Abstract
Background:
Chronic insomnia is associated with poor asthma control. Cognitive-behavioral treatment for insomnia (CBT-I) is an efficacious and durable treatment for comorbid insomnia in medical and psychiatric disorders. However, the efficacy and potential accompanying mechanisms of CBT-I have not been examined in asthma. The purpose of this study is to test the efficacy of a CBT-I intervention on sleep and asthma control in adults with insomnia and asthma. We will also explore airway inflammation (i.e., exhaled nitric oxide, blood eosinophils) as a potential biological mechanism linking improvements in sleep with improvements in asthma control.
Methods:
The study is a single center, parallel group, randomized controlled trial. Two hundred and ten adults with insomnia and asthma that is not well-controlled will be randomized to either a 9-week Internet-based CBT-I program (Sleep Healthy Using the Internet (SHUTi)) or an enhanced usual care condition which utilizes an online educational video about insomnia. The primary sleep outcome is insomnia severity measured by the Insomnia Severity Index. Secondary sleep outcomes are sleep quality and wrist actigraph-recorded sleep parameters. Asthma control will be assessed by the Asthma Control Test, Asthma Quality of Life Questionnaire, pulmonary function testing, and self-report of asthma exacerbations and asthma-related healthcare utilization. Treatment outcomes will be measured at baseline, 9 weeks, and 6 months.
Discussion:
This trial has the potential to identify a novel strategy for improving asthma control. Findings may advocate for the inclusion of treatment of comorbid insomnia into current asthma management practice guidelines.
Keywords: Asthma, Insomnia, Cognitive-behavioral treatment
1. Introduction
Insomnia is the most common sleep disorder, comprising sleep-specific complaints, such as difficulty falling asleep, difficulty staying asleep, or poor sleep quality, and associated daytime symptoms. Insomnia has a prevalence of 10–15% in the adult population [1], and is even more common among those with medical conditions [2]. For instance, approximately 37% of adults with asthma have insomnia [3]. Asthma is highly prevalent, affecting at least 19 million adults in the United States [4]. The cost and morbidity of asthma, including health care costs and utilization, activity limitations, reduced quality of life, and increased risk for mortality, inflict a considerable burden on patients, families, and health care systems [5–7]. Asthma exacerbations, characterized by acute occurrences of increasing symptoms including wheezing, chest tightness, breathlessness, and coughing and decreased lung function, are experienced by at least 10 million adults annually [4]. Achieving asthma control is the goal of asthma therapy and an essential component of asthma management [8]. Reducing current impairment (i.e., frequency and intensity of symptoms and functional limitations) and reducing future risk (i.e., recurrent asthma exacerbations and progressive decline in lung function) are the two main elements of asthma control. Population-based studies suggest that up to 84% of patients fail to achieve good asthma control [9–12]. Identifying targets for intervention that could have substantial impact on asthma control is of great importance for reducing the burden of disease.
Relationships between insomnia and asthma are likely to be bidirectional. Nocturnal awakenings due to nighttime asthma symptoms and the need for quick relief medications can disrupt sleep and potentially exacerbate insomnia severity [13–17]. On the other hand, insomnia can negatively affect asthma control by further limiting engagement in activities and quality of life (current impairment) and by heightening the airway inflammatory response to environmental triggers, thus contributing to worsening of asthma control [3,18–21].
Cognitive-behavioral treatment for insomnia (CBT-I) is a behavioral intervention that uses well-validated techniques including sleep restriction, stimulus control, sleep hygiene, and cognitive techniques to address maladaptive behaviors and thoughts that perpetuate insomnia. CBT-I implemented in medical and psychiatric populations with comorbid insomnia has been shown to improve insomnia severity and general measures of mood, fatigue, and quality of life [22,23]. The efficacy of CBT-I in adults with asthma has not been investigated in a randomized controlled trial (RCT). This trial will examine the effects of CBT-I on sleep and asthma outcomes in adults. We will also explore a mechanistic hypothesis, that insomnia treatment alters a physiological mechanism, inflammation, that links sleep disturbances to inadequate asthma control
2. Methods
The purpose of this study is to test the efficacy of CBT-I on sleep and asthma control in adults with insomnia and asthma. In this trial, the CBT-I intervention will be delivered as a web-based program (Sleep Healthy Using the Internet (SHUTI)) and will be compared to enhanced usual care (EUC) for insomnia, which includes an online educational video about insomnia. The specific aims of this RCT are to (1) compare the efficacy of SHUTi compared to EUC on (la) sleep outcomes (insomnia severity, sleep quality, and wrist actigraph-recorded sleep parameters) and (1b) asthma control at post-treatment (9 weeks) and 6 months; and (2) determine whether changes in sleep mediate the effects of treatment on asthma control at post-treatment and 6 months. An exploratory aim is to determine whether changes in biomarkers of airway inflammation (exhaled nitric oxide, blood eosinophils) at post-treatment and 6-month follow-up account for the relationship between changes in sleep (from baseline to post-treatment) and improvements in asthma control. We hypothesize that SHUTi, compared with EUC, will lead to greater improvements in sleep outcomes and asthma control at post-treatment and 6 months. We also hypothesize that improvements in sleep (from baseline to post-treatment) will mediate the effects of treatment on improvements in asthma control from post-treatment to 6 months.
