA is regulated by cocaine administration. Increased FKBP5 expression may play a role in the dysregulation of the stress axis following chronic cocaine exposure, contributing to the negative affective symptoms of cocaine withdrawal.
Keywords: Stress, Anxiety, Depression, Sex Differences, HPA axis, FKBP51
1. Introduction
Cocaine abuse and dependence are major public health problems with serious economic and social consequences. Preventing cycles of relapse to drug use is the largest challenge for effective treatment of cocaine-dependent individuals, and there are no FDA approved pharmacotherapies for cocaine use disorder. Withdrawal from cocaine produces a negative affective state characterized by anhedonia, anxiety, and depression (Ambrose-Lanci et al., 2010; Gawin and Kleber, 1986; Perrine et al., 2008). This withdrawal-induced negative affective state is mediated by the extended amygdala, a group of interconnected structures including the central nucleus of the amygdala, bed nucleus of the stria terminalis, and the shell of the nucleus accumbens (Koob and Le Moal, 2001; Koob and Volkow, 2010; Stamatakis et al., 2014). The extended amygdala is closely involved with brain stress systems, including the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis governs responses to stress and consists of corticotropin-releasing factor (CRF) released from the paraventricular nucleus of the hypothalamus, adrenocorticotropic hormone (ACTH) released from the pituitary in response to CRF, and subsequent glucocorticoid secretion from the adrenal glands. Both the HPA axis and extra-hypothalamic stress systems are dysregulated by cocaine use and withdrawal (Goeders, 2002; Koob, 2008; Sarnyai et al., 1992).
FKBP5 (FK506-binding protein 51, or FKBP51) is important for the regulation of responses to stress. FKBP5 is a co-chaperone of the glucocorticoid receptor (GR) complex and modulates GR sensitivity to cortisol (Scammell et al., 2001). FKBP5 bound to the receptor complex reduces the affinity of the GR for cortisol and prevents its translocation to the nucleus (Wochnik et al., 2005). However, when cortisol does bind, FKBP5 is exchanged for FKBP4 which promotes nuclear translocation, allowing GR transcriptional activity (Davies et al., 2002; Wochnik et al., 2005). One of the transcriptional targets of the GR is FKBP5. Newly translated FKBP5 then inhibits GR activity (Denny et al., 2000), forming an intracellular feedback loop. Frequent activation of the HPA axis and subsequent cortisol release can lead to increased FKBP5 production, GR resistance, and HPA axis dysregulation. Due to this, FKBP5 has been implicated in the pathology of human psychiatric diseases such as anxiety, depression, and post-traumatic stress disorder (Binder et al., 2008; Ising et al., 2008; Lekman et al., 2008; Minelli et al., 2013; Pérez-Ortiz et al., 2013).
Several studies have investigated drug use and FKBP5 genetic variability in humans. FKBP5 genotype is associated with the severity of withdrawal from nicotine (Jensen and Sofuoglu, 2016), as well as the effect of nicotine on the HPA axis (Koopmann et al., 2016). The interaction of early life trauma and FKBP5 genetic variation predicts heavy drinking in college students (Lieberman et al., 2016), and FKBP5 genetic variability is associated with severity of alcohol withdrawal (Huang et al., 2014). Additionally, two studies found significant associations between the functional FKBP5 SNP rs1360780 and heroin addiction (Levran et al., 2014; Rovaris et al., 2016).
Since cocaine withdrawal and FKBP5 dysregulation produce similar negative affective states, and little is known about how cocaine affects FKBP5, this study investigated regulation of FKBP5 mRNA during withdrawal from chronic cocaine in brain regions important to both the HPA axis and the extended amygdala: the paraventricular nucleus of the hypothalamus, the central nucleus of the amygdala, and the bed nucleus of the stria terminalis. Preliminary studies in the shell of the nucleus accumbens did not find significant differences in FKBP5 mRNA levels, therefore this region was excluded from further study.
2. Methods
2.1. Animals and Drug Administration
Adult male and female Sprague Dawley rats (Charles River) were housed in same sex pairs in ventilated racks on a 12-hour light/dark cycle (lights on 7:00AM) and allowed access to water and standard rat chow ad libitum. All procedures were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by Temple University’s Institutional Animal Care and Use Committee. Cocaine HCl, generously supplied by the NIDA Drug Supply Program, was dissolved in 0.9% saline and injected i.p. in volume of 1ml/kg. Rats were injected with saline (1ml/kg) or cocaine (15mg/kg) in a binge pattern (three times per day, one hour apart, starting at 9:00AM) for 1 or 14 days.
