Abstract
Wernicke encephalopathy (WE) is a neuropsychiatric syndrome caused by thiamine deficiency. In addition to the classical symptoms (oculomotor disorder, confusion, and ataxia), acute polyneuropathy is reported to accompany the clinical condition occasionally. In this case report, we intended to present and attract attention to this rare and significant clinical table which starts with application for WE clinical condition, and is accompanied by acute axonal polyneuropathy in the follow-up stage.
Keywords: Wernicke encephalopathy, thiamine deficiency, acute axonal polyneuropathy
INTRODUCTION
Wernicke’s encephalopathy (WE) is a clinical table that develops due to thiamine (vitamin B1) deficiency and that is characterized by the classical triad of oculomotor dysfunction, confusion and ataxia. It can be caused by chronic malnutrition due to long-term alcohol consumption, prolonged fasting and hyperemesis gravidarum or can occur iatrogenically after bariatric surgery and total parenteral nutrition support (1). In about 80% of patients with Wernicke-Korsakoff encephalopathy, chronic axonal polyneuropathy symptoms such as dominant sensory disorders, decreased muscle strength and decreased deep tendon reflexes can be found in their distal extremities. More rarely, there are cases where acute axonal polyneuropathy is reported to develop simultaneously with the disease (2). In this essay, we wanted to present a case who developed acute axonal polyneuropathy while being followed for WE.
CASE REPORT
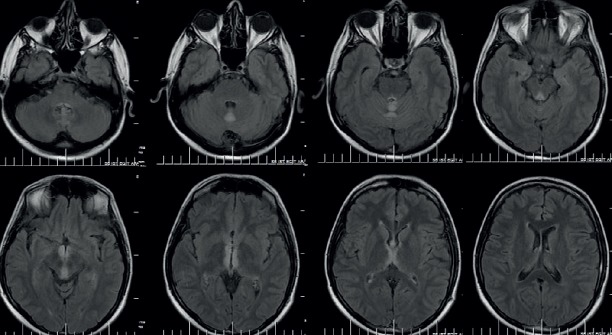
A 32-year-old male patient was admitted to the emergency department of our hospital with complaints of gait impairment, double vision and forgetfulness, which began four days before his admission. The patient, who had no personal history of serious illness, had a history of smoking and chronic daily alcohol consumption (150-200 ml/day for five years), to which was added malnutrition for the past 15 days. There were no abnormal findings on physical examination. In his neurological examination, his eyes were on the midline and his eye movements were severely restricted in all directions. Also, a gaze-evoked horizontal nystagmus, semiptosis, decreased deep tendon reflexes in his lower extremities and truncal ataxia were detected. In the brain magnetic resonance imaging (MRI) that was done in the emergency clinic, a hyperintensity was detected in the cerebellar vermis in the diffusion weighted images (DWI) and around the aqueduct neighboring the third ventricle and in the superior cerebellar vermis and bilateral medial thalamus in the T2 and FLAIR weighted images (Figure 1). The patient was hospitalized for the purpose of examination and treatment. Routine laboratory examinations did not reveal any additional findings, was started on a treatment of 200 mg/day intravenous (IV) thiamine based on his clinical and brain MRI findings which suggested WE. For technical reasons, the erythrocyte transketolase activity and serum thiamine levels could not be measured. In the evaluation pertaining to his amnesia, a significant loss in short term memory (memory subscore 0/3) was observed [mini mental state examination (MMSE): 27/30]. On the third day of treatment, his eye symptoms improved, except for bilateral horizontal nystagmus. While his truncal ataxia continued, weakness in dorsiflexion (0/5) and plantar flexion (3/5) was detected in the bilateral lower extremities a week after the patient’s admission. In the EMG examination done on 10 day of his hospitalization following this, symptoms consistent with an axonal polyneuropathy predominant in the motor fibers of the lower extremities were detected (Table 1). Lumbar puncture could not be done because the patient refused. No special features were detected in the lumbar spinal MRI. IV thiamine treatment was switched to oral on