2.1. Study design
This study is a single-center, parallel group randomized controlled study in which participants are randomized to one of two conditions: an Internet-delivered CBT-I intervention or an EUC condition, consisting of an online educational video about insomnia. The study has been approved by the University of Pittsburgh Institutional Review Board and is registered with ClinicalTrials.gov (Identifier: NCT03327519). Interested individuals will undergo an initial telephone screening which will provide a brief description of the study. Individuals will be asked questions related to demographic information, Internet access, asthma control and quality of life, asthma medications, sleep characteristics, and insomnia symptoms (Table 1). Potential participants who meet the initial screening criteria will be scheduled for a research laboratory visit in the University of Pittsburgh/University of Pittsburgh Medical Center (UPMC) Asthma Institute to review and sign the study consent form and to conduct additional eligibility assessments (Table 1). After eligibility screening and signing informed consent, baseline clinical, sleep, and self-report assessments will be completed, followed by randomization (Fig. 1). Participants will then have access to the Internet-delivered CBT-I program or EUC over the 9-week week intervention period. Follow-up assessments will be conducted immediately post intervention (9 weeks) and at 6 months to determine short and longer-term effects.
Table 1.
Assessment measures.
| Assessment | Telephone screening | Face-to-face screening | Baseline | 9 Weeks | 6 months |
|---|---|---|---|---|---|
| Asthma Control Test | × | × | × | × | |
| Asthma Quality of Life Questionnaire | × | × | × | × | |
| Insomnia Severity Index | × | × | × | × | |
| DSM 5 Insomnia Diagnosis | × | ||||
| Informed Consent | × | ||||
| Multivariate Apnea Prediction Questionnaire | × | ||||
| Epworth Sleepiness Scale | × | ||||
| Patient Health Questionnaire-9 | × | ||||
| GerdQ | × | ||||
| NIDA Quick Screen | × | ||||
| Physical | × | ||||
| Medical History and Medications | × | ||||
| Spirometry | × | × | |||
| Pregnancy test, if applicable | × | ||||
| Demographic variables | × | ||||
| Pittsburgh Sleep Quality Index | × | × | × | ||
| PROMIS Sleep-Related Impairment Scale | × | × | × | ||
| PROMIS Sleep Disturbance Scale | × | × | × | ||
| Asthma Exacerbation and Healthcare Utilization Questionnaire | × | × | × | ||
| Blood eosinophils | × | × | × | ||
| Fractional nitric oxide | × | × | × | ||
| Sleep diary | × | × | × | ||
| Actigraphy | × | × | × |
Fig. 1.
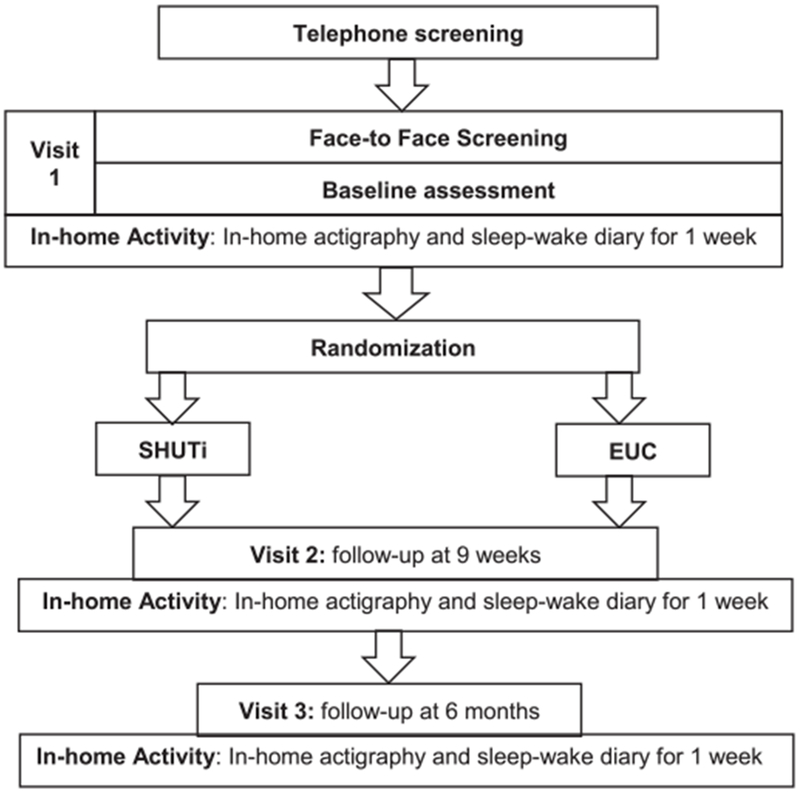
Study flow diagram.