2.2. Quantitative RT-PCR
Rats were euthanized 30 minutes, 24 hours, 48 hours, 7 days, or 14 days following the last cocaine or saline injection by brief CO2 anesthesia followed by decapitation. Brains were removed, rapidly frozen in isopentane (−35–40°C), and stored at −80°C. Frozen brains were sectioned with a cryostat microtome and the paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST) were dissected using 1mm punches from both hemispheres of two adjacent 300 um-thick sections (Paxinos and Watson, 2007).
Total RNA was isolated using the Quick-RNA Miniprep kit (Zymo Research, Irvine, CA), quantified with a NanoDrop 2000 spectrophotometer, and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was carried out with TaqMan Fast Advanced Master Mix and a FKBP5 TaqMan Gene Expression Assay (Rn01768371_m1, Thermo Fisher) using the StepOnePlus Real-Time PCR System (Applied Biosystems). Male and female samples were analyzed together on the same plate for each brain region at the same time point. Relative gene expression levels were measured according to the 2−∆∆Ct method (Livak and Schmittgen, 2001) using 18S Eukaryotic rRNA (Hs99999901_s1, Thermo Fisher) as an internal control.
2.3. Statistical Analyses
Data were analyzed for each time point in each brain region by two-way ANOVA to compare drug and sex main effects, followed by a priori Bonferroni post-hoc tests to identify cocaine-induced changes within each sex. Fold change data are expressed relative to male saline controls.
3. Results and Discussion
3.1. Results
FKBP5 mRNA was measured 30 minutes, 24 hours, 48 hours, 7 days, and 14 days following chronic cocaine administration in male and female rats. In the BNST (Fig 1A) obtained 30 minutes after the last injection, 2-way ANOVA revealed a significant main effect of cocaine (F(1,18)=24.90, p<0.0001), but not sex (F(1,18)=0.0064, p=0.9369), with no significant interaction (F(1,18)=0.0245, p=0.8773). A priori post hoc tests showed FKBP5 mRNA levels were significantly higher in both cocaine-injected males (1.72-fold, p<0.01) and females (1.73-fold, p<0.01) compared to saline-injected controls of the same sex. FKBP5 mRNA was also significantly elevated 24 hours after the last cocaine injection (cocaine: F(1,16)=12.84, p=0.0025; sex: F(1,16)=0.0061, p=0.9385; interaction: F(1,16)=0.4361, p=0.5184) in both males (1.26-fold, p<0.01) and females (1.22-fold, p<0.05).
Figure 1.
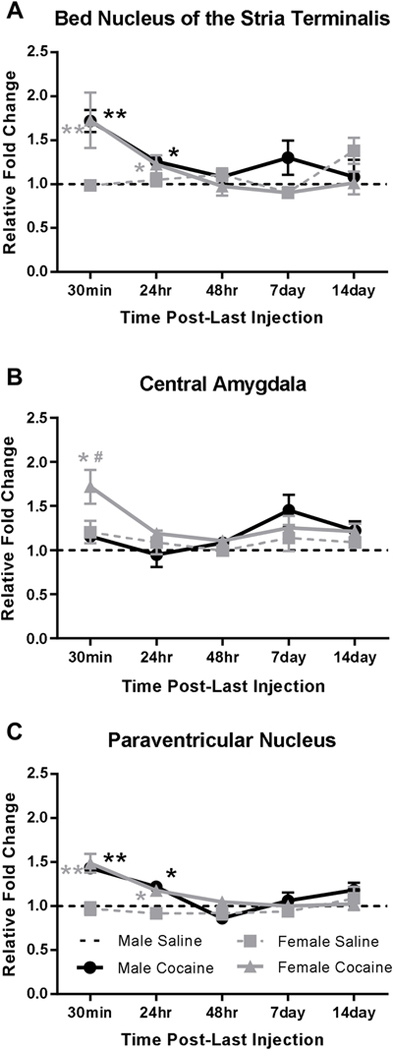
FKBP5 mRNA regulation during withdrawal from chronic cocaine administration in male and female rats.