follow-up. Intermittent (100 mg/day once every other week) IV thiamine supplementation was added to the oral thiamine treatment. In the brain MRI scan that was done a month later, the existing lesions were seen to have regressed, with the exception of the cerebellar vermis (Figure 2). In the check-up EMG examination that was done one and a half months later, abnormal spontaneous activity and a severe pattern of rarefaction were detected particularly in the distally located muscles in the lower extremities in addition to low compound muscle action potential (CMAP) amplitudes in the nerves examined in the lower extremities (Table 2). In the 3rd month examination of the patient whose oral thiamine treatment continued, the motor weakness in the lower extremities had almost completely improved. In the EMG examination done on the 3rd month, the CMAP amplitudes of the nerves examined in the lower extremities were low and in the needle EMG, abnormal spontaneous activity was detected in places during rest in the bilateral tibialis anterior muscle. Spontaneous activity was not detected in the other muscles. In the examination of the patient done at the end of the 6th month, the muscle strength in the lower extremities was full, the CMAP amplitudes of the nerves in the lower extremities had improved compared to the previous examination and while changes in the mild neurogenic motor unit potentials (MUP) were observed during voluntary contraction and a pattern of rarefaction was observed in the distal muscles during full contraction, no abnormal spontaneous activity was observed (Table 3).
Figure 1.
Brain MRI findings of patient initially. Hyperintensity lesions in the superior cerebellar vermis, periaquaduktal region and bilateral medial thalamus in the axial FLAİR sequences.
Table 1.
Nerve conduction studies on 10 day of hospitalization (weakness in the legs after 3 days).
| Latency (ms) | Amplitude (mV) | Velocity (m/s) | |
|---|---|---|---|
| Sensory nerve action potential | |||
| R Median-II.finger | 2.8 | 17 | 42.9 |
| L Median-II.finger | 2.5 | 9.3 | 52 |
| R Ulnar-V.finger | 2.1 | 12 | 47.6 |
| R Sural | 2.7 | 12 | 44.4 |
| Compound motor action potential | |||
| R Median-APB | 3.6 | 7.5 | |
| 7.9 | 7.0 | 53.5 | |
| L Median-APB | 3.7 | 7.4 | |
| 8.2 | 7.0 | 57.8 | |
| R Ulnar-ADM | 1.96 | 7.1 | |
| 7.2 | 5.3 | 49.6 | |
| R Tibialis-AHB | 4.9 | 0.5 | |
| 16.4 | 0.3 | 37.8 | |
| L Peroneal-EDB | 3.5 | 0.7 | |
| 12.0 | 0.5 | 45.9 |
R: Right, L: Left, APB: Abductor pollicis brevis, ADM: Abductor digiti minimi, AHB: Abductor hallucis, EDB: Extensor digitorum brevis. Values in bold type are abnormal results.
Figure 2.
Brain MRI findings at end of the a month. The existing lesions were seen to have regressed, with the exception of the cerebellar vermis in the axial FLAİR sequences.
Table 2.
Concentric needle EMG at the end of the 1,5 months later
| Needle EMG | Pathological spontaneous activity | MUAP | |||||
|---|---|---|---|---|---|---|---|
| Fibrillation | PSW | CRD | Amp | Duration | Polyphasi | IP | |
| R Deltoid | N | N | N | N | N | N | N |
| L Biceps | N | N | N | N | N | N | N |
| L Fleksor carpi ulnaris | N | N | N | N | N | N | N |
| R Paravertebral T12 | N | N | N | N | N | N | N |
| L Gastrocnemius medial head | +2 | N | N | N | N | N | -2 |
| R Gastrocnemius medial head | +2 | N | N | N | N | N | -2 |
| R Peroneus longus | +2 | N | N | N | N | N | -2 |
| L Tibialis anterior | +3 | 1+ | N | N | N | N | -2 |
| R Tibialis anterior | +2 | 1+ | N | N | N | N | -2 |
| L Vastus lateralis | +2 | N | N | N | N | N | -1 |
| R Vastus lateralis | +1 | N | N | N | N | N | -2 |
| R İliopsoas | +1 | N | N | N | N | N | N |
R: Right, L: Left, Amp:Amplitude, MUAP: Motor unit action potantiel, N: Normal PSW: Positive sharp wave, CRD: Complex repetitive discharge, IP:İnterference pattern. Values in bold type are abnormal results.