2.2. Recruitment
Participants will be recruited through two local registries: the University of Pittsburgh Clinical and Translational Science Institute (CTSI) Research Participant Registry (Pitt + Me) and the University of Pittsburgh/UPMC Asthma Institute patient research registry. The Pitt + Me registry is a database of > 107,000 individuals willing to consider participation in research studies. The registry’s software matches participants, based on their demographics and on their ICD-9 or ICD-10 code(s) and/or health preferences, with studies for which they may be eligible. Registry participants receive study alert emails about studies in which they may have interest. If interested, they will contact the Pitt + Me registry screening office, who will initiate a pre-screen and pass on contact information of those who pass the pre-screen to the study researchers. The Asthma Institute has an active asthma patient research registry with over 1600 patients. The registry receives referrals from the General Internal Medicine Division outpatient clinic at UPMC and through the electronic medical record system in the pulmonary and allergy clinics throughout UPMC. In addition, there is a full time recruiter/outreach specialist that advertises and seeks study participants in the community at large. Both registries utilize social media (e.g., Facebook, Twitter) to advertise studies. Alternative recruitment strategies (e.g., adding recruitment sites and intensifying and expanding recruitment efforts) will be explored if enrollment lags are detected.
2.3. Participants
The study will enroll a total of 210 men and women ages 18 and over with diagnoses of insomnia and asthma that is not well controlled. Eligible participants must have reliable internet access because the intervention and EUC condition occur via the Internet. Patients at high risk for obstructive sleep apnea will be excluded as defined by a Multivariate Apnea Prediction Questionnaire [24] score ≥0.48 and an Epworth Sleepiness Scale [25] score > 10. Patients using medications known to affect sleep or wake function (e.g., caffeine, alcohol, hypnotics, benzodiazepines, antidepressants, anxiolytics, antipsychotics, decongestants and sedating antihistamines, and beta blockers) will not be excluded, since these medications are commonly used among insomnia patients and will be addressed as part of the intervention. We will monitor participants’ use of these medications via the sleep-wake diaries. Inclusion and exclusion criteria are summarized in Table 2.
Table 2.
Inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
|
|
FEV1 forced expiratory volume in one second; PC20, provocative concentration of methacholine required to cause a 20% reduction in FEV1.
2.4. Randomization and blinding
Participants will be randomly assigned to either SHUTi or EUC using stratified permuted-block design, with random block sizes of 2, 4, or 6. Participants will be stratified prior to randomization by sex and age (18–40, 41–60, > 60). A randomization list for each sex-age combination will be computer generated by the study statistician. Study personnel involved in recruitment, screening, data collection, and data entry will be blinded to group assignment.
2.5. SHUTi
The intervention condition will utilize an online insomnia intervention (SHUTi). SHUTi is based on CBT-I and incorporates the main components of CBT-I (sleep restriction, stimulus control, cognitive restructuring, sleep hygiene, and relapse prevention) into 6 modules or Cores. Users obtain access to a new Core one week after completion of the previous Core, which is analogous to the schedule used during traditional face-to-face CBT-I. Participants complete daily sleep diaries throughout their use of SHUTi. The sleep diary data is used to track progress, to tailor treatment recommendations (e.g., assign a sleep restriction window), and to assess adherence to the sleep restriction window. Each Core follows the same structure of the weekly sessions of traditional CBT-I: (1) core objectives (what will be learned), (2) review of previous week’s homework and sleep diary data, (3) new intervention material, (4) assignment of homework (treatment strategies for improving sleep over the coming week), and (5) summary of the Core’s main points. Each Core takes 45–60 min to complete. Intervention content is presented through interactive elements including personalized goal-setting, graphics, animation, quizzes to test user knowledge, and vignettes. Automated emails are sent to encourage adherence. No modifications were made to SHUTi for this trial. For a complete description of the SHUTi program, see Thorndike et al., [26].
2.6. EUC
The EUC condition will use an online educational video on insomnia which was developed by Emmi Solutions in consultation with two of the investigators (DJB, PJS). The 25-minute video contains educational information on physiological controls of sleep, sleep hygiene practices, healthy sleep behaviors (e.g., reduce time in bed, get up at the same time every day, go to bed only if sleepy, and do not stay in bed unless asleep), and sleep medications, a self-efficacy assessment, and enables patients to set individual goals. However, it does not present participants with an individualized intervention strategy.
