Abstract
Introduction:
There is inconsistent evidence of interaction between childhood adversities and a serotonin transporter promoter polymorphism (5-HTTLPR) in depression. It is hypothesized that genetic sensitivity to stress could be more specific to recurrent major depressive disorder (MDD). The aim of the study is to replicate a recent study which provided preliminary evidence of interaction between severity of childhood maltreatment and the 5-HTTLPR polymorphism in recurrent MDD.
Methods:
Participants included a well-characterized clinical sample of 70 recurrent MDD cases and 67 never psychiatrically ill controls, aged 18 years or over. Socio-demographic and clinical information form, Composite International Diagnostic Interview (CIDI), Childhood Trauma Questionnaire (CTQ), Beck Depression Inventory (BDI) were applied to both groups, along with genotyping.
Results:
There was no interaction between childhood maltreatment and the 5-HTTLPR in relation to recurrent MDD. All forms of childhood maltreatment were reported as more severe by cases than controls, and there was an independent association between maltreatment and recurrent MDD.
Conclusion:
The path forward to detect genetic risk loci for depression remains challenging. Taking childhood maltreatment history into account could lead to a richer understanding of differences in biological correlates, genetic underpinnings, and outcomes.
Keywords: Recurrent depression, childhood maltreatment, 5-HTTLPR
INTRODUCTION
There has been extensive evidence for the negative impact of adverse childhood experiences on mental health in adulthood (1). Epidemiological studies indicate that children exposed to early adverse experiences such as childhood maltreatment are at increased risk for depression (2). However, not everyone who encounter adversities in childhood succumb to their negative effect, there are substantial individual differences in outcome (3). Therefore, it has been hypothesized that gene by environment (GxE) interactions are of importance in the etiology of major depressive disorder (MDD).
SLC6A4 gene which regulates the uptake of serotonin from the synaptic cleft (4) contains a sequence with varying numbers of repeats which have different transcriptional efficiencies. Although there may be as many as 10 alleles in humans, by far the most common are what are generally termed short (S) and long (L) alleles. Research in GxE interactions has culminated in the demonstration that S allele confers risk for the development of MDD in the presence of adverse life events (5). This potential etiological role of an interaction between stressful life experiences and 5-HTTLPR in MDD has sparked further debate and an array of inconsistent findings (6, 7). Interaction between childhood maltreatment and S allele of 5-HTTLPR has been shown to be stronger amongst individuals suffering from persistent MDD rather than onsets of new episodes (8, 9). Recently, Fisher et al. (10) provided preliminary evidence of interaction between childhood sexual abuse and the 5-HTTLPR in relation to recurrent MDD.
The aim of this study was to replicate Fisher et al.’s study (10) and investigate the interaction between specific forms of childhood maltreatment and the 5-HTTLPR in a clinical sample.
METHODS
Participants
Out of the 448 patients diagnosed with MDD at Başkent University Faculty of Medicine, Department of Psychiatry, 84 patients were included in the study. This study was approved by Başkent University Ethical Board for Clinical Research (KA. 14.201), and all participants provided written informed consent.
After obtaining a detailed medical and family history, patients who had psychotic features (n=23), serious or unstable medical illness (n=46), a history or a current diagnosis of a neurological illness (n=14), a DSM-IV (11)/ICD-10 (12) diagnosis other than recurrent MDD (n=217), clinical and laboratory evidence of thyroid dysfunction (n=24), anemia, vitamin B12 and folic acid deficiency (n=40) were excluded to achieve a homogenous study group. Blood samples of 5 patients were not available, genotyping failed for 3 participants, and 6 patients didn’t complete the assessment tools. As a result, 70 recurrent MDD patients were recruited (56 female, 14 male; mean age 37.76±8.46 years). 67 healthy individuals matched for age, sex and level of education (52 female, 15 male; mean age 39.79±9.39 years) who were responders to verbal announcement were enrolled in the study under identical conditions to those of the patients. None of the controls had a personal history of a psychiatric disorder and/or a neurological illness. All participants were Caucasian, and aged 18 years or over.
Assessment
Socio-demographic and clinical information form: In the form that was designed by the researchers, there were questions regarding age, sex, educational, marital and working status, medical illness and history of mental disorder in the family.