Table 3.
Nerve conduction studies at the end of the 6th month
| Latency (ms) | Amplitude (mV) | Velocity (m/s) | |
|---|---|---|---|
| Sensory nerve action potential | |||
| R Median-II. finger | 2.3 | 17 | 56.5 |
| R Ulnar-V. finger | 2.1 | 12 | 52.4 |
| R Sural | 2.4 | 10 | 45.8 |
| L Sural | 2.4 | 7.7 | 45.8 |
| Compound motor action potential | |||
| R Median-APB | 3.0 | 9.1 | |
| 7.7 | 9.4 | 52.1 | |
| R Ulnar - ADM | 2.6 | 7.3 | |
| 7.2 | 6.9 | 57.6 | |
| R Tibialis-wrist-AHB | 5.9 | 2.3 | |
| 16.9 | 1.6 | 37.3 | |
| L Tibialis-AHB | 7.3 | 1.4 | |
| 17.5 | 0.6 | 39.2 | |
| L Peroneal - EDB | 3.4 | 1.5 | |
| 12.5 | 1.4 | 39.6 |
R:Sağ, L:Sol, APB:Abductor pollicis brevis, ADM:Abductor digiti minimi, AHB:Abductor hallucis, EDB:Ekstensor digitorum brevis. Values in bold type are abnormal results.
DISCUSSION
Wernicke’s encephalopathy is a neuropsychiatric syndrome that occurs as a result of thiamine deficiency. This condition, which can often be overlooked in the clinic, can lead to permanent neurological deficits and even death in some cases (3). The classic signs are oculomotor dysfunction, confusion and ataxia. However, these three signs are seen simultaneously in only 16% of patients (1). Heavy alcohol consumption, severe malnutrition, hyperemesis gravidarum, post-gastrectomy and total parenteral nutrition support (TPN) can lead to the Wernicke-Korsakoff syndrome, peripheral neuropathy, heart failure and lactic acidosis by causing thiamine deficiency (2). In a healthy adult, the thiamine reserve is only 30-50 mg. Therefore, any kind of malnutrition lasting for more than 3-4 weeks causes the depletion of vitamin stores (4).
The main clinical sign in Wernicke’s encephalopathy is changes in the mental state. These changes consist of cognitive disorders ranging from confusion, apathy, lack of memory and concentration and coma to death. This clinical table occurs as a result of the thalamic nuclei and mammillary body being affected (3). Horizontal nystagmus is the most common among ocular disorders. Decreased visual acuity, diplopia, conjugate gaze palsy resulting from paralysis of both lateral recti or other ocular muscles and lesions of the pontine tegmentum, VI. cranial nerve (CN) and III. CN nuclei, reduced pupillary reaction to light, retinal hemorrhages, papilledema, anisocoria and ptosis are other signs that can be observed (3). Gait impairment can cause anything ranging from a mild gait abnormality to ataxia so significant as to be unable to stand, in connection with cerebellar vermis and vestibular dysfunction (5).
For diagnosis, hyperintensities found typically in the thalamus, mammillary body and tectal and periaqueductal regions and atypically in the cerebellum, cranial nerve nuclei and cerebral cortex in the brain MRI scan T2 and FLAIR images are important findings (3). Blood thiamine concentration and erythrocyte transketolase activity can be of help for diagnosis but these parameters are not measured most of the time due to specificity and technical problems (6).