2.7. Assessments
Participants will complete assessments to evaluate insomnia/sleep variables, asthma control, and biomarkers of airway inflammation at baseline, post-treatment, and 6 months (Table 1). Actigraphy and sleep diary will be completed for 1 week in participants’ homes at each assessment point. In order to maintain contact and promote retention, telephone phone calls at 4 week intervals between baseline, posttreatment, and 6-month research laboratory visits will be conducted to collect information on adverse events and medication changes. Additionally, participants will receive reminders for follow-up visits via email or telephone call. We will provide $25 for completion of baseline research laboratory visit and upon return of the Actiwatch following baseline assessment; $37.50 for completion of post-treatment research laboratory visit and upon return of the Actiwatch following posttreatment assessment; and $62.50 for completion of 6-month research laboratory visit and upon return of the Actiwatch following 6-month assessment. Total compensation can total $250 via reloadable debit card.
2.7.1. Sleep
The primary outcome will be insomnia severity assessed by the Insomnia Severity Index (ISI) [27,28]. Secondary outcomes will include the Pittsburgh Sleep Quality Index (PSQI) [29], a global measure of subjective sleep quality, Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance and Sleep-Related Impairment Scales [30,31], which are well-validated, and sleep parameters, including sleep onset latency (minutes to fall asleep after lights out), sleep efficiency (time slept asleep divided by time in bed), wake after sleep onset (minutes spent wake after sleep onset), and total sleep time using an online version of the Consensus Sleep Diary [32] and actigraphy. Actigraphy provides a method to objectively and prospectively estimate sleep-wake patterns. Actiwatch 2® actigraphs (Philips-Respironics, Bend, OR) will be used in this study.
2.7.2. Asthma control
The primary outcome will be asthma-related quality of life assessed by the Asthma Quality of Life Questionnaire (AQLQ) [33,34], a validated condition-specific health-related quality of life measure that evaluates four life domains impacted by asthma: activity limitations, emotional distress, symptoms, and environmental stimuli. Asthma-related quality of life is a critical element of asthma control because it is associated with patients’ perceived control of asthma and predicts future asthma-related health care utilization [35–37]. Secondary outcomes will include the Asthma Control Test (ACT) [38,39], pulmonary function, asthma exacerbations, and asthma-related healthcare use. Spirometry will be performed according to American Thoracic Society guidelines after appropriate withholding of medications [40]. Maximum bronchodilation will be performed with spirometry to assess bronchodilator reversibility. Participants will receive four inhalations of albuterol from a metered dose inhaler and spirometry will be repeated 15 min later. Pre- and post-bronchodilator forced expiratory volume in 1 s (FEV1), FEV1% predicted, forced vital capacity (FVC), and FEV1/FVC ratio, in addition to bronchodilator responsiveness (change in FEV1 after inhaled bronchodilator), will be evaluated.
Biomarkers of airway inflammation will include exhaled nitric oxide and blood eosinophils. Exhaled nitric oxide measure is a noninvasive method for assessing the severity of airway inflammation in asthma [41]. Fractional exhaled nitric oxide will be measured according to manufacturer’s instructions for the Aerocrine NIOX VERO®. Blood will be drawn for complete blood count with differential count to measure eosinophils.
2.8. Sample size justification
The total sample size of 210 (105 per group) was determined focusing on the hypothesis testing of between-group differences (SHUTi vs. EUC) in the mean changes in the primary outcomes of insomnia severity and asthma-related quality of life, and the correlational/regression analyses to assess changes in insomnia severity with changes in asthma quality of life; adjustment of the level of significance for the repeated testing at post-treatment (9 weeks) and 6 months for each primary outcome to 0.025, and considering the expected rate of attrition by 6 months follow-up. To date, no previous trial has specifically examined insomnia symptom response to SHUTi among individuals with asthma. Sample size was therefore estimated by examining SHUTi response in the primary study outcomes of interest among individuals with medical conditions in terms of the standardized mean difference, d. Ritterband et al. [42] demonstrated a large effect for SHUTi compared to a waitlist control group (in favor of SHUTi) on the order of d = 1.85 for insomnia severity (assessed by the ISI) at post-treatment (9 weeks) among cancer survivors with insomnia. Moreover, moderate to large effects for the ISI of d = 0.95 at post-treatment and d = 0.52 at 6-month follow-up (in favor of SHUTi) were observed in a subsample of 38 adults with asthma who were randomly assigned as part of a larger study to either SHUTi (n = 20) or patient education (n = 18) (L.M. Ritterband, pH.D., unpublished data). In terms of asthma-related quality of life, moderate treatment effects, on the order of d = 0.48, were observed for SHUTi compared to a waitlist control group for mental health quality of life (based on the Mental Component Summary of the 12-item Short-Form Health Survey [43]) among cancer survivors with insomnia [42]. Moreover, through a secondary analysis of data from the SHUTi trial with cancer survivors [42], moderate sized correlations of r = 0.345 between changes in insomnia severity and changes in mental health quality of life from baseline to post-treatment in the total sample, largely due to changes in the SHUTi participants. Given these findings, we plan to enroll 105 participants per treatment group (210 total), allowing for up to 20% attrition at 6-month follow-up (i.e., 168 participants with complete follow-up) (L.M. Ritterband, pH.D., unpublished data). This sample size will ensure that we have at least 80% power to: 1) detect between-group differences in the changes in a) insomnia severity from baseline to post-treatment (power > 99%) and 6-month follow-up (power = 86.5%) and b) asthma-related quality of life from baseline to post-treatment (power = 80.1%) and 6-month follow-up (power = 80.1%); and 2) assess changes in insomnia severity as a mediating variable of the effect of SHUTi on asthma-related quality of life (power > 99%).