Fold changes in FKBP5 mRNA compared with male saline-injected controls are shown for 5 time points after the last injection of saline or cocaine in the bed nucleus of the stria terminalis (A), central amygdala (B), and the paraventricular nucleus (C). * = p<0.05 significant cocaine difference compared to saline controls of the same sex; # = p<0.05 significant sex difference compared to cocaine-injected males. N=5–6
In the CeA (Fig 1B) 30 minutes after the last injection, 2-way ANOVA revealed a significant main effect of both sex (F(1,16)=8.651, p=0.0096) and cocaine (F(1,16)=6.630, p=0.0204; interaction: F(1,16)=2.147, p=0.1622). A priori comparisons indicated FKBP5 mRNA was higher in the CeA of females (1.72-fold, p<0.05), but not males (1.16-fold, p>0.05) exposed to repeated cocaine.
In the PVN (Fig 1C), 2-way ANOVA revealed a significant main effect of cocaine (F(1,17)=40.57, p<0.0001), but not sex (F(1,17)=0.0106, p=0.9193) nor an interaction (F(1,17)=0.3486, p=0.5627) thirty minutes after the last injection. Compared to saline controls of the same sex, FKBP5 mRNA levels were significantly elevated in both cocaine-injected males (1.49-fold, p<0.01) and females (1.43-fold, p<0.01). Similar to the BNST, FKBP5 mRNA levels in the PVN were elevated 24 hours after the last cocaine injection (F(1,16)=14.59, p=0.0015; sex: F(1,16)=1.233, p=0.2833; interaction: F(1,16)=0.1503, p=0.7034) in both males (1.22-fold, p<0.05) and females (1.18-fold, p<0.05). FKBP5 mRNA levels were not significantly different 2, 7, or 14 days after the last cocaine injection as compared with saline-injected controls in the BNST, CeA or PVN (Fig 1).
FKBP5 mRNA levels were measured in males and females 30 minutes or 24 hours following acute cocaine or saline administration (i.e. one day of 3 binge-pattern injections). No significant differences in FKBP5 mRNA levels were found in males or females at 30 minutes, or in males at 24 hours. In the BNST at 24 hours after the last injection (Fig 2A), 2-way ANOVA revealed significant main effects of cocaine (F(1,13)=11.80, p=0.0044) and sex (F(1,13)=14.52, p=0.0022) but not interaction (F(1,13)=0.0219, p=0.8844). A priori post-hoc comparison between cocaine- and saline-injected females demonstrates that FKBP5 mRNA levels were significantly higher in the BNST of cocaine- versus saline-injected females (p<0.05) at the 24 hour time-point. Two-way ANOVAs revealed no significant drug or interaction main effects in the CeA (Fig 2B) or PVN (Fig 2C) at either time point.
Figure 2.
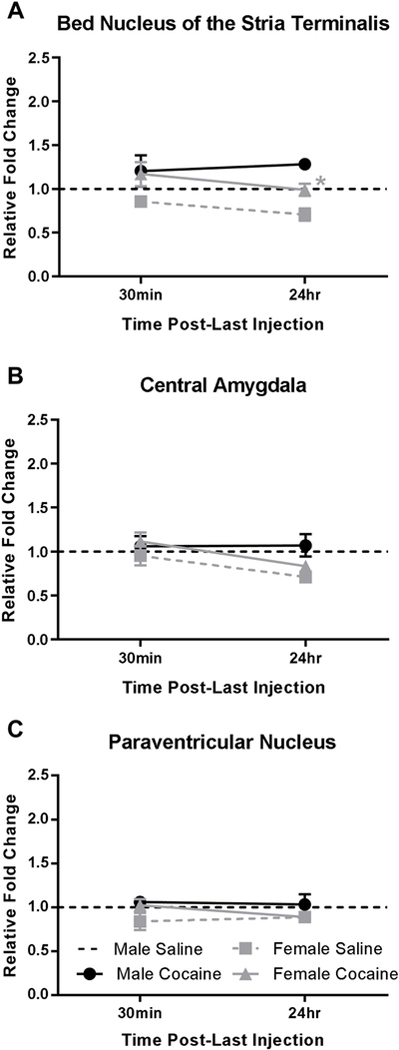
FKBP5 mRNA regulation following acute cocaine administration in male and female rats. Fold changes in FKBP5 mRNA levels compared with male saline-injected controls were measured 30 minutes and 24 hours following acute cocaine or saline administration in the bed nucleus of the stria terminalis (A), central amygdala (B), and the paraventricular nucleus (C). * = p<0.05 significant cocaine difference compared to saline controls of the same sex. N=4–5
3.2. Discussion
Negative mood states experienced during cocaine withdrawal are a challenge for prevention of relapse. Although many studies suggest involvement of the extended amygdala in the negative affective state produced by drug withdrawal (reviewed in Koob and Volkow, 2010), the specific mechanisms underlying withdrawal-induced anxiety, depression, and anhedonia remain unclear. Identification of molecules and pathways involved in negative withdrawal symptoms is crucial for development of treatment strategies for cocaine dependence and will enhance the understanding of the pathogenesis of mood and stress-related disorders. This study investigated the effect of chronic and acute exposure to cocaine on the regulation of FKBP5 expression, a molecule implicated in stress responses and psychiatric disorders. Using quantitative RT-PCR to measure mRNA at five time points following