Composite International Diagnostic Interview (CIDI): Diagnosis of recurrent MDD and absence of any other mental disorder was confirmed by using CIDI. CIDI is a standardized, fully-structured interview developed by the World Health Organization (WHO) that provides current and life-time ICD-10 and DSM-IV diagnoses (13). It is widely used as a research diagnostic interview and has been adapted to Turkish population by Kılıç and Göğüş (14).
Childhood Trauma Questionnaire (CTQ): CTQ was developed by Bernstein et al. (15) for screening the experiences of childhood maltreatment before age 18. It is a Likert type self-report questionnaire which is widely used in clinical and general population samples. Aslan and Alparslan (16) adapted CTQ into Turkish and reported that Turkish version consisted of 40-items with a 3-factor structure: Physical abuse (PA), emotional abuse and emotional neglect (EA-EN) and sexual abuse (SA).
Beck Depression Inventory (BDI): BDI was used to measure the participants’ severity of depressive symptomatology. It is a Likert type self-report inventory developed by Beck et al. (17) and consists of 21 questions about how the subject has been feeling in the last week. BDI was adapted for the Turkish population by Hisli (18).
Genotyping
DNA was extracted from a 10 ml sample of peripheral blood collected from all participants and using NucleoSpin DNA isolation kit (Macherey-Nagel GmbH, Germany). Polymerase chain reaction (PCR) was performed on samples to amplify the 44 base-pair repeat sequence on the promoter region of the SLC6A4 gene (19). Previously published primer sequences, 5’-GGCGTTGCCGCTCTGAATGC-3’(Forward) and 5’-GAGGGACTGAGCTGGACAACCA-3’(Reverse) were used for amplification (20). The products were run on 3% agarose gel electrophoresis to determine L and S polymorphisms and allele frequencies.
Statistical Analysis
Descriptive analyses were presented in mean ± standard deviation for continuous, and in n (%) for categorical variables. Inferential analyses were performed using t-test for continuous variables and a chi-square test for categorical variables.
Main effects of the 5-HTTLPR and childhood trauma were tested with a logistic regression analysis. All analyses were adjusted for age and sex. We first explored the main effects of the CTQ score and genotype on recurrent MDD status using logistic regression analysis. Then, the effects of the 5-HTTLPR and childhood trauma on presence of recurrent MDD were tested with statistical interaction model. Statistical analyses were performed for additive, dominant and recessive models. All analyses were performed with the IBM SPSS 21.0. A p-value threshold of 0.05 was used for statistical significance.
RESULTS
There was no difference in terms of age, gender, marital status and education between groups (Table 1). In control group, more participants had regular income when compared to patients (p=0.003). In recurrent MDD group, more participants had medical illness (p<0.01), and family history of mental disorder (p<0.001). The BDI score (p<0.001), total CTQ score (p<0.001) and subdomain scores (PA p<0.001; EA-EN p<0.001; SA p=0.003) were higher in recurrent MDD group compared to controls.
Table 1.