Our case was diagnosed with WE due to his history, examination and brain MRI findings and who benefited from IV thiamine infusion. In the early stage, a hyperintensity was detected in the cranial MRI scan around the aqueduct neighboring the third ventricle, in the superior cerebellar vermis and bilateral thalamus. In the follow-up after 3 months, the MRI findings were seen to have almost completely improved. The polyneuropathy manifestation in our patient was detected in the examination that was done due to the loss of strength that occurred in the lower extremities while he was being followed for WE. The manifestation of neuropathy was believed to develop acutely because the findings developed acutely and the clinical and electrophysiological findings regressed in the follow-up.
Whether associated with Wernicke’s encephalopathy or not, acute progressive polyneuropathy linked to alcohol consumption has been reported, although it is less common than chronic neuropathy (7). It is important to distinguish this acute axonal pathology from the axonal forms of the Guillain-Barre syndrome (2). Ethanol toxicity and vitamin deficiency are believed to play a role together in the pathogenesis of this clinical portrait that is similar to the Guillain-Barre syndrome, which develops due to chronic alcohol consumption (8). Low serum thiamine levels are also stated to accelerate the progression of polyneuropathy (9). Clinical history, examination findings, progression and cerebrospinal fluid (CSF) examination is important in the differential diagnosis of these clinical conditions. The fact that findings were predominant in the lower extremities in our case suggested possible manifestations of lumbosacral plexopathy or radiculopathy. No findings in favor or radiculopathy were observed in the imaging scans that were done. Normal sensory responses on the follow-up EMGs detracted from a diagnosis of plexopathy.
Looking at the literature, there are few case reports of concurrent acute polyneuropathy and WE (Table 4). In the study that Ishibashi et al. conducted on three patients who developed acute axonal polyneuropathy simultaneously with WE, the patients were given 100 mg/day of IV thiamine infusions and a significant improvement was observed in the nerve conduction studies of two patients after a month and a patient after two weeks (2). Due to improvements being observed in patients within in a short period like a month, it was stated that the process may be caused by decreased ATP due to decreased pyruvate dehydrogenase activity as a result of thiamine deficiency at a cellular level, which in turn leads to the decreased activity of Na-K-ATPase, which plays a role in peripheral nerve depolarization and axonal excitability (2). While significant improvement was observed in the 3rd and 6th month check-up examination of our patient, a partial improvement in his EMG findings was noted. In a case with severe acute axonal polyneuropathy accompanied by WE and concurrent autonomic dysfunction with a history of chronic alcoholism published by Lehman et al., it is stated that IV immunoglobulin treatment was applied in addition to IV thiamine infusion (7).
Table 4.
Wernicke’s encephalopathy and acute polyneuropathy associated with cases in the literature
| Age, gender | Application complaint | Background, habits | Neurological examination | First EMG findings | Treatment | Clinical course |
|---|---|---|---|---|---|---|
| 41 age, M (2) | Acute gastritis caused by nausea-vomiting, muscle weakness, numbness in the lower extremities, double vision. | Depression, prolonged malnutrition, nausea and vomiting due to acute gastritis. | Drowsiness, ophthalmoplegia, nystagmus, distal dominant muscle weakness and loss of sensation in the extremities, decreased DTR. | -CMAPs and SNAPs markedly decreased, nerve conduction velocity was normal. -Sympathetic skin reponses were no response. -Positive sharp waves, rarely large, long duration MUPs |
100 mg / day IV thiamine (for a month) | İmproved the clinical signs and laboratory findings at the end of first month |
| 44 age, M (2) | İmpairment of consciousness after developing symptoms of the common cold. | Taking 100 ml of alcohol a day over 20 years, gastric ulcer. | Drowsiness, nystagmus, distal dominant muscle weakness, decreased DTR. | -CMAPs and SNAPs markedly decreased, nerve conduction velocity was normal. | 100 mg / day IV thiamine (for a month) | İmproved clinical findings at the end of two weeks, and improved EMG findings after first month. |