cessation of cocaine administration, significant increases in FKBP5 expression were found during early withdrawal from chronic cocaine. Overall, FKBP5 expression was not affected by acute administration of cocaine, with the exception of an increase in FKBP5 mRNA in the BNST of female rats 24 hours following cocaine. The sex differences in FKBP5 mRNA levels in the BNST and CeA are notable given that females escalate cocaine use more quickly than males and also find it more difficult to quit (Becker and Hu, 2008; Griffin et al., 1989; Lynch et al., 2002). Exactly what underlies the sex differences in response to cocaine remains unclear. Since FKBP5 mRNA in the BNST and CeA is increased following cocaine in female but not male rats, FKBP5 in these regions may play a role in the greater sensitivity to the negative effects of cocaine in females.
Limited literature exists on the regulation of FKBP5 following cocaine exposure. Congruent with the results of the present study, acute cocaine did not alter FKBP5 expression in the striatum of male mice (Piechota et al., 2010), nor was FKBP5 mRNA altered in the amygdala of male rats 10–11 days following cocaine self-administration (Hadad et al., 2016). In contrast, FKBP5 protein levels are lower in the prefrontal cortex of adolescent male rats in early withdrawal from chronic cocaine (Caffino et al., 2015); the prefrontal cortex was not investigated in the present study.
Repeated cocaine exposure and withdrawal cause persistent over-activation of the HPA axis, leading to increased cortisol levels and impaired negative feedback control (Goeders, 2002; Mantsch et al., 2007). Loss of negative feedback may contribute to negative affective states including anxiety, depression and anhedonia. Withdrawal from chronic cocaine results in affective symptoms similar to psychiatric and stress-related disorders. For example, using binge-pattern cocaine administration similar to the current study, our lab has found increases in anxiety- and depression-like behavior at 24 hours withdrawal in male rats (Perrine et al., 2008). Others have reported similar studies in female rats (Ambrose-Lanci et al., 2010). Human FKBP5 polymorphisms are associated with depression (Appel et al., 2011; Binder et al., 2004; Lekman et al., 2008) and anxiety (Attwood et al., 2011; Ising et al., 2008; Minelli et al., 2013), two of the negative affective states experienced during cocaine withdrawal (Gawin and Kleber, 1986). Thus, targeting FKBP5 may restore negative feedback of the HPA axis and reduce negative affect associated with cocaine withdrawal.
Several preclinical studies demonstrate the antidepressant and anxiolytic properties of FKBP5 inhibition. Attwood et al. (2011) demonstrate that silencing the FKBP5 gene in the amygdala reduces stress-induced anxiety in mice. Additionally, an FKBP5 inhibitor, SAFit2, increases struggling time and reduces floating time in the forced swim test, indicating antidepressant actions (Gaali et al., 2014), and reduces anxiety in mice as measured by the elevated plus maze and dark-light box tests (Hartmann et al., 2015). In addition, chronic stress in rats increases FKBP5 mRNA in the ventral hippocampus and prefrontal cortex, which is reduced by administration of the antidepressant duloxetine (Guidotti et al., 2013). Therefore, it is possible that inhibition of FKBP5 will mitigate anxiety and depression experienced during early withdrawal from chronic cocaine, which is supported by the present findings of upregulation of FKBP5 during early cocaine withdrawal. Further behavioral studies are needed to test this hypothesis. The studies presented here are recognizably limited, and in future studies it will be important to investigate other facets of FKBP5 regulation following chronic cocaine exposure, including measurement of protein levels of FKBP5 and examination of cellular trafficking of the glucocorticoid receptor.