Group Comparisons for Sociodemographics, BDI and CTQ Scores, and Genotypes
| Recurrent MDD (n=70) | Controls (n=67) | Statistical Analysis | ||
|---|---|---|---|---|
| χ2 (df)/t (df) | P | |||
| Age, mean ± SD | 37.76±8.46 | 39.79±9.40 | -1.33 (135) | 0.90 |
| Sex, n (%) | 0.12 (1) | 0.73 | ||
| Women | 56 (80%) | 52 (77.6%) | ||
| Men | 14 (20%) | 15 (22.4%) | ||
| Marital Status, n (%) | 2.60 (1) | 0.11 | ||
| Married/partnered | 48 (68.6%) | 54 (80.6%) | ||
| Single | 22 (31.4%) | 13 (19.4%) | ||
| Education, n (%) | 2.57 (1) | 0.11 | ||
| Primary or secondary | 29 (41.4%) | 19 (28.4%) | ||
| Tertiary | 41 (58.6%) | 48 (71.6%) | ||
| Working status, n (%) | 8.57 (1) | 0.003 | ||
| Regular income | 50 (71.4%) | 61 (91%) | ||
| No regular income | 20 (28.6%) | 6 (9%) | ||
| Medical illness, n (%) | ||||
| Present | 25 (35.7%) | 10 (14.9%) | 7.78 (1) | <0.01 |
| Absent | 45 (64.3%) | 57 (85.1%) | ||
| Family history, n (%) | ||||
| Present | 38 (54.3%) | 5 (7.5%) | 25.409 (1) | <0.001 |
| Absent | 32 (45.7%) | 62 (92.5%) | 24.72 (87.54) | <0.001 |
| BDI score, mean ± SD | 28.70±8.29 | 2.58±3.00 | ||
| Total CTQ score, mean ± SD | 79.94±24.75 | 53.64±9.21 | 8.31 (88.50) | <0.001 |
| PA score, mean ± SD | 28.49±10.51 | 20.39±3.94 | 6.76 (88.72) | <0.001 |
| EA-EN score, mean ± SD | 44.54±15.59 | 28.12±6.96 | 8.02 (96.40) | <0.001 |
| SA score, mean ± SD | 5.91±1.81 | 5.19±0.68 | 3.11 (88.90) | 0.003 |
| Genotype, n (%) | ||||
| LL | 16 (22.9%) | 17 (25.4%) | ||
| SL | 33 (47.1%) | 29 (43.3%) | ||
| SS | 21 (30%) | 21 (31.3%) | ||
MDD, major depressive disorder; BDI, Beck depression inventory; CTQ, childhood trauma questionnaire; PA, physical abuse, EA-EN, emotional abuse-emotional neglect; SA, sexual abuse; SD, standard deviation
The 5-HTTLPR genotypes in patients with recurrent MDD (LL=16 (22.9%), SL=33 (47.1%), SS=21 (30%) were in Hardy-Weinberg equilibrium (c2=0.19, p=0.66). In controls, the 5-HTTLPR genotypes (LL=17 (25.4%), SL=29 (43.3%), SS=21 (31.3%) were also in Hardy-Weinberg equilibrium (c2=1.15, p=0.28). We analyzed three genetic models: additive (LL=0, SL=1, SS=2), dominant (LL=0, SL=1, SS=1) and recessive (LL=0, SL=0, SS=1).
There was a significant independent association between CTQ scores and recurrent MDD (p<0.001) even after adjustment for age and gender (Table 2). On the other hand, we detected no genetic main effect. The total CTQ score for LL, LS and SS genotypes by recurrent MDD status was given in Figure 1 indicating a possible interaction. As seen in the figure, total CTQ scores were higher in participants with recurrent MDD regardless of the genotype. However, participants with an S allele had a steeper slope when compared to participants without an S allele. Statistical interaction was tested for additive model only as this model is considered best model to test biological interaction (21). There was not a statistically significant interaction detected in regression analysis (Table 2).
Table 2.
Main effects and interaction models of the 5-HTTLPR and CTQ scores on presence of recurrent MDD
| OR | 95% CI | P | |
|---|---|---|---|
| CTQ total | 1.10 | 1.06–1.15 | <0.001 |
| Additive model | |||
| 5-HTTLPR | 1.03 | 0.65–1.63 | 0.90 |
| 5-HTTLPR x Trauma | 1.91 | 0.59–6.23 | 0.28 |
| Dominant model | |||
| 5-HTTLPR | 1.15 | 0.52–2.53 | 0.73 |
| Recessive model | |||
| 5-HTTLPR | 0.95 | 0.46–1.98 | 0.89 |
All analyses were adjusted for age and sex.
CTQ, childhood trauma questionnaire; MDD, major depressive disorder; OR, odds ratio; CI, confidence interval.
Figure 1.
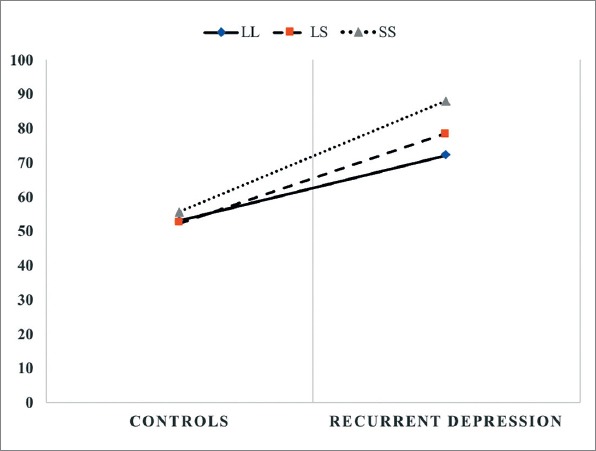
CTQ scores for LL, LS, SS genotypes by depression status.