2.9. Statistical analysis
Initial analyses will involve data description and screening for anomalies (e.g., outliers, missing data, non-normality). Results from these analyses will be used to (1) describe univariate and bivariate distributions, (2) identify group imbalances and associations between dependent variables and suspected covariates/confounders, (3) evaluate missing data, and (4) check for violation of statistical assumptions. If assumptions are violated, data transformations or more statistically robust procedures will be considered. Covariates/confounders (e.g., age at study entry, use of asthma medications) will be included in models secondarily, and their effects on primary predictors will be evaluated.
For comparison of the efficacy of SHUTi compared to EUC on sleep outcomes, an intent-to-treat approach will be used, in which all participants will be included in the groups to which they were randomly assigned, regardless of whether they actually received it. The primary sleep outcome variable will be insomnia severity as measured by the ISI total score. Secondary sleep outcomes include the PSQI and PROMIS Sleep Scales and measures of sleep parameters from the sleep diary and actigraphy which include sleep onset latency, sleep efficiency, wake after sleep onset, and total sleep time. Average sleep onset latency, sleep efficiency, wake after sleep onset, and total sleep time across all days during each assessment period was calculated. Linear mixed-effects modeling with linear contrasts will be used to examine the effect of treatment assignment (SHUTi vs. EUC) on each sleep outcome, with treatment group assignment as the between-subjects factor, and an interaction term between time and group. Random effects for subjects will also be included. Fixed and/or time-dependent covariates (including baseline values) may be included secondarily to adjust for group imbalances or variables related to the dependent variables. Variables having at least moderate associations (i.e., r > 0.30 or odds ratio > 2.5) or medium-sized differences (d > 0.50) with treatment group and/or dependent variables will be considered secondarily when fitting linear mixed effects models and the relative change in parameter estimates and the values of test statistics will be noted. Linear contrasts will be specified and estimated in the repeated measures models to test whether SHUTi will demonstrate greater improvements in sleep measures at post-treatment and 6 months relative to baseline value compared to EUC. Marginal modeling with generalized estimating equations (assuming an unstructured covariance structure) will also be used to analyze each sleep measure because it tends to be more robust to misspecification of the covariance structure and violations in normality assumptions. Results from the application of each modeling approach will be compared via sensitivity analyses, noting changes in parameter estimates and the conclusions drawn based on hypothesis testing. To explore sex as a moderator of the treatment efficacy of SHUTi relative to EUC, the linear mixed models for each sleep measure will be expanded to include sex and its interactions with the other model terms (treatment group, time, and treatment group by time). The linear contrasts will also be re-specified and re-estimated to explore the interaction of sex and treatment group on changes in sleep measures from baseline to post-treatment and 6-months. As the investigation of sex as moderating factor is exploratory, no multiplicity adjustment will be applied.
To explore unique subgroups of SHUTi treatment non-responders, descriptive analyses will provide the proportion of participants who achieve sleep treatment response (defined as a clinically significant change in insomnia severity based on the ISI [i.e., ISI change > 6 points] at post-treatment). Two-sample t-tests and chi-square tests of independence will be conducted to explore differences in demographic (age, sex, marital status, employment status, smoking status), insomnia-related (duration, severity, sleep medication usage), and asthma-related (duration, severity, quick relief inhaler use, exacerbations) variables between treatment responders and non-responders.
For comparison of the efficacy of SHUTi compared to EUC on asthma control, a similar analysis plan will be used as described above. The primary asthma control outcome will be asthma-related quality of life based on the AQLQ total score. Secondary asthma control outcomes include the ACT, spirometry, asthma exacerbations, and asthma-related healthcare use. Descriptive analyses will be conducted to provide the proportion of participants who achieve a clinically important improvement on the Asthma Quality of Life Questionnaire (i.e., score change ≥0.5 points). Characteristics of improvers and non-improvers will be examined.