3.3. Conclusions
Although it is well recognized that cocaine exposure and withdrawal activates the HPA axis (Goeders, 1997; Rivier and Vale, 1987), this is the first demonstration of regulation of FKBP5 expression by chronic cocaine administration. Since FKBP5 can regulate HPA function and has been linked to depression, anxiety, and other affective disorders, its upregulation may play a role in cocaine withdrawal-induced negative affect. In addition, targeting FKBP5 may be therapeutic for cocaine users, and this warrants further study.
Highlights.
FKBP5 mRNA is upregulated by chronic cocaine in the brains of male and female rats
Sex-specific upregulation of FKBP5 was found in the central amygdala of females
FKBP5 regulation occurs during early, but not late, withdrawal from chronic cocaine
Acknowledgements
The authors would like to thank Dr. Thomas Rogers and Dr. William Cornwell for their technical assistance
Role of Funding Source
This work was supported by the National Institutes of Health T32 DA007237, P30 DA013429, and R01 DA018326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Conflict of Interest
No conflict to declare
References
- Ambrose-Lanci LM, Sterling RC, Van Bockstaele EJ, 2010. Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J. Neurosci. Res 88, 816–24. 10.1002/jnr.22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Völzke H, Freyberger HJ, Grabe HJ, 2011. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacol 36, 1982–1991. 10.1038/npp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood BK, Bourgognon J-M, Patel S, Mucha M, Schiavon E, Skrzypiec AE, Young KW, Shiosaka S, Korostynski M, Piechota M, Przewlocki R, Pawlak R, 2011. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature 473, 372–5. 10.1038/nature09938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M, 2008. Sex differences in drug abuse. Front. Neuroendocrinol 29, 36–47. 10.1016/j.yfrne.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ, 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299, 1291–305. 10.1001/jama.299.11.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Künzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Köhnlein O, Dabitz H, Brückl T, Müller N, Pfister H, Lieb R, Mueller JC, Lõhmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B, 2004. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet 36, 1319–1325. 10.1038/ng1479 [DOI] [PubMed] [Google Scholar]
- Caffino L, Giannotti G, Malpighi C, Racagni G, Fumagalli F, 2015. Short-term withdrawal from developmental exposure to cocaine activates the glucocorticoid receptor and alters spine dynamics. Eur. Neuropsychopharmacol 25, 1832–1841. 10.1016/j.euroneuro.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning Y-M, Sánchez ER, 2002. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem 277, 4597–600. 10.1074/jbc.C100531200 [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG, 2000. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 141, 4107–13. 10.1210/endo.141.11.7785 [DOI] [PubMed] [Google Scholar]
- Gaali S, Kirschner A, Cuboni S, Hartmann J, Kozany C, Balsevich G, Namendorf C, Fernandez-Vizarra P, Sippel C, Zannas AS, Draenert R, Binder EB, Almeida OFX, Rühter G, Uhr M, Schmidt MV, Touma C, Bracher A, Hausch F, 2014. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol 11, 33–37. 10.1038/nchembio.1699 [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD, 1986. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch. Gen. Psychiatry 43, 107–13. [DOI] [PubMed] [Google Scholar]
- Goeders NE, 2002. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology 27, 13–33. 10.1016/S0306-4530(01)00034-8 [DOI] [PubMed] [Google Scholar]
- Goeders NE, 1997. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology 22, 237–259. 10.1016/S0306-4530(97)00027-9 [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U, 1989. A Comparison of Male and Female Cocaine Abusers. Arch. Gen. Psychiatry 46, 122 10.1001/archpsyc.1989.01810020024005 [DOI] [PubMed] [Google Scholar]
- Hadad NA, Wu L, Hiller H, Krause EG, Schwendt M, Knackstedt LA, 2016. Conditioned stress prevents cue-primed cocaine reinstatement only in stress-responsive rats. Stress 19, 1–49. 10.1080/10253890.2016.1189898 [DOI] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Gaali S, Kirschner A, Kozany C, Rühter G, Dedic N, Häusl AS, Hoeijmakers L, Westerholz S, Namendorf C, Gerlach T, Uhr M, Chen A, Deussing JM, Holsboer F, Hausch F, Schmidt M V, 2015. Pharmacological Inhibition of the Psychiatric Risk Factor FKBP51 Has Anxiolytic Properties. J. Neurosci 35, 9007–16. 10.1523/JNEUROSCI.4024-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M-C, Schwandt ML, Chester JA, Kirchhoff AM, Kao C-F, Liang T, Tapocik JD, Ramchandani VA, George DT, Hodgkinson CA, Goldman D, Heilig M, 2014. FKBP5 moderates alcohol withdrawal severity: human genetic association and functional validation in knockout mice. Neuropsychopharmacol 39, 2029–38. 10.1038/npp.2014.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Depping A-M, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Mller-Myhsok B, Holsboer F, 2008. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci 28, 389–398. 10.1111/j.1460-9568.2008.06332.x [DOI] [PubMed] [Google Scholar]