| 58 age, M (2) | Mild dysesthesia in the lower extremities, frequent nausea and vomiting followed by 3 weeks after developing double vision, gait disturbance. | Subtotal gastrectomy for gastric cancer. | Ophthalmoplegia, nystagmus, mild muscle weakness in the lower extremities and decreased DTR, dominant distal sensory disturbances | - Nerve conduction was normal. | 100 mg / day IV thiamine | İmproved clinical findings, CMAP and SNAP amplitudes increased at the end of two weeks. |
| 43 age, F (8) | Gait disturbance, muscle weakness, numbness in the legs. | Obesity, drinking 1-2 bottles of wine per day. | Ophthalmoplegia, nystagmus, severe flaccid tetrapareses decreased DTR, hypoesthesia of the lower extremities and reduced vibration. | -CMAPs and SNAPs markedly decreased, nerve conduction velocity was normal. -common abnormal spontaneous activity in proximal and distal muscles. |
IVIG treatment in addition to IV thiamine | Died from sepsis respiratory failure on hospital day 8. |
F: Female, M: Male, CMAP: Compound motor action potential, SNAP: Sensory nerve action potential, MUP: Motor unit potential, IV: Intravenoz,
IVIG: Intravenoz immunoglobulin
In the treatment of WE, dextrose infusion should be avoided as soon as it is clinically suspected and IV thiamine infusion should be started. The recommended dose and route of administration is 200 mg three times a day for the first two days (500 mg three times a day for alcohol dependents) and 200 mg/day (500 mg/day for alcohol dependents) for the following five days, inside a 100 or 250 ml saline solution for 30 minutes in the form of an infusion. At the end of one week, a switch is made to a 100 mg/day oral maintenance dose (9).
In conclusion, WE and acute neuropathy that may occur in association should be kept in mind for patients with a history of alcohol consumption and malnutrition. Especially in these patients, a manifestation of polyneuropathy can be overlooked due to imbalance stemming from ataxia if a neurological examination is not done. It should be remembered that with early diagnosis and rapidly administered IV thiamine treatment, all symptoms can be reversible and that the development process of acute axonal polyneuropathy can be reduced
Footnotes
Informed Consent: Verbal consent was obtained from the patient.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - CS, UE; Design - CS, UE, AT; Supervision - OÖY, UE; Resource - CS, UE; Materials - CS, AT; Data Collection and/ or Processing - CS, OÖY, UE; Analysis and/or Interpretation - CS, AT; Literature Search - UE, CS; Writing - CS, UE; Critical Reviews - OÖY, AT.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Sechi G, Serra A. Wernicke's encephalopathy:new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- 2.Ishibashi S, Yokota T, Shiojiri T, Matunaga T, Tanaka H, Nishina K, Hirota H, Inaba A, Yamada M, Kanda T, Mizusawa H. Reversible acute axonal polyneuropathy associated with Wernicke-Korsakoff syndrome:impaired physiological nerve conduction due to thiamine deficiency? J Neurol Neurosurg Psychiatry. 2003;74:674–676. doi: 10.1136/jnnp.74.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manzo G, Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke's encephalopathy:a review. BioMed Res Int. 2014;2014:503596. doi: 10.1155/2014/503596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke's encephalopathy:review of the literature. AJR Am J Roentgenol. 2009;192:501–508. doi: 10.2214/AJR.07.3959. [DOI] [PubMed] [Google Scholar]
- 5.Victor M. The Wernicke-Korsakoff syndrome. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology 28, Part 2. Amsterdam, The Netherlands: North-Holland; 1976. pp. 243–270. [Google Scholar]
- 6.Dreyfus PM. Clinical application of blood transketolase determinations. New Engl J Med. 1962;267:596–598. doi: 10.1056/NEJM196209202671204. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann HC, Lindenberg R, Arendt G, Ploner M. Acute axonal neuropathy and Wernicke's encephalopathy. J Neurol. 2006;253:1516–1517. doi: 10.1007/s00415-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbulcke M, Janssens J. Acute axonal polyneuropathy in chronic alcoholism and malnutrition. Acta Neurol Belg. 1999;99:198–201. [PubMed] [Google Scholar]
- 9.Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]