To determine if improvements in sleep from baseline to post-treatment mediate the effects of treatment on improvements in asthma control from post-treatment to 6 months, simple mediational models (i.e., models containing a single predictor, single mediator, and single outcome) will be specified and estimated. The predictor variable will be treatment group assignment (SHUTi versus EUC), the mediator variable will be the change in a single sleep measure from baseline to posttreatment, and the outcome variable will be the change in a single asthma control measure from post-treatment to 6 months. The primary sleep outcome will be insomnia severity based on the ISI total score and the primary asthma control outcome will be asthma-related quality of life based on the AQLQ total score. Observed variable path analysis will be used to fit the proposed mediation models. Path coefficients with standard errors and R2 values for the proximal (sleep) and distal (asthma control) endogenous variables will be estimated. The observed variable path analysis via bias-corrected bootstrapping (with 5000 bootstrapped samples) will be used to test for the mediation effect and to yield point and interval estimates of indirect, direct, and total effects. In addition to better accommodating non-normality and having greater statistical power than traditional approaches to mediation analyses (e.g., Baron-Kenny approach, Sobel test), bias-corrected bootstrapping will be employed. Goodness-of-fit will be assessed using the recommended fit indices including the root mean square error of approximation and comparative fit index. Residual analyses will be performed for each path analysis model to identify sources of model misspecification, outliers, and influential observations.
To determine whether changes in biomarkers of airway inflammation across post-treatment and 6-month follow-up account for the relationship between changes in sleep (from baseline to post-treatment) and improvements in asthma control, a similar analytic approach using simple mediational analyses as outlined above will be applied. The predictor variable will be the change in a single sleep outcome, the mediator variable will be the change in a single biomarker of airway inflammation, and the outcome variable will be the change in a single asthma control outcome.
3. Discussion
This study aims to evaluate the effects of CBT-I (compared to EUC) on insomnia severity, subjective and objective sleep parameters, and asthma control reflected by ACT score, asthma-related quality of life, and asthma exacerbations and related healthcare utilization among adults with not well-controlled asthma and comorbid insomnia. We will also explore markers of airway inflammation as a potential physiological mechanism linking improvements in sleep with improvements in asthma control. Implementing a targeted treatment for insomnia would enable us to see if it is possible to significantly reduce insomnia as well as improve asthma control with this targeted treatment. Furthermore, it will provide insight into the complex relationships between insomnia and asthma control.
Given the demands of our evolving health care system, a low cost, scalable method for delivering CBT-I in clinical settings is needed to increase access to care [44]. Our study will utilize a self-guided Internet-based CBT-I (SHUTi), an efficacious and low cost treatment that can be delivered in a real-world clinical setting. Use of an Internet-based CBT-I overcomes the limitations of face-to-face CBT-I, which relies on patient motivation to attend six to eight weekly in-person sessions, remember to keep sleep diaries, and implement changes between sessions without support [45]. SHUTi provides 24/7 accessibility and support, which may enable individuals to better adhere to the intervention and successfully implement tailored recommendations for improving sleep in real life.
Although trials of Internet-based CBT-I included individuals with medical conditions with the exceptions of major psychiatric disorders, other sleep disorders, and unstable chronic disorders, condition-specific clinical indices were not assessed, other than general measures of mood, fatigue, and quality of life [46,47]. This trial will expand upon prior studies examining Internet-based CBT-I by including an extensive assessment of condition-specific (i.e., asthma) clinical outcomes and collection of preliminary data on possible biological mechanisms.
Our results could offer an innovative strategy for improving asthma control that could have a substantial impact on asthma care and quality of life for patients. Ultimately, findings from our study could encourage the inclusion of insomnia—as a comorbid condition that affects asthma control—into the current guidelines for the proper management of asthma.
Funding/Support
This work was supported by the National Institutes of Health [grant number R01HL131587-01A1].
Conflicts of interest disclosures
This was not an industry funded study. X.S., S.M.S. and F.S.L have indicated no financial conflicts of interest. L.M.R. has equity ownership in BeHealth Solutions, LLC, a company developing and making available products related to the research reported in this publication. Specifically, BeHealth Solutions, LLC, has licensed the SHUTi program and the software platform on which it was built from the University of Virginia. The terms of this arrangement have been reviewed and approved by the University of Virginia in accordance with its conflict of interest policy. D.J.B. reports receiving consultation fees from Bayer Healthcare, BeHealth Solutions, Ebb Therapeutics, CME Institute, and Emmi Solutions. In addition, D.J.B. receives licensing fees (royalties) for the Pittsburgh Sleep Quality Index (PSQI), which is copyrighted by the University of Pittsburgh. S.E.W. reports grants and personal fees from AstraZeneca, grants from GSK, grants from Boehringer-Ingelheim, grants and personal fees from Sanofi Regeneron, grants from Novartis, grants from Merck, outside the submitted work. P.J.S. reports grants from Inspire Medical Systems and Jazz Pharmaceuticals, outside the submitted work, and receiving consultation fees from Inspire Medical Systems, Jazz Pharmaceuticals, Itamar Medical, National Football League, and Emmi Solutions.