- Jensen KP, Sofuoglu M, 2016. Stress response genes and the severity of nicotine withdrawal. Pharmacogenomics 17, 1–3. 10.2217/pgs.15.149 [DOI] [PubMed] [Google Scholar]
- Koob G, Moal M Le, 2001. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol 24, 97–129. 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59, 11–34. 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacol 35, 217–38. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann A, Bez J, Lemenager T, Hermann D, Dinter C, Reinhard I, Schuster R, Wiedemann K, Winterer G, Kiefer F, 2016. The effect of nicotine on HPA Axis activity in females is modulated by the FKBP5 Genotype. Ann. Hum. Genet 80, 154–161. 10.1111/ahg.12153 [DOI] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJM, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S, 2008. The FKBP5-gene in depression and treatment response—An association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol. Psychiatry 63, 1103–1110. 10.1016/j.biopsych.2007.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Jeanne Kreek M, 2014. Drug addiction and stress-response genetic variability: Association study in African Americans. Ann. Hum. Genet 78, 290–298. 10.1111/ahg.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R, Armeli S, Scott DM, Kranzler HR, Tennen H, Covault J, 2016. FKBP5 genotype interacts with early life trauma to predict heavy drinking in college students. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 171, 879–887. 10.1002/ajmg.b.32460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. 10.1006/METH.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME, 2002. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology (Berl) 164, 121–37. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR, 2007. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res 1167, 101–111. 10.1016/j.brainres.2007.05.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Maffioletti E, Cloninger CR, Magri C, Sartori R, Bortolomasi M, Congiu C, Bignotti S, Segala M, Giacopuzzi M, Gennarelli M, 2013. Role of allelic variants of FK506-binding protein 51 (FKBP5) gene in the development of anxiety disorders. Depress. Anxiety 30, 1170–1176. 10.1002/da.22158 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates, sixth ed. Academic Press, New York. [Google Scholar]
- Pérez-Ortiz JM, García-Gutiérrez MS, Navarrete F, Giner S, Manzanares J, 2013. Gene and protein alterations of FKBP5 and glucocorticoid receptor in the amygdala of suicide victims. Psychoneuroendocrinology 38, 1251–1258. 10.1016/J.PSYNEUEN.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM, 2008. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology 54, 355–64. 10.1016/j.neuropharm.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Solecki W, Gieryk A, Slezak M, Bilecki W, Ziolkowska B, Kostrzewa E, Cymerman I, Swiech L, Jaworski J, Przewlocki R, 2010. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol 11, R48 10.1186/gb-2010-11-5-r48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Vale W, 1987. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res 422, 403–406. 10.1016/0006-8993(87)90953-X [DOI] [PubMed] [Google Scholar]
- Rovaris DL, Aroche AP, da Silva BS, Kappel DB, Pezzi JC, Levandowski ML, Hess ARB, Schuch JB, de Almeida RMM, Grassi-Oliveira R, Bau CHD, 2016. Glucocorticoid receptor gene modulates severity of depression in women with crack cocaine addiction. Eur. Neuropsychopharmacol 26, 1438–1447. 10.1016/j.euroneuro.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró É, Penke B, Telegdy G, 1992. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res 589, 154–156. 10.1016/0006-8993(92)91176-F [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF, 2001. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol 124, 152–65. 10.1006/gcen.2001.7696 [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD, 2014. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology 76, 320–328. 10.1016/j.neuropharm.2013.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T, 2005. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem 280, 4609–16. 10.1074/jbc.M407498200 [DOI] [PubMed] [Google Scholar]