DISCUSSION
In this study which was conducted in a clinical sample, we found no interaction between childhood maltreatment and 5-HTTLPR polymorphism in relation to recurrent MDD.
There are several studies reporting carriers of either one or two copies of the S allele of 5-HTTLPR were more likely to develop MDD after encountering adversities in childhood (22, 23). These studies also reported that carriers of the S allele have more depressive symptoms in response to childhood maltreatment than were individuals homozygous for the L allele. Furthermore, there was evidence of a dose-response relationship that is the risk of depression is higher amongst those with two copies of the S allele compared to individuals with only one copy in the presence of stress. But inconsistent results like ours have also been reported (6).
It is hypothesized that genetic sensitivity to stress could be more specific to recurrent MDD which is more heritable (10) and is more strongly associated with childhood maltreatment (24). Consequently, we designed the study specifically for investigating GxE interaction for recurrent MDD. Furthermore, following recommendations of Duncan et al. (25) we tried to replicate Fisher et al.’s study (10) in a well-characterized clinical sample by taking into account variables such as psychiatric comorbidity, serious or unstable medical illness, a history or a current diagnosis of neurological illness, thyroid dysfunction, anemia, vitamin B12 and folic acid deficiency, all of which have a potential to yield an unreliable phenotype. For the same reason, in addition to clinical evaluation we applied CIDI to all participants in order to increase the diagnostic validity and reliability of recurrent MDD in cases, and the lifetime absence of psychiatric disorder in controls. Also, we conducted statistical tests for all three genetic models. Still, we failed to demonstrate the interaction between the serotonin transporter gene polymorphism and specific forms of childhood maltreatment in the causation of recurrent MDD, despite a strong indication of possible interaction (Figure 1).
There are several major limitations of this study. First, our sample size was small which decreases statistical power. Although smaller samples allow more precise measures, they are underpowered to establish robust interaction (26). Large samples on the other hand often lack the depth necessary to capture data on environmental or phenotype measures. Second, depression is a phenotypically complex disorder, and it is possible to meet DSM-IV-TR or DSM-5 diagnostic criteria for a MDD episode through at least 227 different symptom combinations (27). Third, there might be confounding factors which we failed to take into consideration. For instance, because we conducted this study at a university out-patient clinic there is a possibility that underprivileged individuals with severe childhood maltreatment and severe depressive symptomatology were unable to have access.
Nevertheless, the latest collaborative meta-analysis reported no evidence of a strong interaction between childhood maltreatment and 5-HTTLPR genotype contributing to the development of depression (28). To determine the magnitude of the interaction and the conditions under which it might be observed, authors performed new analyses on 31 data sets containing 38802 European ancestry subjects genotyped for 5-HTTLPR and assessed for depression and childhood maltreatment, and meta-analyzed the results. Based on their findings Culverhouse et al. (28) conclude that if an interaction exists in which the S allele of 5-HTTLPR increases risk of depression only in stressed individuals, then it is not a broadly generalizable effect, but must be of modest effect size and only observable in limited situations.
Our finding for the main effect of childhood maltreatment in recurrent MDD is consistent with the literature. Some of the strongest evidence for an association between exposure to childhood maltreatment and the development of MDD is found in the Adverse Childhood Experiences (ACE) study (29) which showed that risk for depression increases in a graded dose-dependent fashion with the number of maltreatment-related adverse childhood experiences. Recent epidemiological studies also provide findings that maltreated individuals are twice as likely as those without a history of childhood maltreatment to develop recurrent depression (30).
We found that family history of mental disorder was higher in the patient group. This result is consistent with the literature reporting that presence of positive family history for psychiatric conditions was associated with recurrent MDD (31). Medical illness was also higher in the recurrent MDD group compared to controls.