Abbreviations
- ACT
Asthma Control Test
- AQLQ
Asthma Quality of Life Questionnaire
- CBT-I
cognitive-behavioral treatment for insomnia
- CTSI
Clinical and Translational Science Institute
- EUC
enhanced usual care
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- ISI
Insomnia Severity Index
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PSQI
Pittsburgh Sleep Quality Index
- RCT
randomized controlled trial
- SHUTi
Sleep Healthy Using the Internet
- UPMC
University of Pittsburgh Medical Center
References
- [1].Ohayon MM, Epidemiology of insomnia: what we know and what we still need to learn, Sleep Med. Rev. 6 (2002) 97–111. [DOI] [PubMed] [Google Scholar]
- [2].Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL, Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders, Sleep. 34 (2011) 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Luyster FS, Strollo PJ, Holguin F, Castro M, Dunican EM, Fahy J, et al. , Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III, CHEST J. 150 (2016) 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moorman JE, Akinbami LJ, Bailey C, Zahran H, King M, Johnson C, et al. , National Surveillance of asthma: United States, 2001-2010 Vital & health statistics series 3, analytical and epidemiological studies/US Dept of health and human services, public health service, National Center for Health Statistics; (2012) 1–67. [PubMed] [Google Scholar]
- [5].Ali Z, Dirks CG, Ulrik CS, Long-term mortality among adults with asthma longterm asthma mortalitya 25-year follow-up of 1,075 outpatients with asthma, CHEST J. 143 (2013) 1649–1655. [DOI] [PubMed] [Google Scholar]
- [6].Barnett SBL, Nurmagambetov TA, Costs of asthma in the United States: 2002-2007, J. Allergy Clin. Immunol. 127 (2011) 145–152. [DOI] [PubMed] [Google Scholar]
- [7].Guilbert TW, Garris C, Jhingran P, Bonafede M, Tomaszewski KJ, Bonus T, et al. , Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life, J. Asthma 48 (2011) 126–132. [DOI] [PubMed] [Google Scholar]
- [8].Expert Panel Report 3, Guidelines for the Diagnosis and Management of Asthma, National Heart, Lung, and Blood Institute, Bethesda, 2007. [Google Scholar]
- [9].Colice GL, Ostrom NK, Geller DE, Anolik R, Blaiss M, Marcus P, et al. , The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States, Ann. Allergy Asthma Immunol. 108 (2012) 157–162 ( e1). [DOI] [PubMed] [Google Scholar]
- [10].Fuhlbrigge A, Reed ML, Stempel DA, Ortega HO, Fanning K, Stanford RH. The status of asthma control in the US adult population Allergy Asthma Proc.: OceanSide Publications, Inc; 2009. p. 529–33. [DOI] [PubMed] [Google Scholar]
- [11].Murphy KR, Meltzer EO, Blaiss MS, Nathan RA, Stoloff SW, Doherty DE. Asthma management and control in the United States: results of the 2009 Asthma Insight and Management survey Allergy Asthma Proc.: OceanSide Publications, Inc; 2012. p. 54–64. [DOI] [PubMed] [Google Scholar]
- [12].Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin S-L, Globe G, et al. , Asthma control in the United States, 2008-2010: indicators of poor asthma control, J. Allergy Clin. Immunol. 133 (2014) 1579–1587. [DOI] [PubMed] [Google Scholar]
- [13].Braido F, Baiardini I, Ghiglione V, Fassio O, Bordo A, Cauglia S, et al. , Sleep Disturbances and Asthma Control: A Real Life Study, (2009). [PubMed]
- [14].Krouse HJ, Yarandi H, McIntosh J, Cowen C, Selim V, Assessing sleep quality and daytime wakefulness in asthma using wrist actigraphy, J. Asthma 45 (2008) 389–395. [DOI] [PubMed] [Google Scholar]
- [15].Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, et al. , Sleep quality and asthma control and quality of life in non-severe and severe asthma, Sleep Breath. 16 (2012) 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM, Centers ALAACR, Sleep quality in asthma: results of a large prospective clinical trial, J. Asthma 45 (2008) 183–189. [DOI] [PubMed] [Google Scholar]
- [17].Sundbom F, Lindberg E, Bjerg A, Forsberg B, Franklin K, Gunnbjörnsdottir M, et al. , Asthma symptoms and nasal congestion as independent risk factors for insomnia in a general population: results from the GA 2 LEN survey, Allergy. 68 (2013) 213–219. [DOI] [PubMed] [Google Scholar]
- [18].Axelsson J, Rehman J.-u., Akerstedt T, Ekman R, Miller GE, Höglund CO, et al. , Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/T helper 2 balance in humans, PLoS One 8 (2013) e82291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dimitrov S, Lange T, Tieken S, Fehm HL, Born J, Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans, Brain Behav. Immun. 18 (2004) 341–348. [DOI] [PubMed] [Google Scholar]
- [20].Lange T, Dimitrov S, Fehm H-L, Westermann J, Bom J, Shift of monocyte function toward cellular immunity during sleep, Arch. Intern. Med 166 (2006) 1695–1700. [DOI] [PubMed] [Google Scholar]
- [21].Sakami S, Ishikawa T, Kawakami N, Haratani T, Fukui A, Kobayashi F, et al. , Coemergence of insomnia and a shift in the Thl/Th2 balance toward Th2 dominance, Neuroimmunomodulation 10 (2002-2003) 337–343. [DOI] [PubMed] [Google Scholar]