Cross-sectional design of our study is a salient limitation since it obstructs setting a cause and effect relation. Another limitation is that we relied on retrospective self-reports of childhood maltreatment. The potential influence that mood-congruent memory biases could exert on the salience of childhood memories is a concerning issue. Depressed adults might remember more negative versus positive childhood memories, whereas mentally well adults might show the opposite trend. Such memory biases could confound or inflate the association between childhood maltreatment and adult psychopathology, which raises concerns over the validity of findings (32). However, results of the latest study comparing prospective informant-reports and retrospective self-reports notify that retrospective self-reports are potentially a more useful indicator of clinical presentation (33).
In conclusion, our lack of replication coincides with findings of the latest collaborative meta-analysis in GxE interaction research (28). Although the path forward to detect genetic risk loci for depression remains challenging, what is certain is that an extensive understanding of the etiology of depression is needed. Childhood maltreatment is also a complex etiological agent that appears to vary in impact coupled with a number of susceptibility and resilience co-factors. Still, taking maltreatment history into account could lead to a richer understanding of differences in clinical presentation, genetic underpinnings, biological correlates, and outcomes.
Footnotes
Ethics Committee Approval: Ethical approval was obtained from the Ethics Committee of Başkent University (Decision No. 2014/KA. 14.201)
Informed Consent: Written informed consent form was obtained from all patients.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept– GÖ, AE, HTY; Design– GÖ, AE, HTY; Supervision– GÖ, FŞ; Resource– GÖ, FŞ; Materials– YT, HTY; Data Collection and/or Processing– HTY, YT; Analysis and/or Interpretation– AE, ND, GÖ, FŞ; Literature Search– GÖ, HTY; Writing– GÖ, ND; Critical Reviews– FŞ, AE, ND.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The present study was supported by Başkent University with the project number KA. 14.201.
REFERENCES
- 1.Haliburn J. The links between early childhood trauma and major mental illness:Psychiatry's response? Aust N Z J Psych. 2014;48:580–581. doi: 10.1177/0004867414527178. [DOI] [PubMed] [Google Scholar]
- 2.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 3.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment:the case of serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 5.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression:a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of depression. Mol Psych. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 7.Uher R, Caspi A, Houts R, Sugden K, Williams B, Poulton R, Moffitt TE. Serotonin gene moderates childhood maltreatment's effects on persistent but not single-episode depression:replications and implications for resolving inconsistent results. J Affect Disord. 2011;135:56–65. doi: 10.1016/j.jad.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown GW, Ban M, Craig T, Harris TO, Herbert J, Uher R. Serotonin transporter length polymorphism, childhood maltreatment, and chronic depression:A specific gene-environment interaction. Depress Anxiety. 2012;30:5–13. doi: 10.1002/da.21982. [DOI] [PubMed] [Google Scholar]
- 9.Fisher HL, Cohen-Woods S, Hosang GM, Korszun A, Owen M, Craddock N, Craig IW, Farmer AE, McGuffin P, Uher R. Interaction between specific forms of childhood maltreatment and the serotonin transporter gene (5-HTT) in recurrent depressive disorder. J Affect Disord. 2013;145:136–141. doi: 10.1016/j.jad.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition (DSM-IV) Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 11.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 12.Andrews G, Peters L. The psychometric properties of the Composite International Diagnostic Interview. Soc Psychiatry Psychiatric Epidemiol. 1998;33:80–88. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- 13.Kılıç C, Göğüş A. Composite International Diagnostic Interview:CIDI (Turkish version) Ankara: The Collaboration with World Health Organization Centre Release; 1997. [Google Scholar]