- [22].Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M, Cognitive behavioral therapy in persons with comorbid insomnia: a metaanalysis, Sleep Med. Rev. 23 (2015) 54–67. [DOI] [PubMed] [Google Scholar]
- [23].Wu JQ, Appleman ER, Salazar RD, Ong JC, Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis, JAMA Intern. Med. 175 (2015) 1461–1472. [DOI] [PubMed] [Google Scholar]
- [24].Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, et al. , A survey screen for prediction of apnea, Sleep. 18 (1995) 158–166. [DOI] [PubMed] [Google Scholar]
- [25].Johns MW, A new method for measuring daytime sleepiness: the Epworth sleepiness scale, Sleep. 14 (1991) 540–545. [DOI] [PubMed] [Google Scholar]
- [26].Thorndike FP, Saylor DK, Bailey ET, Gonder-Frederick L, Morin CM, Ritterband LM, Development and perceived utility and impact of an internet intervention for insomnia, E J Appl Psychol. 4 (2008) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bastien CH, Vallières A, Morin CM, Validation of the insomnia severity index as an outcome measure for insomnia research, Sleep Med. 2 (2001) 297–307. [DOI] [PubMed] [Google Scholar]
- [28].Morin CM, Belleville G, Bélanger L, Ivers H, The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response, Sleep. 34 (2011) 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research, Psychiatry Res. 28 (1989) 193–213. [DOI] [PubMed] [Google Scholar]
- [30].Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. , Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments, Sleep. 33 (2010) 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. , Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks, Behav. Sleep Med. 10 (2012) 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. , The consensus sleep diary: standardizing prospective sleep self-monitoring, Sleep. 35 (2012) 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR, Validation of a standardized version of the asthma quality of life questionnaire, CHEST Journal. 115 (1999) 1265–1270. [DOI] [PubMed] [Google Scholar]
- [34].Leidy N, Coughlin C, Psychometric performance of the asthma quality of life questionnaire in a US sample, Qual. Life Res. 7 (1998) 127–134. [DOI] [PubMed] [Google Scholar]
- [35].Eisner MD, Ackerson LM, Chi F, Kalkbrenner A, Buchner D, Mendoza G, et al. , Health-related quality of life and future health care utilization for asthma, Ann. Allergy Asthma Immunol. 89 (2002) 46–55. [DOI] [PubMed] [Google Scholar]
- [36].Katz PP, Yelin EH, Eisner MD, Blanc PD, Perceived control of asthma and quality of life among adults with asthma, Ann. Allergy Asthma Immunol. 89 (2002) 251–258. [DOI] [PubMed] [Google Scholar]
- [37].Magid DJ, Houry D, Ellis J, Lyons E, Rumsfeld JS, Health-related quality of life predicts emergency department utilization for patients with asthma, Ann. Emerg. Med 43 (2004) 551–557. [DOI] [PubMed] [Google Scholar]
- [38].Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. , Development of the asthma control test: a survey for assessing asthma control, J. Allergy Clin. Immunol. 113 (2004) 59–65. [DOI] [PubMed] [Google Scholar]
- [39].Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. , Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists, J. Allergy Clin. Immunol. 117 (2006) 549–556. [DOI] [PubMed] [Google Scholar]
- [40].Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. , Standardisation of spirometry, Eur. Respir. J 26 (2005) 319–338. [DOI] [PubMed] [Google Scholar]
- [41].Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. , An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications, Am. J. Respir. Crit. Care Med. 184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD, Initial evaluation of an internet intervention to improve the sleep of cancer survivors with insomnia, Psycho-Oncology. 21 (2012) 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ware JE Jr., Kosinski M, Keller SD, A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity, Med. Care 34 (1996) 220–233. [DOI] [PubMed] [Google Scholar]
- [44].Smith M, Saunders R, Stuckhardt L, McGinnis JM, Best Care at Lower Cost: The Path to Continuously Learning Health Care in America, National Academies Press, 2013. [PubMed] [Google Scholar]
- [45].Espie CA, Hames P, McKinstry B, Use of the internet and mobile media for delivery of cognitive behavioral insomnia therapy, Sleep Med. Clin. 8 (2013) 407–419. [Google Scholar]
- [46].Seyffert M, Lagisetty P, Landgraf J, Chopra V, Pfeiffer PN, Conte ML, et al. , Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis, PLoS One 11 (2016) e0149139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zachariae R, Lyby MS, Ritterband LM, O’Toole MS, Efficacy of internet-delivered cognitive-behavioral therapy for insomnia–a systematic review and meta-analysis of randomized controlled trials, Sleep Med. Rev. 30 (2016) 1–10. [DOI] [PubMed] [Google Scholar]