- 14.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of childhood abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 15.Aslan SH, Alparslan ZN. The reliability, validity and factor structure of the Childhood Traumatic Questionnaire among a group of university students. Turk J Psychiatry. 1999;10:275–285. [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psych. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Hisli N. A study on the validity of Beck Depression Inventory. Turk Psikol Derg. 1988;22:118–126. [Google Scholar]
- 18.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- 19.Manor I, Eisenberg J, Tyano S, Sever Y, Cohen H, Ebstein RP, Kotler M. Family-based association study of the serotonin transporter promotor region polymorphism (5-HTTLPR) in attention deficit hyperactivity disorder. Am J Med Genet. 2001;105:91–95. [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression:Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 22.Harkness KL, Strauss J, Bagby R, Stewart JG, Larocque C, Mazurka R, Ravindran A, Wynn-Edwards KE, Rector NA, Kennedy J. Interactions between childhood maltreatment and brain-derived neurotrophic factor and serotonin transporter polymorphisms on depression symptoms. Psychiatry Res. 2015;229:609–612. doi: 10.1016/j.psychres.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 23.Wiersma JE, Hovens JG, van Oppen P, Giltay EJ, van Schaik DJ, Beekman AT, Penninx BW. The importance of childhood trauma and childhood life events for chronicity of depression in adults. J Clin Psychiatry. 2009;70:983–989. doi: 10.4088/jcp.08m04521. [DOI] [PubMed] [Google Scholar]
- 24.Duncan LE, Pollastri AR, Smoller JW. Mind the gap:Why many geneticists and psychological scientists have discrepant views about gene-environment interaction (GxE) research. Am Psychol. 2014;69:249–268. doi: 10.1037/a0036320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013;8:651–662. doi: 10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- 26.Dunn EC, Brown RC, Dai Y, Rosand J, Nugent NR, Amstadter AB, Smoller JW. Genetic determinants of depression:recent findings and future directions. Harv Rev Psychiatry. 2015;23:1–18. doi: 10.1097/HRP.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Culverhouse RC, Saccone NL, Horton AC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T, Burmeister M, Cohen-Woods S, Etain B, Fisher HL, Goldman N, Guillaume S, Horwood J, Juhasz G, Lester KJ, Mandelli L, Middeldorp CM, Olié E, Villafuerte S, Air TM, Araya R, Bowes L, Burns R, Byrne EM, Coffey C, Coventry WL, Gawronski KAB, Glei D, Hatzimanolis A, Hottenga JJ, Jaussent I, Jawahar C, Jennen-Steinmetz C, Kramer JR, Lajnef M, Little K, Zu Schwabedissen HM, Nauck M, Nederhof E, Petschner P, Peyrot WJ, Schwahn C, Sinnamon G, Stacey D, Tian Y, Toben C, Van der Auwera S, Wainwright N, Wang JC, Willemsen G, Anderson IM, Arolt V, Åslund C, Bagdy G, Baune BT, Bellivier F, Boomsma DI, Courtet P, Dannlowski U, de Geus EJC, Deakin JFW, Easteal S, Eley T, Fergusson DM, Goate AM, Gonda X, Grabe HJ, Holzman C, Johnson EO, Kennedy M, Laucht M, Martin NG, Munafò MR, Nilsson KW, Oldehinkel AJ, Olsson CA, Ormel J, Otte C, Patton GC, Penninx BWJH, Ritchie K, Sarchiapone M, Scheid JM, Serretti A, Smit JH, Stefanis NC, Surtees PG, Völzke H, Weinstein M, Whooley M, Nurnberger JI, Jr, Breslau N, Bierut LJ. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol Psychiatry. 2018;23:133–142. doi: 10.1038/mp.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 29.Suija K, Aluoja A, Kalda R, Maaroos HI. Factors associated with recurrent depression:a prospective study in family practice. Fam Prac. 2011;28:22–28. doi: 10.1093/fampra/cmq076. [DOI] [PubMed] [Google Scholar]
- 30.Serafini G, Gonda X, Monacelli F, Pardini M, Pompili M, Rihmer Z, Amore M. Possible predictors of age at illness onset and illness duration in a cohort study comparing younger adults and older major affective patients. J Affect Disord. 2018;225:691–701. doi: 10.1016/j.jad.2017.08.077. [DOI] [PubMed] [Google Scholar]
- 31.Susser E, Widom CS. Still searching for lost truths about the bitter sorrows of childhood. Schizophr Bull. 2012;38:672–675. doi: 10.1093/schbul/sbs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newbury JB, Arseneault L, Moffitt TE, Caspi A, Danese A, Baldwin JR, Fisher HL. Measuring childhood maltreatment to predict early-adult psychopathology:Comparison of prospective informant-reports and retrospective self-reports. J Psychiatr Res. 2018;96:57–64. doi: 10.1016/j.jpsychires.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


